Abstract
Objective:
This systematic review aimed to investigate the effects of interventions intended for retraining leg somatosensory function on somatosensory impairment, and secondary outcomes of balance and gait, after stroke.
Data sources:
Databases searched from inception to 16 January 2019 included Cochrane Library, PubMed, MEDLINE, CINAHL, EMBASE, PEDro, PsycINFO, and Scopus. Reference lists of relevant publications were also manually searched.
Review methods:
All types of quantitative studies incorporating interventions that intended to improve somatosensory function in the leg post stroke were retrieved. The Quality Assessment Tool for Quantitative Studies was used for quality appraisal. Standardised mean differences were calculated and meta-analyses were performed using preconstructed Microsoft Excel spreadsheets.
Results:
The search yielded 16 studies, comprising 430 participants, using a diverse range of interventions. In total, 10 of the included studies were rated weak in quality, 6 were rated moderate, and none was rated strong. Study quality was predominantly affected by high risk of selection bias, lack of blinding, and the use of somatosensory measures that have not been psychometrically evaluated. A significant heterogeneous positive summary effect size (SES) was found for somatosensory outcomes (SES: 0.52; 95% confidence interval (CI): 0.04 to 1.01; I2 = 74.48%), which included joint position sense, light touch, and two-point discrimination. There was also a significant heterogeneous positive SES for Berg Balance Scale scores (SES: 0.62; 95% CI: 0.10 to 1.14; I2 = 59.05%). Gait SES, mainly of gait velocity, was not significant.
Conclusion:
This review suggests that interventions used for retraining leg somatosensory impairment after stroke significantly improved somatosensory function and balance but not gait.
Keywords: Systematic review, somatosensory, retraining, lower limb, stroke
Introduction
Somatosensory impairment is common after stroke, occurring in up to 89% of stroke survivors.1 Proprioception and tactile somatosensation are more impaired in the leg than in the arm post stroke,2 with the frequency increasing with increasing level of weakness and stroke severity.2,3 Leg somatosensory impairment also has a significant impact on independence in daily activities3 and activity participation in stroke survivors,4 as well as predicts longer hospital stays and lower frequency of home discharges.5
Leg somatosensory impairment negatively influences balance and gait. Post-stroke plantar tactile deficits correlate with lower balance scores and greater postural sway in standing.6 Tactile and proprioceptive feedback provide critical information about weight borne through the limb.7 Accordingly, tactile and proprioceptive somatosensory deficits may hinder paretic limb load detection ability, potentially leading to reduced weight-bearing and contributing to balance impairment and falls post stroke.8 Indeed, stroke survivors with somatosensory impairment have a higher falls incidence compared to those without somatosensory impairment.3 In addition to reduced balance, impaired load detection may also contribute to gait asymmetry, particularly in the push-off phase.8 In addition, leg proprioception influences variance in stride length, gait velocity,9 and walking endurance in stroke survivors.10 In fact, leg somatosensory impairment has been shown to be the third most important independent factor for reduced gait velocity in stroke survivors.11
Two systematic reviews have previously investigated the effects of interventions for retraining somatosensory function after stroke.12,13 In the first review, published more than a decade ago, only four of the 14 included studies targeted the leg,12 while the second only included studies of the arm.13 Nevertheless, both reviews reported that there were insufficient data to determine the effectiveness of these interventions. A third systematic review evaluating the effectiveness of proprioceptive training14 only included 16 studies with stroke-specific populations, of which only two specifically addressed the leg. From these three reviews, the effects of interventions for post-stroke leg somatosensory impairment remain unclear. In addition, the first review12 was critiqued for including studies with participants without somatosensory impairment, and that did not report somatosensory outcomes.15 Therefore, a targeted systematic review, addressing the limitations of previous reviews, is required to elucidate the effects of interventions for post-stroke leg somatosensory impairment.
It is of interest to clinicians and researchers to evaluate the effects of leg somatosensory retraining on factors that may ultimately influence activity and participation, as this could change practice. Therefore, this systematic review aimed to examine the effects of post-stroke leg somatosensory retraining on somatosensory impairment, balance, gait, motor impairment, and leg function.
Methods
A protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews16 (registration no. CRD42017058993) prior to commencement of this systematic review. The PRISMA guidelines17 were utilised in the process and reporting of this review.
For the purpose of this review, the authors defined somatosensory function as the ability to detect, discriminate, and recognise body sensations.18 Somatosensory modalities affected by stroke that have been previously reported were considered, including detection or localisation of tactile stimuli, proprioception or kinaesthesia, stereognosis or object recognition, pressure or weight discrimination, detection of vibration, texture discrimination, and two-point discrimination.3,13,18–21 Retraining of somatosensory function was defined as any interventions that addressed the remediation of the above-mentioned somatosensory modalities. Intervention methods included elements of education; repetitive practice and feedback in detecting, localising, discriminating, or recognising different sensory stimuli, pressure, or objects; proprioceptive training; and somatosensory stimulation.12,22,23
Electronic databases including Cochrane Library, PubMed, MEDLINE, CINAHL, EMBASE, PEDro, PsycINFO, and Scopus were searched to identify relevant publications, from inception to 16 January 2019. The search strategy (Supplemental Table S1) was developed in collaboration with a librarian by breaking down the review question into components: population, interventions, comparators, outcomes, and study design (PICOS).24 Identification of key search terms was followed, using synonyms and variants of the search terms. The search strategy was trialled on several databases and adjusted accordingly to maximise the sensitivity of the search. Two reviewers (F.S.F.C., S.K.) independently screened titles and abstracts of the studies yielded from the searches to assess for eligibility. Full-text publications of potentially eligible studies were retrieved and further evaluated by the same two reviewers. In addition, reference lists of relevant publications, including available systematic reviews, the included studies, and narrative reviews, were manually searched for eligible articles.
Literature search was restricted to humans and adults (19 years and above), and only studies published in English were included. There were no restrictions to publication date and study setting. Studies were included if participants had leg somatosensory impairment following a stroke, with no restrictions to the stage (acute, subacute, or chronic), category (ischaemic or haemorrhagic), or anatomical location of stroke. All types of quantitative studies incorporating interventions that aimed to improve leg somatosensory function after stroke were included. Studies that did not measure somatosensory impairment or employed somatosensory stimulation that produced muscle contraction were excluded, as muscle contraction could have been a confounding factor. Other reasons for exclusion were studies evaluating assessment tools, observational studies not investigating outcome of interventions, descriptive studies, expert opinions, qualitative studies, systematic reviews, conference abstracts, and unpublished studies.
The primary outcome was somatosensory impairment. Secondary outcomes were balance, gait, motor impairment, and leg function. Any measure of somatosensory impairment was considered, including modality-specific measures (e.g. Semmes–Weinstein monofilaments),25 global measures of sensation (e.g. Nottingham Sensory Assessment),26 and sensory subscales of impairment-based measures (e.g. Fugl-Meyer Assessment (FMA)).27
Data were extracted by one author (F.S.F.C.) using a purpose-designed spreadsheet. A second author (S.K.) checked the data for accuracy. The following data were extracted from each study: study information (author(s), year of publication, location of study, study design), participant baseline information (demographics and characteristics), details of training intervention and dosage, details of control conditions (if any), follow-up period (if any), adverse effects, primary and secondary outcomes, and study results. Missing information required for data analysis was requested from the study authors.
Each included study was assessed for quality using the Quality Assessment Tool for Quantitative Studies,28 which is valid and reliable.29 Two reviewers (F.C.F.S., S.K.) assessed the quality of the articles independently, and discrepancies were discussed until consensus was reached. The Quality Assessment Tool for Quantitative Studies Dictionary30 was utilised to guide ratings. Assessment components were selection bias, study design, confounders, blinding, data collection methods, withdrawals and drop-outs, intervention integrity, and analyses. All components except intervention integrity and analyses were given a rating of strong, moderate, or weak. A global rating was awarded based on ratings of the six components – strong for no weak ratings, moderate for one weak rating, and weak for two or more weak ratings.
Descriptive analyses of the included studies were summarized. Effect sizes, pooled standard deviations (SDs), and P-values (two-tailed) of controlled clinical trials were calculated using a preconstructed Microsoft Excel spreadsheet called Effect Size Calculator.31 Standardised mean difference (SMD) was used as the effect size to enable analysis of similar outcome measures with different scales.32 Calculations for each study were based on post-intervention outcomes, at the latest time points, as recommended in the Cochrane handbook for systematic reviews of interventions.33 Effect size bias was corrected using Hedges’ g,34 from which the 95% confidence intervals (CIs) were derived. The null hypothesis was rejected if the P-value was less than 0.05. Heterogeneity between studies was assessed by calculating the I2 statistic35 using another preconstructed Microsoft Excel spreadsheet.36 An I2 value greater than 50% was considered of substantial heterogeneity.35 A meta-analysis was conducted by pooling the Hedges’ g values to calculate the summary effect size (SES).24 A random-effects model of meta-analysis was applied as it was expected that heterogeneity between studies would be relatively high. Subgroup analyses were conducted for studies using similar outcome measures. In studies that had more than one outcome measure using the same assessment tool within the same sample (e.g. light touch measured at multiple sites of the limb), a hierarchy of the preferred measure was set up a priori and only the SMD based on the measure highest on the list was calculated.37 A narrative summary was provided for data not statistically analysed (e.g. studies without a control group or used a paired design, data not available, discrete data).
Results
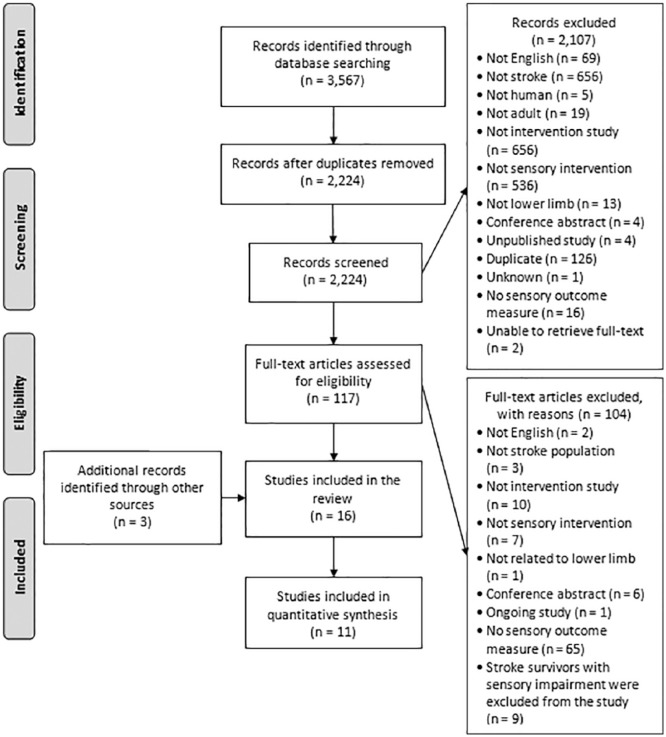
A flow diagram of the study selection process is presented in Figure 1. A total of 16 studies were included at the end of the selection process.
Figure 1.
PRISMA flow diagram.17
Description of included studies
Characteristics of the 16 included studies are displayed in Table 1. There were a total of 430 participants ranging in age from 18 to 82 years. Time since stroke ranged from seven days38 to nearly 16 years.39 Eight studies were set in inpatient rehabilitation,22,38,40–45 and the included studies were executed in nine different countries.
Table 1.
Characteristics of included studies.
| Primary author (year) | Study design and country/setting | Participants | Intervention and dosage | Control and dosage | Outcome measures | Global quality rating |
|---|---|---|---|---|---|---|
| Aruin (2012)46 | RCT; USA/NR | • n = 18 (9 exp/9 con) • Age = 35–75 years • Time since stroke = 1.1–14.1 years |
Compelled body weight shift (0.6 cm shoe lift in unaffected foot) + CPT All days, 6 weeks |
CPT 1 hour, 1×/week |
• FMA-LE • BBS • %BW on affected side • 10MWT gait velocity Follow-up = 3 months |
Weak |
| Cordo (2009)39 | Pretest–posttest; USA/community | • n = 12a
• Age = 38–75 years • Time since stroke = 1.9–15.6 years |
Assisted Movement with Enhanced Sensation robotic treatment (cycled at 5°/second through ±17.5°) + electromyography feedback on affected ankle 30 minutes/day, 6 months |
NA | • Days to 90% recovery of JPS and ankle strength • %BW on affected side • Gait velocity, cadence, and stride length Follow-up = 6 months |
Weak |
| Da Silva Ribeiro (2015)51 | RCT; Brazil/NR | • n = 30 (15 exp/15 con) • Age = 18–60 years • Time since stroke = mean 42.1 months (exp), 60.4 months (con) |
Nintendo Wii virtual rehab 60 minutes, 2×/week, 2 months |
CPT 60 minutes, 2×/week, 2 months |
• FMA-LE (sensation, balance, leg motor, and leg coordination subscores) No follow-up |
Moderate |
| Han (2013)40 | CCT; Korea/inpatient rehab | • n = 62 (31 exp/31 con) • Mean age = 56.1 years (exp), 56.6 years (con) • Time since stroke = mean 15.2 months (exp), 16.1 months (con) |
Unstable surface (proprioceptive sense) exercise underwater 40 minutes, 3×/week, 6 weeks |
Unstable surface exercise on land 40 minutes, 3×/week, 6 weeks | • JPS error (Biodex electrogoniometer) • BBS • Postural sway area EO and EC No follow-up |
Weak |
| Hillier (2006)23 | Single-case, repeated measures; Australia/NR | • n = 3 • Age = 63–73 years • Time since stroke = >2 years |
Education, detection, localisation, discrimination, recognition, and proprioception training on affected foot 45 minutes/session 3×/week, 2 weeks |
NA | • SWM light touch • DPT • Postural sway path length No follow-up |
Weak |
| Huzmeli (2017)41 | CCT; Turkey/inpatient rehab | • n = 26 (13 exp/13 con) • Age = 45–65 years • Time since stroke = mean 40.2 months (exp), 20.1 months (con) |
Proprioception, localisation, vibration, pressure discrimination, and TENS (parameters not described) on affected posterior thigh + neurodevelopmental rehab 45 minutes/session 10 sessions, 2 weeks |
Neurodevelopmental rehab 45 minutes/session 10 sessions, 2 weeks |
• SWM light touch • 2-point discrimination • DPT • Heat/cold senses • BBS • FRT • Barthel Index Follow-up = 2 weeks |
Weak |
| Kwon (2013)52 | RCT; Korea/NR | • n = 45 • (RPM 15/RAM 15/Con 15) •Mean age = 58.7 years (RPM), 61.2 years (RAM), 63.3 years (con) • Time since stroke = mean 10.1 months (RPM), 10.6 months (RAM), 10.9 months (con) |
RPM or RAM exercises on affected leg; angle speed 120°/second; 10°–100° 15 minutes in total, 3 sets of 60 repetitions |
No exercise | • Knee JPS error – passive angle repositioning Knee JPS error – active angle repositioning (Biodex electrogoniometer) No follow-up |
Weak |
| Lee (2015)42 | RCT; Korea/inpatient rehab | • n = 36 (18 exp/18 con) • Age = <65 years (n = 28), ≥65 years (n = 8) • Time since stroke = mean 11.5 months (exp), 11.6 months (con) |
Motor imagery (5 minutes) + proprioceptive training (25 minutes) 30 minutes/session 5×/week, 8 weeks |
Proprioceptive training (30 minutes) 30 minutes/session 5×/week, 8 weeks |
• JPS error (Dualer IQ inclinometer) • BBS • Affected/unaffected WB ratio • TUG No follow-up |
Weak |
| Lynch (2007)22 | RCT; Australia/inpatient rehab | • n = 21 (10 exp/11 con) • Age = 21–82 years • Time since stroke = 13–122 days |
Education, detection, localisation, discrimination, and proprioception on affected big toe and ankle 30 minutes/session 10 sessions, 2 weeks |
Relaxation + standing with EC 30 minutes/session 10 sessions, 2 weeks |
• SWM light touch • DPT • BBS • 10MWT gait velocity • ILAS Follow-up = 2 weeks |
Moderate |
| Mazuchi (2018)47 | RCT; Brazil/NR | • n = 12 • Age = 40–65 years • Time since stroke = mean 67.6 months (exp), 56.6 months (con) |
Aerobic deep water walking training (stationary gait) 30 minutes/session 1st week (50% heart rate), 40 minutes/session 2nd-9th week (60% heart rate); 5-minute warm-up and 5-minute cool-down; 3×/week; 9 weeks |
Aerobic treadmill walking training 30 minutes/session 1st week (50% heart rate), 40 minutes/session 2nd-9th week (60% heart rate); 5-minute warm-up and 5-minute cool-down; 3×/week; 9 weeks |
• Knee JPS absolute error (Biodex isokinetic dynamometer) No follow-up |
Moderate |
| Mohapatra (2012)38 | RCT; USA/inpatient rehab |
n = 11 (5 exp/6 con) Mean age = 49.2 years Time since stroke = 8–13 days (exp), 7–45 days (con) |
Compelled body weight shift (0.6 cm shoe lift in unaffected foot) + CPT 90 minutes/session weekdays, 30 minutes/session Saturdays, 6×/week, 2 weeks |
CPT 90 minutes/session weekdays, 30 minutes/session Saturdays, 6×/week, 2 weeks |
• FMA-LE • BBS • %BW on affected side • Gait velocity No follow-up |
Moderate |
| Moon (2015)43 | CCT; Korea/inpatient rehab |
n = 30 (15 exp/15 con) Mean age = 55.7 (exp), 50.1 (con) Time since stroke = mean 33.3 months (exp), 36.0 months (con) |
Treadmill training with EC 3 × 10 minutes/session 3×/week, 4 weeks |
Treadmill training with EO 3 × 10 minutes/session 3×/week, 4 weeks |
• Knee JPS error (Biodex isokinetic dynamometer) No follow-up |
Weak |
| Morioka (2003)44 | RCT; Japan/inpatient rehab |
n = 26 (12 exp/14 con) Age = 51–79 years Time since stroke = 31–111 days |
Hardness discrimination perceptual learning exercises (3 different levels of rubber sponge hardness) + CPT 10 trials/session 10 sessions, 2 weeks |
CPT NR |
• Hardness discrimination • 2-point discrimination • Postural sway locus length EO and EC No follow-up |
Moderate |
| Peurala (2002)45 | CCTb; Finland/inpatient rehab |
n = 59c
(foot 19 exp/0 con; hand 32 exp/8 con) Mean age = 54.4 years Time since stroke = 7 months–14 years |
TENS (monophasic constant, frequency 50 Hz) using sock electrode on affected foot + CPT 20 minutes/session 2×/day, 3 weeks |
NA (no subjects for foot con) | • Limb skin sensitivity (VAS) • SEP to posterior tibial nerve • 10MWT gait velocity • MMAS No follow-up |
Weak |
| Tyson (2013)49 | Paired-sample randomised cross-over trial; UK/research facility |
n = 29 Age = 28–82 years Time since stroke = NR |
TENS (biphasic symmetrical, phase duration 50 μs, frequency 70–130 Hz, 5 seconds/cycle) using sock electrode on affected foot; Not specifiedd |
Placebo TENS Not specifiedd |
• Ankle proprioception detection threshold (Biodex isokinetic dynamometer) • FRT • 10MWT gait velocity • Ankle strength No follow-up |
Moderate |
| Zankel (1969)48 | CCT; Columbia/NR |
n = 40e (10 exp/10 con) Age = 39–79 years Time since stroke = NR |
Vibration (120 cycles/second) on affected sole 30 minutes, 10 stimuli/minute, 1 month |
No vibration NA |
• SWM light touch • FMA-LE • BBS • 10MWT gait velocity No follow-up |
Weak |
RCT: randomized controlled trial; CCT: controlled clinical trial; NR: not reported; exp: rehab: rehabilitation; experimental group; con: control group; RPM: repeated passive movement; RAM: repeated active movement; CPT: conventional physiotherapy treatment; TENS: transcutaneous electrical nerve stimulation; NA: not applicable; EC: eyes closed; EO: eyes open; FMA-LE: Fugl-Meyer Assessment for lower extremity; BBS: Berg Balance Scale; %BW: percentage of body weight; 10MWT: 10-metre walk test; JPS: joint position sense; SWM: Semmes–Weinstein monofilaments; DPT: distal proprioception test; FRT: Functional Reach Test; TUG: Timed Up and Go Test; ILAS: Iowa Level of Assistance Scale; VAS: visual analogue scale; SEP: somatosensory evoked potentials; MMAS: Modified Motor Assessment Scale.
Only outcomes of subjects receiving lower limb treatment were extracted.
Note that there was no control group for the foot.
Only data of the foot were analysed in this review.
Until subjects felt comfortable with the sensation and outcome measures were obtained while stimulated.
Characteristics of 40 recruited subjects were reported in the article, although only 20 subjects were selected for the study.
Three studies used a similar retraining approach, which included a combination of education, detection, localization, discrimination, recognition, and proprioception of the hemiparetic leg.22,23,41 There was a range of proprioceptive training strategies, including treadmill training with visual deprivation,43 compelled body weight shift,38,46 and aquatic gait training.47 Other interventions included vibration stimulation41,48 and transcutaneous electrical nerve stimulation (TENS).41,45,49 Intervention dosages ranged from a single dose lasting up to approximately two hours49 to 30 minutes daily for six months.39
Outcome measures used in the included studies are described in Table 1. Common somatosensory modalities were light touch, measured with Semmes–Weinstein monofilaments in three studies, and joint position sense (JPS), measured in five studies with the Biodex equipment,50 and in three other studies with the distal proprioception test (DPT). The most common measure for balance was the Berg Balance Scale (BBS), used in five studies. Gait velocity was the main outcome measure for gait. Motor impairment measures included the modified Motor Assessment Scale and ankle strength. Leg function measures included Timed Up and Go (TUG), Barthel Index, and the Iowa Level of Assistance Scale.
Quality appraisal
A summary of the quality appraisal of included studies is presented in Supplemental Table S2. Of the 16 included studies, 10 were rated as weak and six rated as moderate. None of the studies had a strong rating. In the selection bias component, four studies scored somewhat likely to be representative of the target population,22,38,44,46 and none of the studies scored very likely, due to incomplete reporting of recruitment processes. A total of 14 studies were rated strong in study design for being controlled trials. However, only five of these trials described the randomisation method.22,44,47,49,51 The four studies that were rated as weak in the confounders component (i.e. confounders not accounted for) either did not provide sufficient information to ascertain whether or not there were important between-group baseline differences,23,46,48 or there were important differences that were unaccounted for including sex and age.45 Only three studies had blinding of both outcome assessors and study participants.22,44,47 In total, 10 studies were rated as strong for data collection methods and one received a weak rating.44 Five studies were rated separately for somatosensory measures and secondary outcomes due to the use of measures with a range of psychometric properties, all receiving a strong rating for balance or gait measures, and a weak rating for somatosensory measures.22,23,39,41,45 The majority of studies reported 80%–100% of participants completing the study and scored a strong rating in withdrawals and drop-outs. Only two studies reported percentage of compliance with treatment protocol,39,47 and only two of the randomised trials performed an intention-to-treat analysis.22,47
Intervention effects
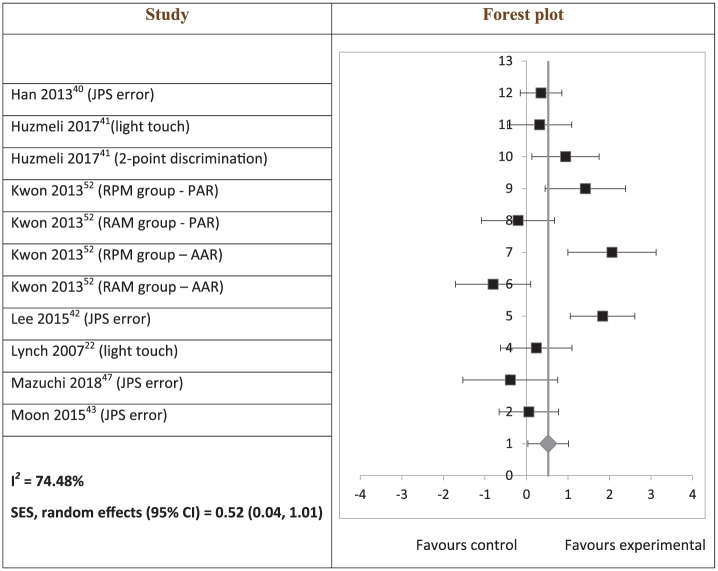
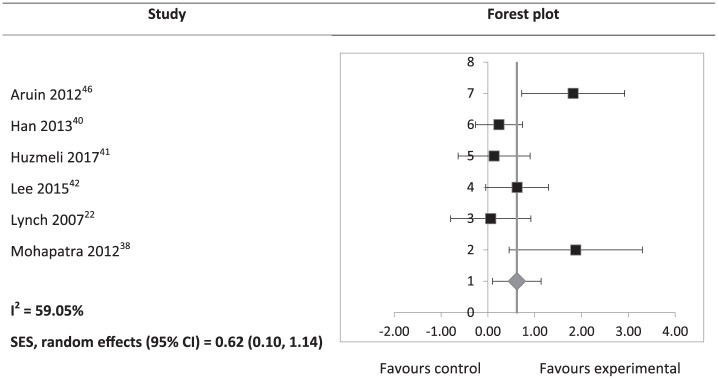
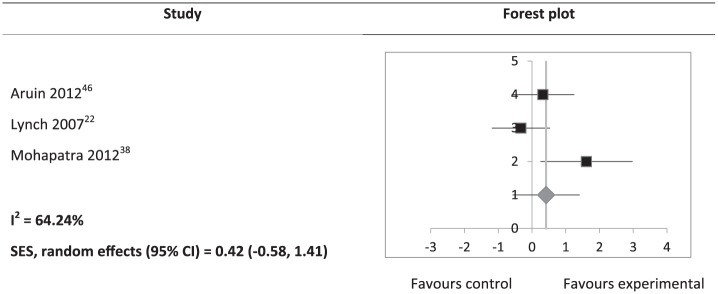
Study results, calculated effect sizes, and CIs are summarised in Supplemental Table S3. Somatosensory outcomes, which included JPS, light touch, and two-point discrimination, showed a significant heterogeneous positive SES (SES: 0.52; 95% CI: 0.04 to 1.01; Figure 2). However, subgroup analyses of JPS (SES: 0.36; 95% CI: –0.25 to 0.96) and light touch (SES: 0.28; 95% CI: –0.86 to 1.41) were not significant. A significant heterogeneous positive SES was found for BBS scores (SES: 0.62; 95% CI: 0.10 to 1.14; Figure 3). SES of weight-bearing on the affected side was not significant (SES: 1.52; 95% CI: –1.71 to 4.74). There was an outlying data set in weight-bearing distribution on the affected side (Supplemental Table S3). A sensitivity analysis was conducted with the exclusion of this data set, and a consistently non-significant SES was found (SES: 1.09; 95% CI: –0.06 to 2.24). Gait velocity SES was not significant (SES: 0.42; 95% CI: –0.58 to 1.41; Figure 4).
Figure 2.
Hedges’ g (95% CI) and summary of effect size (95% CI) on somatosensory outcomes.
SES: summary effect size; CI: confidence interval; RPM: repeated passive movement; RAM: repeated active movement; PAR: passive angle repositioning; AAR: active angle repositioning.
The squares on the forest plot are of the same size, instead of proportional to study weight, as the forest plot was generated on Microsoft Excel.
Figure 3.
Hedges’ g (95% CI) and summary effect size (95% CI) on Berg Balance Scale scores.
SES: summary effect size; CI: confidence interval.
The squares on the forest plot are of the same size, instead of proportional to study weight, as the forest plot was generated on Microsoft Excel.
Figure 4.
Hedges’ g (95% CI) and summary effect size (95% CI) on gait velocity.
SES: summary effect size; CI: confidence interval.
The squares on the forest plot are of the same size, instead of proportional to study weight, as the forest plot was generated on Microsoft Excel.
High clinical heterogeneity and insufficient data prevented meaningful pooling of postural sway area, motor impairment, and leg function outcomes (Supplemental Table S3). Effect sizes of postural sway area, both eyes open and closed, were significant in one study,40 but not significant in the other.44 For results pertaining to motor impairment and leg function, only the Barthel Index effect size was significant (SMD: 1.07; 95% CI: 0.24 to 1.89; P = 0.01).41 Non-significant findings included the FMA for lower extremity (P-values ranging from 0.13 to 0.61),38,46,51 Iowa Level of Assistance Scale (SMD: 0.00; 95% CI: –0.86 to 0.86; P = 1.00),22 and TUG (SMD: 0.19; 95% CI: –0.46 to 0.85; P = 0.56).42 Details of data synthesis are available from the corresponding author.
Data from five studies were not included in data synthesis due to having a cross-over design,49 data being unavailable,48 or having no control group.23,39,45 Non-significant findings were reported for two-point discrimination,41,44 vibration,48 skin sensitivity,45 and DPT.22,23,41 Significant improvements were reported for hardness discrimination,44 configuration of somatosensory evoked potentials,45 and in two out of three subjects for light touch.23 The TENS cross-over trial reported significant improvement post intervention in ankle plantarflexion JPS and plantarflexor strength, but not ankle dorsiflexion JPS and dorsiflexor strength.49 The assisted movement with enhanced sensation robotic therapy trial39 reported that 100% of subjects had 10% or more improvement in ankle JPS, 73% of subjects had 10% or more improvement in ankle dorsiflexor strength, and 91% of subjects had 10% or more improvement in ankle plantarflexor strength post intervention. Significant improvements were reported in weight-bearing on the affected side39 and Forward Reach Test49 post intervention, and a downward trend over time in postural sway area.23 A significant improvement in gait velocity was reported by two studies,39,49 but not significant in one study.45
Adverse effects
Four studies addressed adverse effects.39,46,47,49 Two studies reported no adverse effects.46,47 Reported adverse effects were skin abrasion from self-over-treatment (one subject)39 and one subject reported a day of pain post treatment.49
Discussion
This review aimed to examine the effects of interventions for leg somatosensory impairment after stroke primarily on somatosensory impairment and secondarily on balance, gait, motor impairment, and leg function. Results of meta-analyses suggest that there is evidence that these interventions improve somatosensory function and balance, but not gait, outcomes. However, it may be premature to make firm conclusions about gait outcomes as pertinent variables other than gait velocity, such as gait symmetry, have not been assessed. The effects of these interventions on motor impairment and leg function remain unclear because pooling of data was not possible due to a high degree of clinical heterogeneity and insufficient data.
The findings in this review suggest that interventions for post-stroke leg somatosensory impairment improve somatosensory function. Meta-analysis of somatosensory function was limited to the proprioception (JPS error), light touch, and two-point discrimination modalities of the leg. Although JPS error subgroup analysis was not significant, all but one study52 included in the analysis reported significant improvements post intervention. Although unlikely, it is possible that this one study52 may have skewed the results due to their mixed findings: a significant decrease in JPS error in the repeated passive movement group and an increase in JPS error in the repeated active movement group. Studies that measured proprioception using the DPT, unable to be included in the subgroup analysis, reported no statistically significant improvement,22,23 although one of them reported clinical improvement.23 This apparent lack of improvement was attributed to the lack of sensitivity of DPT.22,23 Light touch training effects appear ambiguous as there were inconsistent findings among the three included studies. One study reported between-group difference in only one (first metatarsal) of seven points of the foot,22 one reported significant improvement in two out of three subjects,23 and the third41 showed a non-significant effect size. Two-point discrimination similarly demonstrated no improvement associated with retraining of somatosensory impairment,44 although this may also be due to the lack of sensitivity of the instrument.41 However, the overall positive findings support the incorporation of interventions for addressing leg somatosensory impairment in stroke rehabilitation. Specifically, the JPS modality may be a suitable starting point of retraining.
Results of this review also suggest that interventions for post-stroke leg somatosensory impairment improve balance. Although pooling was not possible for postural sway area and a non-significant finding was found for weight-bearing on the affected side, three of seven studies that reported these outcomes showed significant positive effect sizes.38,40,46 The remaining studies reported either significant improvements post intervention39,42,44 or a downward trend over time23 (see Supplemental Table S3). A potential reason for improvement in balance, as a result of addressing leg somatosensory impairment, may be the improved perceptive ability through perceptual and motor learning, which is transferred to the motor performance of improved postural control.44 Improvement in balance may in turn reduce falls risk in stroke survivors. This is especially important given the association between somatosensory impairment and a higher falls incidence in stroke survivors compared to those without somatosensory impairment.3
There are a few possible reasons for the finding that interventions for post-stroke leg somatosensory impairment had no effect on gait outcomes. First, gait post stroke can be influenced not only by somatosensory information,11,53 but also by other factors including muscle strength,11,53–55 spasticity,55 cognition,56 visuospatial perception,57 motor function,53,56 and balance.53,54 Changes to somatosensation alone may not be enough to influence gait. It may be necessary to retrain somatosensory function in conjunction with interventions that address these other factors. Second, the interventions in most of the studies included in this review may not have been applied in tasks specific to gait. There is strong evidence for effectiveness of task-specific training for recovery after stroke,58 as a result of neuroplasticity.59 The use of intensive gait-specific training has been recommended for improving gait ability after stroke.60,61 An example of gait-specific retraining of somatosensory function may be ankle proprioceptive discrimination throughout a gait cycle. Third, results may have been influenced by the small sample sizes and varying methodology of the included studies.
Only gait velocity was measured in the three studies included in the meta-analysis. Another variable pertaining to gait that could be considered in assessing treatment effectiveness is symmetry.7 Gait symmetry can be measured using step length, or temporal measures such as stance or swing times.62 It has been suggested that leg proprioceptive and tactile information provides critical feedback that is able to modify gait patterns,7 potentially improving gait symmetry in stroke survivors. Improved somatosensory feedback can contribute to more accurate timing and amplitude of muscle contractions in response to the external environment,63,64 thereby improving gait symmetry. It is not possible to make the conclusion that retraining of leg somatosensory function would not affect gait at all, as none of the studies statistically analysed gait symmetry. One study assessed stride length and reported a significant within-group improvement,39 but data were not included in the meta-analysis due to a lack of a control group. Gait symmetry should be assessed when evaluating effectiveness of leg somatosensory interventions in future trials.
Two previous systematic reviews12,13 that investigated the effects of interventions for somatosensory impairment in the stroke population, although not specific to the leg, reported that there had been insufficient evidence to determine their effects and highlighted the need for high-quality controlled trials. Results from this review suggest that although several additional controlled trials examining the effects of interventions for somatosensory impairment, particularly in the leg, have been undertaken in recent years, the quality of these recent trials either remains poor or is difficult to assess due to incomplete reporting. In view of this, future studies should adhere to reporting guidelines for transparent reporting, such as CONSORT for randomised trials65 and TREND for non-randomised trials.66 Contrary to these previous reviews, the medium summary effects of this review provide preliminary evidence to support retraining of somatosensory function in the leg after stroke, for improving somatosensory function and balance.
Quantifiable and precise somatosensory assessment measures are vital in order to diagnose impairment, evaluate the extent of impairment and treatment effectiveness, and facilitate clinical decision-making about outcomes being achieved.67 The psychometric properties of many of the somatosensory outcome measures used in studies included in this review have not been established or were not reported in the studies, which is consistent with an observation about the dearth of literature examining frequently-used somatosensory assessment tools.68 This had implications for quality ratings of the included studies. Several studies used a range of measures; some such as the timed 10-metre walk test69 and the BBS70 have demonstrated good psychometric properties,71,72 while others such as the DPT73 have not had psychometric properties established. In order to provide a fair rating, five studies were given two different ratings: a strong rating for balance or gait measures and a weak rating for somatosensory measures.22,23,39,41,45 Furthermore, there are concerns raised about the lack of standardisation, responsiveness, and generalisability of somatosensory measures used in stroke rehabilitation.68 For example, a study examining the psychometric properties of the sensory subscale of the Fugl-Meyer Assessment (FMA) found that the high ceiling effect, and poor to moderate reliability, validity, and responsiveness, did not support its clinical use in stroke rehabilitation.74 The FMA was used in three of the included studies in this review.38,46,51 On a positive note, several leg proprioception assessment tools included in this review have been tested for their psychometrics, including electrogoniometers75 and digital inclinometers.76,77 However, these tools have not been tested in the stroke population, and their usage in clinical settings remains limited. Future research should focus on establishing the psychometric properties of these tools in stroke rehabilitation. Development of leg somatosensory measures that are quantifiable, sensitive to change, and available for clinical use may also be required for assessing treatment effectiveness and enabling better quality trials in somatosensory rehabilitation.
There was a diverse range of interventions used in the studies included. This highlights a need for developing standardised retraining methods of leg somatosensory function that can be reliably replicated across trials and in clinical settings, to increase consistency of interventions. An approach for retraining of somatosensory function has been developed,78 which has been demonstrated to be effective albeit only in arm studies.79–81 The approach is derived from theories of perceptual learning and somatosensory processing neurophysiology, and are consistent with the learning-dependent principles of neuroplasticity.78 The key elements of the approach are task-based (goal-directed), guided attentive exploration with vision occluded, immediate and precise feedback, calibration (within and across different sensory modalities, e.g. other limb and vision), anticipation trials, repetition, graded progression, and transfer of training effects to novel stimuli. Further exploration regarding the application and effectiveness of these principles, particularly in the leg, may be beneficial in establishing a standardised approach to addressing somatosensory impairment.
Somatosensory information, both from the joint (proprioception) and from the skin (tactile), has been demonstrated to be associated with perception of verticality,82 which in turn is related to balance.83 It is possible that enhanced proprioceptive and tactile feedback contributes to a more accurate perception of verticality, thereby positively influencing balance. Furthermore, increased weight-bearing on the affected side has been found to be associated with a reduction in postural sway.84 It was suggested that a reduction in postural sway could be due to enhancement of somatosensory information enabled by increased weight-bearing on that leg.84 Based on this review’s meta-analysis, interventions aimed to improve somatosensory function did not appear to increase weight-bearing on the affected side. However, in two studies38,46 where interventions specifically targeted weight-bearing on the affected side, there were larger improvements of weight-bearing on the affected side in the experimental group compared to the control group. Further research into interventions aimed to increase weight-bearing on the affected side may be useful in clarifying its role in reduction of postural sway.
There are obvious strengths and limitations in this review. The main strength is the use of the PRISMA guidelines,17 which enable transparent and complete reporting. One of the limitations is that the inclusion of non-randomised as well as randomised trials of varying quality permitted inclusion of a high risk of bias across studies. However, the reviewers wanted to report all available studies on post-stroke interventions that aimed to improve leg somatosensory function. All the included studies had small sample sizes with the highest being 62 participants.40 These small sample sizes, plus the high risk of selection bias within studies as noted in quality appraisal, mean that outcomes from these studies may not be representative of the wider stroke population. In addition, interpretation of the results may have been influenced by the heterogeneity of interventions and outcomes included. A risk of publication bias also exists due to the exclusion of non-English publications and unpublished studies.24 Nonetheless, the inclusion of non-English studies of randomised trials in systematic reviews found that language restrictions did not appear to bias results of conventional interventions.85
Clinical messages.
Interventions aimed at retraining leg somatosensory function post stroke are shown to be effective for improving somatosensory impairment and balance, but not gait.
Many of the somatosensory assessment tools used in the leg have not been tested for their validity and reliability in stroke rehabilitation.
There is a varied range of intervention methods intended for retraining leg somatosensory function after stroke.
Supplemental Material
Supplemental material, Supplemental_Material for Sensory retraining of the leg after stroke: systematic review and meta-analysis by Fenny SF Chia, Suzanne Kuys and Nancy Low Choy in Clinical Rehabilitation
Acknowledgments
We thank the authors of the included studies who responded to our requests for information.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Fenny SF Chia  https://orcid.org/0000-0002-9243-8776
https://orcid.org/0000-0002-9243-8776
Supplemental material: Supplemental material for this article is available online.
References
- 1. Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehabil 2008; 22(8): 758–767. [DOI] [PubMed] [Google Scholar]
- 2. Tyson SF, Hanley M, Chillala J, et al. Sensory loss in hospital-admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehabil Neural Repair 2008; 22(2): 166–172. [DOI] [PubMed] [Google Scholar]
- 3. Tyson SF, Crow JL, Connell L, et al. Sensory impairments of the lower limb after stroke: a pooled analysis of individual patient data. Top Stroke Rehabil 2013; 20(5): 441–449. [DOI] [PubMed] [Google Scholar]
- 4. Carey LM, Matyas TA, Baum C. Effects of somatosensory impairment on participation after stroke. Am J Occup Ther 2018; 72(3): 7203205100p1–7203205100p10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rand D, Gottlieb D, Weiss P. Recovery of patients with a combined motor and proprioception deficit during the first six weeks of post stroke rehabilitation. Phys Occup Ther Geriatr 2001; 18: 69–87. [Google Scholar]
- 6. Parsons SL, Mansfield A, Inness EL, et al. The relationship of plantar cutaneous sensation and standing balance post-stroke. Top Stroke Rehabil 2016; 23(5): 326–332. [DOI] [PubMed] [Google Scholar]
- 7. Wutzke CJ, Mercer VS, Lewek MD. Influence of lower extremity sensory function on locomotor adaptation following stroke: a review. Top Stroke Rehabil 2013; 20(3): 233–240. [DOI] [PubMed] [Google Scholar]
- 8. Duysens J, Massaad F. Stroke gait rehabilitation: is load perception a first step towards load control. Clin Neurophysiol 2015; 126(2): 225–226. [DOI] [PubMed] [Google Scholar]
- 9. Lin S-I. Motor function and joint position sense in relation to gait performance in chronic stroke patients. Arch Phys Med Rehabil 2005; 86(2): 197–203. [DOI] [PubMed] [Google Scholar]
- 10. Lee M-J, Kilbreath SL, Refshauge KM. Movement detection at the ankle following stroke is poor. Aust J Physiother 2005; 51(1): 19–24. [DOI] [PubMed] [Google Scholar]
- 11. Hsu A-L, Tang P-F, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil 2003; 84(8): 1185–1193. [DOI] [PubMed] [Google Scholar]
- 12. Schabrun SM, Hillier S. Evidence for the retraining of sensation after stroke: a systematic review. Clin Rehabil 2009; 23: 27–39. [DOI] [PubMed] [Google Scholar]
- 13. Doyle S, Bennett S, Fasoli SE, et al. Interventions for sensory impairment in the upper limb after stroke. Cochrane Database Syst Rev 2010; 6: CD006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aman JE, Elangovan N, Yeh IL, et al. The effectiveness of proprioceptive training for improving motor function: a systematic review. Front Hum Neurosci 2014; 8: 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCluskey A, Logan P, Carey L, et al. Critically appraised papers. Evidence for the retraining of sensation after stroke remains limited. Aust Occup Ther J 2010; 57: 200–202. [DOI] [PubMed] [Google Scholar]
- 16. National Institute for Health Research. PROSPERO: international Prospective Register of Systematic Reviews, https://www.crd.york.ac.uk/PROSPERO/ (2017, accessed 10 February 2017).
- 17. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carey LM. Touch and body sensations. In: Carey LM. (ed.) Stroke rehabilitation: insights from neuroscience and imaging. New York: Oxford University Press, 2012, pp.157–172. [Google Scholar]
- 19. Borstad AL, Nichols-Larsen DS. Assessing and treating higher level somatosensory impairments post stroke. Top Stroke Rehabil 2014; 21(4): 290–295. [DOI] [PubMed] [Google Scholar]
- 20. Carey LM. Somatosensory loss after stroke. Crit Rev Phys Rehabil Med 1995; 7: 51–91. [Google Scholar]
- 21. Kim JS, Choi-Kwon S. Discriminative sensory dysfunction after unilateral stroke. Stroke 1996; 27(4): 677–682. [DOI] [PubMed] [Google Scholar]
- 22. Lynch EA, Hillier SL, Stiller K, et al. Sensory retraining of the lower limb after acute stroke: a randomized controlled pilot trial. Arch Phys Med Rehabil 2007; 88(9): 1101–1107. [DOI] [PubMed] [Google Scholar]
- 23. Hillier S, Dunsford A. A pilot study of sensory retraining for the hemiparetic foot post-stroke. Int J Rehabil Res 2006; 29(3): 237–242. [DOI] [PubMed] [Google Scholar]
- 24. Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: CRD University of York, 2009. [Google Scholar]
- 25. Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg 2009; 50(3): 675–682.e1. [DOI] [PubMed] [Google Scholar]
- 26. University of Nottingham. Nottingham sensory assessment. https://www.nottingham.ac.uk/medicine/documents/publishedassessments/nsainstructionsrevised.pdf (2007, accessed 15 June 2017).
- 27. Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 28. Effective public health practice project. Quality assessment tool for quantitative studies, https://merst.ca/wp-content/uploads/2018/02/quality-assessment-tool_2010.pdf (2009, accessed 9 March 2017).
- 29. Thomas BH, Ciliska D, Dobbins M, et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004; 1(3): 176–184. [DOI] [PubMed] [Google Scholar]
- 30. Effective public health practice project. Quality assessment tool for quantitative studies dictionary, https://merst.ca/wp-content/uploads/2018/02/qualilty-assessment-dictionary_2017.pdf (1998, accessed 9 March 2017).
- 31. Centre for Evaluation Monitoring. Effect size calculator, http://www.cem.org/effect-size-calculator (2017, accessed 18 July 2017).
- 32. Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons, 2009, pp.21–32. [Google Scholar]
- 33. Higgins JPT, Deeks JJ. Selecting studies and collecting data. In: Higgins J, Green S. (eds) Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: John Wiley & Sons, 2008, pp.151–185. [Google Scholar]
- 34. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Behav Stat 1981; 6: 107–128. [Google Scholar]
- 35. Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins J, Green S. (eds) Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: John Wiley & Sons, 2008, pp.243–296. [Google Scholar]
- 36. Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a Microsoft Excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cross Validated. Combining outcome measures for meta-analysis, https://stats.stackexchange.com/questions/92134/combining-outcome-measures-for-meta-analysis (2014, accessed 10 January 2019).
- 38. Mohapatra S, Eviota AC, Ringquist KL, et al. Compelled body weight shift technique to facilitate rehabilitation of individuals with acute stroke. ISRN Rehabil 2012; 2012: 328018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cordo P, Lutsep H, Cordo L, et al. Assisted movement with enhanced sensation (AMES): coupling motor and sensory to remediate motor deficits in chronic stroke patients. Neurorehabil Neural Repair 2009; 23(1): 67–77. [DOI] [PubMed] [Google Scholar]
- 40. Han SK, Kim MC, An CS. Comparison of effects of a proprioceptive exercise program in water and on land the balance of chronic stroke patients. J Phys Ther Sci 2013; 25(10): 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dogru Huzmeli E, Yildirim SA, Kilinc M. Effect of sensory training of the posterior thigh on trunk control and upper extremity functions in stroke patients. Neurol Sci 2017; 38(4): 651–657. [DOI] [PubMed] [Google Scholar]
- 42. Lee H, Kim H, Ahn M, et al. Effects of proprioception training with exercise imagery on balance ability of stroke patients. J Phys Ther Sci 2015; 27(1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moon S-J, Kim YW. Effect of blocked vision treadmill training on knee joint proprioception of patients with chronic stroke. J Phys Ther Sci 2015; 27(3): 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morioka S, Yagi F. Effects of perceptual learning exercises on standing balance using a hardness discrimination task in hemiplegic patients following stroke: a randomized controlled pilot trial. Clin Rehabil 2003; 17(6): 600–607. [DOI] [PubMed] [Google Scholar]
- 45. Peurala SH, Pitkanen K, Sivenius J, et al. Cutaneous electrical stimulation may enhance sensorimotor recovery in chronic stroke. Clin Rehabil 2002; 16(7): 709–716. [DOI] [PubMed] [Google Scholar]
- 46. Aruin AS, Rao N, Sharma A, et al. Compelled body weight shift approach in rehabilitation of individuals with chronic stroke. Top Stroke Rehabil 2012; 19(6): 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mazuchi FDAES, Bigongiari A, Francica JV, et al. Aerobic training in aquatic environment improves the position sense of stroke patients: a randomized clinical trial. Motriz: J Phys Ed 2018; 24: 1–7. [Google Scholar]
- 48. Zankel HT. Pallesthesia studies in stroke patients. South Med J 1969; 62: 8–11. [DOI] [PubMed] [Google Scholar]
- 49. Tyson SF, Sadeghi-Demneh E, Nester CJ. The effects of transcutaneous electrical nerve stimulation on strength, proprioception, balance and mobility in people with stroke: a randomized controlled cross-over trial. Clin Rehabil 2013; 27(9): 785–791. [DOI] [PubMed] [Google Scholar]
- 50. Biodex. Dynamometers, http://www.biodex.com/physical-medicine/products/dynamometers (2019, accessed 9 January 2019).
- 51. Da Silva Ribeiro NM, Ferraz DD, Pedreira É, et al. Virtual rehabilitation via Nintendo Wii® and conventional physical therapy effectively treat post-stroke hemiparetic patients. Top Stroke Rehabil 2015; 22: 299–305. [DOI] [PubMed] [Google Scholar]
- 52. Kwon OS, Lee SW. Effect of continuing repeated passive and active exercises on knee’s position senses in patients with hemiplegia. Neurorehabilitation 2013; 33(3): 391–397. [DOI] [PubMed] [Google Scholar]
- 53. Nadeau S, Arsenault AB, Gravel D, et al. Analysis of the clinical factors determining natural and maximal gait speeds in adults with a stroke. Am J Phys Med Rehabil 1999; 78(2): 123–130. [DOI] [PubMed] [Google Scholar]
- 54. Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil 2007; 88(1): 115–119. [DOI] [PubMed] [Google Scholar]
- 55. Lin P-Y, Yang Y-R, Cheng S-J, et al. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch Phys Med Rehabil 2006; 87(4): 562–568. [DOI] [PubMed] [Google Scholar]
- 56. Cho KH, Lee JY, Lee KJ, et al. Factors related to gait function in post-stroke patients. J Phys Ther Sci 2014; 26(12): 1941–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roh H. Effect of visual perceptual disturbance on gait and balance. J Phys Ther Sci 2015; 27(10): 3109–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 2014; 9(2): e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Richards LG, Stewart KC, Woodbury ML, et al. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia 2008; 46(1): 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert Rev Neurother 2007; 7(10): 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pascal M, Barnable E, Bisset R, et al. Task-specific gait training for individuals with chronic CVA: a systematic review. Arch Phys Med Rehabil 2017; 98: e142. [Google Scholar]
- 62. Patterson KK. Gait asymmetry post-stroke. PhD Thesis, University of Toronto, Toronto, ON, Canada, 2010. [Google Scholar]
- 63. Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain 2002; 125(Pt 12): 2626–2634. [DOI] [PubMed] [Google Scholar]
- 64. Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 2006; 86(1): 89–154. [DOI] [PubMed] [Google Scholar]
- 65. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Br Med J 2010; 340: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 2004; 94(3): 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sullivan EJ, Andrews WA, Lanzino AD, et al. Outcome measures in neurological physical therapy practice: part II. J Neurol Phys Ther 2011; 35(2): 65–74. [DOI] [PubMed] [Google Scholar]
- 68. Sullivan JE, Hedman LD. Sensory dysfunction following stroke: incidence, significance, examination, and intervention. Top Stroke Rehabil 2008; 15(3): 200–217. [DOI] [PubMed] [Google Scholar]
- 69. Wade DT, Wood VA, Heller A, et al. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med 1987; 19: 25–30. [PubMed] [Google Scholar]
- 70. Berg KO, Wood-Dauphinee SL, Williams JI, et al. Measuring balance in the elderly: validation of an instrument. Can J Public Health 1992; 83: S7–S11. [PubMed] [Google Scholar]
- 71. Tyson SF, Connell L. The psychometric properties and clinical utility of measures of walking and mobility in neurological conditions: a systematic review. Clin Rehabil 2009; 23(11): 1018–1033. [DOI] [PubMed] [Google Scholar]
- 72. Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med 1995; 27(1): 27–36. [PubMed] [Google Scholar]
- 73. Bohannon RW. Evaluation and treatment of sensory and perceptual impairments following stroke. Top Geriatr Rehabil 2003; 19: 87–97. [Google Scholar]
- 74. Lin J-H, Hsueh I-P, Sheu C-F, et al. Psychometric properties of the sensory scale of the Fugl-Meyer Assessment in stroke patients. Clin Rehabil 2004; 18(4): 391–397. [DOI] [PubMed] [Google Scholar]
- 75. Smith TO, Davies L, Hing CB. A systematic review to determine the reliability of knee joint position sense assessment measures. Knee 2013; 20(3): 162–169. [DOI] [PubMed] [Google Scholar]
- 76. Romero-Franco N, Montano-Munuera JA, Jimenez-Reyes P. Validity and reliability of a digital inclinometer to assess knee joint position sense in a closed kinetic chain. J Sport Rehabil 2017; 26(1): 1–5. [DOI] [PubMed] [Google Scholar]
- 77. Suner-Keklik S, Cobanoglu-Seven G, Kafa N, et al. The validity and reliability of knee proprioception measurement performed with inclinometer in different positions. J Sport Rehabil 2017; 26(6): 1–15. [DOI] [PubMed] [Google Scholar]
- 78. Carey LM. Loss of somatic sensation. In: Selzer M, Clarke S, Cohen L, et al. (eds) Textbook of neural repair and rehabilitation. Cambridge: Cambridge University Press, 2006, pp.231–247. [Google Scholar]
- 79. Carey L, Macdonell R, Matyas TA. SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation: a randomized controlled trial. Neurorehabil Neural Repair 2011; 25(4): 304–313. [DOI] [PubMed] [Google Scholar]
- 80. Carey LM, Matyas TA. Training of somatosensory discrimination after stroke: facilitation of stimulus generalization. Am J Phys Med Rehabil 2005; 84(6): 428–442. [DOI] [PubMed] [Google Scholar]
- 81. Turville M, Carey LM, Matyas TA, et al. Change in functional arm use is associated with somatosensory skills after sensory retraining poststroke. Am J Occup Ther 2017; 71(3): 7103190070p1–7103190070p9. [DOI] [PubMed] [Google Scholar]
- 82. Saeys W, Vereeck L, Truijen S, et al. Influence of sensory loss on the perception of verticality in stroke patients. Disabil Rehabil 2012; 34(23): 1965–1970. [DOI] [PubMed] [Google Scholar]
- 83. Bonan IV, Guettard E, Leman MC, et al. Subjective visual vertical perception relates to balance in acute stroke. Arch Phys Med Rehabil 2006; 87(5): 642–646. [DOI] [PubMed] [Google Scholar]
- 84. Ju SK, Yoo WG. The effect of somatosensory and cognitive-motor tasks on the paretic leg of chronic stroke patients in the standing posture. J Phys Ther Sci 2014; 26(12): 1869–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Moher D, Pham B, Lawson ML, et al. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess 2003; 7(41): 1–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Sensory retraining of the leg after stroke: systematic review and meta-analysis by Fenny SF Chia, Suzanne Kuys and Nancy Low Choy in Clinical Rehabilitation