Abstract
Background:
In our previous dose-escalation study, we uncovered the maximum tolerated dose (MTD) of weekly irinotecan was escalated to 80 mg/m2 and 65 mg/m2 for UDP glucuronosyltransferase family 1 member A1 (UGT1A1) *1*1 and *1*28 rectal cancer patients in neoadjuvant chemoradiotherapy (nCRT). This is an expansion study for *1*1 patients.
Methods:
Patients with clinical stage T3–4, N0–2 rectal cancer eligible for preoperative chemoradiotherapy were screened for the UGT1A1*28 genotype. A total of 52 patients with the *1*1 genotype were enrolled. Whole-pelvic intensity-modulated radiation therapy was given in 50 Gy/25 fractions. Concurrently, irinotecan of 80 mg/m2 and capecitabine of 625 mg/m2 twice daily from Monday to Friday were administered weekly. Primary endpoint was toxicities; secondary endpoints included pathological complete response (pCR), tumour-regression grading, treatment compliance, overall survival, local recurrence and disease-free survival.
Results:
All patients completed capecitabine-based radiotherapy as scheduled, and 42 (81%) patients completed more than three cycles of weekly irinotecan. Overall, grade 3/4 toxicities were observed in 20 cases, including 11 leucopenia, 10 neutropenia and 12 diarrhoea. Forty-three patients (83%) underwent a radical surgery, and 12 were evaluated as pCR. Another four patients accepted a watch-and-wait strategy because of clinical complete response (CCR).
Conclusions:
Our data demonstrated manageable toxicities and an encouraging CCR rate for UGT1A1 *1*1 genotype in an enhanced neoadjuvant therapy. A phase III trial is ongoing to evaluate the value of irinotecan in neoadjuvant therapy (CinClare) [ClinicalTrials.gov identifier: NCT02605265].
Keywords: intensity-modulated radiation therapy, irinotecan, neoadjuvant chemoradiotherapy, rectal cancer, UGT1A1*28
Introduction
Neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) is the standard treatment for locally advanced rectal cancer (LARC). nCRT is usually associated with high rates of sphincter preservation and local control, but distant metastasis is still a common failure form. Therefore, it needs to be determined whether the integration of newer, more active chemotherapy or molecular targeted agents can significantly improve the clinical outcomes of LARC.
As an effective agent, irinotecan was investigated in recent studies in the nCRT setting. Some studies with a small sample size reported pathological complete response (pCR) rates ranging from 10% to 35% with a weekly irinotecan dose of 50–60 mg/m2 in combination with fluoropyrimidine (FU)-based CRT. The Radiation Oncology Group (RTOG) 0247 trial compared capecitabine plus irinotecan with capecitabine plus oxaliplatin while combined with pelvic radiotherapy and demonstrated no differences in tumour downstaging or toxicities between the two groups. Interestingly, inconsistent results were observed in both short-term and long-term outcomes. Irinotecan demonstrated a poorer pCR but a better long-term survival. Therefore, the authors suggested the need for further study on irinotecan.1,2
The UGT1A1 gene is located on chromosome 2, and it encodes a protein that modifies hepatic bilirubin to allow its excretion. Single-nucleotide polymorphisms that reduce the activity of the UGT1A1 gene therefore tend to increase serum bilirubin levels. UGT1A1*1 [rs8175347, (TA)6TAA] is the wild-type allele and is associated with normal enzyme activity. One particular variation in the promoter region of this gene is known as the UGT1A1*28 variant [rs8175347, (TA)7TAA]. The UGT1A1*28 allele had aroused some attention, which reduced UGT1A1-mediated inactivation of SN-38, the active metabolite of irinotecan, which is associated with the risk of myelosuppression and severe diarrhoea.3–7 In two dose-escalation phase I trials, the maximum tolerated dose (MTD) of irinotecan in the FOLFIRI regimen could be significantly increased based on the UGT1A1*28 genotype.8,9
Our previous phase I study also tested a new approach for concurrent nCRT in the era of genomic medicine, and used the UGT1A1*28 genotype to guide the escalation of the weekly irinotecan dose in patients with LARC.10 The MTD of weekly irinotecan was escalated to 80 mg/m2 in *1*1 genotype and 65 mg/m2 in *1*28 genotype in combination with capecitabine and pelvic intensity-modulated radiation therapy (IMRT) of 50 Gy/25 fractions. This is the expansion phase for patients with the *1*1 allele, the most common genotype of UGT1A1*28. The aim is to further identify effect and safety for the recommended dose of weekly irinotecan for rectal cancer patients with UGT1A1*1*28.
Materials and methods
Eligibility criteria
Patients with histologically confirmed, locally advanced rectal adenocarcinoma (cT3/4 or N+, M0) located within 12 cm of the anal verge were screened for UGT1A1*28 genotype. The eligibility criteria included the UGT1A1 *1*1 genotype, age 18–75 years, Karnofsky Performance status⩾ 60, adequate bone marrow function (leucocyte count > 4000/ml, platelet count > 100,000/ml), adequate hepatic function [alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels no more than twice the upper limit of normal] and adequate renal function (creatinine clearance > 50 ml/min).
Patients were excluded if they harboured the *1*28 or *28*28 allele, were previously diagnosed with other malignant tumours, had received pelvic radiotherapy (RT) or systemic chemotherapy, or had malabsorption syndrome, inflammatory bowel disease, ischaemic heart disease or any other condition not suitable for CRT.
Baseline evaluation
Evaluation was performed within 2 weeks before treatment. Baseline evaluation included a complete history, physical examination (digital rectal examination and other necessary examinations), complete blood count, hepatic and renal functional analysis, tumour marker measurement, colonoscopy and biopsy, computed tomography (CT) of the thorax and abdomen, high-resolution magnetic resonance imaging (MRI) of the pelvis. Pelvic MRI was used to establish the pretreatment staging.
UGT1A1 genotyping assay
Patients enrolled in this study were screened for UGT1A1 genotypes. Blood samples were collected using DNA extraction kits (QIAamp DNA Blood Midi Kit, QIAGEN Co., Ltd.). The forward primer 5′-TCCCTGCTACCTTTGTGGAC-3′ and the reverse primer 5′-AGCAGGCCCAGGACAAGT-3′ were used for pCR, which was performed in a 25 µl volume containing 2.5 µl 15 mmol/l Mg2+, 2 µl 2.5 mmol/l deoxynucleoside triphosphate, 0.2 µl 5 U Taq polymerase and 30 ng deoxyribonucleic acid (DNA). The amplification reaction was run for 40 cycles. Genotypes were assigned based on the number of T–A repeats in each allele.
Concurrent chemoradiotherapy
IMRT was delivered with a linear accelerator using 6-MeV photons and five to seven coplanar fields. All patients received a CT scan in the treatment position (supine or prone position), with 5 mm slices from the L3–L4 junction to 2 cm below the perineum. The clinical target volume (CTV) included the entire mesorectum (perirectal fascia), presacral space, the internal iliac lymph nodes and high-risk anatomical and nodal subsites, based on the distance of the tumour from the anal margin. Based on our institution setup data, the planning target volume was defined as the CTV with 10 mm margins superiorly and inferiorly and 8 mm margins in all other directions. A total irradiation dose of 50 Gy was given in daily fractions of 2.0 Gy, 5 days per week. The positioning and isocentre of each patient were verified on electronic portal imaging device films for the anterior and lateral gantry positions by visually comparing the digitally reconstructed radiographs.
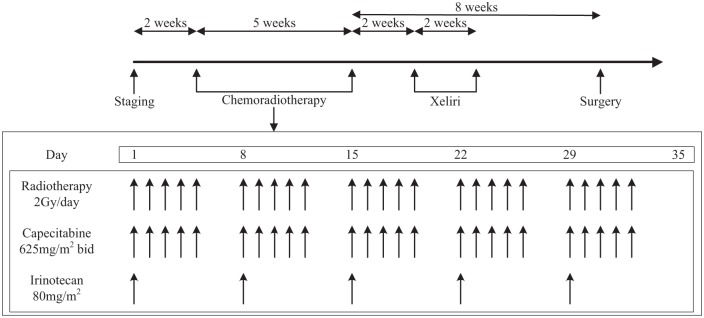
All patients were scheduled to receive weekly irinotecan of 80 mg/m2, administered as a 30–90 min intravenous infusion from day 1 of RT and continued for 5 consecutive weeks. Capecitabine was administered of 625 mg/m2 twice daily (b.i.d.) from Monday to Friday during the whole course of CRT (Figure 1).
Figure 1.
The workflow of this study.
b.i.d., twice daily.
Before starting irinotecan, patients were pretreated with standard doses of atropine, dexamethasone and 5-HT3 receptor antagonist. Diarrhoea was promptly treated with 4 mg loperamide at the onset and then with 2 mg every 2 h until the patient was diarrhoea free for at least 12 h. G-CSF (granulocyte colony-stimulating factor) were given to treat ⩾ grade 2 leucopenia/neutropenia events.
Surgery and pathology
At 2 weeks after the completion of CRT, one cycle of XELIRI (irinotecan 200 mg/m2 on day 1 and capecitabine 1000 mg/m2 b.i.d. day 1–14) was administered. A mandatory TME was scheduled 8 weeks after the completion of CRT, whereas the surgery type (anterior resection or abdominal–perineal resection) and whether a temporary colostomy was necessary were decided by the surgeon. All resected lymph nodes were examined according to standard procedures. If the number of lymph nodes was less than 12, two pathologists came to a consensus to ensure reliability of the detection result. pCR was defined as the absence of tumour cells in the surgical specimen both at the primary tumour site and at regional lymph nodes. The pathologic stage and tumour-regression grading (TRG) were evaluated according to the criteria of the American Joint Committee on Cancer (AJCC).11
Toxicity and measurement
Blood counts were determined twice weekly during nCRT. Tumour marker measurements and renal and hepatic functional tests were performed before and after treatment or as needed. Patients were questioned regarding changes in appetite, mucositis, malaise, vomiting, nausea and diarrhoea.
Toxicities were evaluated and recorded on a weekly basis according to the National Cancer Institute Common Toxicity Criteria (CTCAE 4.0). If grade 3 toxicities occurred, the physicians determined causes and decided on the response. Patients with nonhaematologic grade 3–4 toxicity or haematologic grade 4 toxicity could continue a lower dose of irinotecan, as decided by physicians, without changing the dose of capecitabine. Capecitabine and irinotecan could be discontinued based on patients’ refusal to continue treatment, physician assessment, intolerable side effects and disease progression. If the patient experienced a severe or persistent adverse event related to radiation, the RT schedule was interrupted or modified. RT was withheld until the related adverse events were resolved to grade 0 or 1.
Follow up
All patients were recommended to receive postoperative chemotherapy regardless of pathological result. A total of six cycles of capecitabine-based chemotherapy were recommended during the perioperative period.
Patient follow up was scheduled every 3 months during the first 2 years, and then every 6 months over the next 3 years. After 5 years, the frequency of follow up was extended to once each year.
Endpoints and statistics
The primary objective of this study was to identify the rate of grade ⩾ 3 toxicities. Secondary endpoints included pCR, TRG, treatment compliance, overall survival (OS), local recurrence and disease-free survival (DFS). DFS was defined as the time from the date of radical surgery to the date of a first relapse, the diagnosis of a secondary cancer after the initial diagnosis, or death from any cause, whichever occurred first. Patients without surgery were not included in the DFS analysis.
The sample size consideration was based on the grade ⩾ 3 toxicities. With 53 analysable patients, we had 90% power to reject the null hypothesis that the true toxicity rate was less than 30% with a type I error level of 5% if the number of patients with grade ⩾ 3 toxicities was 22 or more.
All characteristics were described by the frequency for categorical variables, by mean and standard deviations for normal distributional continuous data, and by the median for non-normal distributional continuous data. Survival curves were estimated using the Kaplan–Meier method and compared with Log-rank test.
The study was performed according to the Declaration of Helsinki, and the protocol was reviewed and approved by the local ethical institutional review board (Fudan University Shanghai Cancer Centre). All patients provided written informed consent before being enrolled into this trial [ClinicalTrials.gov identifier: NCT01474187].
Results
Patient characteristics
Between June 2012 and July 2015, 53 patients were enrolled in this expansion study. One patient withdrew the informed consent without any treatment. Thus, 52 cases were analysed finally. The patient characteristics are listed in Table 1: 31 of the patients were male and 21 were female, the median age was 57.5 years (range from 24 to 69 years). Fifty patients (96.2%) were diagnosed with cT3–4 and 50 patients had positive lymph nodes. Furthermore, 20 cases demonstrated a positive mesorectal fascia (MRF) status. The median distance from the anal verge was 4.8 cm (range 1.6–11cm). Elevated baseline carcinoembryonic antigen and CA19-9 were observed in 34.6% and 30.8% cases, respectively.
Table 1.
Demographic and baseline clinical characteristics distribution.
| No. | % | |
|---|---|---|
| Sex | ||
| Male | 31 | 59.6% |
| Female | 21 | 40.4% |
| Age, years | ||
| Median (range) | 57.5 (24–69) | |
| Mean (SD) | 54.3 (9.9) | |
| Clinical T stage | ||
| T2 | 2 | 3.8% |
| T3a | 18 | 34.6% |
| T3b | 15 | 28.8% |
| T3c | 4 | 7.7% |
| T4a | 6 | 11.5% |
| T4b | 7 | 13.5% |
| Clinical N stage | ||
| N0 | 2 | 3.8% |
| N1 | 22 | 42.3% |
| N2a | 12 | 23.1% |
| N2b | 16 | 30.8% |
| MRF | ||
| Negative | 32 | 61.5% |
| Positive | 20 | 38.5% |
| Location from anal verge, cm | ||
| Median (range) | 4.8 (1.6–11) | |
| Mean (SD) | 4.8 (1.8) | |
| Length of the tumour, cm | ||
| Median (range) | 5.1 (2.7–11.4) | |
| Mean (SD) | 5.4 (1.9) | |
| CEA | ||
| Normal | 33 | 63.5% |
| Abnormal | 18 | 34.6% |
| Missing | 1 | 1.9% |
| CA19-9 | ||
| Normal | 34 | 65.4% |
| Abnormal | 16 | 30.8% |
| Missing | 2 | 3.8% |
| Total | 52 | 100.0% |
CEA, carcinoembryonic antigen; MRF, mesorectal fascia; SD, standard deviation.
Treatment and toxicities
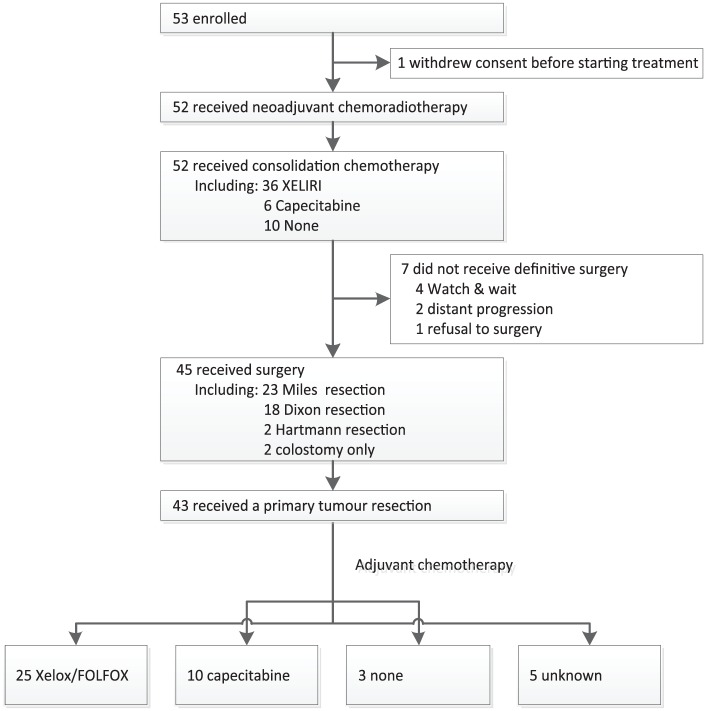
Fifty-two patients completed a full dose of radiotherapy and concurrent capecitabine. When it came to irinotecan, 11 patients received 5 cycles of weekly irinotecan, and 21, 10, 7 and 3 cases received 4, 3, 2 and 1 cycles of weekly irinotecan during the course of CRT, respectively. The CONSORT flow diagram is listed in Figure 2.
Figure 2.
The CONSORT flow diagram.
Overall, grade 3/4 toxicities were observed in 20 cases (38.5%), including 11 leucopenia, 10 neutropenia and 12 diarrhoea (Table 2). During the course of CRT, a total of 40 patients (76.9%) received G-CSF to relieve the grade of leucopenia and neutropenia.
Table 2.
Toxicities during the course of neoadjuvant chemoradiotherapy.
| Adverse events | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Diarrhoea | 5 | 9.6 | 4 | 7.7 | 12 | 23.1 | 0 | 0 |
| Anaemia | 20 | 38.5 | 9 | 17.3 | 1 | 1.9 | 1 | 1.9 |
| Leucopenia | 8 | 15.4 | 26 | 50 | 6 | 11.5 | 5 | 9.6 |
| Neutropenia | 18 | 34.6 | 8 | 15.4 | 5 | 9.6 | 5 | 9.6 |
| Thrombocytopenia | 6 | 11.5 | 0 | 0 | 1 | 1.9 | 0 | 0 |
| Abdominal cramping | 7 | 13.5 | 5 | 9.6 | 0 | 0 | 0 | 0 |
| Proctitis | 5 | 9.6 | 10 | 19.2 | 0 | 0 | 0 | 0 |
| Fatigue or asthenia | 12 | 23.1 | 13 | 25 | 0 | 0 | 0 | 0 |
| Anorexia | 14 | 26.9 | 9 | 17.3 | 1 | 1.9 | 0 | 0 |
| Total | 12 | 23.1 | 18 | 34.6 | 15 | 28.8 | 5 | 9.6 |
At 2 weeks after the end of CRT, a total of 36 patients received consolidation chemotherapy with the XELIRI regimen (33 in 1 cycle, 2 in 2 cycles and 1 in 3 cycles). The other 16 cases, including 6 in capecitabine alone and 10 in no chemotherapy, reduced the consolidation chemotherapy dose due to poor tolerance.
A total of 45 patients underwent surgery according to the schedule (Table 3). Twenty-three patients underwent a Miles resection, 18 and 2 cases received Dixon and Hartmann surgery, respectively, and the other 2 cases only received a colostomy because of unresectable disease. Of the 43 patients receiving a primary tumour resection, pCR was observed in 12 (27.9%) patients. Of the 45 patients, 12, 14, 15 and 2 were marked as TRG 0/1/2/3, respectively (27.9%, 32.6%, 34.9% and 4.7%). No perioperative deaths were observed. After a radical surgery, 35 patients receive adjuvant chemotherapy (25 in XELOX/FOLFOX and 10 in capecitabine alone).
Table 3.
Surgery and pathologic characteristics (n = 43).
| n | % | |
|---|---|---|
| Type of surgery | ||
| Miles | 23 | 44.2 |
| Dixon | 18 | 34.6 |
| Hartmann | 2 | 3.8 |
| ypT stage | ||
| ypT0 | 12 | 27.9 |
| ypT1 | 6 | 14.0 |
| ypT2 | 12 | 27.9 |
| ypT3 | 11 | 25.6 |
| ypT4 | 2 | 4.7 |
| ypN stage | ||
| ypN0 | 34 | 79.1 |
| ypN1 | 8 | 18.6 |
| ypN2 | 1 | 2.3 |
| yp stage | ||
| 0 | 12 | 27.9 |
| I | 15 | 34.9 |
| II | 7 | 16.3 |
| III | 9 | 20.9 |
| TRG | ||
| 0 | 12 | 27.9 |
| 1 | 14 | 32.6 |
| 2 | 15 | 34.9 |
| 3 | 2 | 4.7 |
| Total | 43 | 100 |
TRG, tumour-regression grading.
Four patients accepted a watch-and-wait strategy and received consolidation chemotherapy after a clinical complete response (CCR), which was evaluated by digital rectal exam, pelvic MRI, and endoscopy according to Memorial Sloan Kettering Cancer Centre criteria.12 For the other three patients, one refused to undergo a definitive surgery despite non-CCR status, and two were found having distant progression during the course of neoadjuvant therapy.
Follow up
With median follow up of 24.8 months (4.2–60.3 months), the 3-year DFS was 64.1% for patients with primary tumour resection, including one local failure, seven distant metastases, one secondary malignant tumour and two deaths. Four patients achieving CCR received watch-and-wait strategy and maintained a disease-free status, with a median follow up of 26.3 months (18.2–37.6 months).
Discussion
This is an expansion study following a previous dose-escalation trial. A total of 52 patients with UGT1A1*1*1 genotype were enrolled in this study. Our data demonstrate an encouraging tumour regression with acceptable acute toxicities. During the course of nCRT, overall grade 3/4 toxicities were observed in 20 cases (38.5%), including 11 leucopenia, 10 neutropenia and 12 diarrhoea. When it came to tumour regression, 12 out of 43 patients who underwent a TME surgery were evaluated as pCR. In addition, another four cases received a watch-and-wait strategy because of clinical complete response. Therefore, the total complete response (CR) rate should be 30.8%, comprising pCR and CCR.
Several previous studies with small sample sizes have tested the dose and effectiveness of irinotecan for nCRT1,2,13–18 (Table 4). Although the weekly dose of irinotecan was set at a low value of 50 mg/m2 concurrently with FU-based CRT in most early studies with small sample sizes, encouraging pCR rates ranging from 10% to 35% have been observed. The RTOG 0247 trial was a head-to-head phase II study comparing oxaliplatin and irinotecan in an FU-based nCRT setting. In order to control the overall toxicities during the course of CRT, the weekly doses of irinotecan and capecitabine were significantly reduced in the irinotecan arm. The preliminary results showed similar toxicities and tumour downstaging between the two arms, although a lower rate of pCR was observed in the irinotecan arm (10% versus 21%). Interestingly, after a median 4-year follow-up period, the irinotecan group demonstrated a higher OS and DFS than the oxaliplatin group.1,2 These inconsistent results suggest the presence of an unknown mechanism of action of irinotecan in combination with CRT, which warrants further study.
Table 4.
Some phase II trials of irinotecan-based neoadjuvant chemoradiotherapy.
| Patients, n | Concurrent chemo type (mg/m2) | RT (Gy/fractions) | pCR (%) | Survival (%) | Toxicity | Grade 3–4 toxicity (%) | |
|---|---|---|---|---|---|---|---|
| Navarro et al.13 | 74 | CPT-11 (50 mg/m2 qw*5) | 45/25 | 13.7 | N/A | Diarrhoea | 14 |
| 5-fu (225 mg/m2 q.i.d.) | Lymphocytopenia | 47 | |||||
| Willeke et al.14 | 36 | Irinotecan (50 mg/m2 qw*5) | 45/25 | 15 | 2-year OS 83 | Diarrhoea | 11 |
| Capecitabine (500 mg/m2 b.i.d.) | Leucocytopenia | 25 | |||||
| Shin et al.15 | 36 | Irinotecan (40 mg/m2 qw*4) | 50.4/28 | 21 | 3-year OS 94.3 | Diarrhoea | 8.3 |
| S-1 (70 mg/m2 q.i.d.) | 3-year DFS 72.1 | Haematologic | 13.9 | ||||
| 3-year LR 9.5 | |||||||
| Klautke et al.16 | 37 | Irinotecan (40 mg/m2 qw*6) | 50.4/28+5.4/3 boost | 22 | 5-year DFS 70 | Diarrhoea | 32 |
| CIV 5-fu (250 mg/m2 day 1–43) | 5-year LR 7 | Haematologic | 11 | ||||
| 28 | Irinotecan (40 mg/m2 qw*6) | 16 | 3-year DFS 73 | Diarrhoea | 39 | ||
| Capecitabine (1500 mg/m2 day 1–43) | 5-year LR 4 | Haematologic | 11 | ||||
| 20 | Irinotecan (50 mg/m2 qw*4) | 0 | N/A | Diarrhoea | 10 | ||
| Capecitabine (1500 mg/m2 day 1–43) | Haematologic | 0 | |||||
| 20 | Irinotecan (60 mg/m2 qw*4) | 35 | N/A | Diarrhoea | 15 | ||
| Capecitabine (1500 mg/m2 day 1–43) | Haematologic | 11 | |||||
| Sato et al.17 | 67 | Irinotecan (40 mg/m2 qw*4) | 45/25 | 34.7 | N/A | Diarrhoea | 4.5 |
| S-1 (80 mg/m2 q.i.d.) | Leucopenia | 4.5 | |||||
| Hong et al.18 | 48 | Irinotecan (40 mg/m2 qw*5) | 45/25+5.4/3 boost | 25 | 5-year OS 93.6 | Diarrhoea | 2.1 |
| Capecitabine (1650 mg/m2 q.i.d.) | 5-year DFS 75 | Leucopenia | 6.3 | ||||
| Wong et al.1,2 | 48 | Irinotecan (50 mg/m2 qw*4) | 50.4/28 | 10 | 4-year OS 85 | Total toxicity | 26.9 |
| Capecitabine (1200 mg/m2 q.i.d.) | 4-year DFS 68 |
b.i.d., twice daily; CIV, continuous intravenous infusion; CPT, camptothecin; DFS, disease-free survival; 5-fu, 5-fluorouracil; LR, local recurrence; N/A, nonapplicable; OS, overall survival; pCR, pathological complete response; q.i.d., four times daily; qw*4, four times weekly; qw*5, five times weekly; RT, radiotherapy.
Recently, a series of preclinical and clinical studies have shown that the UGT1A1 genotype is associated with the risk of severe toxicities to irinotecan and can guide the dose of irinotecan.3,6,7 The UGT1A1*28 allele can reduce the inactivation of SN-38, which is the active metabolite of irinotecan, and thus increase the risk of grade 3–4 irinotecan-related toxicities.19 Two phase I trials sought to escalate the dose of irinotecan in the FOLFIRI regimen in metastatic colorectal cancer patients stratified by the UGT1A1 genotype.8,9 The MTD of irinotecan in the FOLFIRI regimen was escalated nearly twofold in patients with the *1*1 genotype. However, in a subsequent phase II study, although high-dose FOLFIRI plus bevacizumab achieved an expected overall response rate in UGT1A1 *1*1 and *1*28 patients, it was stopped at the interim analysis because the number of unacceptable toxicities was higher than the number defined in the stopping rules in the statistical analysis plan (⩾20%).20 It suggested that the escalated irinotecan dose required more evidence for validation.
In nCRT, we deduced that an escalated dose of irinotecan guided by the UGT1A1*28 genotype might increase tumour response, too, and designed this dose-escalation study. The data showed that 80 mg/m2 and 65 mg/m2 of weekly irinotecan were the MTDs for the UGT1A1 *1*1 and *1*28 groups, respectively.10 On account of the MTD small sample-size result not being stable enough, we started this expansion phase to recruit additional 52 cases with *1*1 genotype.
However, our study had some limitations. First, only patients with UGT1A1 *1*1 genotype were enrolled in this expansion study. Patients with *1*28 genotype were excluded because of a low accruing speed. In the report by Liu et al., UGT1A1*1*28 was identified in around 20% of Asian cases, which is significantly lower than that in Western population.21 Second, the true incidence of haematologic toxicity might be underestimated because 76.9% enrolled patients received G-CSF during the course of CRT. Furthermore, the frequent use of G-CSF led to a delay of weekly chemotherapy, which is partly the reason for only 21% patients completing five cycles of weekly irinotecan as planned. Third, according to the concurrent guideline, irinotecan was not recommended in adjuvant chemotherapy based on three phase III trials;22–24 however, it is not clear how to determine the chemotherapy regimen for those patients who had a good response to irinotecan-based nCRT. Thus, further studies are necessary to optimize the postoperative chemotherapy regimen in future.
With encouraging clinical outcomes from this study, a phase III multicentre trial is ongoing to validate the benefit of irinotecan in an nCRT setting guided by the UGT1A1 genotype (CinClare).25 Together with the ARISTOTLE study, the other UK phase III trial using irinotecan-based nCRT, we believe that our study will provide strong evidence to guide future treatment decisions.
In conclusion, our data demonstrated manageable toxicities and an encouraging CR rate in patients with the UGT1A1 *1*1 genotype. Thus, irinotecan warrants more attention in nCRT.
Acknowledgments
We thank all of the patients and their families participating in this research.
YG, YS, YX and JZ, ZZ designed and performed the trial and wrote the manuscript. CL and JW helped in data collection and cowrote the manuscript. JZ performed the statistical analysis. WG, PL and SC provided surgical treatment for patients. DH provided pathological evaluation. JZ and ZZ were the principal investigators of the clinical trial.
Yun Guan, Yunzhu Shen and Ye Xu contributed equally to this work.
Footnotes
Funding: The work is financially supported by Natural Science Foundation of Shanghai (Grant no. 13ZR1408100) for Ji Zhu.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Ji Zhu  https://orcid.org/0000-0001-7134-9419
https://orcid.org/0000-0001-7134-9419
Contributor Information
Yun Guan, Department of Radiation Oncology, Fudan University Shanghai Cancer Centre, Shanghai, China; Department of Oncology, Fudan University, Shanghai, China; Department of Neurosurgery, Fudan University, Shanghai, China; Department of Cyberknife, Fudan University, Shanghai, China.
Yunzhu Shen, Department of Oncology, Nanjing Medical University, Nanjing, China.
Ye Xu, Department of Colorectal Surgery, Fudan University Shanghai Cancer Centre, Shanghai, China; Department of Oncology, Fudan University, Shanghai, China.
Chao Li, Department of Radiation Oncology, Fudan University Shanghai Cancer Centre, Shanghai, China; Department of Oncology, Fudan University, Shanghai, China.
Jingwen Wang, Department of Radiation Oncology, Fudan University Shanghai Cancer Centre, Shanghai, China; Department of Oncology, Fudan University, Shanghai, China.
Weilie Gu, Department of Colorectal Surgery, Fudan University Shanghai Cancer Centre, Shanghai, China; Department of Oncology, Fudan University, Shanghai, China.
Peng Lian, Department of Colorectal Surgery, Fudan University Shanghai Cancer Centre, Shanghai, China; Department of Oncology, Fudan University, Shanghai, China.
Dan Huang, Department of Pathology, Fudan University Shanghai Cancer Centre, Shanghai, China; Department of Oncology, Fudan University, Shanghai, China.
Sanjun Cai, Department of Colorectal Surgery, Fudan University Shanghai Cancer Centre, Shanghai, China; Department of Oncology, Fudan University, Shanghai, China.
Zhen Zhang, Department of Radiation Oncology, Fudan University Shanghai Cancer Centre, No. 270, Dong’An Road, Shanghai 200032, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China.
Ji Zhu, Department of Radiation Oncology, Fudan University Shanghai Cancer Centre, No. 270, Dong’An Road, Shanghai 200032, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, 200032, China.
References
- 1. Wong SJ, Winter K, Meropol NJ, et al. Radiation Therapy Oncology Group 0247: a randomized phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2012; 82: 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong SJ, Moughan J, Meropol NJ, et al. Efficacy endpoints of radiation therapy group protocol 0247: a randomized, phase 2 study of neoadjuvant radiation therapy plus concurrent capecitabine and irinotecan or capecitabine and oxaliplatin for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2015; 91: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biason P, Masier S, Toffoli G. UGT1A1*28 and other UGT1A polymorphisms as determinants of irinotecan toxicity. J Chemother 2008; 20: 158–165. [DOI] [PubMed] [Google Scholar]
- 4. Hoskins JM, Goldberg RM, Qu P, et al. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 2007; 99: 1290–1295. [DOI] [PubMed] [Google Scholar]
- 5. Kim TW, Innocenti F. Insights, challenges, and future directions in irinogenetics. Ther Drug Monit 2007; 29: 265–270. [DOI] [PubMed] [Google Scholar]
- 6. Toffoli G, Cecchin E, Corona G, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol 2006; 24: 3061–3068. [DOI] [PubMed] [Google Scholar]
- 7. Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004; 22: 1382–1388. [DOI] [PubMed] [Google Scholar]
- 8. Toffoli G, Cecchin E, Gasparini G, et al. Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 2010; 28: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marcuello E, Páez D, Paré L, et al. A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer 2011; 105: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu J, Li X, Shen Y, et al. Genotype-driven phase I study of weekly irinotecan in combination with capecitabine-based neoadjuvant chemoradiation for locally advanced rectal cancer. Radiother Oncol 2018; 129: 143–148. [DOI] [PubMed] [Google Scholar]
- 11. Edge SB, Compton CC, et al. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 12. Smith JJ, Chow OS, Gollub MJ, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 2015; 15: 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Navarro M, Dotor E, Rivera F, et al. A phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil, for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2006; 66: 201–205. [DOI] [PubMed] [Google Scholar]
- 14. Willeke F, Horisberger K, Kraus-Tiefenbacher U, et al. A phase II study of capecitabine and irinotecan in combination with concurrent pelvic radiotherapy (CapIri-RT) as neoadjuvant treatment of locally advanced rectal cancer. Br J Cancer 2007; 96: 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin SJ, Kim NK, Keum KC, et al. Phase II study of preoperative chemoradiotherapy (CRT) with irinotecan plus S-1 in locally advanced rectal cancer. Radiother Oncol 2010; 95: 303–307. [DOI] [PubMed] [Google Scholar]
- 16. Klautke G, Küchenmeister U, Foitzik T, et al. Intensified irinotecan-based neoadjuvant chemoradiotherapy in rectal cancer: four consecutive designed studies to minimize acute toxicity and to optimize efficacy measured by pathologic complete response. Radiother Oncol 2007; 85: 379–384. [DOI] [PubMed] [Google Scholar]
- 17. Sato T, Ozawa H, Hatate K, et al. A phase II trial of neoadjuvant preoperative chemoradiotherapy with S-1 plus irinotecan and radiation in patients with locally advanced rectal cancer: clinical feasibility and response rate. Int J Radiat Oncol Biol Phys 2011; 79: 677–683. [DOI] [PubMed] [Google Scholar]
- 18. Hong YS, Kim DY, Lim SB, et al. Preoperative chemoradiation with irinotecan and capecitabine in patients with locally advanced resectable rectal cancer: long-term results of a phase II study. Int J Radiat Oncol Biol Phys 2011; 79: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 19. Marsh S, Hoskins JM. Irinotecan pharmacogenomics. Pharmacogenomics 2010; 11: 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manfredi S, Bouché O, Rougier P, et al. High-dose FOLFIRI plus bevacizumab in the treatment of metastatic colorectal cancer patients with two different UGT1A1 genotypes: FFCD 0504 study. Mol Cancer Ther 2015; 14: 2782–2788. [DOI] [PubMed] [Google Scholar]
- 21. Liu JY, Qu K, Sferruzza AD, et al. Distribution of the UGT1A1*28 polymorphism in Caucasian and Asian populations in the US: a genomic analysis of 138 healthy individuals. Anticancer Drugs 2007; 18: 693–696. [DOI] [PubMed] [Google Scholar]
- 22. Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007; 25: 3456–3461. [DOI] [PubMed] [Google Scholar]
- 23. Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009; 27: 3117–3125. [DOI] [PubMed] [Google Scholar]
- 24. Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802). Ann Oncol 2009; 20: 674–680. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Z, Sun X, Liu A, et al. A multicenter randomized phase III trial of capecitabine with or without irinotecan driven by UGT1A1 in neoadjuvant chemoradiation of locally advanced rectal cancer (CinClare). J Clin Oncol 2019; 37(15s): Supplement Abstract: 3510. [DOI] [PMC free article] [PubMed] [Google Scholar]