Abstract
Objectives:
This study aimed to investigate serum chemerin concentrations in obese children and adolescents and to investigate the associations of chemerin with body mass index (BMI), lipid levels, and insulin sensitivity.
Methods:
Forty-eight obese and 40 nonobese Chinese children and adolescents were included in the study. BMI and levels of chemerin, lipids, glucose, and insulin were measured following an overnight fast. The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and BMI standard deviation score (BMI-SDS) were determined for all participants.
Results:
Serum chemerin levels were found to be significantly higher in obese children and adolescents than in control group members (94.83 ± 5.99 ng/mL vs 56.43 ± 4.16 ng/mL, P < .001). There were significant correlations between chemerin and age, BMI, BMI-SDS, total triglyceride (TG) levels, insulin levels, and HOMA-IR. After controlling for age, we found that chemerin levels were also significantly correlated with BMI-SDS (r =+ 0.284, P = .008) and HOMA-IR (r =+ 0.241, P = .034). In a stepwise multiple regression analysis, we observed only BMI-SDS to be an important determinant of chemerin level.
Conclusions:
In our sample of Chinese children and adolescents, chemerin levels were significantly higher in the obese group than in the control group. Chemerin levels were positively correlated with BMI-SDS and HOMA-IR and negatively correlated with age. We thus believe that further study is necessary to investigate the risk of metabolic abnormalities in young obese children and adolescents.
Keywords: chemerin, child, obesity, insulin resistance
Introduction
The rapid increase in the prevalence of childhood obesity has alarmed public health agencies, health care clinicians, health care researchers, and the general public.1 Obesity in childhood is associated with a variety of disorders2–5 and long-term cardiovascular complications.6 Furthermore, obese children tend to become obese adults.7 For these reasons, research on obesity during childhood is especially important to prevent adult obesity and associated metabolic disorders.
Recent studies have identified the important role played by adipose tissue hormones, adipokines, in obesity-associated complications. Of the adipokines, considerable research has been performed on leptin,8 adiponectin,9 and resistin,10 while another adipokine, chemerin, has been studied in recent years.11,12 The expression of chemerin has been found to be increased in adipose tissue of obese and type 2 diabetic animals,11 and chemerin has been reported to modulate adipogenesis.13,14 Moreover, recent studies have shown that serum chemerin is positively correlated with body weight and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) in overweight pediatric patients,15 and impacts factors of metabolic syndrome in obese children.16 Furthermore, other studies have also reported that chemerin may play a role in the development of cardiovascular diseases in children and adolescents.17,18
Collectively, these results suggest that chemerin may play an important role in regulating obesity and metabolic syndrome. However, are few studies on chemerin in obese children and adolescents, particularly in China.
The aims of the present study were to evaluate chemerin levels in a population of nonobese and obese children and adolescents in China and to evaluate whether chemerin levels were associated with obesity, metabolic parameters, and/or the insulin resistance (IR) index.
Patients and Methods
Study population and anthropometric measurements
Between June 2013 and June 2015, 88 (48 obese and 40 control) Chinese children and adolescents were enrolled in a cross-sectional study. The age range of participants included in the study was 5 to 14 years. All participants were from Guangzhou, China, and were recruited from among patients who had been referred for evaluation of obesity and for regular health check-ups. Demographic and clinical data were analyzed retrospectively. Obesity was defined as body mass index (BMI) for age- and sex-specific categories at the 95th percentile or higher.19 The inclusion criteria for the control group was a BMI between the 15th and 85th percentile.19 Exclusion criteria included endocrine disease, infections, chronic illnesses, and the use of prescription medication. BMI was calculated as weight in kilograms divided by height in meters squared. Using the least mean squares (LMS) method which compares the calculated BMI with the distribution of BMI in a Chinese standard population adjusted for age and gender, the standardized BMI (BMI-SDS) was calculated.20 Waist circumference (WC) was measured to the nearest 0.1 cm using a tape measure at the level of the umbilicus.21 Each participant had a detailed medical and family history taken and underwent a complete physical examination, as well as determination of the stage of puberty according to Tanner criteria.22 Fifteen milliliters of venous blood was drawn after an overnight fast (12 hours) at 8:00 to 9:00 a.m. on the day of a clinical visit, and these were collected according to the recommendations of the kits’ manufacturers. Serum and plasma samples were frozen and stored at −70°C.
The nature and purpose of the study was carefully explained to parents and participants before written consent was obtained from the parents and voluntary assent from the children/adolescents. This study was approved by the Institutional Ethical Board of the First Affiliated Hospital of Sun Yat-sen University.
Biochemical tests
Fasting plasma glucose (FPG, mmol/L), total cholesterol (TC, mmol/L), high-density lipoprotein cholesterol (HDL, mmol/L), and triglyceride (TG, mmol/L) levels were determined for all subjects, using standard laboratory methods and commercially available test kits (Roche Diagnostics GmbH, Mannheim, Germany). Low-density lipoprotein (LDL, mmol/L) cholesterol values were obtained using the Friedewald formula. Insulin levels (μU/mL) were measured using an enzyme-linked immunoassay kit (DRG Instruments GmbH, Marburg, Germany), with a lower limit of sensitivity of 1.76 μU/mL, and intra- and interassay coefficients of variation of 2.2% and 4.4%, respectively.
The index of IR, HOMA-IR, was calculated according to the homeostasis model formula as follows23: HOMA-IR = insulin × FPG / 22.5, where insulin is the fasting insulin level (μU/mL), and FPG is the fasting plasma glucose level (mmol/L).
Chemerin was measured in serum using a sandwich enzyme-linked immunosorbent assay (ELISA) (Human Chemerin DuoSet ELISA Kit, catalog No. DY2324, R&D Systems, Inc, Minneapolis, MN, USA) following the manufacturer’s instructions. The respective intra- and interassay coefficients of variation were 4.5% and 4%. The sensitivity of the ELISA assay was 0.0625 to 2 ng/mL.
Statistical analyses
All data were recorded on a computer database and analyzed using SPSS 13.0 software (IBM SPSS, Chicago, IL, USA). Results were expressed as mean ± standard deviation (SD) or median interquartile range (IQR) according to the data distribution type. Kolmogorov-Smirnov tests were used to test the normality of distribution for different variables. For variables that were normally distributed (demographic, anthropometric, and metabolic), differences between subgroups were calculated using Student t tests for independent samples; for parameters that were not normally distributed, Mann-Whitney U tests were performed. Pearson or Spearman correlation analysis was used to analyze bivariate relations and to test for associations between chemerin concentration and obesity measures, metabolic parameters, and IR index. Where necessary, partial correlations and stepwise multiple linear regressions were used to determine which parameters affected chemerin levels. Statistical significance was set at P < .05.
Results
Subjects’ characteristics
Characteristics and anthropometric parameters of the subjects used in this study are summarized in Table 1. A total of 48 obese and 40 nonobese age- and sex-matched control group subjects were studied. We found a significant difference in BMI and WC between the obese and control groups. Moreover, the obese group also had significantly higher TC, TG, LDL-c, and insulin levels, and HOMA-IR.
Table 1.
The general characteristics, anthropometric, and biochemical variables of the study population.
| Variables | Control(n = 40) | Obesity(n = 48) | P value |
|---|---|---|---|
| Age, years | 10.86 ± 2.23 | 10.42 ± 2.03 | .334 |
| Sex | .874 | ||
| Girls | 16 (40%) | 20 (41.7%) | |
| Boys | 24 (60%) | 28 (58.3%) | |
| BMI, kg/m2 | 16.80 ± 1.83 | 25.65 ± 4.18 | <.001* |
| WC, cm | 80.90 ± 4.99 | 66.55 ± 4.58 | <.001* |
| Tanner stage | .085 | ||
| 1 | 23 (57.5%) | 18 (37.5%) | |
| 2 | 7 (17.5%) | 11 (22.9%) | |
| 3 | 4 (10%) | 11 (22.9%) | |
| 4 | 4 (10%) | 3 (6.3%) | |
| 5 | 2 (5%) | 5 (10.4%) | |
| TC, mmol/L | 4.08 ± 0.76 | 4.69 ± 0.64 | .003* |
| TG, mmol/L | 0.89 ± 0.30 | 1.27 ± 0.62 | .003* |
| LDL-c, mmol/L | 2.38 ± 0.75 | 2.90 ± 0.73 | .037* |
| HDL-c, mmol/L | 1.56 ± 0.23 | 1.46 ± 0.57 | .579 |
| Insulin, μU/mL | 7.62 ± 4.93 | 23.63 ± 17.57 | <.001* |
| FPG, mol/L | 4.86 ± 0.52 | 4.80 ± 0.54 | .593 |
| HOMA-IR | 1.72 ± 1.10 | 4.73 ± 3.17 | <.001* |
| Chemerin, ng/mL | 94.83 ± 5.99 | 56.43 ± 4.16 | <.001* |
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride; WC, waist circumference.
Indicates significant difference between groups (P < .05). P values derived from unpaired Student t test for normally distributed continuous variables, Mann-Whitney U test for nonnormally distributed continuous variables, and chi-square test for categorical variables.
Serum chemerin levels were significantly higher in the obese group relative to the control group
Circulating chemerin levels were measured in serum samples from 88 individuals; of these 48 were in the obese and 40 in the control group. Serum chemerin concentrations were significantly higher in the obese than in the control group (94.83 ± 5.99 ng/mL and 56.43 ± 4.16 ng/mL, respectively, P < .001; Table 1).
Serum chemerin levels were associated with characteristics of metabolic syndrome in all subjects
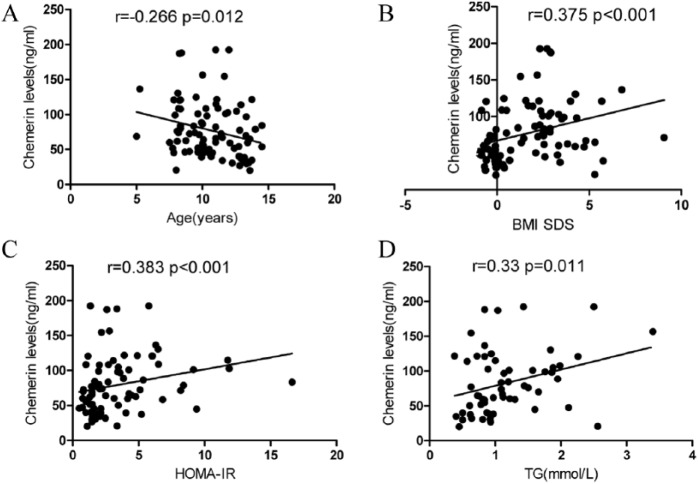
When chemerin levels were correlated with the anthropometric and biochemical indices of all 88 subjects (regardless of obesity status, sex, and Tanner stage); significant correlations were found between chemerin levels and age, BMI, BMI-SDS, TG, and insulin levels, and HOMA-IR (Table 2 and Figure 1). There was no significant correlation between chemerin levels and WC. After controlling for age, chemerin levels were still significantly correlated with BMI-SDS (r =+ 0.284, P = .008) and HOMA-IR (r =+ 0.241, P = .034). After we controlled for age and BMI-SDS, we found no significant correlations between chemerin and age, BMI, TG, and insulin levels, or HOMA-IR. In a multiple regression analysis with chemerin as the dependent variable and BMI-SDS, TG, and HOMA-IR as independent variables, we observed that only BMI-SDS was an important determinant of chemerin level (Δr2 = 0.37, P = .005).
Table 2.
Correlation of chemerin serum levels with anthropometric and metabolic parameters in children.
| Parameter | r | p | rb |
p
b
|
rb |
p
b
|
|---|---|---|---|---|---|---|
| (adjusted for age) | (adjusted for age, BMI SDS) | |||||
| Agea | –0.266 | .012 | ||||
| BMIa | 0.341 | .001 | –0.034 | .757 | ||
| BMI SDSa | 0.375 | <.001 | 0.284 | .008 | ||
| WC | 0.103 | .06 | 0.127 | .106 | 0.113 | .17 |
| Tanner stagea | –0.171 | .112 | –0.034 | .757 | –0.165 | .128 |
| TC, mmol/La | 0.325 | .015 | 0.123 | .37 | 0.113 | .414 |
| TG mmol/La | 0.33 | .011 | –0.02 | .884 | –0.038 | .776 |
| LDL-c, mmol/La | 0.143 | .343 | 0.031 | .84 | 0.025 | .874 |
| HDL-c mmol/La | –0.062 | .675 | 0.101 | .5 | 0.103 | .519 |
| Insulin, μU/mLa | 0.366 | .001 | 0.171 | .118 | 0.028 | .803 |
| FPG, mmol/La | 0.058 | .604 | 0.017 | .883 | 0.027 | .815 |
| HOMA-IRa | 0.383 | <.001 | 0.241 | .034 | 0.122 | .289 |
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LDL, low-density lipoprotein; SDC, standard deviation score; TC, total cholesterol; TG, triglyceride; WC, waist circumference.
Spearman correlation analysis was performed for chemerin serum levels and the indicated parameter.
Partial correlation analysis was performed.
Figure 1.
Association between chemerin levels and (A) age, (B) BMI SDS, (C) HOMA-IR, and (D) TG in all subjects.
TG indicates triglycerides; HOMA-IR, homeostasis model assessment of insulin resistance; BMI SDS, body mass index SD score.
Discussion
To our knowledge, no other studies to date have examined the effects of chemerin serum levels in obese children and adolescents in China. In this study, chemerin serum concentrations were found to be markedly elevated in obese subjects and were positively correlated with BMI-SDS. In addition, we also observed that serum chemerin was negatively correlated with age. Even after controlling for age, chemerin levels were still correlated with BMI-SDS. We also found a better correlation between chemerin and BMI-SDS than with BMI, suggesting that BMI-SDS was more reflective of increased body fat in obese children. It has been firmly established that adipose tissue is the main source of chemerin production. However, no negative correlation between age and chemerin has been reported, and the reasons for this relationship should be analyzed further. Our study and other studies have suggested that chemerin levels are closely related to insulin resistance and hyperlipidemia.15,24 Moreover, another study found chemerin levels to be associated with vascular endothelial damage.18 These results may suggest that the younger the obese children are, the greater the damage risk. Therefore, it is necessary to further investigate the risk of metabolic abnormalities in young obese children and adolescents.
It has been reported that chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes.25 In addition, chemerin has been positively related to HOMA-IR at baseline in young obese adults.26 The relationship between chemerin and insulin sensitivity was also investigated in our study. Here, we found a significant positive correlation between serum chemerin and HOMA-IR and fasting serum insulin; these findings agree with those in previous reports.15,21,25 However, after we controlled for BMI-SDS, the statistical significance of this correlation disappeared. Therefore, increasing chemerin concentration in obese children and adolescents may play a role in the pathological processes leading to obesity.
We also found that serum chemerin was significantly correlated with TG. This supports findings of previous studies24 and is also consistent with the finding that chemerin has a lipolytic effect in differentiated 3T3-L1 adipocytes.13,14 However, we found no significant correlation between chemerin levels and WC, which is inconsistent with results of another study.16 This may be related to a difference in fat distribution of children from China compared with children from other countries. Taken together, chemerin may play a role in insulin resistance and lipid disorders in obese populations. While our results use cross-sectional data and relatively small sample sizes of children and adolescents, future studies with larger sample sizes are needed to clarify the role chemerin plays in the development of obesity and metabolic syndrome.
In summary, in this study, we report that chemerin levels were significantly higher in obese than in control children/adolescents of Chinese origin. Chemerin correlated significantly with BMI-SDS and HOMA-IR in all individuals, suggesting that increased chemerin levels may be involved in pathological mechanisms related to obesity. This study is helpful in understanding the changes and characteristics of chemerin in obese children and adolescents in China, and in providing a theoretical basis for the prevention and treatment of childhood obesity.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Doctoral Scientific Fund from the Ministry of Education of China (20130171110048).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HJB—conceptualized and designed the study, drafted the manuscript, and approved the final manuscript as submitted; LLX—conceptualized and designed the study, drafted the manuscript, and approved the final manuscript as submitted; YZQ—conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted;HSC—conceptualized and designed the study, drafted the initial manuscript, critically reviewed the manuscript, and approved the final manuscript as submitted.
ORCID iDs: Hong-Jun BA  https://orcid.org/0000-0001-5268-211X
https://orcid.org/0000-0001-5268-211X
Hong-Shan CHEN  https://orcid.org/0000-0002-5018-4288
https://orcid.org/0000-0002-5018-4288
References
- 1. Eno Persson J, Bohman B, Tynelius P, Rasmussen F, Ghaderi A. Prevention of childhood obesity in child health services: follow-up of the PRIMROSE trial. Child Obes. 2018;14:99–105. [DOI] [PubMed] [Google Scholar]
- 2. Alissa EM, Sutaih RH, Kamfar HZ, Alagha AE, Marzouki ZM. Serum progranulin levels in relation to insulin resistance in childhood obesity. J Pediatr Endocrinol Metab. 2017;30:1251–1256. [DOI] [PubMed] [Google Scholar]
- 3. Al- Hamad D, Raman V. Metabolic syndrome in children and adolescents. Translational Pediatrics. 2017;6:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vikram NK. Cardiovascular and metabolic complications: diagnosis and management in obese children. Indian J Pediatr. 2018;85:535–545. [DOI] [PubMed] [Google Scholar]
- 5. Donin AS, Nightingale CM, Owen CG, Rudnicka AR, Cook DG, Whincup PH. Takeaway meal consumption and risk markers for coronary heart disease, type 2 diabetes and obesity in children aged 9-10 years: a cross-sectional study. Arch Dis Child. 2018;103:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattsson N, Ronnemaa T, Juonala M, Viikari JS, Raitakari OT. Childhood predictors of the metabolic syndrome in adulthood. The cardiovascular risk in young Finns study. Ann Med. 2008;40:542–552. [DOI] [PubMed] [Google Scholar]
- 7. Pietrobelli A, Espinoza MC, De Cristofaro P. Childhood obesity: looking into the future. Angiology. 2008;59:30S–33S. [DOI] [PubMed] [Google Scholar]
- 8. Van Harmelen V, Reynisdottir S, Eriksson P, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. [DOI] [PubMed] [Google Scholar]
- 9. Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. [DOI] [PubMed] [Google Scholar]
- 10. Devanoorkar A, Kathariya R, Guttiganur N, Gopalakrishnan D, Bagchi P. Resistin: a potential biomarker for periodontitis influenced diabetes mellitus and diabetes induced periodontitis [published online ahead of print February 12, 2014]. Dis Markers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. [DOI] [PubMed] [Google Scholar]
- 12. Maghsoudi Z, Kelishadi R, Hosseinzadeh-Attar MJ. Association of chemerin levels with anthropometric indexes and C-reactive protein in obese and non-obese adolescents. ARYA Atheroscler. 2015;11:102–108. [PMC free article] [PubMed] [Google Scholar]
- 13. Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. [DOI] [PubMed] [Google Scholar]
- 14. Roh SG, Song SH, Choi KC, et al. Chemerin—a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun. 2007;362:1013–1018. [DOI] [PubMed] [Google Scholar]
- 15. Sledzinska M, Szlagatys-Sidorkiewicz A, Brzezinski M, Kazmierska K, Sledzinski T, Kaminska B. Serum chemerin in children with excess body weight may be associated with ongoing metabolic complications: a pilot study. Adv Med Sci. 2017;62:383–386. [DOI] [PubMed] [Google Scholar]
- 16. Niklowitz P, Rothermel J, Lass N, Barth A, Reinehr T. Link between chemerin, central obesity, and parameters of the metabolic syndrome: findings from a longitudinal study in obese children participating in a lifestyle intervention. Int J Obes (Lond). 2018;42:1743–1752. [DOI] [PubMed] [Google Scholar]
- 17. Fontes VS, Neves FS, Candido APC. Chemerin and factors related to cardiovascular risk in children and adolescents: a systematic review. Rev Paul Pediatr. 2018;36:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Landgraf K, Friebe D, Ullrich T, et al. Chemerin as a mediator between obesity and vascular inflammation in children. J Clin Endocrinol Metab. 2012;97:E556–E564. [DOI] [PubMed] [Google Scholar]
- 19. Group of China Obesity Task Force. [Body mass index reference norm for screening overweight and obesity in Chinese children and adolescents]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:97–102. [PubMed] [Google Scholar]
- 20. Chen P. Physical activity, physical fitness, and body mass index in the Chinese child and adolescent populations: an update from the 2016 Physical Activity and Fitness in China—The Youth Study. J Sport Health Sci. 2017;6:381–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sung RY, Yu CC, Choi KC, et al. Waist circumference and body mass index in Chinese children: cutoff values for predicting cardiovascular risk factors. Int J Obes (Lond). 2007;31:550–558. [DOI] [PubMed] [Google Scholar]
- 22. Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. [DOI] [PubMed] [Google Scholar]
- 23. Ten S, Maclaren N. Insulin resistance syndrome in children. J Clin Endocrinol Metab. 2004;89:2526–2539. [DOI] [PubMed] [Google Scholar]
- 24. Maghsoudi Z, Kelishadi R, Hosseinzadeh-Attar MJ. The comparison of chemerin, adiponectin and lipid profile indices in obese and non-obese adolescents. Diabetes Metab Syndr. 2016;10:S43–S46. [DOI] [PubMed] [Google Scholar]
- 25. Ernst MC, Issa M, Goralski KB, Sinal CJ. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151:1998–2007. [DOI] [PubMed] [Google Scholar]
- 26. Lee MK, Chu SH, Lee DC, et al. The association between chemerin and homeostasis assessment of insulin resistance at baseline and after weight reduction via lifestyle modifications in young obese adults. Clin Chim Acta. 2013;421:109–115. [DOI] [PubMed] [Google Scholar]