Abstract
We present a case of chronic myelomonocytic leukemia (CMML) associated with myeloid sarcomas. The CMML also harbored a NPM1 mutation, which is uncommonly described outside the context of acute myeloid leukemia (AML). We describe our treatment strategy, which involved remission-induction chemotherapy that led to rapid resolution of myeloid sarcomas, and we present a literature review highlighting the treatment challenges that similar cases pose.
Keywords: chronic myelomonocytic leukemia, myelodysplastic syndrome/myeloproliferative neoplasm, myeloid sarcoma, NPM1 mutation
Introduction
We present a case report of chronic myelomonocytic leukemia (CMML) harboring an NPM1 mutation associated with extensive myeloid sarcomas. The unusual case highlights the challenges of managing CMML with unusual manifestations and rare mutations.
Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic malignancy characterized by overlapping features of both a myeloproliferative neoplasm and a myelodysplastic syndrome.1 It is a rare hematological malignancy with a reported incidence of 0.3–0.52/100,000 patients.2
The hallmark of myeloid sarcoma (MS) (also referred to as granulocytic sarcoma or chloroma) is the presence of myeloid blasts in the extramedullary tissues.3,4 Lymphadenopathy as a manifestation of MS has been described in case series.5 MS is typically associated with acute myeloid leukemia (AML) but it can also be seen in cases of myelodysplastic syndromes (MDS).6 The association of MS with myeloproliferative neoplasm (MPN) or MDS/MPN overlap syndromes is infrequent.7 In a large series of 452 patients reported by the Mayo Clinic, 119 patients had extramedullary manifestations.8 Of those, 15% had lymphadenopathy, 6% leukemia cutis, 3% gingival infiltrates, and two patients had MS. Few cases of CMML have presented with pericardial effusion or lymph node involvement.9,10
Molecular testing using PCR-based techniques or next-generation sequencing (NGS) mutational analysis has become a valuable tool in the context of hematological disorders. NPM1 mutations have been described predominantly in AML with a potential association with chloromas.11,12 NPM1 mutations have been reported, but less frequently, in MDS, MPN, or MDS/MPN cases.13 NPM1 in the context of CMML is very infrequent; for example, it was reported in only 2% of patients in a series of 383 CMML cases,14 with other reports having similar frequency.15 NPM1 mutations have a prognostic impact at least in AML,16 but the impact of these mutations in other hematological disorders is not well defined.
Case presentation
This is a case of a 46-year-old woman with initial presentation of progressive fatigue and shortness of breath. After multiple visits to different physicians she was noted to have developed abnormalities in peripheral blood counts. Namely, she developed leukocytosis with neutrophilia and monocytosis, accompanied by anemia and thrombocytopenia (Table 1). She had a bone marrow (BM) biopsy for further evaluation by her hematologist (Table 2). The BM was hypercellular with 10% monocytes and approximately 4% blasts. Karyotype was normal. NGS analysis revealed NPM1, NRAS, and ETV6 aberrations. Overall, the findings were considered to represent MDS/MPN unclassifiable (Table 2). Given leukocytosis, patient was placed on cytoreductive therapy with hydrea.
Table 1.
Complete blood count at various points.
| Timeline of CBC | WBC (103 cells/mm3) | Hgb (g/dl) | Platelet count (103 cells/mm3) | Neutrophils (103 cells/mm3) | Monocytes (cells/mm3 ) (percentage) |
% Blasts |
|---|---|---|---|---|---|---|
| 4 months prior to first BM biopsy | 4.9 | 11.7 | 255 | 1.8 | 598 (12.2%) | 0 |
| ~2 weeks prior to first BM biopsy | 27 | 7.8 | 96 | 9.4 | 1890 (14%) | 12 |
| Day 131 post alloHSCT | 4.32 | 6.9 | 50 | 2.68 | 302 (7%) | 0 |
| Day 223 post alloHSCT | 6.41 | 8 | 127 | 3.01 | 448 (7%) | 0 |
CBC, Complete blood count; WBC, white blood cells; Hgb hemoglobin; BM, bone marrow; alloHSCT, matched unrelated donor allogeneic bone marrow transplant.
Table 2.
Summary of bone marrow biopsies.
| BM morphology | FISH/karyotype | NGS mutational analysis | Accompanying CBC | |
|---|---|---|---|---|
| First BM biopsy | Markedly hypercellular marrow with myeloid hyperplasia and approximately 4% blasts and 10% monocytes. Iron stores were adequate. Findings consistent with myelodysplastic/myeloproliferative neoplasm unclassifiable. |
FISH analysis for 5p/5q,7p11/7q31,chromosome 8, t(9;22),t(8;21),t(15;17) was normal. Karyotype: 46 XY[20]. |
Pathogenic alterations in the NPM1 (p.W288Cfs*12 frameshift; VAF: 54%) and NRAS (p.G12D frameshift; VAF:54%) genes. Genomic alteration of uncertain significance was detected on the ETV6 gene (p.P223L missense; VAF: 48%). The NPM1 mutation was an insertion frameshift alteration located in exon 11 and was expected to be pathogenic. | WBC:28.5 (103 cells / mm3) Hgb: 8.3 (gr/dl) Platelets:143 (103 cells/mm3) Differential: 59.9% neutrophils 25.2% lymphocytes 14.9% Mid cells. |
| Second BM Biopsy (2 months after first biopsy) | Markedly hypercellular marrow with myelomonocytic hyperplasia and trilineage myelodysplasia; there was a background of decreased trilineage hematopoiesis. There was mild to focally moderate reticulin fibrosis (MF: 1–2 out of 3). These findings were considered to be most consistent with CMML-2. | FISH analysis N/A. Karyotype:47,XX,+21[11]/46,XX[9]. |
NGS results N/A. | WBC: 67.7 (103 cells/mm3) Hgb: 6.1 (gr/dl) Platelets:91 (103 cells / mm3) Differential: 47% neutrophils 9% lymphocytes 10% monocytes 6% blasts 0% basophils 1% eosinophils |
| Third BM biopsy (2.5 months from first BM biopsy) |
BM biopsy show marked aspiration artifact with focal hypercellular area BM Flow cytometry (5.2% immature myeloid cells) and hematopathology review evaluation of peripheral blood: Morphology and flow cytometry compatible with undefined MPN/MDS syndrome / CMML. |
FISH analysis MDS/AML: 45% of the cells had three copies of the AML1 gene at 21q22. FISH analysis for PDGFRA, PDGFRB, FGFR1, and JAK2 rearrangements were negative. Karyotype: 47,XX,+21[18]/46,XX[4]. |
Mutational analysis was notable for the same molecular aberrations as the initial BM biopsy. | WBC:44.69 (103 cells/mm3) Hgb: 6.5 (gr/dl ) Platelets:34 (103 cells/mm3) Differential: 66% neutrophils 5% lymphocytes 11% monocytes 7% blasts |
| Post remission-induction BM biopsy (at count recovery) | Normal cellular marrow (60%) with trilineage hematopoiesis and sequential maturation. | Karyotype: 46,XX[20]. | NGS was not performed. Note: An NGS that was performed few days prior to this BM revealed persistence of ETV6 p.P223L variant. The allelic fraction was also approximately the same as before (40–50%). According to the report this could represent a germline variant and its clinical significance, if any, is uncertain. |
WBC:6 (103 cells/mm3) Hgb: 9.2 (gr/dl) Platelets:235 (103 cells/mm3) Differential: 64 % neutrophils 18% lymphocytes 11 % monocytes 0% blasts |
| Day 55 post alloHSCT BM biopsy. | Minimal marrow particles present with no blasts noted on the smear. Rare erythroid linage cells noted. The specimen was suboptimal for evaluation. | Karyotype: 46,XX[20]. | NGS was not performed. | WBC:1.09 (103 cells/mm3) Hgb: 7.1 (gr/dl) Platelets:7 (103 cells/mm3) Differential: 74 % neutrophils 24% lymphocytes 0 % monocytes 0% blasts |
BM, Bone marrow; FISH, Fluorescence in situ hybridization; NGS, next generation sequencing; CBC, Complete blood count; MF, marrow fibrosis; CMML, chronic myelomonocytic leukemia; WBC, white blood cells; Hgb hemoglobin; MPN, myeloproliferative neoplasm; MDS, myelodysplastic syndromes; AML, acute myeloid leukemia; alloHSCT, matched unrelated donor allogeneic bone marrow transplant.
The patient developed lymphadenopathy (LAD) soon after. Computed tomography (CT) imaging studies of neck and chest confirmed bilateral axillary, mediastinal, right hilar LAD, and with prominent lymphoid tissue in nasopharynx. A CT scan of the abdomen and pelvis noted hepatosplenomegaly and enlarged bilateral inguinal lymph nodes.
A right axillary lymph node biopsy was obtained approximately 50 days from the initial evaluation by her hematologist and was consistent with MS. The tissue was infiltrated by mildly enlarged, lymphoid-like cells, with a scant amount of cytoplasm, hyperchromatic nuclei, focally irregularly shaped, and with tiny nucleoli. A few inflammatory cells were also noted in the background. Immunohistochemistry stains of the lymph node revealed myeloid and megakaryocytic precursors. Given the disparity between MPO and CD68, it was considered that many of the cells represented monocytes. Furthermore, there was a significant number of CD117-positive cells compatible with promonocytes, but CD34 negative/CD117 positive blasts could not be entirely excluded. Chromosomal analysis was unsuccessful.
The patient had another BM biopsy approximately 2 months after the first (second BM biopsy in Table 2), which revealed a markedly hypercellular BM with myelomonocytic hyperplasia and trilineage myelodysplasia. Karyotype was 47,XX+21[11]/46,XX[9]. Despite being on hydrea, accompanying CBC revealed that leukocytosis persisted, as well as the anemia and thrombocytopenia. Monocytosis also persisted (10% of WBC), and the patient had 6% blasts in the peripheral blood. The overall findings of the BM biopsy supported a diagnosis of CMML-2.
The patient was transferred to the care of our institution and a repeat BM biopsy was performed 2.5 months after the first (third BM biopsy in Table 2). The sample showed marked aspiration artifact with focal hypercellular area (Figure 1). Fluorescence in situ hybridization (FISH) analysis demonstrated three copies of the RUNX1 gene at 21q22 and a karyotype similar to the previous one (47,XX,+21[18]/46,XX[4]). NGS revealed similar aberrations as the report from the initial BM biopsy. Because the patient had monocytosis that persisted for 3 months; was without evidence of another etiology for monocytosis; had an extensive work up, excluding chronic myeloid leukemia, primary myelofibrosis, polycythemia vera; FISH analysis without evidence of PDGFRA, PDGFRB, FGFR1, and JAK2 rearrangements, the diagnosis of CMML was reaffirmed. The blast percentage on the peripheral blood by morphology and the BM flow cytometry did not support progression to AML.
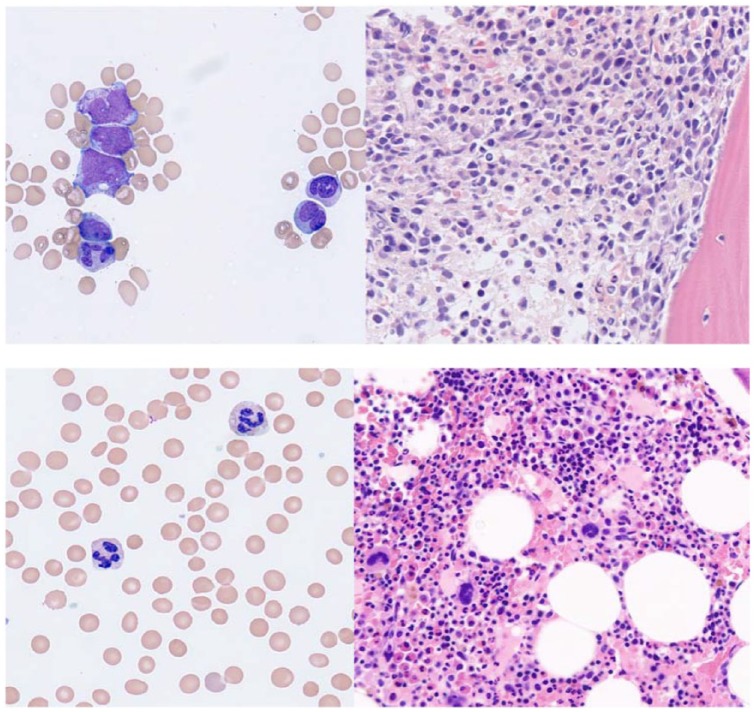
Figure 1.
Top panel (pre-induction). Peripheral blood smear with blasts and immature monocytes (left). BM core biopsy at high magnification (40X) demonstrating early myeloid lineage cells (right). Bottom panel (post-induction at count recovery). Peripheral blood smear without circulating blasts (left). Normocellular bone marrow (right).
Repeat CT imaging of neck, chest, abdomen, and pelvis noted persistent extensive LAD. Due to unusual presentation, needle biopsy of the right inguinal lymph node and excisional biopsy of a neck lymph node were performed and were interpreted as MS. Namely, immunohistochemical stains were performed on the needle biopsy sample of the inguinal node, and the neoplastic cells were positive for CD33 and CD43. CD15+ was noted in a subset of neoplastic cells and CD163 exhibited focal positivity. The neoplastic cells were negative for CD117, CD34, CD14, CD3, CD79a, and CD123. Overall, immunohistochemistry (IHC) and morphology revealed dense infiltration of predominantly immature myeloid cells with some degree of monocytic differentiation. The histologic features of the neck lymph node were similar, and, morphologically, the two cases were identical. Notably, the lymph node biopsy measured more than 4 cm and was completely replaced by immature myeloid infiltrates (Figure 2).
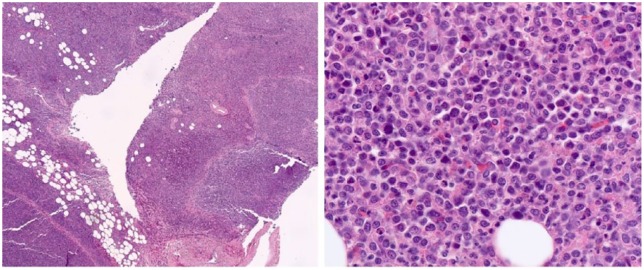
Figure 2.
Lymph node is completely replaced by immature myeloid infiltrate, and flow cytometry was positive for aberrant immature myeloid cells.
Given the findings of MS and presence of the NPM1 mutation, the decision was made to treat with intensive chemotherapy schema. The patient received idarubicin 12 mg/m2 and cytarabine 100 mg/m2 as part of the standard induction protocol [3+7]. Importantly, a CT scan performed at approximately day 14 of induction revealed significant improvement of LAD.
Remission induction was complicated by a Clostridioides difficile infection and necrotizing enteritis of the jejunum. The patient underwent jejunum excision, with pathology negative for any evidence of MS. Upon count recovery, another BM biopsy was done (fourth BM biopsy in Table 2), which revealed resolution of the hyperplasia and normalization of the karyotype. NGS revealed persistence of the ETV6 alteration at a variant allelic frequency of 40–50%, whereas NPM1 and NRAS mutation was not detected. Given persistence with variant allelic frequency 40–50% at remission, this could represent a germline variant. A search of ExAC (Exome Aggregation Consortium) population frequency databases revealed that this variant is reported but is very infrequent. In silico analysis including SIFT, Polyphen, and FATHMM algorithms did not support a detrimental functional consequence. The three cases reported in the COSMIC database involved colon and lung cancer cases. Taken together, the clinical significance of this variant, if any, was deemed uncertain. Notably, repeat CT imaging while in remission revealed resolution of lymphadenopathy.
The patient completed one cycle of consolidation with high dose cytarabine followed by matched unrelated donor allogeneic bone marrow transplant (alloHSCT) utilizing a regimen of fludarabine and melphalan. Her post-transplant course was complicated by CMV and EBV reactivation, and graft-vs-host disease (GVHD) of skin (grade I, stage III) and intestine (grade III). The GVHD manifestation improved with topical and intravenous followed by oral steroids respectively. At day 55 post alloHSCT BM biopsy is reported as fifth biopsy in Table 2. It was suboptimal for evaluation but karyotype remained normal and flow cytometry did not reveal aberrant myeloid population; patient did not have further BM biopsies. Despite her complicated post-transplant course, the patient continues to recover slowly without recurrence of LAD (Table 1 includes CBC at various points post alloHSCT). The patient is approximately 230 days post alloHSCT at the time of the submission of this manuscript.
Discussion
Several reports examined the mutational landscape of CMML and frequently reported mutations are TET2, SRSF2, ASXL1, RUNX 1, NRAS, and CBL.17,18 NPM1 has been rarely reported with CMML,14 and its presence appears to be an ominous marker for progression to AML.15 To the best of our knowledge, our case of NPM1 mutated CMML with extensive extramedullary disease has not been previously reported.
Our case posed significant diagnostic dilemmas. Mutations affecting the NPM1 gene are reported rarely in CMML and frequently in AML. The World Health Organization (WHO) recognize AML with mutated NPM1 as a separate entity; however, the mutation by itself without 20% or more blasts is not enough to classify a case as AML. The mutation that was detected in our case was a 4 bp insertion [c.859_860insTCTG (p.W288Cfs)] and has been previously described in AML.19,20 The particular NPM1 mutation leads to creation of a nuclear export sequence, ultimately leading to loss-of-function due to aberrant localization of the Npm1 protein (Jackson laboratory Clinical Knowledgebase; https://ckb.jax.org/geneVariant/show?geneVariantId=11363). Given the rarity of the cases it is very difficult to determine if NPM1 mutation is associated with favorable outcomes in CMML.
The clinical significance of ETV6 p.P223L, if any, is unknown. It appears to be benign based on a previous report,21 and has been reported in population databases, but infrequently; in silico analysis did not predict detrimental functional consequences. Apart from the unusual molecular aberrations, our case had cytogenetic evolution, with acquisition of an extra chromosome 21. Acquisition of cytogenetic abnormalities has been described in CMML and may be associated with progression to AML.22 Peng and colleagues noted also in their series that two out of eight patients with CMML and NPM1 mutation had trisomy 21; however, this association has not been observed consistently.15 In the same series, one patient had rash, but no definite report of extramedullary manifestations. In another report of NPM1 mutation, a patient developed splenomegaly infiltrated by CMML.23 It will be of interest to examine if NPM1 mutation is associated with propensity for involvement of extramedullary tissues in CMML or MDS/MPN overlap syndromes, but research would be hampered by the rarity of such cases.
Management of patients with CMML or other MDS/MPN overlap syndromes with extramedullary manifestations is challenging as large series are lacking. CMML may have only a transient response to remission induction regimens, such as 3+7, and may not recover normal hematopoiesis. Other regimens reported in the literature include topotecan alone24 or in combination with cytarabine.25,26 Although responses with these regimens could include complete remissions, toxicities limit the application of these regimens in elderly patients with comorbidities. An alternative approach is the use of hypomethylating agents such as 5-azacytidine or decitabine.27 Recent publications highlight and summarize treatment outcomes with the different chemotherapy regimens.28,29
Ultimately, alloHSCT is considered the only curative approach for CMML. Outcomes of CMML patients undergoing alloHSCT were reported to be affected by blast percentage at the time of transplant, cytogenetics, and existing comorbidities.20–23 The reported studies are mostly relatively small and retrospective (summarized in recent publications30,31). Reports are conflicting for the relative impact of factors determining outcomes of alloHSCT, but, given the rarity of CMML, the prospect of large randomized studies is unlikely.32 It remains unclear if pre-transplant treatment with hypomethylating agents is the optimal treatment modality.32,33 Relapse of CMML occurs in a significant percentage of patients that had alloHSCT. In an analysis of 85 patients, Eissa and colleagues report a relapse rate of 24% at 2 years and 27% at 10 years.34
Data regarding MS and CMML are scant. We were able to find case reports of using hypomethylating agents for CMML associated with leukemia cutis,35,36 and a few patients treated with AML induction followed by involved field radiation.37 We employed a strategy similar to MS associated with AML, given that the patient had few comorbidities and a high burden of symptoms. Moreover, given reports that NPM1 is associated with chemo-sensitivity,38,39 and high CR rates, at least on AML, we extrapolated that a similar response might occur in our case. Indeed, it was notable that LAD resolved quickly after initiation of 7+3 induction chemotherapy. There is limited information regarding optimal consolidation approach in patients with MS. Extrapolating from the AML literature, we recommended alloHSCT.40 Notably, the impact of NPM1 mutation in MS associated with AML is unknown.40 The mechanisms leading to formation of MS are not well understood, and include a complex interplay of metalloproteinases with B2-integrin41,42 and it is unclear if mechanisms are common between different diseases.
Conclusion
CMML with MS is not reported often, and optimal treatment has not been established. In the case reported, NPM1 was present and the patient had rapid resolution of LAD and attained remission, highlighting that the NPM1 chemosensitivity noted on AML might be applicable to other hematological conditions.
Acknowledgments
Authors Faris Matanes and Basel M.A. AbdelAzeem contributed equally. We would like to thank Dr Shuko Harada for her help regarding molecular aberrations presented in the manuscript.
Footnotes
Author contributions: Faris Matanes: wrote part of manuscript and reviewed literature.
Basel M.A. AbdelAzeem: wrote part of manuscript and reviewed literature.
Ayman Saad: reviewed and wrote part of the manuscript.
Vishnu Reddy: Reviewed manuscript and provided images.
Guarav Shah: Wrote part of the manuscript.
Nikolaos Papadantonakis: supervised and formulated case report; wrote manuscript. Review of literature.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Consent for publication: The provided information are de-identified and no health protected information is shared on this publication. Consent was provided by patient.
Availability of data and materials: Not applicable.
ORCID iD: Nikolaos Papadantonakis  https://orcid.org/0000-0003-1943-6421
https://orcid.org/0000-0003-1943-6421
Contributor Information
Faris Matanes, Jordan University of Science and Technology, Irbid, Jordan; and Vascular Biology and Hypertension Program, University of Alabama at Birmingham, USA.
Basel M.A. AbdelAzeem, Eldemerdash Hospital, Faculty of Medicine-Ain Shams University, Cairo, Egypt
Gaurav Shah, Internal Medicine, University of Alabama at Birmingham, Birmingham, AL, USA.
Vishnu Reddy, Department of Genetics, University of Alabama at Birmingham, Birmingham, AL, USA.
Ayman Saad, Division of Hematology, Ohio State University, Columbus, OH, USA.
Nikolaos Papadantonakis, Division of Hematology/Oncology, Department of Medicine, University of Alabama at Birmingham, 1720 2nd Avenue South, NP 2540, Birmingham, AL 35294-3300, USA.
References
- 1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127: 2391–2405. [DOI] [PubMed] [Google Scholar]
- 2. Guru Murthy GS, Dhakal I, Mehta P. Incidence and survival outcomes of chronic myelomonocytic leukemia in the United States. Leuk Lymphoma 2017; 58: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 3. Solh M, Solomon S, Morris L, et al. Extramedullary acute myelogenous leukemia. Blood Rev 2016; 30: 333–339. [DOI] [PubMed] [Google Scholar]
- 4. Byrd JC, Edenfield WJ, Shields DJ, et al. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol 1995; 13: 1800–1816. [DOI] [PubMed] [Google Scholar]
- 5. Elyamany G, Khan M, Hag El I, et al. Generalized lymphadenopathy as the first presentation of granulocytic sarcoma: a diagnostic challenge. Case Rep Med 2013; 2013: 483291–483295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horvath E, Demian S, Nagy E. Case report: myelodysplastic syndrome- associated myeloid sarcoma: an unusual clinical presentation of a rare disease. F1000Res 2016; 5: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orofino N, Cattaneo D, Bucelli C, et al. An unusual type of myeloid sarcoma localization following myelofibrosis: a case report and literature review. Leuk Res Rep 2017; 8: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoversten K, Vallapureddy R, Lasho TL, et al. Extramedullary manifestations in world health organization-defined chronic myelomonocytic leukemia: clinical, molecular and prognostic correlates. Blood 2017; 130(Suppl. 1): 4272–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strupp C, Germing U, Trommer I, et al. Pericardial effusion in chronic myelomonocytic leukemia (CMML): a case report and review of the literature. Leuk Res 2000; 24: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 10. Bowen JM, Perry AM, Quist E, et al. Extramedullary hematopoiesis in a sentinel lymph node as an early sign of chronic myelomonocytic leukemia. Case Rep Pathol 2015; 2015: 594970–594974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verhaak RGW, Goudswaard CS, van Putten W, et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 2005; 106: 3747–3754. [DOI] [PubMed] [Google Scholar]
- 12. Falini B, Lenze D, Hasserjian R, et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia 2007; 21: 1566–1570. [DOI] [PubMed] [Google Scholar]
- 13. Ernst T, Chase A, Zoi K, et al. Transcription factor mutations in myelodysplastic/myeloproliferative neoplasms. Haematologica 2010; 95: 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vallapureddy R, Lasho TL, Hoversten K, et al. Nucleophosmin 1 (NPM1) mutations in chronic myelomonocytic leukemia and their prognostic relevance. Am J Hematol 2017; 92: E614–E618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peng J, Zuo Z, Fu B, et al. Chronic myelomonocytic leukemia with nucleophosmin (NPM1) mutation. Euro J Haematol 2016; 96: 65–71. [DOI] [PubMed] [Google Scholar]
- 16. McCurdy SR, Lymphoma MLL. Emerging molecular predictive and prognostic factors in acute myeloid leukemia. Leuk Lymphoma 2017; 61: 1–19. [DOI] [PubMed] [Google Scholar]
- 17. Meggendorfer M, Roller A, Haferlach T, et al. SRSF2 mutations in 275 cases with chronic myelomonocytic leukemia (CMML). Blood 2012; 120: 3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duchmann M, Yalniz FF, Sanna A, et al. Prognostic role of gene mutations in chronic myelomonocytic leukemia patients treated with hypomethylating agents. EBio Med 2018; 31: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiao C, Zhang R, Hong M, et al. Heterogeneous leukemic clones identified by NPM1 mutation analysis in patient with acute monocytic leukemia. Leuk Lymphoma 2012; 53: 886–890. [DOI] [PubMed] [Google Scholar]
- 20. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012; 481: 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moriyama T, Metzger ML, Wu G, et al. Germline genetic variation in ETV6 and risk of childhood acute lymphoblastic leukaemia: a systematic genetic study. Lancet Oncol 2015; 16: 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang G, Fu B, Hu S, et al. Prognostic impact of acquisition of cytogenetic abnormalities during the course of chronic myelomonocytic leukemia. Am J Hematol 2015; 90: 882–887. [DOI] [PubMed] [Google Scholar]
- 23. Oki Y, Jelinek J, Shen L, et al. Induction of hypomethylation and molecular response after decitabine therapy in patients with chronic myelomonocytic leukemia. Blood 2008; 111: 2382–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beran M, Kantarjian H, O’Brien S, et al. Topotecan, a topoisomerase I inhibitor, is active in the treatment of myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 1996; 88: 2473–2479. [PubMed] [Google Scholar]
- 25. Beran M, Estey E, O’Brien S, et al. Topotecan and cytarabine is an active combination regimen in myelodysplastic syndromes and chronic myelomonocytic leukemia. J Clin Oncol 1999; 17: 2819–2830. [DOI] [PubMed] [Google Scholar]
- 26. Weihrauch MR, Staib P, Seiberlich B, et al. Phase I/II clinical study of topotecan and cytarabine in patients with myelodysplastic syndrome, chronic myelomonocytic leukemia and acute myeloid leukemia. Leuk Lymphoma 2004; 45: 699–704. [DOI] [PubMed] [Google Scholar]
- 27. Santini V, Allione B, Zini G, et al. A phase II, multicentre trial of decitabine in higher-risk chronic myelomonocytic leukemia. Leukemia 2018; 32: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2018 update on diagnosis, risk stratification and management. Am J Hematol 2018; 93: 824–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bell JA, Galaznik A, Huelin R, et al. Systematic literature review of treatment options and clinical outcomes for patients with higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia. Clin Lymphoma Myeloma Leuk 2018; 18: e157–e166. [DOI] [PubMed] [Google Scholar]
- 30. Alfonso A, Montalban-Bravo G, Garcia-Manero G. Current management of patients with chronic myelomonocytic leukemia. Curr Opin Oncol 2017; 29: 79–87. [DOI] [PubMed] [Google Scholar]
- 31. Mora E, Sanz GF. Current management of patients with chronic myelomonocytic leukemia. Curr Opin Oncol 2018; 30: 409–417. [DOI] [PubMed] [Google Scholar]
- 32. Sanz GF. A lot to learn about allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biol Blood Marrow Transplant 2017; 23: 713–714. [DOI] [PubMed] [Google Scholar]
- 33. de Witte T, Bowen D, Robin M, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood 2017; 129: 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eissa H, Gooley TA, Sorror ML, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia: relapse-free survival is determined by karyotype and comorbidities. Biol Blood Marrow Transplant 2011; 17: 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leukemia cutis in the setting of CMML: A predictor for leukemic transformation. J Am Acad Dermatol 2014; 70: AB117. [Google Scholar]
- 36. Di Battista V, Matteucci C, Pulini S, et al. Sensitivity of cutaneous chronic myelomonocytic leukaemia lesions to hypomethylating treatment. Eur J Dermatol 2017; 27: 540–542. [DOI] [PubMed] [Google Scholar]
- 37. Hoversten K, Vallapureddy R, Lasho T, et al. Nonhepatosplenic extramedullary manifestations of chronic myelomonocytic leukemia: clinical, molecular and prognostic correlates. Leuk Lymphoma 2018; 2015: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang S, Qin F, Yang L, et al. Nucleophosmin mutations induce chemosensitivity in THP-1 leukemia cells by suppressing NF-κB activity and regulating Bax/Bcl-2 expression. J Cancer 2016; 7: 2270–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Falini B, Martelli MP, Bolli N, et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood 2011; 117: 1109–1120. [DOI] [PubMed] [Google Scholar]
- 40. Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol 2011; 2: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang C, Chen Z, Li Z, et al. The essential roles of matrix metalloproteinase-2, membrane type 1 metalloproteinase and tissue inhibitor of metalloproteinase-2 in the invasive capacity of acute monocytic leukemia SHI-1 cells. Leuk Res 2010; 34: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 42. Stefanidakis M, Karjalainen K, Jaalouk DE, et al. Role of leukemia cell invadosome in extramedullary infiltration. Blood 2009; 114: 3008–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]