Abstract
The SLITs (SLIT1, SLIT2, and SLIT3) are a family of secreted proteins that mediate positional interactions between cells and their environment during development by signaling through ROBO receptors (ROBO1, ROBO2, ROBO3, and ROBO4). The SLIT/ROBO signaling pathway has been shown to participate in axonal repulsion, axon guidance, and neuronal migration in the nervous system and the formation of the vascular system. However, the role of the SLIT/ROBO pathway has not been thoroughly clarified in tumor development. The SLIT/ROBO pathway can produce both beneficial and detrimental effects in the growth of malignant cells. It has been confirmed that SLIT/ROBO play contradictory roles in tumorigenesis. Here, we discuss the tumor promotion and tumor suppression roles of the SLIT/ROBO pathway in tumor growth, angiogenesis, migration, and the tumor microenvironment. Understanding these roles will help us develop more effective cancer therapies.
Keywords: migration, proliferation, SLIT/ROBO pathway, tumor, tumor microenvironment
Introduction
The formation of tumors is modulated by multiple soluble and immobilized molecular factors including proteases, growth factors, extracellular matrix components, etc. Increasing evidence has shown that tumors can mimic embryonic development. Therefore, multiple molecular cytokines that affect embryonic development have been investigated in cancer progression. The SLIT/ROBO pathway is particularly involved in embryonic development.1 Gara et al.2 reviewed the roles of the SLIT/ROBO pathway in different types of cancer, molecular crosstalk, and the modulation of oncogenic signaling pathways. Huang et al.3 summarized the SLIT/ROBO pathway and its biological significance in gastrointestinal cancers. Recent studies showed that the SLIT/ROBO pathway can produce both beneficial and detrimental effects in the growth of malignant cells. It seems that SLIT/ROBO play contradictory roles in tumorigenesis. Therefore, in this brief review, the tumor promotion and tumor suppression roles of the SLIT/ROBO pathway and its biological significance in cancer will be summarized.
Overview of the SLIT/ROBO pathway
It has been shown that SLITs, ligands for Roundabout (ROBO), are required in preventing axons from recrossing the central nervous system midline in Drosophila.4 SLIT proteins are also identified as repulsion ligands for ROBO receptors in axon guidance in vertebrates including mammals.5,6 Thus far, three distinct SLIT genes (SLIT1, SLIT2, and SLIT3) and four distinct ROBO genes (ROBO1, ROBO2, ROBO3, and ROBO4) have been cloned in mammals.7–9
SLITs (SLIT1–SLIT3), are expressed in the nervous system, which have been found in Drosophila,10–12 Caenorhabditis elegans,13 xenopus,13 mice,6,13 chickens,13 humans,13 and rats.14,15 The protein can also be expressed in tumor cells,16 leukocytes,7 luteal cells,17 and other cells. Structurally, SLIT protein, a secreted glycoprotein from the N terminus to C terminus, contains four leucine-rich repeats (LRRs: D1–D4), seven (in Drosophila SLIT) to nine (in vertebrate SLIT) epidermal growth factor (EGF) repeats, a laminin G-like module, and a cysteine rich knot (Figure 1).12,18 The LRRs are sufficient for SLIT interaction with the receptor ROBO.19 Proteolytic processing of the human SLIT-2 protein gives rise to an N-terminal fragment (SLIT-N) and a C-terminal fragment (SLIT-C).5,6 SLIT-N contains all four LRRs and five of the EGF repeats (amino acids 1–1117) and SLIT-C contains the rest of the protein.5 Both the full-length protein and the fragments of SLIT can be secreted extracellularly.5,10–12 Studies have confirmed that SLIT2 is a large glycoprotein of approximately 200 kD that is typically processed into a 55–60 kD diffusible C-terminal fragment (SLIT2-C), a 140 kD N-terminal fragment (SLIT2-N), and a number of unknown fragments that are more tightly associated with cells. All SLIT fragments show different functional activities in vivo.20
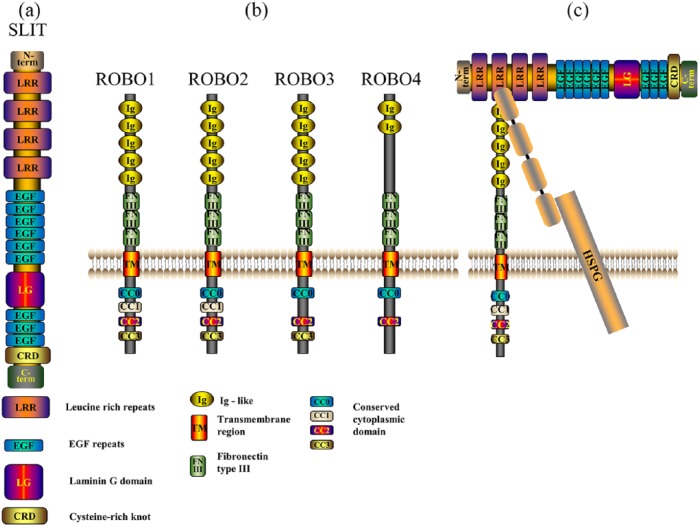
Figure 1.
Structure of the SLIT/ROBO protein family. (a) Structure of SLIT protein, a secreted glycoprotein from the N terminus to C terminus, contains four leucine-rich repeats (LRRs, D1–D4), seven to nine epidermal growth factor (EGF) repeats, a laminin G-like module and a cysteine-rich knot. (b) The ROBO family contain five immunoglobulin (Ig) domains, three fibronectin type III modules in the extracellular region, one transmembrane region and one intracellular conserved cytoplasmic domain including CC0, CC1, CC2, and CC3. (c) SLIT D2 binds on its concave surface to the Ig1 domain of ROBO through electrostatic and hydrophobic contact regions such as the heparan sulfate proteoglycan (HSPG) syndecan.
The ROBO family, which contains four members (ROBO1, ROBO2, ROBO3/Rig-1, and ROBO4/Magic), is a class of conserved transmembrane receptor proteins (Figure 1). They are mainly expressed in the nervous system but can also be expressed elsewhere such as in vascular endothelial cells and muscle cells.16 ROBO1, ROBO2, and ROBO3, which have been identified in organisms including Drosophila,21 C. elegans,22 zebrafish,23 human,21 and mouse,24 contain five Ig-like (immunoglobulin-like) domains, three fibronectin type III modules in the extracellular region, one transmembrane region, and one intracellular conserved cytoplasmic domain including CC0, CC1, CC2, and CC3.21,22 ROBO4 contains only two Ig-like functional areas and three fibronectin type III modules in the extracellular region, and one transmembrane region and one intracellular region including CC0 and CC2 small areas only.8,9
The SLIT-ROBO interaction is mediated by the second LRR domain of SLIT (D2) and the first two N-terminal Ig domains of ROBO,19 heparin sulfate (HS) disaccharide units are required to support SLIT-ROBO signaling.25 SLIT D2 binds on its concave surface to the Ig1 domain of ROBO through electrostatic and hydrophobic contact regions.19 By binding to their receptor ROBOs, SLITs activate intracellular signal transduction pathways in order to mediate biological effects on cells.26
In the nervous system, SLIT/ROBO signaling has been shown to be involved in axonal repulsion,4,27 neuronal migration,28 and axon guidance.29 SLITs and ROBOs also have an evolutionarily conserved role in preventing axons from migrating to inappropriate locations during the assembly of the nervous system.20 The SLIT/ROBO signal from the floor plate repels longitudinal axons away from the ventral midline and maintains straight longitudinal growth.30 In addition, SLIT proteins have also been shown to stimulate branching and elongation of sensory axons and cortical dendrites in vitro.5 It was reported that SLIT1 protein promotes neurite outgrowth and elongation when added to both adult rat dorsal root ganglion (DRG) and cultured DRG, where SLIT1/ROBO2 mRNA and protein were detected.31 SLIT/ROBO signaling also contributes to the patterning of both the peripheral and central branches of sensory neurons with distinct positive branching and negative guidance actions, respectively.32
SLITs and ROBOs are also expressed in non-neuronal tissues, such as mouse lung and kidney.33 Several SLITs and ROBOs have also been found to be aberrantly expressed during the development of ovarian, endometrial, cervical, and prostate cancer in the reproductive system.34 The SLIT/ROBO pathway in tumors have gradually received more attention. However, the roles of SLIT/ROBO pathway in tumors should be further discussed.
SLIT/ROBO pathway as an oncogene in tumor progression
In the last decade, studies have demonstrated the dual roles of SLITs and ROBOs as axon guidance cues in the developing nervous system, where they both attract and repel neuronal migration. This bifunctionality is also observed in cancers as both oncogenes and tumor suppressor genes. The expression of SLIT and ROBO is altered in a wide variety of cancer types, identifying them as potential therapeutic targets35 (Table 1).
Table 1.
Oncogenic and tumor-suppressive role of SLIT/ROBO signaling in human cancers.
| Tumor type | SLIT/ROBO pathway status | Function | References |
|---|---|---|---|
| Pancreatic cancer | ROBO3↑ | Oncogenic | Han et al.45 |
| Pancreatic cancer | SLIT2↓ | Tumor-suppressor | Goehrig et al.48 |
| Lung cancer | SLIT3↓ | Tumor-suppressor | Zhang et al.49 |
| Lung cancer | USP33 ↓ | Tumor-suppressor | Wen et al.50 |
| Colorectal cancer (CRC) | SLIT2↓; USP33 ↓ | Tumor-suppressor | Huang et al.51 |
| Colorectal cancer | ROBO1 ↑; ROBO4 ↑. | Oncogenic | Groene et al.36 |
| Colorectal cancer | SLIT2 ↑; ROBO1↑ | Oncogenic | Zhou et al.37 |
| Colorectal cancer | SLIT2↑; ROBO2↑ | Oncogenic | Sanz-Pamplona et al.52 |
| Breast cancer | srGAP3↓ | Tumor-suppressor | Lahoz and Hall53 |
| Mucoepidermoid carcinoma | SLIT2 ↑; ROBO1↑ | Oncogenic | Han et al.42 |
| Ovarian cancer | SLIT2↓; SLIT3↓; ROBO1↓ ROBO2↓; ROBO4↓ | Tumor-suppressor | Dickinson et al.54 |
| Oral squamous cell carcinoma | SLIT2↓ | Tumor-suppressor | Bauer et al.55 |
| Nasopharyngeal cancer | ROBO1↑ | Oncogenic | Alajez et al.56 |
| Hepatocellular cancer | SLIT1, SLIT2, and SLIT3 genes were methylated | Tumor-suppressor | Zheng et al.57 |
| Hepatocellular cancer | ROBO1↑; ROBO2↑; ROBO4↓; SLIT3↓ |
Oncogenic | Avci et al.41 |
| Cervical cancer | SLIT1 ↓; SLIT2 ↓; SLIT3 ↓ ROBO1 ↓; ROBO3 ↓ | Tumor-suppressor | Narayan et al.58 |
| Gliomas | SLIT2 ↓ | Tumor-suppressor | Dallol et al.59 |
| Prostate cancer | SLIT1 ↑; ROBO1 ↓ | Oncogenic | Latil et al.40 |
Previous studies have shown that high levels of SLITs and ROBOs are expressed in many types of tumors and SLIT/ROBO signaling has a positive effect on tumor growth. For instance, by means of microarray analysis and real-time polymerase chain reaction (PCR), Groene et al.36 showed that the expression of ROBO1 and ROBO4 was significantly upregulated in colorectal carcinoma compared with normal tissue, while SLIT2 showed no differential expression between colorectal carcinoma and normal tissue. ROBO1 expression was mainly in tumor cells, whereas ROBO4 was located primarily in the endothelial cells of tumor vessels.36 Zhou et al.37 demonstrated that the expression of SLIT2 and ROBO1 was significantly associated with an increased metastatic risk and poorer overall survival in colorectal carcinoma patients. ROBO1 and SLIT2 mRNAs were detected in breast cancer cell lines and breast cancer tissues, and ROBO1 expression was elevated in breast carcinoma compared with normal breast mammary epithelial cells.38,39 There was also a striking increase in SLIT1 expression in prostate tumors.40 Owing to the overexpression of ROBO1 in hepatocellular carcinoma and the shedding of ROBO1 into serum in humans, this receptor of SLITs was thought to be a potential new serological marker for hepatocellular carcinoma.41 In addition, SLIT2 and ROBO1 were overexpressed in human mucoepidermoid carcinoma Mc3 cells.42
SLIT2/ROBO1 pathway promoted the Mc3 cells proliferation and the treatment of Mc3 cells with the monoclonal antibody R5 which can interrupt the SLIT2/ROBO1 pathway caused significantly suppressed cell growth and proliferation and markedly lowered the expression of PCNA.42 Furthermore, SLIT2 expression was correlated with the loss of basement membrane in the samples of human skin squamous cell carcinoma at different stages of disease progression. The SLIT2-Tg mice were found to develop significantly more skin tumors than wild-type mice, the skin tumors that occurred in SLIT2-Tg mice were significantly larger than those in the wild-type mice after 7,12-dimethylbenz[a]anthracene initiation until the end of the experiment. SLIT2 also could promote the invasive ability of the squamous cell carcinoma cell line A431 and this effect could be significantly repressed by the antibody R5.43
Tumor metastasis, the process of tumor cells migrating to other distant organs, invading blood and lymphatic vessels and leading to secondary tumor formation, is important for cancer development. Recent studies indicated that the SLIT/ROBO signaling pathway plays a role in promoting tumor cell migration and, thus, promotes tumor metastasis. SLIT2 functions as a potent chemo-attractant for breast cancer cells, inducing migration of cells expressing ROBO1. Furthermore, the SLIT2/ROBO1 signal was shown to upregulate MMP-9 to enhance breast cancer cell invasion.39 Low mRNA expression of the ROBO2 was associated with poor patient survival, whereas high mRNA expression of ROBO3, a known inhibitor of ROBO2 signaling, demonstrated an appropriate reciprocal inverse association with poor survival in pancreatic cancer.44 ROBO3 expression is upregulated in pancreatic cancer tissue samples and pancreatic cancer cell lines. Overexpression of ROBO3, which was associated with activated Wnt/β-catenin and GSK-3β, and other markers indicating epithelial–mesenchymal transition (EMT), promotes pancreatic cancer cell growth, invasion, and metastasis in vitro and in mouse xenograft tumor models. MiR-383 was also identified as a suppressor of ROBO3, and its expression was inversely correlated with ROBO3.45 To further explore the SLIT/ROBO signaling in tumor progression, Yang et al.46 intercrossed SLIT2 transgenic mice with a nonmetastatic RIP1-Tag2 mouse tumor model. They found that transgenic overexpression of SLIT2 significantly enhanced tumor lymph angiogenesis and subsequently promoted mesenteric lymph node metastasis of pancreatic islet tumors. SLIT2 was expressed minimally in normal and hyperplastic mucosa, moderately in dysplastic mucosa, and highly in neoplastic mucosa obtained from hamster buccal pouch in a multistage model of 7,12-dimethyl-1,2-benzanthracene-induced squamous cell carcinoma, and increased SLIT2 expression was associated with higher tumor angiogenesis.47
Importantly, interruption of the SLIT2–ROBO1 interaction using R5 inhibited tumor angiogenesis and growth in an in vivo model. Therefore, targeting SLIT/ROBO signaling may offer a novel approach for oral cancer therapy (Figure 2).
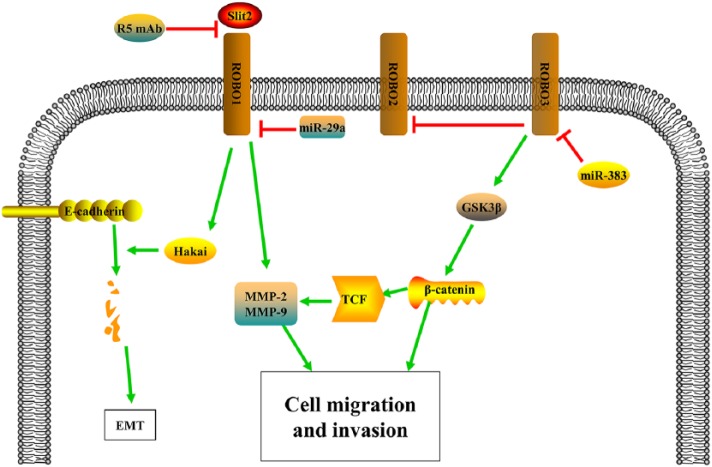
Figure 2.
Mechanisms of the SLIT/ROBO pathway as an oncogene in cancer. The SLIT2/ROBO1 signal upregulates MMP-2 and MMP-9, thus promotes cell migration and invasion. SLIT2/ROBO1 signal recruits a ubiquitin ligase Hakai for E-cadherin ubiquitination and lysosomal degradation and thus promotes the epithelial–mesenchymal transition (EMT). The monoclonal antibody R5, which can interrupt the SLIT2/ROBO1 pathway, causes significantly suppressed cell growth and proliferation. ROBO3 promotes cancer cell growth, invasion and metastasis, which is associated with activated Wnt pathway components, β-catenin and GSK-3β, and other markers indicating the EMT, and miR-383 functions as a suppressor of ROBO3.
As is well known, the EMT is one of the initiating steps that play a key role during tumor invasion and metastasis. In colorectal epithelial carcinoma cells, recombinant SLIT2 inducing ROBO1 expression recruited a ubiquitin ligase Hakai for E-cadherin ubiquitination and lysosomal degradation, thus promote the EMT, tumor growth, and liver metastasis. Moreover, this effect can be attenuated by knockdown of Hakai.37
Therefore, the SLIT2, ROBO1, and ROBO3 may function as oncogenes that promote cancer proliferation and metastasis which may provide potential target structures for the antitumorigenic and anti-angiogenic therapy of these special carcinomas.
SLIT/ROBO pathway as tumor suppressor genes
Compared with several studies on promoting tumor progression, SLITs and ROBOs are tumor suppressor genes in some special tumors. Here we further elucidate the anticancer function of the SLIT/ROBO pathway.
It has been shown that SLIT/ROBO pathway genes are frequently inactivated by promoter region’s hypermethylation, resulting in downregulated gene expression in many human cancers.58,60–62 SLIT2 was methylated in 71% (5/7) of glioma cell lines and in 59% (37/63) of other tumors and the SLIT2 expression was downregulated in methylated gliomas tumor samples, which indicated that SLIT2 was frequently inactivated by promoter region CpG island hypermethylation in gliomas and might be a good candidate for a glioma tumor suppressor gene.59 SLIT2 expression was reduced in CRC tissues because of hypermethylation of the SLIT2 gene in CRC cells, and SLIT2 could inhibit CRC cell migration that required USP33 by deubiquitinating and stabilizing ROBO1.51 Another study also showed that USP33 was downregulated in lung cancer patients and that low expression of USP33 was associated with poor prognosis, which may be associated with reduced protein stability of ROBO1 in lung cancer cells.50
SLITs and ROBOs inhibit cancer cell proliferation and invasion
Basal SLIT2, SLIT3, ROBO1, ROBO2, and ROBO4 expression level was lower in primary cultures of ovarian cancer epithelial cells when compared to normal OSE and in poorly differentiated SKOV-3 cells compared with the more differentiated PEO-14 cells. Furthermore, blocking SLIT/ROBO activity reduced apoptosis in both PEO-14 and SKOV-3 tumor cells.54 SLIT/ROBO signaling was shown to decrease the proliferative rate and increase the apoptotic rate of the oral squamous cell carcinoma line Tb through regulating Fas-FasL proteins, and this effect could be interrupted by R5 which could neutralize the binding of ROBO1 to SLIT2.63
The overall expression of SLIT3 is low in lung tumor tissues compared with normal tissues. Silencing of SLIT3 induced EMT by downregulation of E-cadherin and upregulation of vimentin, and enhanced MMP2 and MMP9 expression, thus promoting proliferation, migration, and invasion of A549 cells.49 In addition, treatment with SLIT3 led to strong inhibition of migration in malignant melanoma cells, and downregulation of AP-1 activity and target gene expression contributed to the negative regulation of migration.64 In pancreatic cancer, SLIT2 mRNA expression was reduced in human pancreatic ductal adenocarcinoma (PDAC) and correlated with lymphatic metastasis. SLIT2 inhibited directed migration and invasion of PDAC cells in vitro. To further address the effects of SLIT2 on PDAC growth and progression in vivo, MiaPaCa2-SLIT2 cells were grown as orthotopic xenografts and it was found that SLIT2 and ROBO1 inhibited invasion, metastasis, and angiogenesis of PDAC xenografts in vivo. Recombinant human SLIT2 dose-dependently decreased migration of oral squamous cell carcinoma (OSCC) cells PCI52-PC, which might be associated with an interaction of P-cadherin with ROBO3, a high affinity receptor of SLIT2. Downregulation of ROBO3 expression via siRNA neutralized SLIT2 induced migration block in PCI52-PC cells. This phenomenon demonstrated that the effect of SLIT2 on P-cadherin expressing OSCC cells was supposedly via modulation of ROBO3 interaction.55
SLIT2/ROBO1 pathway interrupts the HGF/c-MET mediating cancer progression
The hepatocyte growth factor (HGF) and its receptor, the transmembrane tyrosine kinase c-MET, promote cell proliferation, survival, motility, and play a crucial role both in tumor progression.65 The shRNA-mediated depletion of SLIT2 or ectopic expression of a soluble decoy ROBO enhance HGF-induced migration, matrix invasion, accompany with the upregulation of Cdc-42 and the downregulation of Rac-1 activities. Accordingly, autocrine overexpression or exogenous administration of SLIT2 prevent HGF-induced motile responses, reduce Cdc-42 activation, and stimulation of Rac–1.66 In addition, medulloblastoma invasion was inhibited after recombinant SLIT2 protein treatment, which was accompanied with downregulation of activated Cdc42.67
SLIT2/ROBO1 pathway prevents the CXCL12/CXCR4 induced cancer progression
Upregulation of CXCR4 is associated with poor prognosis in breast cancer and pancreatic cancer. Marlow et al. showed that loss of SLITs (SLIT2, SLIT3) or their ROBO1 receptor in murine mammary gland or human breast carcinoma cells resulted in coordinate upregulation of the CXCL12 and CXCR4 signaling axis, which was accompanied by hyperplastic changes in cells and desmoplastic alterations in the surrounding stroma. Furthermore, SLIT overexpression down-regulated CXCR4 and dominantly suppressed tumor growth in a xenograft model.68 SLIT2 treatment inhibited CXCL12/CXCR4-induced breast cancer cell metastasis. SLIT2 inhibited CXCL12-induced tyrosine phosphorylation of focal adhesion components such as RAFTK/Pyk2 at residues 580 and 881, focal adhesion kinase at residue 576, and Paxillin. It was also found that SLIT2 inhibited CXCL12-induced phosphatidylinositol 3-kinase (PI3K), p44/42 MAPK, and MMP-2 and MMP-9 activities, but it did not have an effect on JNK and p38 MAPK activities.69 These studies classified SLITs as negative regulators of the CXCL12/CXCR4 pathway and identified a molecular signature in hyperplastic breast lesions that signified inappropriate upregulation of key prometastatic genes.68
In addition, there were some studies that revealed that SLIT/ROBO signaling might not play an important role in regulating human cancer cell proliferation and migration. Dai et al. found that three major members (SLIT2, SLIT3, and ROBO1) of the SLIT/ROBO family were widely expressed in the human normal and malignant ovarian tissues and in OVCAR-3 and SKOV-3 cells.70 However, recombinant human SLIT2 did not significantly affect SKOV-3 cell migration, and OVCAR-3 and SKOV-3 cell proliferation. SLIT2 also did not induce ERK1/2 and AKT1 phosphorylation in OVCAR-3 and SKOV-3 cells. Sanz-Pamplona et al. also demonstrated that no significant association between SLIT2 or SLIT3 level of expression and prognosis was found in colon cancer.52
In short, these inconsistent results showed the complexity of the role of the SLIT/ROBO signaling pathway in tumor proliferation. More thorough and careful studies are needed to clarify the precise role of this pathway (Figure 3).
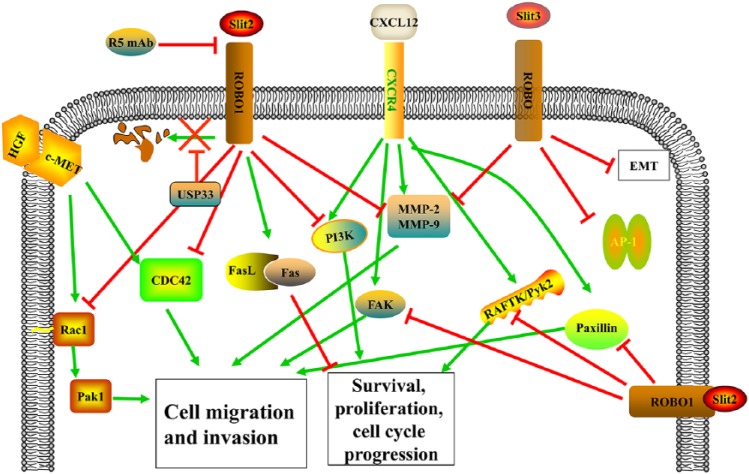
Figure 3.
Mechanisms of the SLIT/ROBO pathway as a tumor-suppressor gene in tumor progression. SLIT2/ROBO1 signal decreases the proliferative rate and increases the apoptotic rate through regulating Fas-FasL proteins and PI3K/AKT pathway. USP33 can inhibit the lysosomal degradation of ROBO1, and SLIT3 leads to strong inhibition of migration through downregulation of AP-1 activity and targeting vimentin, MMP2, and MMP9. SLIT2/ROBO1 signal prevents hepatocyte growth factor (HGF)-induced motile responses, reduces Cdc-42 activation, and stimulates Rac-1 activities and, thus, inhibits cell proliferation, survival, and motility. SLIT2/ROBO1 functions as negative regulators of CXCL12/CXCR4 pathway through inhibiting CXCL12-induced tyrosine phosphorylation of focal adhesion components such as RAFTK/Pyk2, FAK, and Paxillin.
Downstream and crosstalk of the SLIT/ROBO pathway
SLIT/ROBO signaling is important for tumor development, but its mechanisms in tumors remain unclear. There are several molecules that have been confirmed to be downstream factors of the SLIT/ROBO pathway. A study in Drosophila provided evidence that both the Abelson (Abl) tyrosine kinase and its substrate Enabled (Ena), which binds directly to ROBO’s cytoplasmic domain, played opposing roles in ROBO signal transduction. Abl and Ena may bind CC2, which was partly responsible for ROBO repulsion in the midline, whereas Abl antagonized ROBO-mediated repulsion by phosphorylating a tyrosine residue in CC1.71 According to their work, Wong et al. showed SLIT bound ROBO (ROBO1) through the CC3 motif and the C-terminal sequence of ROBO to interact with SLIT–ROBO GTPase activating proteins (srGAPs), a new member of the Rho GTPase activating proteins (GAPs). They further showed that srGAPs inactivated Cdc42, resulting in reconstruction of cytoskeletal proteins via regulation of actin polymerization to inhibit cell migration in the mammalian nervous system. Ubiquitin-specific protease 33 (USP33)/VDU1 was also shown to be involved in ROBO1–USP33 interaction and participate in SLIT/ROBO signaling in cancer cell migration.72 Another study also showed that srGAP1 is an important downstream molecule of Slit2 signaling in CRC, and mediates the antimigration function of Slit2 by inhibiting Cdc42.73 SrGAP2 protein expression is reduced or absent in a subset of primary osteosarcoma samples, srGAP2 and other axon guidance proteins likely play a role in osteosarcoma metastasis.74
The intracellular signal transduction pathway that includes Abelson kinase, Enabled protein, GAPs, and the Rho family of small GTPases may also play a role in endothelial cells. In addition, PI3K is important for endothelial cell responses to SLIT2, but the mechanism remains unknown.75 Apart from this, it was shown that cellular protrusive activity was inhibited via a SLIT2–ROBO4–paxillin–GIT1 network.71
The study led by Zhang and Zhou demonstrated that downregulation of ROBO1 using small interfering RNA inhibited mesenchymal stem cell (MSC) proliferation.76 In addition, four miRNAs (miR), including miR-218, miR-29a, miR-146, and miR-148, inhibited the protein expression of ROBO1 in the MSCs, with miR-29 having the most marked effect. ROBO1 was identified as a novel target of miR-29a with a luciferase reporter assay. Overexpression of miR-29a suppressed the protein expression levels of ROBO1 and SLIT2 and inhibited the viability and proliferation of the MSCs. By contrast, overexpression of ROBO1 partly rescued these inhibitory effects of miR-29a on the MSCs. These results indicated that the miR29a/ROBO1 axis was crucial for the regulation of MSC viability and proliferation, suggesting that miR29a may serve as a potential clinical target for MSC expansion and stem cell transplantation. In addition, miR-218 suppressed nasopharyngeal cancer progression through downregulating the SLIT2-ROBO1 pathway in a negative feedback loop manner.56
The role of SLIT/ROBO in the tumor microenvironment
The tumor microenvironment is composed of tumor cells, stromal cells, and other cellular components as well as extracellular matrix and various molecules that mediate intercellular interactions. These components form a complex tumor microenvironment that prominently affects the occurrence, growth, invasion, metastasis, and drug resistance.77 Here we further investigate the role of SLIT/ROBO pathway in the tumor microenvironment.
SLIT/ROBO pathway in tumor angiogenesis
The SLIT/ROBO signal is critical for axon guidance and neuronal precursor cell migration in the nervous system. Evidence suggested that classical neuronal guidance cues also regulated vascular development.78 Expression of ROBO1 and ROBO4 has been observed in vascular endothelial cells. They may serve different functions in SLIT/ROBO signaling due to differential expression in various phenotypes of endothelial cells, ROBO1 induced long and thin actin fibers, whereas ROBO4 induced short and thick actin bundles along with membrane ruffles.79 SLIT3, which was also observed in endothelial cells and vascular smooth muscle cells, promoted angiogenesis but decreased neurogenesis.80 Here, we focus on SLIT/ROBO signaling in angiogenesis via SLIT/ROBO1 and SLIT/ROBO4. The SLIT2 was expressed in a broad spectrum of tumor cell lines and interacted with human umbilical vein endothelial cells (HUVECs) and tumor-associated endothelial cells in the presence of ROBO1. Tumor cells appeared to secrete the SLIT2 protein, which formed a gradient field for the attraction of endothelial cells through interaction with ROBO1 on endothelial cell surface. Thus, endothelial cells migrated toward tumor and formed new blood vessels.16 The SLIT/ROBO signaling inhibited tumor angiogenesis and growth in a model of chemically induced squamous cell carcinoma when blocked by R5, a monoclonal antibody against the first immunoglobulin domain of ROBO1.47 The observation that ROBO1 was highly expressed in the early postnatal days of C57BL/6J mice correlated with superficial vascular and deep vascular plexus formation, indicating that ROBO1 might participate in retinal neovascularization.81 Thus, SLIT/ROBO1 signaling may promote angiogenesis in tumors.
ROBO4 is a vascular-specific receptor and mainly expressed in active angiogenesis, especially tumor vessels.8,9,82 Previous studies showed that ROBO4 inhibited endothelial cell migration through interaction with SLIT2, suggesting that ROBO4 might negatively regulate new vessel formation. This point was supported by several studies focusing on HUVECs, microvascular endothelial cells,83–85 and an animal model of ocular angiogenesis.86 In addition, Acevedo et al. suggested that SLIT2 could both positively and negatively regulate angiogenesis by binding to ROBO1 and ROBO4, respectively, and activation of ROBO4 blocked vascular endothelial growth factor (VEGF)-induced angiogenesis and vascular permeability.87 However, a study by Huang et al. suggested that ROBO4 expressed much in fibrovascular membranes (FVMs), and loss of ROBO4 disturbed tube formation.88 Knockdown or overexpression of ROBO4 impaired intersomitic vessels formation in zebrafish showed contradictory functions of ROBO4 in angiogenesis.89 Furthermore, using siRNA knockdown of both ROBO1 and ROBO4 decreased endothelial motility and disrupted tube formation.79
Taking these studies together, it is suggested that SLIT/ROBO1 and SLIT/ROBO4 signaling might interact with each other and cooperate in tumor angiogenesis (Figure 4).
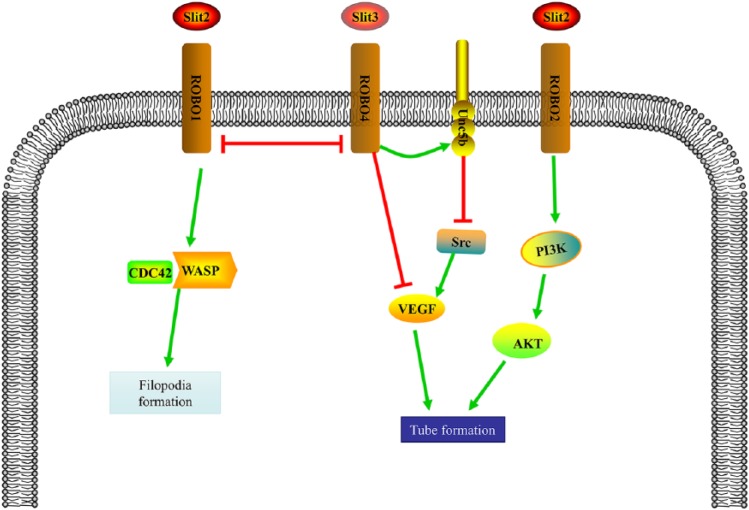
Figure 4.
Mechanisms of the SLIT/ROBO pathway in tumor angiogenesis. SLIT2 and SLIT3 lead to bidirectional adjusting of angiogenesis. Through modulating the activity of ROBO1, ROBO2, ROBO4, Src, and vascular endothelial growth factor (VEGF) pathway, endothelial cell behaviors, including tube formation and filopodia formation, could be regulated.
SLIT/ROBO signaling in inflammation
SLIT/ROBO signaling influences neuronal migration, axon guidance, and also functions in a similar way in directing inflammatory cells discrepantly. SLIT2 is involved in inflammation modulating in several ways. SLIT2/ROBO1 selectively impaired directional migration of neutrophils and T cells toward chemoattractant especially CXCL12/CXCR4-induced chemotaxis90,91 and neutrophil recruitment,92 and also mediated inhibition of Langerhans cell migration resulting in suppression of contact hypersensitivity responses.93 However, during lung inflammation, SLIT2/ROBO1 led to enhancement of eotaxin-induced eosinophil chemotaxis, and exaggeration of allergic airway inflammation.94 Furthermore, through SLIT2/ROBO4 interaction, SLIT2 could regulate endothelial related inflammation by inhibiting vascular leak and stabilizing the vasculature by downregulating lipopolysaccharide or VEGF.85,95 In patients with acute kidney injury (AKI), higher levels of plasma ROBO4 suggested a link between endothelial dysregulation and onset of AKI.96 SLIT3 took a role in increasing the spontaneous and chemoattractant-induced migration of primary monocytes by inducing a chemokinetic effect.97
SLIT/ROBO particles have shown their potentials as markers or targets in treating inflammatory diseases such as periodontitis, AKI, and crescentic glomerulonephritis (Figure 5).96,98
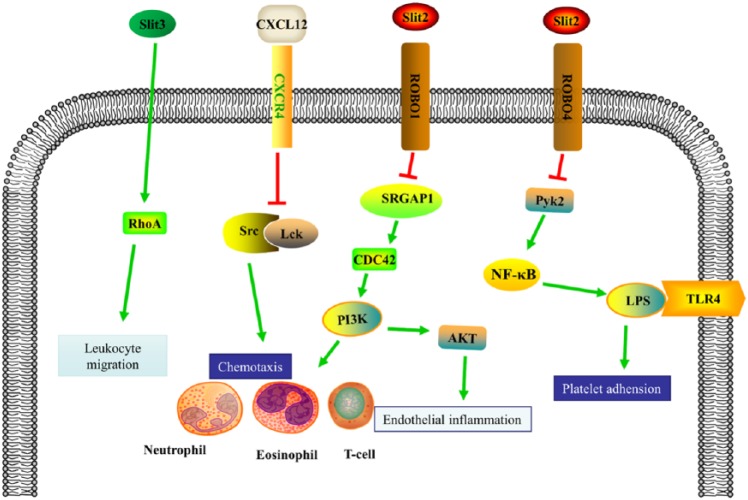
Figure 5.
Mechanisms of the SLIT/ROBO pathway in tumor inflammation process. SLIT2 is involved in inflammation modulated by ROBO1, ROBO4, and AKT pathway. The downstream pathways of ROBO1 and ROBO4 including PI3K and nuclear factor (NF)-kB pathways involve in chemotaxis of inflammatory cells (T cells, Langerhans cells, neutrophils, and eosinophils) and angio-associated components (endothelial cells, platelets). SLIT3 regulates CXCL12/CXCR4 and RhoA in modulating inflammation, chiefly monitoring inflammatory migration and chemotaxis.
SLIT/ROBO plays a dual role in tumor neural invasion
Perineural invasion (PNI) is considered as an alternative route for the metastatic spread of pancreatic cancer cells; however, the molecular changes leading to PNI are still poorly understood. Andreas et al. showed that disrupting SLIT2–ROBO signaling in PDAC might enhance metastasis and PDAC cells to neural invasion. It has been found that a reduction in SLIT2–ROBO pathway activity existed in PDAC and restoring the SLIT2 expression in SLIT2-deficient PDAC cells inhibited their bidirectional chemoattraction with neural cells.48 However, another group showed that SLIT2 was expressed by cancer-associated fibroblasts (CAFs), increasing neurite outgrowth from dorsal root ganglia neurons as well as from Schwann cell migration or proliferation. Inhibition of SLIT2/ROBO signaling disrupted this stromal/neural connection.99
The controversial effects of SLIT/ROBO on tumor neural invasion reflect its complicated role in tumor progression and need to further be explored.
Conclusion
There is a close relationship between abnormal signaling and tumor development. To develop effective cancer therapeutics, it is useful to understand how SLIT/ROBO signaling affects tumor formation and angiogenesis and the mechanisms by which SLIT/ROBO signaling exerts these effects. To develop an effective therapeutic approach for cancer treatment, additional studies are required, also taking into account the important regulation mediated by SLIT/ROBO pathway.
Footnotes
Author Contributions: Zhengdong Jiang and Gang Liang conceived the concept for this paper. All authors (Ying Xiao, Tao Qin, and Xin Chen) collected the available literature. Erxi Wu, Qingyong Ma, and Zheng Wang revised the manuscript critically. Zhengdong Jiang and Gang Liang contributed equally to this work.
Funding: This work was supported by the National Natural Science Foundation of China (Grant Numbers 81672434 and 81472248).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Qingyong Ma  https://orcid.org/0000-0001-8977-320X
https://orcid.org/0000-0001-8977-320X
Contributor Information
Zhengdong Jiang, Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China.
Gang Liang, Department of Hepatobiliary Surgery, No. 215 Hospital of Shaanxi Nuclear Industry, Xianyang, Shaanxi, China.
Ying Xiao, Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China.
Tao Qin, Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China.
Xin Chen, Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China.
Erxi Wu, Department of Neurosurgery, Neuroscience Institute, Baylor Scott and White Health, Temple, TX, USA; Neuroscience Institute, Baylor Scott & White Health, Temple, TX, USA; Department of Surgery, Texas A & M University Health Science Center, College of Medicine, TX, USA; Department of Pharmaceutical Sciences, Texas A & M University College of Pharmacy, College Station, TX, USA; LIVESTRONG Cancer Institutes, Dell Medical School, the University of Texas at Austin, Austin, TX, USA.
Qingyong Ma, Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, China.
Zheng Wang, Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, China.
References
- 1. Li Y, Zhang XT, Wang XY, et al. Robo signaling regulates the production of cranial neural crest cells. Exp Cell Res 2017; 361: 73–84. [DOI] [PubMed] [Google Scholar]
- 2. Gara RK, Kumari S, Ganju A, et al. Slit/Robo pathway: a promising therapeutic target for cancer. Drug Discov Today 2015; 20: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang T, Kang W, Cheng AS, et al. The emerging role of Slit-Robo pathway in gastric and other gastro intestinal cancers. BMC Cancer 2015; 15: 950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McConnell RE, van Veen JE, Vidaki M, et al. A requirement for filopodia extension toward Slit during Robo-mediated axon repulsion. J Cell Biol 2016; 213: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang KH, Brose K, Arnott D, et al. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 1999; 96: 771–784. [DOI] [PubMed] [Google Scholar]
- 6. Brose K, Bland KS, Wang KH, et al. Slit proteins bind robe receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999; 96: 795–806. [DOI] [PubMed] [Google Scholar]
- 7. Geutskens SB, Andrews WD, van Stalborch AMD, et al. Control of human hematopoietic stem/progenitor cell migration by the extracellular matrix protein Slit3. Lab Invest 2012; 92: 1129–1139. [DOI] [PubMed] [Google Scholar]
- 8. Huminiecki L, Gorn M, Suchting S, et al. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics 2002; 79: 547–552. [DOI] [PubMed] [Google Scholar]
- 9. Dickinson RE, Hryhorskyj L, Tremewan H, et al. Involvement of the SLIT/ROBO pathway in follicle development in the fetal ovary. Reproduction 2010; 139: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hummel T, Schimmelpfeng K, Klambt C. Commissure formation in the embryonic CNS of Drosophila I. Identification of the required gene functions. Dev Biol 1999; 209: 381–398. [DOI] [PubMed] [Google Scholar]
- 11. Kidd T, Brose K, Mitchell KJ, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998; 92: 205–215. [DOI] [PubMed] [Google Scholar]
- 12. Rothberg JM, Jacobs JR, Goodman CS, et al. Slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF And LRR domains. Genes Dev 1990; 4: 2169–2187. [DOI] [PubMed] [Google Scholar]
- 13. Hao JC, Yu TW, Fujisawa K, et al. C-elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron 2001; 32: 25–38. [DOI] [PubMed] [Google Scholar]
- 14. Yi X-N, Zheng L-F, Zhang J-W, et al. Dynamic changes in Robo2 and Slit1 expression in adult rat dorsal root ganglion and sciatic nerve after peripheral and central axonal injury. Neurosci Res 2006; 56: 314–321. [DOI] [PubMed] [Google Scholar]
- 15. Han X, Zhang M-C. Potential anti-angiogenic role of Slit2 in corneal neovascularization. Exp Eye Res 2010; 90: 742–749. [DOI] [PubMed] [Google Scholar]
- 16. Su X, Ren Y, Yu N, et al. Thymoquinone inhibits inflammation, neoangiogenesis and vascular remodeling in asthma mice. Int J Immunopharmacol 2016; 38: 70–80. [DOI] [PubMed] [Google Scholar]
- 17. Dickinson RE, Myers M, Duncan WC. Novel regulated expression of the SLIT/ROBO pathway in the ovary: possible role during luteolysis in women. Endocrinology 2008; 149: 5024–5034. [DOI] [PubMed] [Google Scholar]
- 18. Holmes GP, Negus K, Burridge L, et al. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mechan Dev 1998; 79: 57–72. [DOI] [PubMed] [Google Scholar]
- 19. Morlot C, Thielens NM, Ravelli RBG, et al. Structural insights into the Slit-Robo complex. Proc Natl Acad Sci U S A 2007; 104: 14923–14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chedotal A. Slits and their receptors. In:Bagnard D. (ed.) Advances in experimental medicine and biology. Vol. 621 New York: Springer, 2007, pp.65–80. [DOI] [PubMed] [Google Scholar]
- 21. Evans TA, Bashaw GJ. Functional diversity of robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr Biol 2010; 20: 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J, Ding M. Robo and Ror function in a common receptor complex to regulate Wnt-mediated neurite outgrowth in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2018; 115: E2254–E2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devine CA, Key B. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev Biol 2008; 313: 371–383. [DOI] [PubMed] [Google Scholar]
- 24. Jaworski A, Tom I, Tong RK, et al. Operational redundancy in axon guidance through the multifunctional receptor Robo3 and its ligand NELL2. Science 2015; 350: 961–965. [DOI] [PubMed] [Google Scholar]
- 25. Fukuhara N, Howitt JA, Hussain S-A, et al. Structural and functional analysis of Slit and heparin binding to immunoglobulin-like domains 1 and 2 of Drosophila Robo. J Biol Chem 2008; 283: 16226–16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dascenco D, Erfurth M-L, Izadifar A, et al. Slit and receptor tyrosine phosphatase 69D confer spatial specificity to axon branching via Dscam1. Cell 2015; 162: 1140–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li HS, Chen JH, Wu W, et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 1999; 96: 807–818. [DOI] [PubMed] [Google Scholar]
- 28. Carr L, Parkinson DB, Dun XP. Expression patterns of Slit and Robo family members in adult mouse spinal cord and peripheral nervous system. PLoS One 2017; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown HE, Reichert MC, Evans TA. In vivo functional analysis of drosophila Robo1 fibronectin type-III repeats. G3 2018; 8: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mastick GS, Farmer WT, Altick AL, et al. Longitudinal axons are guided by Slit/Robo signals from the floor plate. Cell Adh Migr 2010; 4: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang HY, Zheng LF, Yi XN, et al. Slit1 promotes regenerative neurite outgrowth of adult dorsal root ganglion neurons in vitro via binding to the Robo receptor. J Chem Neuroanat 2010; 39: 256–261. [DOI] [PubMed] [Google Scholar]
- 32. Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci 2007; 27: 6843–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xian J, Clark KJ, Fordham R, et al. Inadequate lung development and bronchial hyperplasia in mice with a targeted deletion in the Dutt1/Robo1 gene. Proc Natl Acad Sci U S A 2001; 98: 15062–15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction 2010; 139: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ballard MS, Hinck L. A. roundabout way to cancer. In: Daar IO. (ed.) Advances in cancer research. San Diego: Academic Press, 2012, pp.187–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Groene J, Doebler O, Loddenkemper C, et al. Robo1/Robo4: differential expression of angiogenic markers in colorectal cancer. Oncol Rep 2006; 15: 1437–1443. [PubMed] [Google Scholar]
- 37. Zhou W-J, Geng ZH, Chi S, et al. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res 2011; 21: 609–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhattacharya R, Mukherjee N, Dasgupta H, et al. Frequent alterations of SLIT2-ROBO1-CDC42 signalling pathway in breast cancer: clinicopathological correlation. J Genet 2016; 95: 551–563. [DOI] [PubMed] [Google Scholar]
- 39. Schmid BC, Rezniczek GA, Fabjani G, et al. The neuronal guidance cue Slit2 induces targeted migration and may play a role in brain metastasis of breast cancer cells. Breast Cancer Res Treat 2007; 106: 333–342. [DOI] [PubMed] [Google Scholar]
- 40. Latil A, Chene L, Cochant-Priollet B, et al. Quantification of expression of netrins, slits and their receptors in human prostate tumors. Int J Cancer 2003; 103: 306–315. [DOI] [PubMed] [Google Scholar]
- 41. Avci ME, Konu O, Yagci T. Quantification of SLIT-ROBO transcripts in hepatocellular carcinoma reveals two groups of genes with coordinate expression. BMC Cancer 2008; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han B, Wang L, Wang J, et al. Role of Slit-Robo signaling in the proliferation of human mucoepidermoid carcinoma Mc3 cells. J South Med Univ 2012; 32: 37–39. [PubMed] [Google Scholar]
- 43. Qi C, Lan H, Ye J, et al. Slit2 promotes tumor growth and invasion in chemically induced skin carcinogenesis. Lab Invest 2014; 94: 766–776. [DOI] [PubMed] [Google Scholar]
- 44. Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016; 531: 47. [DOI] [PubMed] [Google Scholar]
- 45. Han S, Cao C, Tang T, et al. ROBO3 promotes growth and metastasis of pancreatic carcinoma. Cancer Lett 2015; 366: 61–70. [DOI] [PubMed] [Google Scholar]
- 46. Yang X-M, Han H-X, Sui F, et al. Slit-Robo signaling mediates lymphangiogenesis and promotes tumor lymphatic metastasis. Biochem Biophys Res Commun 2010; 396: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang L-J, Zhao Y, Han B, et al. Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Sci 2008; 99: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goehrig A, Detjen KM, Hilfenhaus G, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res 2014; 74: 1529–1540. [DOI] [PubMed] [Google Scholar]
- 49. Zhang C, Guo H, Li B, et al. Effects of Slit3 silencing on the invasive ability of lung carcinoma A549 cells. Oncol Rep 2015; 34: 952–960. [DOI] [PubMed] [Google Scholar]
- 50. Wen P, Kong R, Liu J, et al. USP33, a new player in lung cancer, mediates Slit-Robo signaling. Protein Cell 2014; 5: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang Z, Wen P, Kong R, et al. USP33 mediates Slit-Robo signaling in inhibiting colorectal cancer cell migration. Int J Cancer 2015; 136: 1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanz-Pamplona R, Berenguer A, Cordero D, et al. Aberrant gene expression in mucosa adjacent to tumor reveals a molecular crosstalk in colon cancer. Mol Cancer 2014; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lahoz A, Hall A. A tumor suppressor role for srGAP3 in mammary epithelial cells. Oncogene 2013; 32: 4854–4860. [DOI] [PubMed] [Google Scholar]
- 54. Dickinson RE, Fegan KS, Ren X, et al. Glucocorticoid regulation of SLIT/ROBO tumour suppressor genes in the ovarian surface epithelium and ovarian cancer cells. PLoS One. 2011; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bauer K, Dowejko A, Bosserhoff AK, et al. Slit-2 facilitates interaction of P-cadherin with Robo-3 and inhibits cell migration in an oral squamous cell carcinoma cell line. Carcinogenesis 2011; 32: 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alajez NM, Lenarduzzi M, Ito E, et al. miR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res 2011; 71: 2381–2391. [DOI] [PubMed] [Google Scholar]
- 57. Zheng D, Liu B-B, Liu Y-K, et al. Analysis of the expression of Slit/Robo genes and the methylation status of their promoters in the hepatocellular carcinoma cell lines. Zhonghua Gan Zang Bing Za Zhi 2009; 17: 198–202. [PubMed] [Google Scholar]
- 58. Narayan G, Goparaju C, Arias-Pulido H, et al. Promoter hypermethylation-mediated inactivation of multiple Slit-Robo pathway genes in cervical cancer progression. Mol Cancer 2006; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dallol A, Krex D, Hesson L, et al. Frequent epigenetic inactivation of the SLIT2 gene in gliomas. Oncogene 2003; 22: 4611–4616. [DOI] [PubMed] [Google Scholar]
- 60. Dickinson RE, Dallol A, Bieche I, et al. Epigenetic inactivation of SLIT3 and SLIT1 genes in human cancers. Br J Cancer 2004; 91: 2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dunwell TL, Dickinson RE, Stankovic T, et al. Frequent epigenetic inactivation of the SLIT2 gene in chronic and acute lymphocytic leukemia. Epigenetics 2009; 4: 265–269. [DOI] [PubMed] [Google Scholar]
- 62. Prasad A, Paruchuri V, Preet A, et al. Slit-2 induces a tumor-suppressive effect by regulating beta-catenin in breast cancer cells. J Biol Chem 2008; 283: 26624–26633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ma YG, Wang LJ, Han B, et al. Effect of Slit-Robo signal on apoptosis of oral cancer cell line Tb. Zhonghua Kou Qiang Yi Xue Za Zhi 2006; 41: 232–235. [PubMed] [Google Scholar]
- 64. Denk AE, Braig S, Schubert T, et al. Slit3 inhibits activator protein 1-mediated migration of malignant melanoma cells. Int J Mol Med 2011;28(5):721–726. [DOI] [PubMed] [Google Scholar]
- 65. Blumenschein GR, Jr., Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol 2012; 30: 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stella MC, Trusolino L, Comoglio PM. The Slit/Robo system suppresses hepatocyte growth factor-dependent invasion and morphogenesis. Mol Biol Cell 2009; 20: 642–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Werbowetski-Ogilvie TE, Sadr MS, Jabado N, et al. Inhibition of medulloblastoma cell invasion by Slit. Oncogene 2006; 25: 5103–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marlow R, Strickland P, Lee JS, et al. SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4 within breast epithelium. Cancer Res 2008; 68: 7819–7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Prasad A, Fernandis AZ, Rao Y, et al. Slit protein-mediated inhibition of CXCR4-induced chemotactic and chemoinvasive signaling pathways in breast cancer cells. J Biol Chem 2004; 279: 9115–9124. [DOI] [PubMed] [Google Scholar]
- 70. Dai CF, Jiang YZ, Li Y, et al. Expression and roles of Slit/Robo in human ovarian cancer. Histochem Cell Biol 2011; 135: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bashaw GJ, Kidd T, Murray D, et al. Repulsive axon guidance: Abelson and enabled play opposing roles downstream of the roundabout receptor. Cell 2000; 101: 703–715. [DOI] [PubMed] [Google Scholar]
- 72. Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, et al. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci U S A 2009; 106: 14530–14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Feng Y, Feng L, Yu D, et al. srGAP1 mediates the migration inhibition effect of Slit2-Robo1 in colorectal cancer. J Exp Clin Cancer Res 2016; 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marko TA, Shamsan GA, Edwards EN, et al. Slit-Robo GTPase-Activating Protein 2 as a metastasis suppressor in osteosarcoma. Sci Rep 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang B, Xiao Y, Ding BB, et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003; 4: 19–29. [DOI] [PubMed] [Google Scholar]
- 76. Zhang Y, Zhou S. MicroRNA-29a inhibits mesenchymal stem cell viability and proliferation by targeting Roundabout 1. Mol Med Rep 2015; 12: 6178–6184. [DOI] [PubMed] [Google Scholar]
- 77. Wang Z, Li J, Chen X, et al. Disrupting the balance between tumor epithelia and stroma is a possible therapeutic approach for pancreatic cancer. Med Sci Monit 2014; 20: 2002–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Paul JD, Coulombe KLK, Toth PT, et al. SLIT3-ROBO4 activation promotes vascular network formation in human engineered tissue and angiogenesis in vivo. J Mol Cell Cardiol 2013; 64: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sheldon H, Andre M, Legg JA, et al. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J 2009; 23: 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Greaves E, Collins F, Esnal-Zufiaurre A, et al. Estrogen receptor (ER) agonists differentially regulate neuroangiogenesis in peritoneal endometriosis via the repellent factor SLIT3. Endocrinology 2014; 155: 4015–4026. [DOI] [PubMed] [Google Scholar]
- 81. Huang L, Yu W, Li X, et al. Robo1/Robo4: different expression patterns in retinal development. Exp Eye Res 2009; 88: 583–588. [DOI] [PubMed] [Google Scholar]
- 82. Cai H, Xue Y, Li Z, et al. Roundabout4 suppresses glioma-induced endothelial cell proliferation, migration and tube formation in vitro by inhibiting VEGR2-Mediated PI3K/AKT and FAK signaling pathways. Cell Physiol Biochem 2015; 35: 1689–1705. [DOI] [PubMed] [Google Scholar]
- 83. Yang Y-C, Chen P-N, Wang S-Y, et al. The differential roles of Slit2-exon 15 splicing variants in angiogenesis and HUVEC permeability. Angiogenesis 2015; 18: 301–312. [DOI] [PubMed] [Google Scholar]
- 84. Tian R, Liu Z, Zhang H, et al. Investigation of the regulation of roundabout4 by hypoxia-inducible factor-1 alpha in microvascular endothelial cells. Invest Ophthalmol Vis Sci 2015; 56: 2586–2594. [DOI] [PubMed] [Google Scholar]
- 85. Jones CA, London NR, Chen H, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nature Med 2008; 14: 585–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen H, Zhang M, Tang S, et al. Slit-Robo signaling in ocular angiogenesis. Adv Exp Med Biol 2010; 664: 457–463. [DOI] [PubMed] [Google Scholar]
- 87. Marlow R, Binnewies M, Sorensen LK, et al. Vascular Robo4 restricts proangiogenic VEGF signaling in breast. Proc Natl Acad Sci U S A 2010; 107: 10520–10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huang L, Yu W, Li X, et al. Expression of Robo4 in the fibrovascular membranes from patients with proliferative diabetic retinopathy and its role in RF/6A and RPE cells. Mol Vis 2009; 15: 1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 89. Bedell VM, Yeo SY, Park KW, et al. roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci U S A 2005; 102: 6373–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tole S, Mukovozov IM, Huang Y-W, et al. The axonal repellent, Slit2, inhibits directional migration of circulating neutrophils. J Leukoc Biol 2009; 86: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 91. Prasad A, Qamri Z, Wu J, et al. Pivotal advance: Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. J Leukoc Biol 2007; 82: 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chaturvedi S, Yuen DA, Bajwa A, et al. Slit2 prevents neutrophil recruitment and renal ischemia-reperfusion injury. J Am Soc Nephrol 2013; 24: 1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Guan HB, Zu GR, Xie Y, et al. Neuronal repellent Slit2 inhibits dendritic cell migration and the development of immune responses. J Immunol 2003; 171: 6519–6526. [DOI] [PubMed] [Google Scholar]
- 94. Ye B-Q, Geng ZH, Ma L, et al. Slit2 regulates attractive eosinophil and repulsive neutrophil chemotaxis through differential srGAP1 expression during lung inflammation. J Immunol 2010; 185: 6294–6305. [DOI] [PubMed] [Google Scholar]
- 95. Zhao H, Anand AR, Ganju RK. Slit2-Robo4 pathway modulates lipopolysaccharide-induced endothelial inflammation and its expression is dysregulated during endotoxemia. J Immunol 2014; 192: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Burke-Gaffney A, Svermova T, Mumby S, et al. Raised plasma Robo4 and cardiac surgery-associated acute kidney injury. PLoS One. 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Geutskens SB, Hordijk PL, van Hennik PB. The chemorepellent Slit3 promotes monocyte migration. J Immunol 2010; 185: 7691–7698. [DOI] [PubMed] [Google Scholar]
- 98. Zhao Y, Su Y, Ye L. Slit-Robo: a potential way to treat periodontitis. Med Hypotheses 2012; 79: 186–188. [DOI] [PubMed] [Google Scholar]
- 99. Secq V, Leca J, Bressy C, et al. Stromal SLIT2 impacts on pancreatic cancer-associated neural remodeling. Cell Death Dis 2015; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]