Abstract
Background:
Osteosarcoma (OSA) is a highly metastatic pediatric bone tumor. Adjuvant chemotherapy and surgical resection represent standard treatments; however, the prognosis is still poor. Effective strategies are urgently needed. Chondroitin sulfate proteoglycan (CSPG)4 is a transmembrane proteoglycan with a low expression in normal tissues but high expression in several solid tumors, where it plays a central tumorigenic role. Therefore, it represents a promising therapeutic target. The high homology between human and canine CSPG4 and the recognized translational power of canine tumors as preclinical models for human malignancies prompted us to evaluate CSPG4 expression and the consequences of its immune-targeting for both human and canine OSA treatment.
Methods:
We analyzed CSPG4 overexpression in human and canine OSA samples and its significance for the survival of OSA patients. We exploited functional in vitro experiments to assess the antitumor potential of CSPG4 immune-targeting.
Results:
CSPG4 is overexpressed in OSA and has possible clinical implications as suggested by an evident correlation between CSPG4 overexpression and a shorter survival for both OSA-affected humans and dogs. The potential of CSPG4 immune-targeting for OSA treatment came from the ability of anti-CSPG4 monoclonal antibodies and sera, derived from human-CSPG4-DNA vaccinated canine patients, to significantly inhibit human and canine CSPG4-positive OSA cell proliferation, migration, and osteospheres generation. Moreover, CSPG4 immune-targeting has been shown to potentiate the effect of doxorubicin.
Conclusions:
Overall, these results provide the rationale to investigate the CSPG4 immune-targeting as a promising weapon for the treatment of CSPG4-positive OSA canine patients, to be successfully translated to a human setting.
Keywords: antibodies, comparative oncology, CSPG4, osteosarcoma
Introduction
Osteosarcoma (OSA) is the most common primary malignant tumor of the bones. Of note, OSA is the sixth most-frequent pediatric cancer and represents the second most common cause of cancer-related death in this age group; a second peak is observed in adults after the sixth decade of life.1,2 Currently, primary OSA is classified into low- (Grade I) and high-grade subtypes (Grade II and III in the presence of metastasis), high-grade OSA being the most prevalent and aggressive variant.3
At initial diagnosis, almost 20% of OSA patients present evidence of metastatic spreading commonly involving lungs (90%), sites within the same affected bones (8–10%) or, more rarely, lymph nodes.4,5 However, considering that the vast majority of patients without overt metastasis at diagnosis develop lung metastasis within 6–36 months, it is presumed that these apparently nonmetastatic patients actually have micrometastases already at diagnosis.6,7
Conventional treatments consist mainly in the surgical resection of the primary tumor, in association with neoadjuvant and adjuvant chemotherapy with doxorubicin, methotrexate, and cisplatin. On the basis of the percentage of tumor necrosis after neoadjuvant chemotherapy, patients can be classified as poor responders (Huvos Grades I–II), responders (Huvos Grade III), or good responders (Huvos Grade IV). A strong correlation between the Huvos Grade and the subsequent effectiveness of postoperative chemotherapy and disease-free survival has been observed. This standard of care treatment is quite effective in the setting of localized OSA. Indeed, nowadays, the 5-year survival rate for localized OSA is about 65–70%, while only 20% of patients with multifocal disease or metastasis at diagnosis are still alive after 5 years.2,7,8
Therefore, it is evident that OSA is still a very aggressive and fatal disease for which no significant therapeutic advances have been achieved in the last 30 years and for which the identification of novel and more effective approaches is needed to improve patient survival.
As a step forward, during the last few decades, researchers have addressed to the identification of potentially therapeutically targetable OSA-associated antigens. Several tyrosine kinase receptors have been identified as over-expressed in OSA, including KIT, vascular endothelial growth factor receptor (VEGFR)-2 and VEGFR-3, platelet-derived growth factor (PDGFR)-β, and MET, and found to be correlated with metastasis development and poor survival.9–12 As a consequence, in the last few years several targeted therapies have been investigated, which unfortunately showed only limited efficacy in advanced OSA.11,13 The need for novel and more clinically relevant targets is therefore critically evident.
The chondroitin sulfate proteoglycan (CSPG)4 is a cell surface proteoglycan, considered as an ideal tumor-associated antigen, that is, an oncoantigen,14 because it is poorly expressed in healthy tissues,15–23 whereas in a vast range of human neoplasms it is in overexpressed by tumor cells, the tumor microenvironment and, cancer stem cells (CSCs). It has been widely described that CSPG4 has a key role in several oncogenic pathways required for malignant progression and metastasization.24 We have already demonstrated the clinical relevance of CSPG4 immune-targeting by means of DNA vaccination for the treatment of canine malignant melanoma (MM);25,26 however, to the best of the authors’ knowledge very few investigations have been undertaken on the involvement of CSPG4 in OSA.27,28
The aim of this study was to evaluate CSPG4 as a potential immunotherapeutic target for both human and canine OSA patients. This could offer an appealing opportunity to exploit spontaneous occurring OSA in pet dogs as model of human OSA tumors and for predicting the clinical efficacy of therapeutic approaches targeting CSPG4. Indeed, canine OSA is nearly indistinguishable from the human disease, presenting the same risk factors, histological tumor grading, similar standard treatments, and clinical responses.29,30 All these shared features make OSA-bearing dogs a valuable translational model of human OSA for the investigations of novel immunotherapies that could benefit both species.
Based on these considerations, we investigated CSPG4 expression in both human and canine OSA. We aimed to evaluate the effects of anti-CSPG4 targeting, alone or in combination with doxorubicin, on both human and canine OSA tumor cells and on osteospheres, enriched in CSC, which are considered responsible for recurrences and metastasis. Overall, our results provide the rationale for testing the clinical effectiveness of an anti-CSPG4 immunotherapy in dogs affected by spontaneous OSA, with the final aim of its translation for the treatment of human OSA patients.
Material and methods
Sample collection and clinical follow up
Tissue samples from 50 cases of spontaneous canine appendicular OSA collected via routine care between 2008 and 2014 at the Veterinary Teaching Hospital, Department of Veterinary Science of the University of Turin, were examined in this study.
Client-owned dogs affected by spontaneous OSA were treated according to the European guidelines established in the Principles of Laboratory Animal Care (directive 86/609/EEC). The Ethical Committee of the Department of Veterinary Science (University of Turin) approved the experimental protocol, which follows the best practice of veterinary care; written consent for entry into the study was obtained from dog owners. For all canine OSA patients, the initial data collected included history, physical examination, complete blood count, serum biochemical profile, urinalysis and abdominal ultrasound. Limb [lateral–lateral (LL) and anterior–posterior (AP) views] and chest [LL, right and left, and dorso–ventral (DV) views] radiographic evaluation was performed to examine the features and the extent of the tumor in addition to the presence of lung metastasis. From 2010 evaluation with computer tomography (CT) was included. In cases where regional lymph nodes were enlarged, they were aspirated, cytologically examined, and then removed for histological evaluation. All dogs included in this study were surgically treated (amputation or limb sparing) before receiving adjuvant chemotherapy. The Kaplan–Meier method was used to estimate overall and disease-free survival times. Differences in survivals were tested for significance using log-rank tests.
Immunohistochemical analysis
Immunohistochemical (IHC) analysis was performed as described previously24,31 on collected canine OSA. Samples were fixed in 4% neutral buffered formalin, embedded in paraffin, and sectioned at 4 μm. Then, samples were stained with hematoxylin and eosin to establish the histological diagnosis. The histological grade was determined according to the systems proposed by Loukopoulos and Robinson.32 The grading was evaluated as I (low), II, or III (high). IHC for CSPG4 in the 50 samples was performed on 4 μm sections of formalin-fixed, paraffin-embedded tissues. Sections were exposed to high-temperature for antigen unmasking by incubation at 98°C with citric acid buffer, pH 6.0. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 30 min at room temperature. Tissue sections were incubated for 12 h at room temperature with a polyclonal anti-CSPG4 antibody (diluted 1:40, Sigma Aldrich, St. Louis, MO, USA), then 30 min with biotinylated-secondary antibody (Vectastain Elite ABC) and revealed with the ImmPACT DAB kit for peroxidase, both from Vector Laboratories Inc. (Burlingame, CA). A total score considering the proportion of positively stained tumor cells and the average staining intensity was assigned as previously described.31 Briefly, the score indicating the positivity of tumor cells was assigned as follows: 0 (none); 1 (<1/100 or <1%); 2 (1/100–1/10 or 1–10%); 3 (1/10–1/3 or 10–30%); 4 (1/3–2/3 or 30–70%); and 5 (>2/3 or >70%). The score representing the estimated average staining intensity of positive tumor cells encompass 0 if none, 1 weak, 2 intermediate, or 3 strong. The two scores were then added to each other to obtain a final score of CSPG4 expression ranging from 2 to 8. A total score ⩾4 was used as cut-off.
Cell lines and osteospheres
Human OSA cell lines (MG-63, SAOS-2, U2OS) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Sigma Aldrich) or RPMI (Sigma Aldrich) supplemented with 20% fetal bovine serum (FBS, Sigma Aldrich). Penny cells, derived from the tail biopsy of a primary grade III canine OSA, were grown in ISCOVE Modified Dulbecco’s Medium (Sigma Aldrich) supplemented with 10% FBS. All cells were grown in medium supplemented with penicillin/streptomycin (Sigma Aldrich) and maintained at 37°C and 5% carbon dioxide in a humidified incubator.
Both human and canine osteospheres were generated following the protocol described in Conti et al.33 Briefly, cells were detached and plated in ultra-low-attachment 75 cm flasks (Sigma Aldrich) at 6 × 104 viable cells/ml in serum-free DMEM-F12 (Sigma Aldrich) supplemented with 0.4% bovine serum albumin (BSA), 20 ng/ml basic fibroblast growth factor (bFGF), 20 ng/ml epidermal growth factor (EGF), 5 mg/ml insulin, all from Sigma Aldrich. Non-adherent spherical cells’ clusters, named osteospheres, were collected after 48 h or 5 days for further analysis. Photographs of osteospheres were taken using a CCD-300-RC camera, and images were processed using Fiji Software (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, MD) and PowerPoint (Microsoft, Redmond, WA).
MTT cell proliferation assay
3-(4,5-dimetiltiazol-2-il)-2,5-difeniltetrazol (MTT; Merck Millipore, Burlington, MA, USA) assay was used to assess proliferation of human MG-63 and canine Penny OSA cells. Briefly, epithelial cells were seeded in triplicate in 96-well plates (5 × 103 cells and 8 × 103 cells/100 µl well, respectively) in serum-free medium and allowed to adhere overnight. To evaluate the inhibition of the CSPG4-mediated cell proliferation, four-selected anti-CSPG4 purified mAbs, 225.28, TP32, TP49, and VF20-VT87.41, were mixed in a pool or used as single agents. All the mAbs were produced in the laboratory of Prof. Ferrone (Massachusetts General Hospital, Harvard Medical School, Boston, MA) and are secreted by hybridomas generated from BALB/c mice immunized with human melanoma cells. Specifically, the mAb 225.28 is a mouse IgG2a, while the others (TP32, TP49, and VF20-VT87.41) are all mouse IgG1, which recognize distinct and spatially distant epitopes of human CSPG4. The specificity of mAbs was characterized as described elsewhere.34–36
Cells were incubated with different treatments for 48 h at the following final concentrations or dilutions: control isotypes (Sigma Aldrich; 25 µg/ml), anti-CSPG4 mAbs (225.28, TP32, TP49, and VF20-VT87.41, tested individually at 25 µg/ml or mixed in a pool to a final total concentration of 25 µg/ml), doxorubicin (Sigma Aldrich; 100 nM, 1 µM, or 10 µM), melanoma canine patients’ sera collected pre- and post-vaccination (1:100) with Hu-CSPG4 DNA plasmid.25,26 Cells grown in medium alone were used as control. MTT solution (5 mg/ml) was added to each well following manufacturer’s instruction. Following 4 h incubation at 37°C, 100 µl of dimethyl sulfoxide (DMSO, Sigma Aldrich) were added to dissolve formazan crystals and absorbance was measured on an enzyme-linked immunosorbent assay (ELISA) plate reader (Bio-Rad, Hercules, CA) with a wavelength of 570 nm.
To assess viability of human MG-63 and canine Penny osteosphere-derived cells, 6 × 104 cells/ml were seeded in 96-well plates in non-adherent conditions and incubated with different stimuli as described above and spheres were allowed to generate for 48 h. Following overnight incubation at 37°C, formazan crystals were dissolved by adding 100 µl isopropanol with HCl 0.04 N to each well. Optical density was measured on an ELISA plate reader (Bio-Rad) with a test wavelength of 570 nm and a reference wavelength of 630 nm. Difference between 570 nm and 630 nm readings represents the output value.
Flow cytometry analysis
Human and canine OSA epithelial cells and osteospheres were detached and dissociated by using Cell Dissociation Non Enzymatic 1X solution (Sigma Aldrich) and then resuspended in the culture medium with a concentration of 1 × 105 cells in 100 µl. Cytofluorimetric analysis were performed on cells for the detection of CSPG4 antigen using a mixed pool of the following mAbs (225.28, TP32, TP49, and VF20-VT87.41; 25 µg/ml final concentration) directed towards different epitopes of CSPG4 and produced in the lab of Prof. Ferrone (refer to MTT cell proliferation assay). Pooled mAbs were incubated on OSA cells for 30 min at 4°C and antibody binding was evaluated using a fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse Ig (Dako-Cytomation, Glostrup, Denmark). Flow cytometry was performed with a FACS Verse (BD Biosciences, San Jose, CA, USA) and the results were expressed as percentage of positive cells and mean fluorescence intensity (MFI) and analyzed with BDFacs Suite software.
Western blotting
Human and canine OSA cells were incubated in lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 1% Nonidet P-40, 1 mM Na3VO4, 1 mM NaF, and protease inhibitors, all from Sigma Aldrich) for 30 min at 4°C. Cell lysates were centrifuged for 5 min at 15,000g. Total protein concentration was quantified using the Pierce™ BCA Protein Assay Kit (Thermo-Fisher Scientific, Rockford, IL, USA). Following 5’ denaturation at 95°C in 2-mercaptoethanol-containing Laemmli sample buffer (Bio-Rad, Hercules, CA), equal amounts of protein (70 µg) were separated through electrophoresis in an Any kDa Mini-Protean TGX precast gel (Bio-Rad) and then transferred onto an Immobilion-P PVDF membrane (Merck Millipore, Billerica, MA). Following blocking with 5% nonfat dry milk (Santa Cruz Biotechnology, Dallas, TX) in wash buffer (Tris Buffered Saline - TBS - supplemented with 0.05% Tween-20 from Sigma Aldrich), the membrane was incubated overnight at 4°C with a pool of anti-CSPG4 mAbs (225.28, TP32, TP49, and VF20-VT87.41, 5 µg/ml each), washed three times in TBS and 0.05% Tween-20, and then incubated for 1 h at room temperature with HRP-conjugated rabbit antimouse Ig (Sigma Aldrich). Actin was used as control for equal protein loading. Immunoreacting bands were detected using ECL (Thermo Scientific) according to the manufacturer’s instructions. Band relative intensity was acquired using a ChemiDoc™ Touch Imaging System (Bio-Rad).
Cell migration assay
To measure cell migration, human MG-63 and canine Penny OSA cells were pre-incubated with different treatments at the following final concentrations: control isotypes (Sigma Aldrich; 100 µg/ml), anti-CSPG4 mAbs (225.28, TP32, TP49, and VF20-VT87.41 mixed in a pool to a final total concentration of 100 µg/ml), alone or in combination with doxorubicin (Sigma Aldrich; 10 µM or 100 nM); then seeded (5 × 104 and 3 × 104, respectively, per well) in 100 μl of serum-free medium in the top chamber of a 24-Transwell plate (8-μm pore size; Corning, Amsterdam, Netherlands). Cells incubated with medium alone were used as control. All bottom chambers of the Transwell plates were filled with 10% FBS-supplemented medium (600 μl per well) and cells were incubated at 37°C in a 5% CO2 atmosphere. After 48 h, the nonmigrated cells on the top side of the filter were removed by scrubbing twice with cotton tipped swab. Migrated cells on the bottom side of the filter were fixed with 2.5% glutaraldehyde (Sigma-Aldrich) and stained with 0.2% crystal violet (Sigma-Aldrich). After washing, the migrated cells of four randomly selected fields per well were imaged using an Olympus BX41 microscope (Olympus Corp., Tokyo, Japan) and analyzed using Fiji and Imagej Softwares (Rasband, W.S., ImageJ, US National Institutes of Health).
Dataset
Genome-wide gene expression analysis was based on previously deposited microarray experiments and data preprocessing, described in Kuijjer et al.1,37 The dataset included genome-wide gene expression data of osteoblasts (GEO accession number GSE33382), mesenchymal stem cells (MSCs; GEO accession number GSE28974), and 84 high-grade OSA pre-treatment biopsies (GEO accession number GSE33382). All data were processed together as described by Kuijjer et al.1
Meta-analysis on patient databases
The mRNA expression of CSPG4 in human OSA samples was determined by querying the R2 Kaplan–Meier scanner (https://hgserver1.amc.nl/cgi-bin/r2/main.cgi). For prognostic studies, R2 analysis software was used and patients were stratified by expression of the gene of interest. Overall survival and metastasis-free survival data were presented as Kaplan–Meier plots and tested for significance using log-rank tests. To define the cutoff between high and low gene expression, all percentiles between the lower and upper quartiles were computed, and the best performing threshold was used as a cutoff.
Statistical analysis
Normally distributed data are reported as means ± standard deviation (SD) unless otherwise stated. Other variables are expressed as percentages. Quantita-tive evaluations were carried out using the Student’s t test. The Kaplan–Meier method was used to estimate survival times and differences in survival distribution were analyzed using the log-rank test. Values of p < 0.05 were considered significant. All analyses were conducted using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Results
CSPG4 is overexpressed in human high-grade OSA and is related to a poor prognosis.
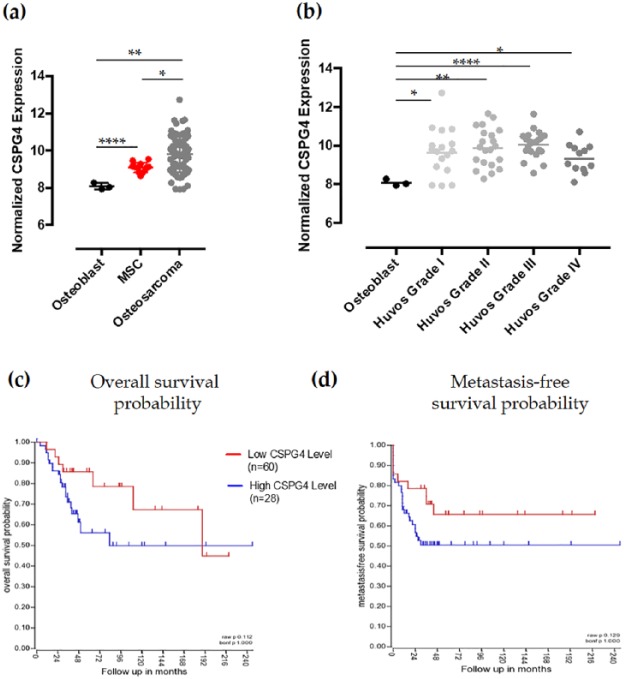
In order to evaluate the potentiality of CSPG4 as a novel target for human OSA, we used a publicly available comprehensive microarray dataset (superseries accession number GSE42352), including normalized gene expression data of osteoblasts (n = 3; GEO accession number GSE33382), MSCs (n = 12, GEO accession number GSE28974), and high-grade OSA pretreatment biopsies (n = 84, GEO accession number GSE33382).1,37 CSPG4 mRNA expression resulted significantly upregulated in MSC, considered potential precursors for OSA development, as compared with normal osteoblasts. CSPG4 mRNA expression was further significantly increased in high-grade OSA biopsies (Figure 1(a)). Taking advantage of the clinicopathological details regarding the high-grade OSA samples included in the analyzed dataset,37 we sought to evaluate whether the CSPG4 expression level in pretreatment OSA biopsies could be related to their response to neoadjuvant chemotherapy, expressed in Huvos Grades. As shown in Figure 1(b), CSPG4 mRNA in pretreatment OSA samples is not related to the Huvos Grade, therefore it cannot be considered as a prognostic factor implicated in the response to the neoadjuvant treatment. Nevertheless, CSPG4 mRNA expression is quite higher in Huvos Grade I–III samples, which include OSA with a lower necrosis in response to neoadjuvant chemotherapy and therefore with a worse prognosis, as compared with Huvos Grade IV samples, characterized by OSA with a 100% necrosis after neoadjuvant chemotherapy and with a better prognosis. In addition, CSPG4 overexpression showed a trend of correlation with a shorter overall survival (OS; Figure 1(c)) and metastasis-free survival (Figure 1(d)) of OSA patients. Taken together, these data suggest the potential role of CSPG4 in human OSA progression and the possible clinical relevance of its adjuvant targeting for high-grade OSA treatment, representing an even more interesting opportunity for those OSA that are not responsive to neoadjuvant chemotherapy.
Figure 1.
Chondroitin sulfate proteoglycan (CSPG)4 mRNA expression in human osteosarcoma (OSA). (a,b) Normalized gene expression levels of CSPG4, in mesenchymal stem cell (MSC) and OSA biopsies as compared with normal osteoblasts in toto (mean ± SD) (a) or divided according to the Huvos system grading (geometric mean) (b). Student’s t test: *p < 0.05; **p < 0.050; ****p < 0.0001. (c,d) Kaplan–Meier curves depicting overall survival (c) and metastasis-free survival (d) probability, in years, for OSA patients stratified by high (blue) or low (red) mRNA CSPG4 expression.
CSPG4 is highly expressed in canine OSA
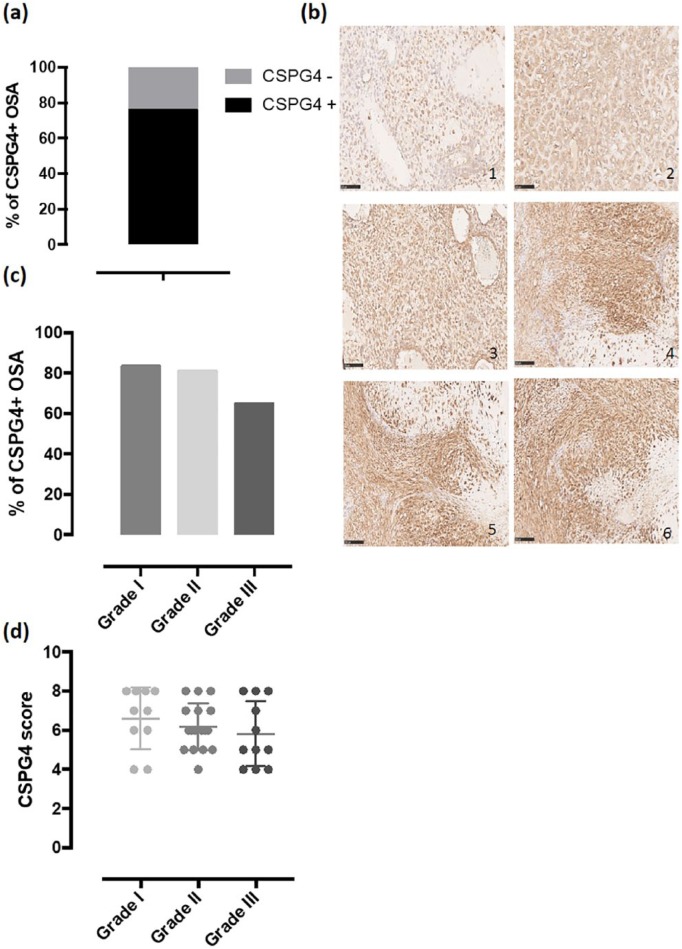
Canine patients affected by spontaneous OSA are considered a highly translational and predictive model to evaluate the clinical response to innovative therapeutic treatments for human OSA, providing several advantages over mouse models.38 On the basis of these considerations and to confirm the predictivity of the canine OSA model also for CSPG4, first we investigated its expression in canine OSA biopsies. Specifically, we evaluated CSPG4 protein expression using IHC in a total of 50 samples of canine appendicular OSA from patients treated between 2008 and 2014 at the Department of Veterinary Science of the University of Turin. IHC staining for CSPG4 detection was performed on all the collected tissues as previously described31 at the moment of the surgery, before any treatments. A score of CSPG4 expression was determined as published previously31 in order to obtain a value of 0 (if negative) or from 2 to 8. There were 38 (76%) CSPG4-positive and 12 (24%) CSPG4-negative primary canine OSA samples (Figure 2(a)). For all CSPG4-positive samples, staining was observed mainly on the membrane surface and in the cytoplasm of neoplastic cells, while no staining was detected in the surrounding healthy tissue (Figure 2(b)). The score of CSPG4 expression was different among positive samples and representative IHC examples are shown in Figure 2(b). Considering the histological classification of OSA biopsies at diagnosis, CSPG4 expression was observed in 10 out of 12 (83%) canine patients of Grade I, 17 out of 21 (81%) of Grade II, and 11 out of 17 (65%) of Grade III (Figure 2(c)). CSPG4 expression score showed to be independent from the tumor grade (Figure 2(d)). Overall, these results labeled the CSPG4 as a tumor-associated antigen overexpressed in canine OSA.
Figure 2.
Expression of chondroitin sulfate proteoglycan (CSPG)4 antigen in canine osteosarcoma (OSA) samples. (a) Percentage of primary OSA that scored positive (black bar) or negative (gray bar) for CSPG4 expression. (b) Immunohistochemical (IHC) staining of canine OSA biopsies with an anti-CSPG4 antibody. Representative sections of primary OSA not expressing (1), expressing low levels (2), or expressing high levels (3–6) of the CSPG4 antigen. (c) Percentage of CSPG4-positive primary OSA considering the different histological grade. (d) CSPG4 score of expression (mean ± SD) of CSPG4-positive primary OSA considering the different histological grade.
CSPG4 overexpression in canine OSA is related to poor survival
For each of the 50 canine patients included in the study, in addition to the OSA biopsy, we collected clinical data at the diagnosis including history, a physical exam, complete blood count, serum biochemical profile, urinalysis, abdominal ultrasound, and clinical follow up. Limb and chest radiographic (or CT) evaluation was performed to examine the features and the extent of the tumor and to exclude lung metastasis at diagnosis. All dogs included in this study were surgically treated (amputation or limb sparing) before receiving adjuvant chemotherapy using doxorubicin (30 mg/m2, 4–5 administrations, 21 days apart) or cisplatin (70 mg/m2, 4–5 administrations, 21 days apart) as single agents or in combination (4 cycles, 21 days apart, each cycle consisting of cisplatin 50 mg/m2 at day 1 and doxorubicin 15 mg/m2 at day 2).
Canine patients were clinically and radiographically examined every 3 months during the first year after the conclusion of chemotherapy and then every 6 months. OS was considered as the days between the surgery and death, while the disease-free interval (DFI) was considered as the number of days between surgery and tumor recurrence and/or evidence of metastatic disease. Considering a CSPG4 score ⩾4 as threshold, canine patients affected by CSPG4-positive OSA displayed a significantly shorter survival as compared with CSPG4-negative OSA (Figure 3(a)), with a median survival time (MST) of 271 and 440 days, respectively (Table 1). As far as the DFI is concerned, a trend is evident (Figure 3(b)), with a median DFI of 237 days for canine patients bearing a CSPG4-positive OSA and 339 days for dogs affected by a CSPG4-negative OSA (Table 1).
Figure 3.
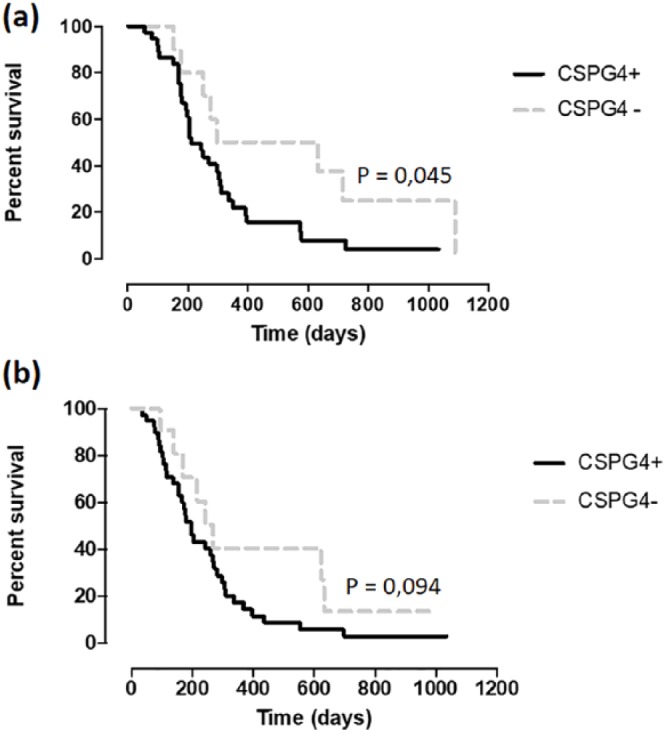
Kaplan–Meier curve comparing median survival times (MSTs) and disease-free intervals (DFIs) in different groups. MST (a) and DFI (b) (in days) in chondroitin sulfate proteoglycan (CSPG)4-positive (black line) and CSPG4-negative (gray dotted line) osteosarcoma (OSA) canine patients (log-rank test *p = 0.045, p = 0.094; Hazard Ratio (Mantel–Haenszel) = 2.021; 95% confidence intervals of ratio = 1013–4034).
Table 1.
Median survival times (MSTs) and disease-free intervals (DFIs) for osteosarcoma canine patients enrolled in the study.
| Group | MST (days) | DFI (days) |
|---|---|---|
| Overall population (n = 50) | 312 (241–382)a | 261 (199–324)a |
| CSPG4 + (n = 38) | 271 (207–336) | 237 (174–300) |
| CSPG4 - (n = 12) | 440 (221–659) | 339 (158–520) |
(LCL95% – UCL95%), lower – upper control limits.
CPSG4, chondroitin sulfate proteoglycan 4.
Overall, these results suggest the potential clinical relevance of CSPG4 for canine OSA progression, highlighting the importance of its targeting for comparative oncology studies for OSA treatment.
CSPG4 immune-targeting significantly inhibits CSPG4-dependent human and canine OSA cells proliferation
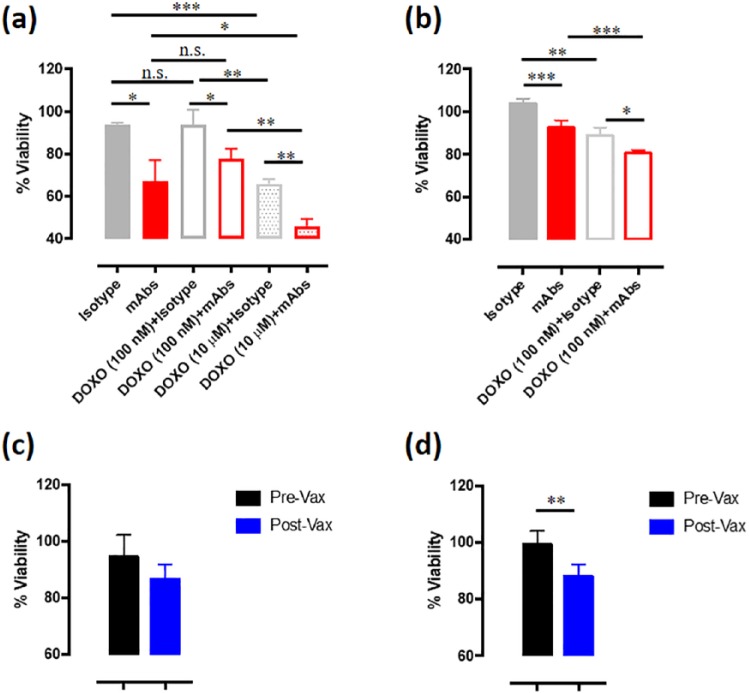
As CSPG4 facilitates constitutive activation of signaling pathways, which promote cell proliferation,24,39 we sought to in vitro evaluate the impact of CSPG4 immune-targeting on the growth of OSA cells. First, we evaluated CSPG4 expression in human (MG-63, U2OS, and SAOS-2) and canine (Penny) OSA cell lines. Flow cytometry analyses were performed using a pool of specific anti-CSPG4 mAbs (225.28, TP32, TP49, and VF20-VT87.41). Interestingly, cytofluorimetric results demonstrated that all the tested cell lines expressed high levels of CSPG4 (Supplemental Figure S1(a)) and this was confirmed also by Western blot analysis (Supplemental Figure S1(b)). For the following experiments, we selected the human MG-63 and the canine Penny cell lines. To understand the role of CSPG4 in OSA growth, MG-63 and Penny cells were incubated with control isotypes or anti-CSPG4 mAbs (225.28, TP32, TP49, and VF20-VT87.41), used as a mixed pool or as single agents. The proliferation of both OSA cell lines was significantly inhibited, as compared with control, by anti-CSPG4 mAbs used as a pool (Figure 4(a) and (b)) or as single agents (Supplemental Figure S2(a) and (b)). For this reason, in the following studies the mixed pool of the four anti-CSPG4 mAbs was used. As doxorubicin is a chemotherapeutic drug commonly used for the treatment of both human and canine OSA, we evaluated whether the CSPG4 targeting through mAbs could enhance its antiproliferative effect. At the lowest selected dose of doxorubicin (100 nM), MG-63 cells resulted resistant to the chemotherapeutic agent (Figure 4(a)), therefore the reduction in cell proliferation obtained by the combination of doxorubicin and anti-CSPG4 mAbs was primarily due to CSPG4 immune-targeting alone. As expected, when resistance is overcome using a higher dose of doxorubicin (10 µM), the MG-63 cell proliferation is significantly inhibited (Figure 4(a)). In this case, the combination with anti-CSPG4 mAbs is more effective in inhibiting human OSA cell proliferation compared with both the highest dose of doxorubicin and mAbs alone (Figure 4(a)). Regarding the canine OSA cells, as shown in Figure 4(b), the addition of doxorubicin at the lowest selected dose (100 nM) resulted per se effective in significantly inhibiting tumor cell proliferation, however a significantly higher inhibition was observed when the combination of mAbs and doxorubicin was applied, being superior to both single agents alone. As we have previously demonstrated the clinical effectiveness of in vivo CSPG4 immune-targeting by means of DNA vaccination in dogs affected by MM,25,26 to evaluate the potential of this strategy for the treatment of OSA, we incubated human and canine OSA cells with sera collected from canine MM patients enrolled in our previous veterinary trial. The sera were collected at the moment of the surgical removal of the primary tumor (pre-Vax) or after the vaccination (post-Vax) with a plasmid coding for the Hu-CSPG4, that induces the production of anti-CSPG4 antibody in the vaccinated dogs, against both the human and the canine CSPG4 protein.25 Interestingly, post-Vax sera were able to inhibit the proliferation of both human and canine OSA cells as compared with pre-Vax sera (Figure 4(c) and (d)). Together, these results suggest that CSPG4 may have a key role in both human and canine OSA cell proliferation, which could be impaired by its immune-targeting. Of note, our data propose the potential impact of adjuvant anti-CSPG4 DNA vaccination, alone or in combination with doxorubicin (Supplemental Figure S3) for the treatment of OSA patients, as we previously demonstrated for another aggressive and CSPG4-positive disease, MM.25,26
Figure 4.
Effects of chondroitin sulfate proteoglycan (CSPG)4 immune-targeting on osteosarcoma (OSA) cell proliferation. Cell proliferation was assessed by using the MTT assay and the results are expressed as the percentage (mean value ± SD) of cell viability in each condition respect to cells grown in the medium alone, considered as 100%. (a) Proliferation of human CSPG4-positive MG-63 cells incubated with medium alone, control isotypes (25 µg/ml final concentration), anti-CSPG4 mAbs pool (225.28, TP32, TP49, and VF20-VT87.41 mixed to a final concentration of 25 µg/ml), alone or in combination with 100 nM or 10 µM doxorubicin (DOXO), for 48 h. (b) Proliferation of canine CSPG4-positive Penny cells incubated with medium alone, control isotypes (25 µg/ml final concentration), anti-CSPG4 mAbs pool (225.28, TP32, TP49, and VF20-VT87.41 mixed to a final concentration of 25 µg/ml), alone or in combination with 100 nM doxorubicin (DOXO), for 48 h. (c,d) Proliferation of CSPG4-positive human (c) and canine (d) OSA cells incubated with medium alone, pre-Vax sera (black bars) or post-Vax sera (blue bars) from five canine malignant melanoma (MM) patients after the fourth cycle of vaccination with the Hu-CSPG4 DNA vaccine. Student’s t test: *p < 0.03; **p < 0.005; ***p < 0.001; ****p < 0.0001.
CSPG4 immune-targeting significantly inhibits CSPG4-positive human and canine osteospheres.
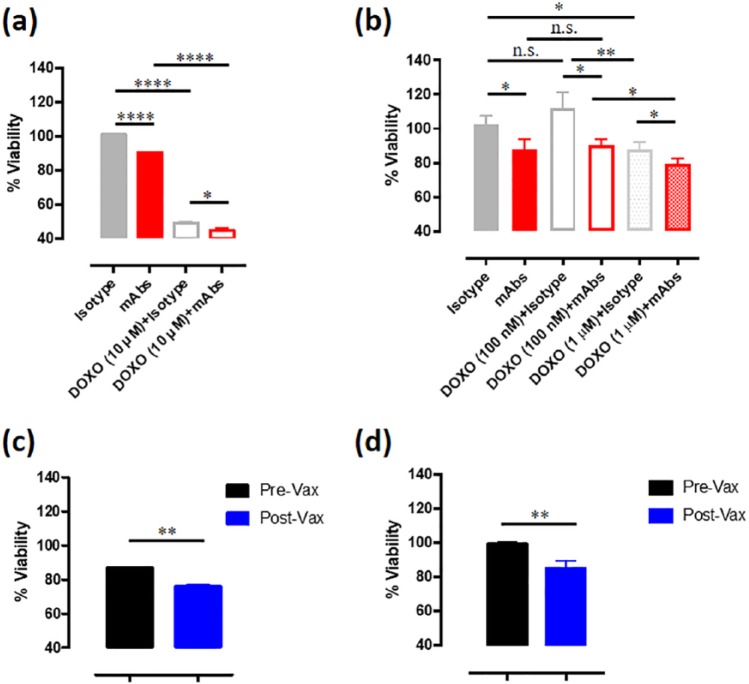
OSA is among the several cancer types in which a relevant role of CSC in tumor initiation and progression is evident. Indeed, CSC are thought to be the main drivers of OSA-related death, being responsible for tumor chemoresistance, finally resulting in recurrence and metastasis.40 Starting from this assumption, we generated MG-63 (Supplemental Figure S4(a)) and Penny (Supplemental Figure S4(b)) derived osteospheres, following the protocol described by Conti and coworkers.33 Interestingly human (Supplemental Figure S4(c)) and canine (Supplemental Figure S4(d)) osteosphere-derived cells expressed high levels of CSPG4, making it an even more interesting antigen for the immune-targeting of both differentiated cancer cells and CSC in OSA. Of note, both human (Figure 5(a)) and canine (Figure 5(b)) osteospheres incubated with anti-CSPG4 mAbs pool (225.28, TP32, TP49, and VF20-VT87.41) displayed reduced spheroid viability as compared with control, with each mAb clone showing a similar effect (Supplemental Figure S2(c) and (d)). In addition, we measured the in vitro growth of osteospheres when incubated with doxorubicin alone or in combination with anti-CSPG4 mAbs. For the human MG-63-derived osteospheres we selected the 10 µM doxorubicin dose, since the lower dose was not even effective on epithelial cells (Figure 4(a)). CSPG4 targeting with mAbs pool significantly increased the inhibitory effect of doxorubicin on osteospheres viability (Figure 5(a)). Regarding the canine Penny-derived osteospheres, the 100 nM doxorubicin dose, successfully used for epithelial canine Penny cell proliferation studies (Figure 4(b)) was not effective alone against osteospheres (Figure 5(b)), confirming previous findings that CSC avail of increased chemoresistance ability.41 Using a higher doxorubicin concentration (1 µM) on canine Penny-derived osteospheres, we observed that doxorubicin alone in this case significantly inhibits the sphere viability (Figure 5(b)), however, the combinatorial approach of doxorubicin plus anti-CSPG4 mAbs further improved the effect of the single treatments alone (Figure 5(b)). Interestingly, post-Vax sera derived from canine MM patients vaccinated with the Hu-CSPG4 plasmid 25 resulted effective in inhibiting human and canine osteosphere viability, as compared with pre-Vax sera (Figure 5(c) and (d)). Overall, these findings suggest that CSPG4 immune-targeting with a mix of anti-CSPG4 mAbs, and most interestingly, with anti-CSPG4 DNA vaccination, could also have an impact on the CSC compartment, which is considered endowed of a high metastatic potential.
Figure 5.
Effects of chondroitin sulfate proteoglycan (CSPG)4 immune-targeting on cancer stem cell (CSC)-enriched osteospheres. Osteospheres viability was assessed by using the MTT assay and the results are expressed as the percentage (mean value ± SD) of cell viability in each condition respect to cells grown in the medium alone, considered 100%. (a) Viability of human MG-63-derived osteospheres incubated with medium alone, control isotypes (25 µg/ml final concentration), or anti-CSPG4 mAbs pool (225.28, TP32, TP49, and VF20-VT87.41 mixed to a final concentration of 25 µg/ml), alone or in combination with 10 µM doxorubicin (DOXO), for 48 h. (b) Viability of canine Penny-derived osteospheres incubated with medium alone, control isotypes (25 µg/ml final concentration) or anti-CSPG4 mAbs pool (225.28, TP32, TP49, and VF20-VT87.41 mixed to a final concentration of 25 µg/ml), alone or in combination with 100 nM or 1 µM doxorubicin (DOXO), for 48 h. (c,d) Viability of human MG-63 (c) and canine Penny-derived (d) osteospheres incubated with medium alone, pre-Vax sera (black bar) or post-Vax sera (blue bar) from five canine malignant melanoma (MM) patients after the fourth cycle of vaccination with the Human CSPG4 DNA vaccine. Student’s t test: *p < 0.05; **p < 0.050; ****p < 0.0001.
CSPG4 immune-targeting significantly inhibits CSPG4-dependent human and canine OSA cells migration.
Migration is a critical property of cancer cells, which could determine their metastatic behavior. As metastasis is one of the major challenges in OSA treatment, we evaluated the potential of CSPG4 immune-targeting in counteracting the migratory ability of human and canine OSA cells. Interestingly, when cells were pre-incubated with anti-CSPG4 mAbs pool (225.28, TP32, TP49, VF20-VT87.41), a significantly reduced migratory potential, as compared with control, was evident for both human MG-63 (Figure 6(a)) and canine Penny (Figure 6(b)) OSA cells. Moreover, cell motility was also affected by doxorubicin; indeed, a significant migratory reduction was observed when MG-63 (Figure 6(a)) and Penny (Figure 6(b)) cells were treated with 10 µM and 100 nM doxorubicin, respectively. However, the combinatorial approach using doxorubicin plus anti-CSPG4 mAbs pool further improved the effect of the single treatments alone in both human MG-63 (Figure 6(a)) and canine Penny (Figure 6(b)) cells, making the possibility of chemotherapy and adjuvant CSPG4 immune-targeting an even more appealing strategy to fight against the metastatic disease.
Figure 6.
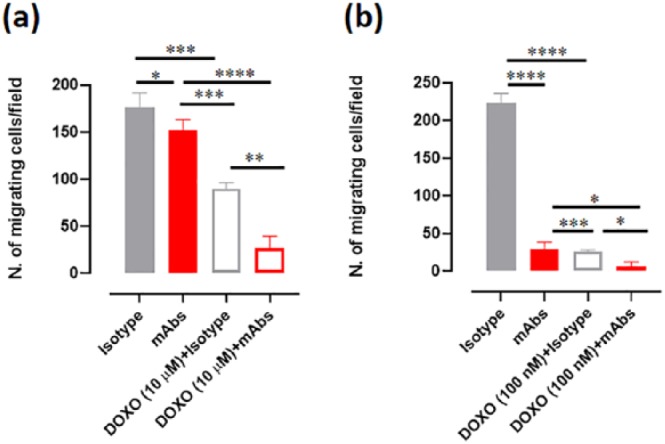
Effects of chondroitin sulfate proteoglycan (CSPG)4 immune-targeting on osteosarcoma (OSA) cell migration. OSA cell migratory ability was assessed by using the Transwell migration assay. Human MG-63 (a) and canine Penny (b) cells were placed in the upper chamber and incubated for 48 h with medium alone, control isotypes (100 µg/ml final concentration) or CSPG4 mAbs pool (225.28, TP32, TP49, and VF20-VT87.41 mixed to a final concentration of 100 µg/ml), alone or in combination with 10 µM or 100 nM doxorubicin (DOXO). Cells migrated to the lower surface of the membrane were stained with crystal violet for microscopical observation. The mean ± SD of the number of migrated cells counted in four different fields were reported in the graph. Student’s t test: *p < 0.05; **p < 0.001; ***p < 0.0004; ****p < 0.0001.
Discussion
CSPG4 is a transmembrane protein involved in several protumorigenic signaling pathways. It is expressed in a wide range of highly aggressive tumors, including MM, triple-negative breast carcinomas, leukemia, gliomas, in which it is associated with those hallmarks linked to tumorigenesis including proliferation, invasion, and metastasization.24,39 The overexpression of CSPG4 on CSC in different tumor histotypes, could suggest its potential implication also in providing a survival advantage to this subpopulation, considered responsible for recurrences and metastasis. Therefore, for all these properties, the CSPG4 is considered an ideal and safe oncoantigen14 for anticancer targeted therapies, being barely expressed on normal healthy tissues.15–23 Indeed, by means of an IHC analysis of a FDA Standard Frozen Tissue Array, including 30 different organs, Rivera et al. demonstrated that no CSPG4 expression was found in healthy tissues.22 In general, a limited CSPG4 expression is associated with stem cells and adult progenitor cells, which however have been suggested to lose its expression during terminal differentiation.19 Moreover, a heterogeneous CSPG4 expression has been detected on activated pericytes but interestingly, only poorly stabilized vascular structures contain CSPG4 expressing pericytes, whereas CSPG4 is downregulated in pericytes associated with quiescent vessels, and absent or not detectable in pericytes of stable vessels in the adult healthy tissues.42 On the basis of these considerations, several immunotherapeutic approaches against CSPG4 for the treatment of melanoma and other CSPG4-expressing tumor histotypes have been tested both in preclinical and clinical settings.24 CSPG4-specific chimeric antigen receptors (CAR) T cells,27 as well as sophisticated mAb-based approaches have been generated,27,43 demonstrating the antitumor potential of CSPG4 immune-targeting. An alternative approach is active immunization, which has demonstrated to bring about effective and long-lasting antitumor responses, without the risk of resistance development. In this direction, some evidence of the effectiveness of active immunization against CSPG4 in melanoma patients was found through vaccination with the anti-idiotypic antibody MK2-23, which bears the internal image of the mAb 763.74 against a defined CSPG4 epitope. Interestingly, the induction of CSPG4-specific antibodies in immunized patients was associated with significantly longer survival and metastasis regression.44,45 However, this approach never ended up in clinics, owing to both the difficulties in standardization of MK2-23 and to side effects associated with Bacille Calmette–Guerin administration, the adjuvant required to break immune tolerance and to induce an efficient immune response.24,46 However, these encouraging data provided a strong rationale for the development of new strategies of active immunization against CSPG4.
Recently, DNA-based vaccines have raised interest as a concrete and viable anticancer strategy.47 In this direction, we have recently focused our attention on the antitumor potential of in vivo electroporation of a DNA vaccine (electrovaccination) coding for the Hu-CSPG4 protein. To confer a high translational power to our study, we tested the safety, immunogenicity, and clinical efficacy of the vaccine in prospectively enrolled client-owned dogs with en bloc surgically resected stage II and III CSPG4-positive spontaneous oral MM.25,26 The results obtained in our studies demonstrated the ability of the xenogeneic DNA electrovaccination against CSPG4 to break the immune tolerance in dogs and to induce a specific humoral response which relates favorably with a significant prolongation of disease-free and overall survival time in vaccinated dogs with surgically resected MM as compared with controls treated with surgery alone.25,26 These results lay the foundation for the evaluation of this immunization strategy for the treatment of other CSPG4-expressing tumors.
To date, OSA still represents a critical challenge in the oncology field, because conventional therapies have demonstrated partial effectiveness only in patients affected by localized tumor, while failing in the treatment of advanced patients. Several strategies have been evaluated to improve the survival of OSA patients without encouraging results. Some clinical trials involving tyrosine kinase targeted therapies or checkpoint inhibitors48 have been assessed, however, considerable improvements in patients’ outcome have not been realized at all.41 Therefore, it is clearly evident the urgent need for novel and effective therapies. In this panorama, the identification of CSPG4 as a potential OSA-associated target could offer new possibilities for the treatment of this disease.
For this purpose, in the present study, we first focused our attention on the evaluation of CSPG4 expression in human OSA. We have analyzed mRNA levels for CSPG4 in previously published1,37 genome-wide expression data of osteoblasts, MSCs, and 84 high-grade OSA pretreatment biopsies. We detected the overexpression of CSPG4 mRNA in human high-grade OSA biopsies, as compared with the hypothesized OSA progenitors, and validated the CSPG4 protein overexpression in human OSA cell lines. We availed of the collected information regarding the clinical evolution of the disease of the 84 high-grade OSA patients included in the dataset.37 All patients underwent neoadjuvant chemotherapy, and for all patients, the Huvos necrosis grading system was applied for the assessment of chemotherapy efficacy. Unfortunately, patients with a poor histologic response to neoadjuvant chemotherapy (mostly Huvos Grade I–II) showed no benefit following distinct postoperative therapies.49 Interestingly, CSPG4 mRNA was found over-expressed independently from the Huvos Grade and a higher degree of expression resulted in lower grades. We further evaluated whether the CSPG4 expression is related to OSA patients’ prognosis. Actually, OSA patients show a shorter overall survival and metastasis-free survival probability when CSPG4 is overexpressed. Therefore, taken as a whole, these results suggest the strong potentiality of adjuvant CSPG4 targeting for both good and poor responders to chemotherapy.
In addition, because of the age of its onset, OSA is socially important and limits the possibility of testing new therapies; the identification of reliable models of human OSA is therefore a critical point. For this reason, we decided to evaluate the feasibility of using spontaneous canine OSA as a preclinical model to test anti-CSPG4 immunotherapies. Indeed, canine patients spontaneously develop tumors as humans do, in a context of an intact immune system, with strong anatomical and physiological similarities with the human counterpart. This is true also for OSA. Moreover, as in humans, treatments for canine OSA includes mainly surgery and chemotherapy, which however are often disappointing with only 20% of canine OSA patients treated using the current standard of care still alive at 1 year and lung metastasis being the most relevant cause of death.50–52 A tangible example of the importance of comparative oncology in OSA came from the early 1990s, when limb-sparing methods pioneered in canine patients with OSA53 have become standard of care for human patients, which today clearly benefit from advances made in both surgical treatment and in the provision of supportive care.
With this in mind, we have demonstrated that CSPG4 expression is detectable, with variable expression levels, in a high percentage of canine OSA biopsies by means of IHC. In addition, also canine OSA cell line showed to highly express CSPG4, representing an interesting tool to be exploited for in vitro studies. As for humans, the Kaplan–Meyer curves suggest that CSPG4 overexpression is also related to a poor prognosis in canine patients. Thus, with this study we highlighted the potential clinical relevance of evaluating anti-CSPG4 strategies in canine OSA patients, with a high translational value. For this reason, we examined the potentiality of CSPG4 immune-targeting against both human and canine OSA cell lines in vitro, in order to consider anti-CSPG4 immunotherapy as a potential new weapon against OSA.
CSPG4 is implicated in several cellular processes,24,39 therefore its targeting could impair simultaneously different steps in the tumorigenic process. First, our results suggest that CSPG4 is involved in OSA cell proliferation. Indeed, we showed that four anti-CSPG4 selected mAbs (225.28, TP32, TP49, and VF20-VT87.41) are able to significantly impair the proliferation of both human and canine OSA tumor cells. This inhibition is evident when mAbs are used in a mixed pool or when used as single agents, suggesting that the engagement of different antigen epitopes by each clone does not seem to have different effects on cancer cell survival. The effect of CSPG4 immune-targeting in vitro is evident although modest. This can be attributed to several reasons: both OSA cell lines are not 100% positive for CSPG4 expression, therefore there will be a CSPG4-negative population able to escape to mAbs treatment; the selected dose of mAbs used is low, so better effects could be achieved increasing the dose. However, an interesting finding is the ability of mAbs treatment to significantly sensitize OSA cells to doxorubicin. Actually, the combination of doxorubicin, one of the most common chemotherapeutic agent used in both human and veterinary setting,41 with anti-CSPG4 mAbs enhanced the inhibition of cancer cells’ growth. Of note, on one side CSPG4 can regulate the AKT–pAKT pathway considered responsible for chemoresistance, on the other side we have previously demonstrated that anti-CSPG4 mAbs can induce a downregulation of the CSPG4 receptor when incubated with CSPG4-positive melanoma cells,26 and this could consequently impair the downstream signaling, leading to the reduction of the AKT-pAKT axis (unpublished data and Rolih et al.24). Therefore, the association of chemotherapy and anti-CSPG4 immune-targeting open up the possibility of increasing the antitumor effect of single agents alone, combining standard of care with novel strategies.
Moreover, the intrinsic OSA chemoresistance may be the result also of a privileged survival of a population of tumor cells, that is, CSC to which are associated tumor recurrence and metastasis development following chemotherapy. For this reason, in this study we evaluated the ability of mAbs alone or in combination with doxorubicin to impair not only epithelial cancer cells but also osteospheres enriched in CSC. First, we demonstrated that subsequent passage of CSC-enriched osteospheres retain the CSPG4 overexpression, making it an interesting antigen to target CSC too. Then, we showed the ability of mAbs, alone or in combination with doxorubicin, to inhibit osteospheres viability. Also in this case, the selected anti-CSPG4 mAbs (225.28, TP32, TP49, VF20-VT87.41) showed a similar effect when used together in a pool or when tested individually. These results propose the potentiality of CSPG4 immune-targeting for the elimination of CSC and the prevention of recurrences and metastasis in OSA. Moreover, our data suggest a potential involvement of CSPG4 in OSA cell migration, because we demonstrated the significant impact of CSPG4 immune-targeting against the migratory ability of both human and canine OSA cell lines, highlighting the pleiotropic effects of anti CSPG4 mAbs. In addition, mAbs treatment significantly increases the antimigratory effect of doxorubicin, as demonstrated by Transwell assays, supporting the relevant clinical consequence of combinatorial anti-CSPG4 immune-targeting and chemotherapy to fight against OSA metastasis. Overall, these results provide an additional step forward in the understanding the impact of CSPG4 in its whole for OSA progression.
Finally, to consider anti-CSPG4 DNA electrovaccination as a new therapy for the adjuvant treatment of OSA, on the basis of our previous positive results obtained for MM, we used sera derived from MM canine patients adjuvantly treated with Hu-CSPG4 DNA plasmid to evaluate the ability of vaccine induced antibodies to inhibit the proliferation of OSA cells and osteospheres in vitro. Interestingly post-vaccination sera were effective in inhibiting cell growth and sphere viability alone or in combination with doxorubicin. These results suggest the potential efficacy of our DNA vaccination strategy also for the treatment of canine OSA patients in vivo, with a strong translational value for human OSA management.
Supplemental Material
Supplemental material, Supplemental_Figure for Identification of CSPG4 as a promising target for translational combinatorial approaches in osteosarcoma by Federica Riccardo, Lidia Tarone, Selina Iussich, Davide Giacobino, Maddalena Arigoni, Federica Sammartano, Emanuela Morello, Marina Martano, Francesca Gattino, Raffaella De Maria, Soldano Ferrone, Paolo Buracco and Federica Cavallo in Therapeutic Advances in Medical Oncology
Acknowledgments
Paolo Buracco and Federica Cavallo contributed equally.
Footnotes
Author contributions: FC, PB, and FR contributed to the conception and design of the study. FC, FR, and LT wrote the manuscript. FR and LT performed the in vitro experiments. SI performed the immunohistochemical analyses. DG, FS, EM, MM, FG, and PB contributed to patient recruitment and clinical follow up. MA performed the meta-analysis and data collection from human datasets. RDM and SF provided reagents and cell lines. All authors contributed to collection, analyses and interpretation of data, reviewed, and approved the final submitted version.
Funding: This work was sponsored by the Fondazione Ricerca Molinette Onlus Torino, Italy, and the Italian Ministry of Health, within the ‘Progetti ordinari di Ricerca Finalizzata’ (grant number RF-2013-02359216). FR was supported by a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC).
Conflict of interest statement: The authors declare that there is no conflict of interests.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Federica Riccardo, University of Torino, Department of Molecular Biotechnology and Health Sciences, Molecular Biotechnology Centre, Via Nizza, 52, Torino, TO, 10126, Italy.
Lidia Tarone, Department of Molecular Biotechnology and Health Sciences, Molecular Biotechnology Center, University of Torino, Torino, Italy.
Selina Iussich, Department of Veterinary Sciences, University of Torino, Grugliasco, Italy.
Davide Giacobino, Department of Veterinary Sciences, University of Torino, Grugliasco, Italy.
Maddalena Arigoni, Department of Molecular Biotechnology and Health Sciences, Bioinformatics and Genomic Unit, Molecular Biotechnology Center, University of Torino, Torino, Italy.
Federica Sammartano, Department of Veterinary Sciences, University of Torino, Grugliasco, Italy.
Emanuela Morello, Department of Veterinary Sciences, University of Torino, Grugliasco, Italy.
Marina Martano, Department of Veterinary Sciences, University of Torino, Grugliasco, Italy.
Francesca Gattino, Department of Veterinary Sciences, University of Torino, Grugliasco, Italy.
Raffaella De Maria, Department of Veterinary Sciences, University of Torino, Grugliasco, Italy.
Soldano Ferrone, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Paolo Buracco, Department of Veterinary Sciences, University of Torino, Grugliasco, Italy.
Federica Cavallo, Department of Molecular Biotechnology and Health Sciences, Molecular Biotechnology Center, University of Torino, Torino, Italy.
References
- 1. Kuijjer ML, Peterse EFP, van den Akker BEWM, et al. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer 2013; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer 2018; 65: 1–9. [DOI] [PubMed] [Google Scholar]
- 3. Kundu Z. Classification, imaging, biopsy and staging of osteosarcoma. Indian J Orthop 2014; 48: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 2006; 106: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 5. Carrle D, Bielack S. Osteosarcoma lung metastases detection and principles of multimodal therapy. Cancer Treat Res 2009; 152: 165–184. [DOI] [PubMed] [Google Scholar]
- 6. Marko TA, Diessner BJ, Spector LG. Prevalence of metastasis at diagnosis of osteosarcoma: an international comparison. Pediatr Blood Cancer 2016; 63: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taran S, Taran R, Malipatil N. Pediatric osteosarcoma: an updated review. Indian J Med Paediatr Oncol 2017; 38: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luetke A, Meyers PA, Lewis I, et al. Osteosarcoma treatment: where do we stand? A state of the art review. Cancer Treat Rev 2014; 40: 523–532. [DOI] [PubMed] [Google Scholar]
- 9. Messerschmitt PJ, Rettew AN, Brookover RE, et al. Specific tyrosine kinase inhibitors regulate human osteosarcoma cells in vitro. Clin Orthop Relat Res 2008; 466: 2168–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pignochino Y, Grignani G, Cavalloni G, et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer 2009; 8: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown HK, Schiavone K, Gouin F, et al. Biology of bone sarcomas and new therapeutic developments. Calcif Tissue Int 2018; 102: 174–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu J, Xie L, Guo W. PDGF/PDGFR effects in osteosarcoma and the “add-on” strategy. Clin Sarcoma Res 2018; 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ehnman M, Larsson O. Microenvironmental targets in sarcoma. Front Oncol 2015; 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cavallo F, Calogero RA, Forni G. Are oncoantigens suitable targets for anti-tumour therapy? Nat Rev Cancer 2007; 7: 707–713. [DOI] [PubMed] [Google Scholar]
- 15. Benassi MS, Pazzaglia L, Chiechi A, et al. NG2 expression predicts the metastasis formation in soft-tissue sarcoma patients. J Orthop Res 2009; 27: 135–140. [DOI] [PubMed] [Google Scholar]
- 16. Nicolosi PA, Dallatomasina A, Perris R. Theranostic impact of NG2/CSPG4 proteoglycan in cancer. Theranostics 2015; 5: 530–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Svendsen A, Kmiecik J, et al. Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS One 2011; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garusi E, Rossi S, Perris R. Antithetic roles of proteoglycans in cancer. Cell Mol Life Sci 2012; 69: 553–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kozanoglu I, Boga C, Ozdogu H, et al. Human bone marrow mesenchymal cells express NG2: possible increase in discriminative ability of flow cytometry during mesenchymal stromal cell identification. Cytotherapy 2009; 11: 527–533. [DOI] [PubMed] [Google Scholar]
- 20. Beard RE, Abate-Daga D, Rosati SF, et al. Gene expression profiling using Nanostring digital RNA counting to identify potential target antigens for melanoma immunotherapy. Clin Cancer Res 2013; 19: 4941–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Birbrair A, Zhang T, Wang ZM, et al. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol - Cell Physiol 2013; 305: C1098–C1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rivera Z, Ferrone S, Wang X, et al. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin Cancer Res 2012; 18: 5352–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ziai MR, Imberti L, Ferrone S, et al. Analysis with monoclonal antibodies of the molecular and cellular heterogeneity of human high molecular weight melanoma associated antigen. Cancer Res 1987; 47: 2474–2480. [PubMed] [Google Scholar]
- 24. Rolih V, Barutello G, Iussich S, et al. CSPG4: a prototype oncoantigen for translational immunotherapy studies. J Transl Med 2017; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riccardo F, Iussich S, Maniscalco L, et al. CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin Cancer Res 2014; 20: 3753–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piras LA, Riccardo F, Iussich S, et al. Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4-antigen electrovaccination. Vet Comp Oncol 2017; 15: 996–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beard RE, Zheng Z, Lagisetty KH, et al. Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. J Immunother Cancer 2014; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sato S, Tang YJ, Wei Q, et al. Mesenchymal tumors can derive from Ng2/Cspg4-expressing pericytes with β-catenin modulating the neoplastic phenotype. Cell Rep 2016; 16: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morello E, Martano M, Buracco P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma. Vet J 2011; 189: 268–277. [DOI] [PubMed] [Google Scholar]
- 30. Simpson S, Dunning MD, de Brot S, et al. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand 2017; 59: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mayayo SL, Prestigio S, Maniscalco L, et al. Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J 2011; 190. [DOI] [PubMed] [Google Scholar]
- 32. Loukopoulos P, Robinson WF. Clinicopathological relevance of tumour grading in canine osteosarcoma. J Comp Pathol 2007; 136: 65–73. [DOI] [PubMed] [Google Scholar]
- 33. Conti L, Lanzardo S, Arigoni M, et al. The noninflammatory role of high mobility group box 1/toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J 2013; 27: 4731–4744. [DOI] [PubMed] [Google Scholar]
- 34. Imai K, Ng A, Glassy M, et al. Differential effect of interferon on the expression of tumor-associated antigens and histocompatibility antigens on human melanoma cells: relationship to susceptibility to immune lysis mediated by monoclonal antibodies. J Immunol 1981; 127: 505–509. [PubMed] [Google Scholar]
- 35. Wilson BS, Imai K, Natali PG, et al. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. Int J Cancer 1981; 28: 293–300. [DOI] [PubMed] [Google Scholar]
- 36. Temponi M, Ferrone S, Gold AM. Binding parameters and idiotypic profile of the whole immunoglobulin and fab’ fragments of murine monoclonal antibody to distinct determinants of the human high molecular weight-melanoma associated antigen. Cancer Res 1992; 52: 2497–2503. [PubMed] [Google Scholar]
- 37. Kuijjer ML, Rydbeck H, Kresse SH, et al. Identification of osteosarcoma driver genes by integrative analysis of copy number and gene expression data. Genes Chromosom Cancer 2012; 51: 696–706. [DOI] [PubMed] [Google Scholar]
- 38. Riccardo F, Aurisicchio L, Impellizeri JA, et al. The importance of comparative oncology in translational medicine. Cancer Immunol Immunother 2014; 64: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Price MA, Wanshura LEC, Yang J, et al. CSPG4, a potential therapeutic target, facilitates malgnant progression of melanoma. Pigment Cell Melanoma Res 2011; 24: 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci 2018; 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harrison DJ, Geller DS, Gill JD, et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 2018; 18: 39–50. [DOI] [PubMed] [Google Scholar]
- 42. Virgintino D, Girolamo F, Errede M, et al. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 2007; 10: 35–45. [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Geldres C, Ferrone S, et al. Chondroitin sulfate proteoglycan 4 as a target for chimeric antigen receptor-based T-cell immunotherapy of solid tumors. Expert Opin Ther Targets 2015; 19: 1339–1350. [DOI] [PubMed] [Google Scholar]
- 44. Mittelman A, Chen GZJ, Wong GY, et al. Human high molecular weight-melanoma associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2–23: modulation of the immunogenicity in patients with malignant melanoma. Clin Cancer Res 1995; 1: 705–713. [PubMed] [Google Scholar]
- 45. Mittelman A, Chen ZJ, Yang H, et al. Human high molecular weight melanoma-associated antigen (HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody MK2–23: induction of humoral anti-HMW-MAA immunity and prolongation of survival in patients with stage IV melanoma. Proc Natl Acad Sci 1992; 89: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang X, Ko EC, Peng L, et al. Human high molecular weight melanoma-associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2–23: enhancement of immunogenicity of anti-idiotypic monoclonal antibody MK2–23 by fusion with interleukin 2. Cancer Res 2005; 65: 6976–6983. [DOI] [PubMed] [Google Scholar]
- 47. Iezzi M, Quaglino E, Amici A, et al. DNA vaccination against oncoantigens: a promise. Oncoimmunology 2012; 1: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lussier DM, Johnson JL, Hingorani P, et al. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer 2015; 19: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goorin AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: pediatric oncology group study POG-8651. J Clin Oncol 2003; 21: 1574–1580. [DOI] [PubMed] [Google Scholar]
- 50. Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J 2014; 55: 69–85. [DOI] [PubMed] [Google Scholar]
- 51. Schmidt AF, Nielen M, Klungel OH, et al. Prognostic factors of early metastasis and mortality in dogs with appendicular osteosarcoma after receiving surgery: an individual patient data meta-analysis. Prev Vet Med 2013; 112: 414–422. [DOI] [PubMed] [Google Scholar]
- 52. Schmidt AF, Groenwold RHH, Amsellem P, et al. Which dogs with appendicular osteosarcoma benefit most from chemotherapy after surgery? Results from an individual patient data meta-analysis. Prev Vet Med 2016; 125: 116–125. [DOI] [PubMed] [Google Scholar]
- 53. Thompson JP, Fugent MJ. Evaluation of survival times after limb amputation, with and without subsequent administration of cisplatin, for treatment of appendicular osteosarcoma in dogs: 30 cases (1979–1990). J Am Vet Med Assoc 1992; 200: 531–533. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Figure for Identification of CSPG4 as a promising target for translational combinatorial approaches in osteosarcoma by Federica Riccardo, Lidia Tarone, Selina Iussich, Davide Giacobino, Maddalena Arigoni, Federica Sammartano, Emanuela Morello, Marina Martano, Francesca Gattino, Raffaella De Maria, Soldano Ferrone, Paolo Buracco and Federica Cavallo in Therapeutic Advances in Medical Oncology