Short abstract
This research analyzes the preparedness of the health care systems in six European countries to ensure timely diagnosis and treatment of patients if an Alzheimer's disease–modifying therapy becomes available.
Keywords: Alzheimer's Disease and Dementias, France, Germany, Health Care Services Capacity, Italy, Mental Health Treatment, Spain, Sweden, United Kingdom
Abstract
No disease-modifying therapy is currently available for Alzheimer's disease, but therapies are in development, and one may become available in the near future. Based on results from early-stage clinical trials, therapeutic development has focused on the hypothesis that Alzheimer's dementia must be prevented rather than cured, because candidate treatments have not been able to reverse the course of dementia. Thus, current trials target patients with early-stage Alzheimer's disease. Were a therapy to become available, patients could undergo first screening for signs of early-stage memory loss or mild cognitive impairment (MCI), testing for the Alzheimer's disease pathology, and then treatment with the aim of halting or slowing progression to Alzheimer's dementia. An important health systems challenge will arise if this new treatment paradigm bears out in late-stage clinical trials. In the 28 European Union countries, we estimate that approximately 20 million individuals over age 55 have MCI, although most people have not been tested for disease pathology. Thus, when a therapy first becomes available, there would be a substantial number of existing (or prevalent) MCI patients who would require screening, diagnosis, and then treatment as quickly as possible to prevent the progression to full-blown Alzheimer's dementia. This research analyzes the preparedness of the health care systems in six European countries—France, Germany, Italy, Spain, Sweden, and the United Kingdom—to ensure timely diagnosis and treatment of patients if a disease-modifying therapy for Alzheimer's becomes available.
Key Findings
The burden of Alzheimer's disease in high-income countries is expected to approximately double between 2015 and 2050. Recent clinical trial results give hope that a disease-modifying therapy might become available in the near future. The therapy is expected to treat early-stage patients to prevent or delay the progression to dementia.
This preventive treatment paradigm implies the need to screen, diagnose, and treat a large population of patients with mild cognitive impairment. There would be many undiagnosed prevalent cases that would need to be addressed initially, and then the longer-term capacity to address incident cases would not need to be as high as the short-term capacity.
We use a simulation model to assess the preparedness of the health care system infrastructure in six European countries—France, Germany, Italy, Spain, Sweden, and the United Kingdom—to evaluate, diagnose, and treat the expected number of patients.
Projected peak wait times range from five months for treatment in Germany to 19 months for evaluation in France. The first years without wait times would be 2030 in Germany and 2033 in France, and 2042 in the United Kingdom and 2044 in Spain. Specialist capacity is the rate-limiting factor in France, the United Kingdom, and Spain, and treatment delivery capacity is an issue in most of the countries.
If a disease-modifying therapy becomes available in 2020, we estimate the projected capacity constraints could result in over 1 million patients with mild cognitive impairment progressing to Alzheimer's dementia while on wait-lists between 2020 and 2044 in these six countries.
A combination of reimbursement, regulatory, and workforce planning policies, as well as innovation in diagnosis and treatment delivery, is needed to expand capacity and to ensure that available capacity is leveraged optimally to treat patients with early-stage Alzheimer's disease.
Introduction
Alzheimer's disease is a progressive neurodegenerative disorder that leads to cognitive and functional decline, resulting in Alzheimer's dementia and premature death. Alzheimer's dementia imposes a substantial burden on patients, their families and caregivers, and the broader society. As the risk of developing Alzheimer's dementia increases with aging populations, the burden of disease is estimated to approximately double in G7 countries and nearly triple in G20 countries between 2015 and 2050 (Prince et al., 2015).
Currently, no disease-modifying treatment is available, but there are therapies in development. With some encouraging early-stage clinical trial results and collaboration efforts after many disappointing trials, there is cautious optimism that a therapy may become available in the near future (Aisen, 2017; “Alzheimer's Disease: A Time for Cautious Optimism,” 2015). Although there have been recent setbacks in trials that raise questions about the disease targets and mechanisms of action (Honig et al., 2018; Murphy, 2018), several therapies remain in clinical trials (Table 1).
Table 1.
Alzheimer's Disease-Modifying Therapy Candidates in Phase 2 and Phase 3 Clinical Trials, as of September 2018
| Candidate | Sponsor | Clinical Trial Phase | Expected Primary Completion Date | National Clinical Trial Identifier |
|---|---|---|---|---|
| Anti–beta-amyloid antibodies | ||||
| Aducanumab (BIIB037) | Biogena | Phase 3 | January 2020 | NCT02477800 |
| Crenezumab | Hoffman-La Roche | Phase 3 | August 2020 | NCT02670083 |
| Gantenerumab | Hoffman-La Roche | Phase 3 | May 2022 | NCT03443973, NCT03444870 |
| BAN2401 | Eisai/Biogen | Phase 2 | July 2018 | NCT01767311 |
| LY3002813b | Eli Lilly | Phase 2 | October 2020 | NCT03367403 |
| Anti-tau antibodies | ||||
| ABBV-8E12 | AbbVie | Phase 2 | December 2020 | NCT02880956 |
| RO7105705 | Genentech | Phase 2 | September 2020 | NCT03289143 |
| BACE inhibitors | ||||
| Elenbecestat (E2609) | Eisai/Biogen | Phase 3 | March 2021 | NCT03036280 |
| CNP520 | Novartis/Amgen/Banner Alzheimer's Institute | Phase 2/3 | July 2024 | NCT03131453 |
| Vaccines | ||||
| CAD106 (anti–beta amyloid) | Novartis | Phase 2/3 | August 2024 | NCT02565511 |
| AADvac1 (anti-tau) | Axon Neuroscience | Phase 2 | June 2019 | NCT02579252 |
SOURCE: RAND analysis of company websites and ClinicalTrials.gov website as of September 21, 2018.
NOTE: BACE = beta-secretase 1.
Aducanumab is jointly developed with Eisai.
LY3002813 is being evaluated alone and in combination with LY3202626, a BACE inhibitor.
Based on results from early-stage clinical trials, therapeutic development has focused on the hypothesis that Alzheimer's dementia must be prevented rather than cured, because candidate treatments have not been able to reverse the course of dementia. Thus, current trials target patients with early-stage Alzheimer's disease by first screening patients for signs of early-stage memory loss, or mild cognitive impairment (MCI), conducting tests for the Alzheimer's disease pathology, and then treating patients with the aim of halting or slowing progression from MCI due to Alzheimer's disease to Alzheimer's dementia.
An important health systems challenge will arise if this new paradigm bears out in late-stage clinical trials. In the 28 European Union (EU) countries, we estimate that approximately 20 million individuals over age 55 have MCI (Eurostat, 2018; Petersen et al., 2018), although most people have not been tested for disease pathology, as there is currently no treatment option. Thus, when a therapy first becomes available, there would be a substantial number of existing (or prevalent) MCI cases that would require screening, diagnosis, and then treatment as quickly as possible to prevent the progression to full-blown Alzheimer's dementia.1
We have previously analyzed the preparedness of the health care system in the United States to handle the potential caseload if and when a disease-modifying therapy becomes available (Liu et al., 2017). With the capacity of the current infrastructure projected forward, we found that there would be long waiting times for dementia specialist visits initially, followed by considerable waits for biomarker testing to confirm the Alzheimer's pathology and moderate waits for infusion delivery of the therapy, resulting in 2.1 million patients developing Alzheimer's dementia between 2020 and 2034 (about 13 percent of the potentially avoidable new cases in this period) while on waiting lists in the United States (Liu et al., 2017).
Other countries may face similar infrastructure challenges in diagnosing and delivering treatment to people with Alzheimer's disease. International attention has been paid to the preparedness of individual countries to support and adopt innovation in dementia diagnosis, treatment, and care. For example, the Global Coalition on Aging and Alzheimer's Disease International for G7 countries developed the Dementia Innovation Readiness Index (Global Coalition on Aging and Alzheimer's Disease International, 2017). The World Health Organization has a global action plan with action area 4 focused on diagnosis, treatment, care and support (WHO, 2017), and a guide for countries to develop dementia plans (WHO, 2018).
The objective of this study is to extend the infrastructure analysis to six European countries: France, Germany, Italy, Spain, Sweden, and the United Kingdom. This selection is made up of six large countries representing 65 percent of the population in the European Union (Eurostat, 2018). As with the U.S. analysis, we assess how each country's infrastructure for diagnosis and treatment lines up with the potential caseload of patients. We use the simulation model developed in the U.S. study and incorporate country-specific data and expert input on clinical practices. Our goal is to demonstrate the potential magnitude of the mismatch between the patient caseload and the available supply of services, not to provide an exact prediction of future therapies and capacity. Our estimates reflect a base case scenario assuming that the current capacities of the health care systems are carried forward.
In the following sections, we briefly describe the conceptual framework and the simulation model. We then present data on the current capacity in each country. We show the effects of capacity constraints on access to diagnosis and treatment of Alzheimer's disease and describe the effects of resolving the capacity constraints in each country. We conclude with a comparison of the preparedness of the health care systems in the six countries and discuss potential efforts to address the expected mismatch between supply and demand. We hope this analysis will help facilitate continued dialogue among stakeholders on how to ensure timely diagnosis and treatment of patients if a disease-modifying therapy for Alzheimer's becomes available.
Conceptual Framework and Simulation Model
The patient journey that we use as the framework for our analysis is depicted in Figure 1. We assume that individuals age 55 and older could undergo cognitive screening via a short instrument, such as the Mini–Mental State Examination or Folstein test in a primary care setting (screening phase) (Folstein, Folstein, and McHugh, 1975); we use the threshold of 55 years based on the inclusion criteria in several late-stage clinical trials currently in progress (e.g., U.S. National Library of Medicine, 2018a; 2018b; 2018c). Patients screening positive would be referred to a dementia specialist for further evaluation and possible referral to testing for the presence of biomarkers related to Alzheimer's disease (diagnostic phase). If testing is positive, patients would return to the dementia specialist for further evaluation to confirm whether treatment is indicated and appropriate. If appropriate, they would be referred to treatment (treatment phase), which would reduce the risk of progression from MCI to manifest dementia due to Alzheimer's disease (outcomes phase). While patients await diagnosis and treatment, the disease continues to progress—that is, patients are at risk of progressing to a later stage of the disease, at which point the treatment would no longer be effective.
Figure 1.
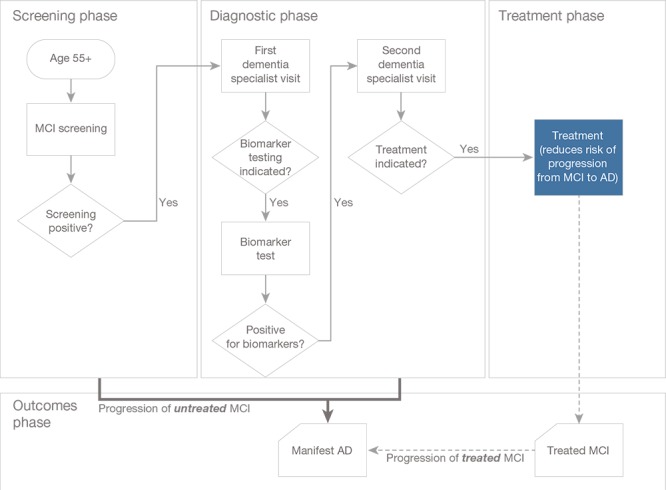
Conceptual Framework for the Patient Journey
SOURCE: Liu et al., 2017.
NOTE: AD = Alzheimer's dementia.
We use the simulation model previously developed for use in the United States to estimate the effect of capacity constraints on access to care for patients with suspected Alzheimer's disease (Liu et al., 2017). The model consists of a Markov model to simulate transitions between disease states and a systems dynamic model to simulate health care system capacity constraints within the MCI state. Patients’ journey through the disease states is guided by transition probabilities from having “no cognitive impairment” (i.e., no MCI and no Alzheimer's dementia) to MCI to Alzheimer's dementia. The transition probabilities are based on reported rates from epidemiological studies. Within the MCI state, patients move through the diagnostic and treatment phases based on a system dynamics model with outflows constrained by infrastructure capacity. We model three capacity constraints: availability of dementia specialists, biomarker testing, and infusion delivery. We optimize the capacity of the dementia specialist workforce to provide the second (confirmatory) visit to a patient such that the maximum number of patients receive their confirmatory visit in the same year as their initial visit—that is, we assume that specialists would not take on a new patient for an initial visit if the specialist would not have the capacity to provide confirmatory visits for existing patients. Figure 2 shows the expected patient demand in the six countries at each stage of the patient journey, assuming no constraints on infrastructure capacity for screening, diagnosis, and treatment. We estimate that 7.1 million MCI patients would seek evaluation by a dementia specialist and 2.3 million would be indicated for treatment in the six countries.
Figure 2.
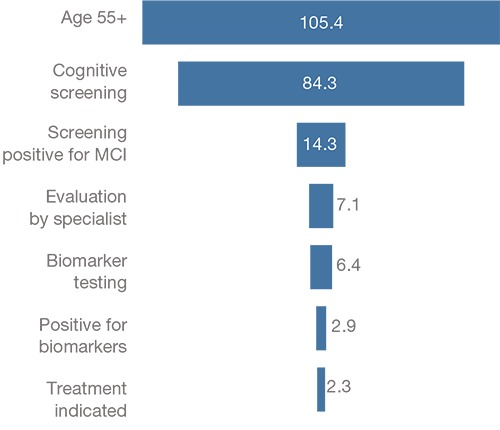
Expected Patient Demand at Each Stage of the Patient Journey in the Six European Countries in 2019 (millions)
NOTE: The number of expected patients is from France, Germany, Italy, Spain, Sweden, and the United Kingdom.
The assumptions about patient uptake, contraindications, and the treatment delivery and effectiveness are based on expert input in the U.S. study as described in Liu et al., 2017. For this analysis of the six European countries, we apply the same general assumptions regarding the treatment, uptake, and disease transitions as in our U.S. study and indicate when different assumptions are used. In summary, the key assumptions in our analysis are as follows:
A disease-modifying therapy for patients with MCI due to Alzheimer's disease becomes available in 2020. We assume the therapy would be an anti–beta-amyloid monoclonal antibody, as candidates that target beta-amyloid are the furthest along in clinical trials, that would be delivered by intravenous administration.
Individuals become eligible for annual cognitive screening at age 55. Annual screenings are conducted by general practitioners, and we assume screening capacity would be unconstrained. We assume that 80 percent of eligible individuals would be screened each year. Screening starts in 2019 as patients and providers anticipate the approval of the therapy. We assume that 50 percent of individuals who screen positive for MCI would continue to receive further evaluation.
Further evaluation would be conducted during a single visit to a dementia specialist—a neurologist, geriatrician, or geriatric (or old-age) psychiatrist, depending on the clinicians involved in dementia diagnosis and care in each country. If the evaluation confirms MCI and does not find an alternative explanation for MCI (e.g., prior stroke) or a reason to not pursue treatment (e.g., presence of another life-limiting disease), individuals would be referred to testing for biomarkers. We assume that 90 percent of patients with confirmed MCI that is possibly due to Alzheimer's disease would undergo biomarker testing.
In the European countries, biomarker testing may be performed by a cerebrospinal fluid (CSF) test or a Positron Emission Tomography (PET) scan for amyloid and/or tau.2 We assume that 90 percent of biomarker tests would be performed using CSF, and 10 percent would be conducted with PET scans when CSF is contraindicated (Alzheimer's Research UK, 2018).3 We assume that 45 percent of MCI patients have clinically relevant biomarker levels.
If a patient tests positive for biomarkers, she or he returns to a dementia specialist for a second visit, during which the treatment indication is confirmed, the patient is consented, and, if no contraindications to the treatment are found, the patient is referred to treatment. We assume that 80 percent of MCI patients testing positive for biomarkers would have no contraindications for treatment.
We assume the following treatment characteristics: Treatment would be delivered by intravenous infusion every four weeks over one year, following protocols for a typical immunotherapy; and treatment would reduce the relative risk of progression from MCI to Alzheimer's dementia by 50 percent.
For country-specific assumptions and data, we consulted a convenience sample of ten experts familiar with clinical practice and patient needs in each country—primarily clinicians and academic researchers specializing in care for patients with Alzheimer's disease and related dementias. The country-specific assumptions include the type of specialists involved in detection and diagnosis of cognitive impairment and dementia and the use of CSF assays and PET imaging in the diagnosis of Alzheimer's disease. The country-specific data include the population, disease prevalence, and mortality; the capacity scenarios are based on historical and projected workforce and infrastructure data.
Current Capacity Estimates and Projections
Specialist Workforce
We estimate the capacity of dementia specialists based on historical workforce data and assumptions about excess capacity. Overall, the dementia specialist workforce consists of neurologists, geriatricians, and geriatric or old-age psychiatrists. Table 2 shows the estimated number of specialists in each country.
Table 2.
Estimated Workforce to Supply Dementia Specialist Visits
| Neurologists | Geriatricians | Geriatric or Old-Age Psychiatrists | Specialists per 100,000 People | |
|---|---|---|---|---|
| France | 2,571 | 1,756 | — | 6.7 |
| Germany | 6,607 | 2,149 | 10,943 | 24.0 |
| Italy | 6,508 | 1,415 | 1,578 | 16.0 |
| Spain | 2,719 | 970 | 735 | 9.5 |
| Sweden | — | 450 | 1,349 | 18.2 |
| United Kingdom | 1,755 | 1,332 | 1,761 | 7.3 |
NOTES: The numbers of neurologists and psychiatrists are based on 2014–2016 data from Eurostat (most recent for each country). The number of geriatricians is based on 2012–2017 data from European Geriatric Medicine Society (most recent for each country). Based on expert input about the specialists involved in diagnosis of MCI due to Alzheimer's disease, we do not include geriatric psychiatrists in France and neurologists in Sweden.
As shown in Table 2, the specialists involved in diagnosing cognitive impairment and dementia vary in each country. We assume the types of specialties involved in this clinical area within each country to be unchanged over time in our projections. For example, diagnoses may be conducted by neurologists or geriatricians in memory clinics or private neurology practices in France. In Sweden, however, neurologists are typically not involved in diagnosis; rather, diagnoses are conducted by geriatricians and psychiatrists.
Another difference across countries is the extent to which psychiatrists have a role in formal diagnosis. In Germany and Sweden, experts suggested that approximately 60 percent of psychiatrists would be involved in formal diagnosis of MCI due to Alzheimer's disease, whereas the remaining 40 percent primarily practice psychotherapy. In contrast, in the United Kingdom, old-age psychiatry is a relatively small subspecialty representing about 15 percent of total psychiatrists (Royal College of Psychiatrists, 2017).
Generally, there is a perceived shortage of specialists, which is expected to worsen with the aging populations, but country-specific workforce projections by specialty are scarce in publicly available reports. Publicly available workforce projections are limited to certain geographies, exhibit different trends by specialties, and show uncertainty in the projections. For example, the Centre for Workforce Intelligence in England estimated the total psychiatrist workforce in four possible scenarios, with a baseline projection of about a 1- to 2-percent decrease between 2015 and 2030 and alternate scenarios ranging from about a 9-percent decrease to a 5-percent increase in the psychiatrist workforce (Centre for Workforce Intelligence, 2014). In Germany, the National Association of Statutory Health Insurance Physicians predicted the number of neurologists to remain relatively stable through 2030, with fluctuation of ±3 percent between 2014 and 2030 (Kassenarztliche Bundesvereinigung, 2016). In France, overall physician density is estimated to decrease slightly between 2015 and 2022 and then increase by over 25 percent by 2050 (Ono, Lafortune, and Schoenstein, 2013); these projections also include alternate scenarios but do not report on specific specialties. Given the variation in these workforce projections and the limited data, we use a simplified baseline assumption of static specialist workforce levels in each country from 2017 through 2050. If the workforce capacity were to change (e.g., if it responded to increased patient demand and expanded capacity), then our results would be different. Our base case scenario shows the effects if capacity is unchanged in the future. Therefore, our base case scenario should be interpreted as projections if the current workforce and capacity were maintained.
The availability of the dementia specialist workforce to see MCI patients in the diagnostic phase depends on the capacity of each specialist to conduct the evaluations. In our scenarios, we assume that each dementia specialist provides the same average number of ambulatory visits per year as neurologists in our U.S. model, 2,860 visits (Dall et al., 2013), because we assume the cognitive assessment and confirmation of the diagnosis would be conducted following the same guidelines across the specialist types.4 We also assume that these specialists devote 5 percent of their capacity to conducting these evaluations of MCI patients; we assume this excess capacity would be relatively low because of increasing demands on physician workforces with the aging population (we also examine scenarios using alternative assumptions with higher capacity, 7.5 percent, and lower capacity, 2.5 percent).
Diagnostic Technology
Diagnosing predementia Alzheimer's in a patient with MCI involves confirming the presence of biomarkers (beta-amyloid and/or tau) (Portet at al., 2006). In Europe, biomarkers may be detected using a CSF test or with neuroimaging. CSF samples are taken via a lumbar puncture (spinal tap) and tested for the presence of amyloid and tau proteins (Olsson et al., 2016; Herukka et al., 2017). Although lumbar punctures are more invasive than imaging tests, CSF tests are commonly used and generally well-accepted in the countries studied. However, if a patient has a contraindication for a lumbar puncture (e.g., due to anticoagulant medication) (Engelborghs et al., 2017), then neuroimaging with a PET scan may be used. In an amyloid PET scan, a radioactive tracer that selectively binds to amyloid is injected into a patient and a positron camera determines the brain areas with abnormal radiation activity, which indicates the presence of amyloid deposits.
We assume that capacity for CSF tests is theoretically unconstrained because lumbar punctures may be performed by general practitioners in various settings.5 We assume that 90 percent of biomarker tests could be conducted by CSF testing, and 10 percent would be conducted by PET scans (Alzheimer's Research UK, 2018).
We project the capacity for conducting PET scans based on the historical number of PET scanners in each country and assumptions about the annual number of scans per scanner and growth in the number of PET scanners. Our projections are based on 2015 Organisation for Economic Co-operation and Development (OECD) data for an installed base of PET scanners in France, Italy, Sweden, and Spain (OECD, 2017b) and country-specific data sources for PET scanners in the United Kingdom and Germany (National Cancer Research Institute PET Core Lab, 2018; Gesundheitsberichterstattung des Bundes, 2018). As with our prior U.S. analysis, we assume that existing devices operate at about 50 percent of their capacity due to scheduling constraints. We assume that newly installed devices would be largely dedicated to amyloid scans and could be run at 80 percent of their capacity.
Infusion Delivery
As mentioned, we assume the disease-modifying therapy would be administered intravenously. Thus, the capacity of the health care system to deliver infusions is a potential constraint to accessing the treatment. Many candidates currently in phase 3 trials are infused every four weeks over one to two years.6 However, this may reflect initial phases of treatment, and it is unknown whether longer-term durations of treatment would be needed. In this analysis, we assume that a course of treatment would consist of 14 infusions over one year. If the therapy required delivery for more than one year, then the capacity required would be even greater.
Our projections of the capacity of infusion centers are based on relative health care system capacity estimates and assumptions about growth rates. Due to lack of data on infusion capacity in the countries studied, we adjust the historical numbers of infusions delivered in the United States to each country based on population size and relative health care system infrastructure information. We develop a general health care capacity index for each country studied based on data on hospital beds, active nurses, MRI scanners, and PET scanners. The rationale for these four components is that the number of hospital beds is an indication of the facility space available, the number of nurses provides information about staff available to administer infusions, and the number of MRI and PET scanners provides an indication about medical technology and the degree of investment in the medical sector. We use OECD data to develop this index for the six countries relative to the United States (OECD, 2017b). We then use this index to scale the projected infusion capacity in each of the countries relative to the United States (based on infusion projections presented in Liu et al., 2017). We applied the same assumptions as in our prior U.S. analysis regarding the excess capacity (10 percent of current infusion capacity and 80 percent of new infusion capacity) that could be devoted to the treatment of MCI patients with Alzheimer's pathology.
Simulation Results for Selected Capacity Scenarios
We estimate the potential impact of capacity constraints on wait times and the progression of Alzheimer's disease under three scenarios: a base case scenario with current capacity estimates projected forward, an alternative scenario without infusion capacity limits, and an alternative scenario without any capacity limits.
Base Case: Current Capacity Projections
Of the three constraints we consider, we estimate that there will be delays in dementia specialist visits and infusion treatment but find that biomarker testing is not a constraint in any of the countries studied. As shown in Figure 3, there are substantial differences in the capacity of the health care systems in the countries studied to handle a new treatment for Alzheimer's disease. As a result, average wait times differ by country and are eliminated at different points in time: The backlog of cases clears as late as 2044 in Spain and as early as 2030 in Germany.
Figure 3.
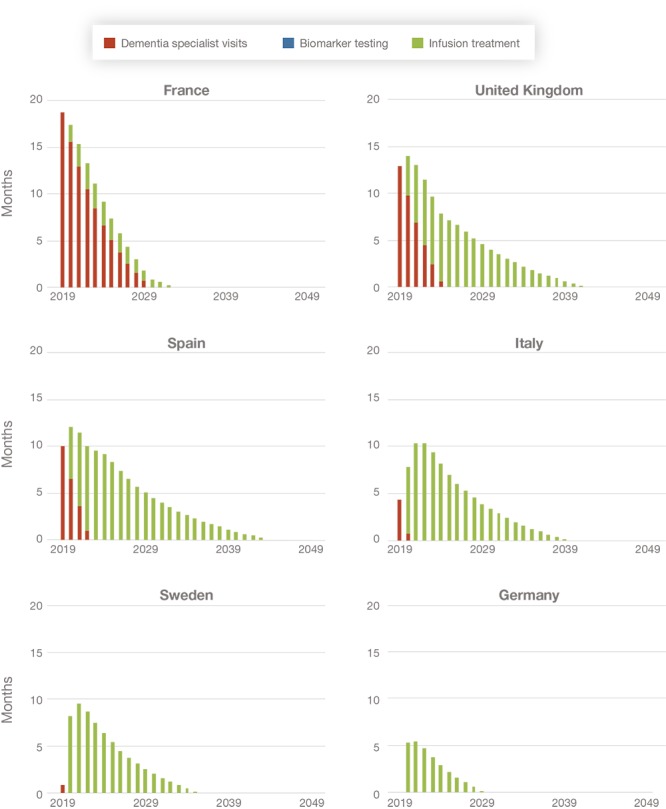
Projected Wait Times for Alzheimer's Disease Diagnosis and Treatment, by Country (average time delay in months)
In France, wait times would be driven primarily by the availability of specialists. While wait times in France are eliminated within a few years, France has the highest capacity constraints in the short run due to the limited number of specialists (and, thus, longer average wait times that result in a higher share of avoidable Alzheimer's dementia cases occurring compared with the other countries). The overall backlog clears by 2033, given the relatively larger infusion capacity in France.
In Spain and the United Kingdom, the wait times are a result of limited capacity for specialist visits and infusions. Because of the limited capacity in both the diagnosis and treatment phases, we estimate that the waiting period would extend to 2044 in Spain and 2042 in the United Kingdom.
In Italy, Sweden, and Germany, the wait times would be driven primarily by the capacity to deliver infusions. Wait times in Italy are expected until 2040. Wait times are much shorter in Sweden and Germany, where there are theoretically enough specialists to diagnose the large number of MCI patients. This results in wait times constrained only by availability of infusion services, which we expect to be relatively well-supplied compared with the other countries. We estimate that wait times extend until 2036 in Sweden and 2030 in Germany.
The estimates for the base case scenario reflect what we believe are moderate assumptions about capacity growth; however, there is substantial uncertainty in these estimates given limited historical data and unknown characteristics of a future breakthrough Alzheimer's therapy. In the following section, we show projections of alternative scenarios assuming that policies are put into effect in response to a new therapy, specifically to address capacity issues. The alternative scenarios are discussed relative to the base case scenario.
Comparison with Alternative Scenarios
In addition to the base case scenario with the current capacity projected forward, we explore two alternative scenarios assuming the capacity constraints are partially or fully addressed. The health care system capacity for diagnosis and treatment could be expanded in response to a new Alzheimer's treatment. We first discuss the specifications of the two alternative scenarios and then compare results of these scenarios for each country.
Alternative Scenario 1: Removing the Constraint on Infusion Capacity
For the first alternative scenario, we remove capacity constraints on infusion delivery in the simulation, leaving only access to dementia specialists as a constraint, as biomarker testing is already not constrained in the base case scenario.
The projected capacity for administering intravenous infusions in our base scenario falls short of potential demand. As described earlier, we assume that patients would require infusions every four weeks for a period of one year. For an estimated 2.3 million MCI patients who could be eligible for treatment in 2020 in the six countries, approximately 32 million infusions would be required to treat the prevalent MCI population.
The historical growth in capacity for immunotherapy and chemotherapy treatments provides precedence for similar capacity growth to accommodate the demand. Similar challenges were addressed for immunomodulating antibodies for inflammatory diseases in the late 1990s, and, more recently, the introduction of biologic therapies for multiple cancers and other conditions, such as multiple sclerosis, have required expansion of infusion capacity (Tralongo et al., 2011).
It is possible that dementia specialists and other operators could also expand capacity in response to demand in a similar way; however, accommodating the large number of patients with MCI due to Alzheimer's disease could be more challenging than treating patients with inflammatory conditions. An Alzheimer's disease–modifying therapy would be first in class, whereas the immunomodulating antibodies were preceded by disease-modifying oral drugs, which allowed physicians to first target the limited infusion capacity to refractory cases. In addition, building infusion capacity for the immunotherapies was more financially viable because they are delivered to patients for the rest of their lives, while the amyloid antibodies are expected to be administered in a finite number of doses. Permanent capacity growth for amyloid antibodies may be less favorable, given that there will be about 14 million prevalent MCI cases initially but about 1 million incident MCI cases each year in the six countries studied.
An option to expand capacity quickly, in addition to infusion centers as extensions of memory clinics or other facilities, would be home infusion delivery. Home infusion services would reduce investment into permanent facilities that may later have idle capacity if the therapy is time-limited. The amyloid antibodies are infused over a relatively short time and are not known to have acute side effects; thus, they may be suitable for home infusion if it proves to be a financially viable option.
Alternative Scenario 2: Removing All Capacity Constraints
In alternative scenario 2, we remove all capacity constraints—that is, the constraints on both infusion delivery and dementia specialist visits. As with the prior scenarios, this is hypothetical; however, this scenario is less likely because medical specialists require long training times, and diagnosing Alzheimer's requires comprehensive assessment. The assessment, including medical history, cognitive testing, neurological testing, and brain imaging, is typically conducted by physicians. We include this scenario to demonstrate the upper bound of Alzheimer's dementia cases that could be potentially avoided if all capacity constraints are overcome.
Comparison of New Alzheimer's Dementia Cases Occurring in the Base Case and Alternative Scenarios
Figure 4 shows how the base case scenario and the two alternative scenarios would change the estimated cumulative incidence of Alzheimer's dementia between 2020 and 2050. Although the incremental benefit of removing capacity constraints is a small proportion of total possible incident cases avoided, it represents over 1 million additional patients in the six countries studied—a significant burden for the respective health care systems.
Figure 4.
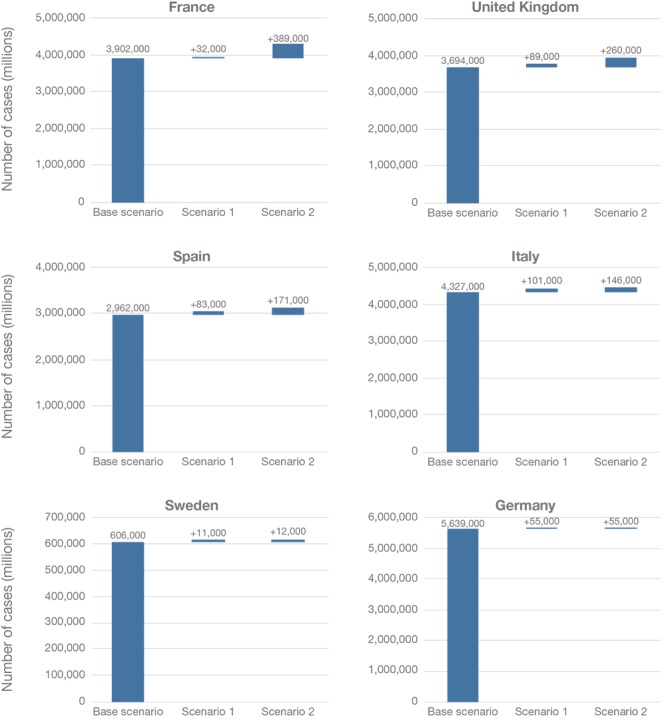
Projected Cumulative Number of New Alzheimer's Dementia Cases Avoided Between 2020 and 2050 Under Different Scenarios, by Country
NOTES: The number of cases in both Scenario 1 and Scenario 2 are shown relative to the base case scenario. For example, in Germany, there are 55,000 additional new Alzheimer's dementia cases averted in Scenario 1 relative to the base scenario, and 55,000 additional cases averted in Scenario 2 relative to the base scenario. In other words, there is no difference between Scenario 1 and Scenario 2, in which the specialist constraint is removed, because there is no projected waiting time for specialists in Germany.
Between 2020 and 2050, we estimate that over 3.9 million incident Alzheimer's dementia cases would be avoided in the base case in France.7 Removing the capacity constraint for infusion delivery (Scenario 1) would avoid an additional 32,000 cases relative to the base case (0.7 percent of potentially avoidable new cases). Removing the specialist constraint as well (Scenario 2) has a larger impact and would avoid 389,000 additional cases relative to the base case (9.0 percent of potentially avoidable new cases).
In the United Kingdom, nearly 3.7 million incident Alzheimer's dementia cases would be avoided in the base case. We estimate that removing the capacity constraint for infusion delivery would avoid an additional 89,000 cases relative to the base case (2.2 percent of potentially avoidable new cases), and when the specialist constraint (Scenario 2) is removed, an additional 260,000 cases would be avoided relative to the base case (6.4 percent of potentially avoidable new cases).
In Spain, we estimate that nearly 3 million incident Alzheimer's dementia cases could be avoided in the base case. Removing the capacity constraint for infusion delivery (Scenario 1) would allow an additional 83,000 cases to be avoided relative to the base case (2.6 percent of potentially avoidable new cases). When the specialist constraint (Scenario 2) is removed, an additional 171,000 cases would be avoided relative to the base case (5.3 percent of potentially avoidable new cases).
In Italy, we estimate over 4.3 million incident Alzheimer's dementia cases would be avoided in the base case, an additional 101,000 cases relative to the base case (2.2 percent of potentially avoidable new cases) would be avoided if the capacity constraint for infusion delivery is removed, and 146,000 additional cases would be avoided relative to the base case (3.2 percent of potentially avoidable new cases) if the specialist and infusion constraints are removed.
In Sweden, over 600,000 incident Alzheimer's dementia cases would be avoided in the base case. Removing the capacity constraint for infusion delivery (Scenario 1) would allow an additional 11,000 cases to be delayed relative to the base case (1.7 percent of potentially avoidable new cases). When the specialist constraint (Scenario 2) is removed, we estimate that 12,000 additional cases would be avoided relative to the base case (1.9 percent of potentially avoidable new cases) between 2020 and 2050.
In Germany, over 5.6 million incident Alzheimer's dementia cases would be avoided in the base case scenario. Removing the capacity constraint for infusion delivery (Scenario 1) would allow an additional 55,000 cases to be avoided relative to the base case (1.0 percent of potentially avoidable new cases). There are no additional avoided cases when the specialist constraint (Scenario 2) is removed relative to the base case because we estimate no waiting times for specialist visits even in the base case, hence the number of avoidable cases relative to the base scenario is also 55,000.
Summary of Results by Country
Table 3 shows a summary of the differences in maximum wait times across the six countries. The longest wait times are experienced in the first few years after a new therapy becomes available. As noted earlier, we find that biomarker testing is not a binding constraint, given the availability of CSF testing in Europe. All six countries are expected to experience infusion wait times less than 12 months, with infusion wait times of less than six months estimated in Germany and France. There is greater variation in specialist wait times, with no wait times expected in Germany and wait times more than 12 months expected in France and the United Kingdom.
Table 3.
Summary of Wait Times for Capacity Constraints, by Country
| Maximum Waiting Time, Months | First Year with No Wait Times | Potentially Avoidable New Alzheimer's Dementia Cases Occurring While Patients Wait, 2020–2044 (percentage of potentially avoidable cases) | |||
|---|---|---|---|---|---|
| Specialists | Infusions | Base Case: Specialist and Infusion Constraints | Scenario 1: Specialist Constraint | ||
| Germany | No wait | < 6 | 2030 | 55,000 (1%) | 0 (0%) |
| Sweden | < 6 | 6–12 | 2036 | 12,000 (2%) | 1,000 (< 1%) |
| Italy | < 6 | 6–12 | 2040 | 146,000 (3%) | 45,000 (1%) |
| Spain | 6–12 | 6–12 | 2044 | 171,000 (5%) | 88,000 (3%) |
| United Kingdom | > 12 | 6–12 | 2042 | 260,000 (7%) | 171,000 (4%) |
| France | > 12 | < 6 | 2033 | 389,000 (9%) | 357,000 (8%) |
| Total | — | — | — | 1,033,000 (5%) | 662,000 (3%) |
NOTE: The potentially avoidable new Alzheimer's dementia cases are measured relative to Scenario 2, in which there are no capacity constraints and, thus, no patients waiting. There are no waiting times for biomarker testing if SCF assays are used for 90 percent of testing and PET scans are used for 10 percent of testing.
Across the six countries, between 1 and 9 percent of potentially avoidable incident cases of Alzheimer's dementia between 2020 and 2044 still occur in the base scenarios (using Scenario 2, without any constraints, as the reference point for the maximum number of avoidable cases). The percentage of avoidable cases is highly dependent on the effectiveness of the therapy; however, the relative differences between countries provide insight on the variation in the infrastructure across countries. The smallest constraint is observed in Germany, with 99 percent of cases avoided already in the base scenario. The elimination of wait times for specialists would have the largest relative impact for Spain, the United Kingdom, and France. Although the vast majority of new Alzheimer's dementia cases are avoided in the base scenarios in all countries, over 1 million cases develop while patients are on waiting lists in the six countries studied. If infusion capacity can be addressed (Scenario 1), the number of potentially avoidable new Alzheimer's dementia cases that occur while patients are on wait lists goes down to 662,000. However, the specialist capacity must be addressed to prevent waits for all possible new dementia cases.
Limitations
There are several limitations in our analysis. As with our prior analysis, the framework for the model is based on a highly stylized patient journey that simplifies patterns of care. We model hypothetical scenarios involving a future Alzheimer's treatment with assumed specifications—such as time of market entry, efficacy, and dosing—that depends on actual clinical trial results and is uncertain at this time. Nonetheless, the goal of this analysis is to describe possible scenarios rather than precisely predict future supply and demand to demonstrate potential challenges and inform policy solutions that could help mitigate the challenges.
Many of our modeling assumptions are based on a therapeutic profile that is unknown at this time. As current clinical trials of Alzheimer's therapies typically include patients age 50 or 55 and older (e.g., U.S. National Library of Medicine, 2018a; 2018b; 2018c), we assume that patients would be screened starting at age 55. We assume uptake rates for screening, evaluation, testing, and treatment based on expert input from our prior U.S. analyses; however, uptake at each step would depend on such factors as patient awareness and acceptance and reimbursement levels in each country.
Our capacity projections are based on extrapolations of historical data and assumptions informed by expert input from clinicians and researchers familiar with the countries studied, and our projections will almost certainly not accurately predict future patterns. Although general practitioners have the technical capability to do basic cognitive screening and lumbar punctures (to collect cerebrospinal fluid for diagnostic tests), not all general practitioners may choose to perform these tests. We do not examine the impact of constraints in primary care settings; any capacity constraints in cognitive screening and lumbar punctures would increase estimated waiting times. While CSF and PET measures are correlated and both can accurately detect early Alzheimer's disease, the tests provide different information about disease state and progression (Palmqvist et al, 2015; Bouallegue et al., 2017; AlzForum, 2017); we did not differentiate between CSF and PET testing in this analysis. We also do not consider capacity issues related to monitoring the disease progression, such as the use of magnetic resonance imaging.
Due to the lack of data on the specialist workforce in the future and on current capacity to provide infusions in each of the countries studied, we make simplified assumptions with a flat workforce projection and based on relative capacity data compared with the United States. We do not distinguish subspecialties within neurology; some general neurologists may require training in dementia diagnosis and care. We also assume that specialists across countries see the same average number of patients per year. Although the number of consultations per doctor varies across countries, the number of doctor consultations depends partly on the role of nurses and other practitioners in providing care (OECD, 2017a). We assume that early detection and diagnosis protocols for cognitive impairment would require a similar amount of time regardless of the country. However, our convenience sample of clinical and patient advocacy experts did not include specific expertise in workforce or infusion market issues.
As with most simulation analyses, we combined data from various sources that may not be consistent with each other. For example, there are multiple sources for dementia and Alzheimer's dementia prevalence estimates. We relied on country-specific estimates over international estimates when possible and verified that our model produces similar projected burden of Alzheimer's dementia as other studies. In addition, we make several simplifications, such as transitions between disease states occurring in one-year time steps and parameterized by annual transition probabilities. As developments in diagnostic and treatment capacity depend on a host of uncertain factors, such as the results of clinical trials, level of demand, and reimbursement levels, we emphasize that our results are meant to show the magnitude of the mismatch between projected demand and capacity rather than to provide a precise estimate of that mismatch.
Discussion
There is guarded optimism among researchers that one or more disease-modifying therapies for Alzheimer's disease could become available in the upcoming years. Such a breakthrough therapy could finally provide patients and their families with a treatment to delay or prevent the progression of this devastating condition. The potential caseload is sizable, given that most therapies in development focus on treating early-stage Alzheimer's disease: We estimate that approximately 7.1 million people with MCI in the six European countries could seek timely diagnosis by a specialist and, when indicated, testing for Alzheimer's pathology to determine their eligibility for treatment.
Our analysis suggests that the health care systems in some of the European countries have insufficient capacity to diagnose and treat the large number of patients with early-stage Alzheimer's disease. While there would be no waiting for biomarker testing if capacity for CSF testing is sufficient, we estimate that all countries could face insufficient capacity for infusion services, and some countries also have limited availability of specialists under our base case capacity assumptions. In Germany and Sweden, the main infrastructure constraint would be infusion capacity, resulting in less than 2 percent of potentially avoidable new Alzheimer's dementia cases developing in each country while patients wait. However, in the other four countries, wait times due to both specialist availability and infusion capacity would delay treatment for more significant numbers of patients, resulting in up to about 9 percent of potentially avoidable Alzheimer's dementia cases while patients are waiting in France, 6 percent in the United Kingdom, 5 percent in Spain, and 3 percent in Italy. As Alzheimer's disease is a progressive neurodegenerative disorder, we estimate that the combined effect of the specialist and infusion wait times could result in over 1 million new cases of Alzheimer's dementia that could be avoided between 2020 and 2044, were a treatment to exist and be delivered immediately to all eligible patients.
We emphasize that our projections assess the potential capacity to deliver an Alzheimer's therapy, whereas regulations, reimbursement levels, and volume agreements between regulators, payers, and providers will determine the actual capacity devoted. In addition, the projections hinge on assumptions that are uncertain, given limited information on how capacity will evolve over time. For example, resource allocation in the National Health Service (NHS) of the United Kingdom is determined by centrally mandated requirements and decisions by clinicians in the local NHS provider organizations. In Germany, capacity planning and volume agreements at the state and regional levels will influence available capacity and payment levels, which, in turn, will affect the degree to which providers will devote activity to dementia-related care.
Causes of Differing Wait Times Between Countries
The different patterns in wait times are driven by the relative capacity for specialist visits and infusion delivery in each health care system. Notably, the limited capacity for both specialists and infusion delivery in Spain and the United Kingdom resulted in the longest period before wait times are eliminated (i.e., until the backlog of cases is cleared). However, the peak wait times from diagnosis to treatment were the longest in France, due to the substantial wait times for specialist visits in the early years. In contrast, we estimate wait times for infusions but minor wait times for specialists in Italy. While capacity for infusion delivery results in delayed access to treatment in Sweden and Germany, overall wait times are much shorter there than in the other countries.
The range of specialties involved in dementia care is the critical factor in determining wait times for specialist visits. Psychiatry is a much larger specialty than neurology and geriatrics in all six countries. Thus, countries like Germany and Sweden, where a larger share of psychiatrists is involved, tend to have short wait times. Conversely, wait times are long in countries, like the United Kingdom and France, where fewer or no psychiatrists are involved.
In countries that currently rely on the relatively small neurology and geriatric specialties to conduct the diagnosis and evaluation of early-stage Alzheimer's patients, it may be possible to expand the role of physicians from larger specialties. For example, more internal medicine physicians and general psychiatrists could be trained in Alzheimer's diagnosis. Such training and coaching by specialists may be complemented by telemedicine and telemonitoring efforts currently under way in many European countries (eHealth Stakeholder Group on Implementing the Digital Agenda for Europe, 2014).
Some Countries Have Initiated Efforts to Improve Access to Dementia Care
In addition to training more providers, the establishment of graduated clinical pathways that rely on general practitioners to screen and identify higher-risk patients out of the large pool of prevalent MCI patients could relieve the burden on specialists. For example, France has a national plan that includes aims to improve care pathways for patients with neurodegenerative disorders (see Box 1). Italy has launched a project to identify patients with the highest risk of developing Alzheimer's dementia to improve coordination and timeliness of care (see Box 2). An understanding of the characteristics of patients with early-stage Alzheimer's disease is vital to efficiently identify patients who could benefit from a new treatment.
Box 1.
Plan Maladies Neurodégénératives 2014–2019, France
The French Ministers of Health, Ageing and Family and of Research have published a five-year strategic plan to improve care for neurodegenerative disorders (Alzheimer Europe, 2016). The plan emphasizes the need for care pathways for these disorders generally, and specifically for Alzheimer's disease. It points out the need for screening and early detection, proper diagnostic evaluation, and management of comorbidities, even in the absence of a disease-modifying treatment. The plan calls for the development of better tools for screening and diagnosis, access to lifelong quality care, wherever patients live, and the need to build interdisciplinary capacity and capabilities, mainly through centers of excellence and diffusion into the community.
Box 2.
Interceptor Project, Italy
In December 2017, Italy launched the Interceptor Project to help identify people at higher risk of developing Alzheimer's disease, with the goal of improving their quality of care (Alzheimer Europe, 2017). The initiative aims to enroll 400 patients ages 50 to 85 with mild cognitive impairment and develop strategies to identify patients with the highest risk of developing Alzheimer's dementia and thus the greatest likelihood of benefiting if a new treatment becomes available (“Italy Launches Pioneering Project to Identify Alzheimer's Risk,” 2017). The Italian Ministry of Health expects to collect comprehensive data based on neuropsychological assessments, CSF tests, and PET and MRI scans over a period of three years, which is expected to help inform the selection of the most appropriate biomarkers for nationwide screening for patients with a higher risk of progressing toward Alzheimer's dementia. One of the stated goals, moreover, is to better understand which patients are most likely to benefit from a novel therapy, thus increasing the system's sustainability (Ministero della Salute, 2017).
Other Countries Have Explored New Models for Infusion Delivery
If a therapeutic agent requires near-monthly visits to administer an intravenous infusion, most European countries may not have the capacity to provide infusions to all eligible patients in a timely manner. In contrast to the specialist limitations, the inadequacy of infusion capacity may have two relatively near-term solutions: building dedicated infusion centers as extensions to existing facilities and using home infusions, if allowable based on the therapy's safety profile.
Dedicated outpatient infusion centers may be built in cooperation with hospitals, memory clinics, and other health care facilities. In the past, specialized infusion centers have been established to provide outpatient infusion therapy administered by nurses to multiple sclerosis patients in Germany (see Box 3). Similar centers could be established for MCI patients if a new therapy becomes available, with specialized nurses trained in intravenous administration, oversight by physicians, and patient education.
Box 3.
Multiple Sclerosis Specialist Nurses in Germany and Other EU Countries
The advent of new therapies for multiple sclerosis, a severe neurological condition, has necessitated new drug delivery pathways, including the education of nurses to provide specialized care for patients with regular antibody infusions. In Germany, it is possible to receive infusions for multiple sclerosis therapy in outpatient settings with specialized nurse assistance, increasing the quality of life of patients while reducing the cost and burden on existing health care facilities in caring for these patients (Giedigkeit, 2018). Where relevant, nurses may also provide training for self-administered injections and the education about potential side effects (Sachsisches Krankenhaus Großschweidnitz, 2018). Across European countries, an online training program for nurses that aims to consolidate standards in multiple sclerosis care has been offered in multiple languages and offers six different modules: understanding the disease, clinical presentation, diagnosis and assessment, treatment, care and support, and rehabilitation (European Multiple Sclerosis Platform, 2018).
Although there is currently limited experience with home infusions in Europe, a mix of facility-based and home infusions may ultimately offer patients and physicians therapeutic options when resources are constrained, assuming both are implemented in compliance with relevant regulations and are found to be financially viable.
EU-Wide Efforts Can Provide Guidance and Promulgate Best Practices
In addition to increasing specialist and infusion capacity in each country, joint actions and planning in Europe can help provide better coordinated and more timely care for Alzheimer's patients. For example, the Second European Joint Action on Dementia aims to identify best practices in dementia care (see Box 4), and several national plans and strategies have been drafted in countries including France, Italy, and Sweden to improve the diagnosis and care of dementia patients, improve their quality of life (and that of their caregivers), and better coordinate research (Alzheimer Europe, 2016; Di Fiandra, 2015; Alzheimer Europe, 2013).
Box 4.
European Joint Action on Dementia
The second European Joint Action on Dementia was launched in 2016, building on a previous joint action ALCOVE (Alzheimer Cooperative Valuation in Europe) that sought to improve epidemiological data on dementia, improve the timeliness and reliability of dementia diagnosis, understand support systems for behavioral and psychological symptoms, improve the rights and dignity of dementia patients, and reduce the use of antipsychotics to improve dementia patients’ quality of life (Alzheimer Europe, 2018a; Leperre Desplanques et al., 2013).
The most recent effort has brought together experts from multiple European countries with the goal of discussing “practical guidance for policy makers developing and implementing their national dementia plans, policies and strategies” (Alzheimer Europe, 2018a). In November 2017, for instance, they published a report on the risks and benefits associated with dementia diagnosis (Krolak-Salmon et al., 2017). In early 2018, a pilot program addressing diagnosis and post-diagnostic support was launched, focusing on destigmatization, nurse–general practitioner cooperation, and telemedicine to improve dementia detection in nursing homes (Act on Dementia, 2018).
Additionally, the Joint Action on Dementia aims to generate evidence on improving outcomes in dementia patients and their caregivers; enhance EU-wide collaboration on dementia diagnosis, services, and support; and inform an international collaboration on care for dementia patients (Alzheimer Europe, 2018b).
Conclusions
Positive early clinical trial results have led to guarded optimism that a disease-modifying therapy for Alzheimer's disease may become available in the coming years. The ability to halt or slow the progression of this devastating and common disease would represent a significant breakthrough. We find variation in the preparedness of the health care systems in six European countries studied: All six systems may encounter wait times due to lack of capacity, but some systems have less infrastructure than others to diagnose and treat early-stage Alzheimer's patients. Our analysis suggests that the health care systems in some European countries would lack adequate capacity to provide patients with access to treatment within a reasonable time frame, mainly because of constraints in the capacity of specialists to diagnose patients and of infusion delivery services. If a disease-modifying therapy becomes available in 2020, we estimate that failure to increase capacity means that over 1 million patients with MCI due to Alzheimer's disease could develop dementia while waiting for evaluation and treatment in the six countries between 2020 and 2044. Longer wait times and thus higher shares of potentially avoidable dementia cases are expected for France, the United Kingdom, and Spain.
Addressing the health care capacity constraints will require combining payment, regulatory, and workforce planning policies with broad education campaigns to increase patient awareness of the importance of timely detection of early-stage disease. We hope that this report helps facilitate discussions among multiple stakeholders on how to address the potential obstacles to delivering an Alzheimer's disease therapy in a timely way.
Notes
The research described in this article was sponsored by Biogen and conducted within RAND Health.
The caseload would lessen after the prevalent cases are treated, as the number of patients who newly develop MCI (incident cases) each year would be a fraction of the prevalent cases. In this study, we focus on cases in six European countries, in which we estimate approximately 14.3 million individuals over age 55 currently have MCI and about 1.0 to 1.4 million incident cases of MCI will develop each year between 2020 and 2050, suggesting the long-term capacity would not need to be as high as the short-term capacity needed when a new treatment is introduced.
This assumption differs from our prior analysis in the United States, where a PET scan is the only currently Food and Drug Administration–approved modality for clinical use.
Although we assume that 90 percent of biomarker tests could be conducted using CSF in each of the six countries in this analysis, there is within-country regional variation in the acceptability of using CSF for diagnostic testing.
The evaluation of MCI patients may include a brain magnetic resonance imaging (MRI), which we do not assume to be constrained by capacity. Although the average number of consultations per doctor varies across countries, cognitive assessment and diagnosis following standardized protocols could reduce the variation in length of visits. If fewer consultations per doctor are possible, then the capacity projections would be lower.
Although our convenience sample of experts from the six countries confirmed that general practitioners are trained to perform lumbar punctures in various settings, there is variability in acceptance and norms across and within countries.
The actual treatment duration would depend on the results from later-stage clinical trials.
In our scenarios, we assume treatment reduces the relative risk of progression from MCI to Alzheimer's dementia by 50 percent. We refer to the difference in Alzheimer's dementia cases that occur with the treatment without any infrastructure constraints and those that occur without the treatment as “potentially avoidable” cases within a given time period. Although we refer to these cases as avoidable, in reality it is unknown whether a treatment would delay the progression of Alzheimer's disease for some time or prevent dementia from occurring.
References
- Act on Dementia. “Make It Happen”. 2018. http://www.actondementia.eu/make-it-happen webpage, European Commission, As of May 25, 2018:
- Aisen P. S. “Continuing Progress in Alzheimer's Disease Trials: Cause for Optimism,”. Journal of Prevention of Alzheimer's Disease. 2017;Vol. 4(No. 4):211–212. doi: 10.14283/jpad.2017.34. pp. [DOI] [PubMed] [Google Scholar]
- Alzforum. “CSF and Brain Markers Highlight Different Facets of Dementia”. August 12, 2017. https://www.alzforum.org/news/conference-coverage/csf-and-brain-markers-highlight-different-facets-dementia Alzheimer's Association International Conference 2017 proceedings, London, July 16–20, As of July 20, 2018:
- Alzheimer Europe. “Sweden: Background Information About the National Dementia Strategy”. 2013. http://www.alzheimer-europe.org/Policy-in-Practice2/Country-comparisons/2012-National-Dementia-Strategies-diagnosis-treatment-and-research/Sweden fact sheet, Luxembourg, As of May 25, 2018:
- Alzheimer Europe. “France: National Dementia Strategies”. 2016. http://www.alzheimer-europe.org/Policy-in-Practice2/National-Dementia-Strategies/France fact sheet, Luxembourg, As of May 25, 2018:
- Alzheimer Europe. “Italy Initiates New Project to Identify AD Risk”. December 6, 2017. http://www.alzheimer-europe.org/News/Policy-watch/Wednesday-06-December-2017-Italy-initiates-new-project-to-identify-AD-risk press release, Luxembourg, As of May 25, 2018:
- Alzheimer Europe. “2016 [Launch] of 2nd Joint Action on Dementia”. January 19, 2018a. http://www.alzheimer-europe.org/Policy-in-Practice2/EU-Action-on-Dementia/2016-Lunch-of-2nd-Joint-Action-on-Dementia webpage, Luxembourg, As of May 25, 2018:
- Alzheimer Europe. “EU Joint Action on Dementia 2015–2018: Background and Overview”. 2018b. https://www.alzheimer-europe.org/content/download/100875/637885/file/PL2.1%2520-%2520Huggins%2520Geoff.pdf&usg=AOvVaw02itT7wDCVQuRd2A_NZSfb briefing slides, As of May 25, 2018:
- “Alzheimer's Disease: A Time for Cautious Optimism,”. Lancet Neurology. 2015;Vol. 14(No. 8):779. doi: 10.1016/S1474-4422(15)00154-4. p. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Research UK. Thinking Differently: Preparing Today to Implement Future Dementia Treatments. March 2018. https://www.alzheimersresearchuk.org/wp-content/uploads/2018/04/thinking_differently_report-180328-single.pdf Cambridge, UK, As of May 25, 2018:
- Bouallegue F. B., Mariano-Goulart D., Payoux P. Alzheimer's Disease Neuroimaging Initiative. “Comparison of CSF Markers and Semi-Quantitative Amyloid PET in Alzheimer's Disease Diagnosis and in Cognitive Impairment Prognosis Using the ADNI-2 Database,”. Alzheimer's Research and Therapy. 2017;Vol. 9(No. 32):1–13. doi: 10.1186/s13195-017-0260-z. and the. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Workforce Intelligence. In-Depth Review of the Psychiatrist Workforce. London: Mouchel Management Consulting; November 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/507557/CfWI_Psychiatrist_in-depth_review.pdf As of May 25, 2018: [Google Scholar]
- Dall T. M., Storm M. V., Chakrabarti R., Drogan O., Keran C. M., Donofrio P. D. and Vidic T. R. “Supply and Demand Analysis of the Current and Future US Neurology Workforce,”. Neurology. 2013;Vol. 81(No. 5):470–478. doi: 10.1212/WNL.0b013e318294b1cf. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiandra Teresa, Canevelli Marco, Di Pucchio Alessandra, Vanacore Nicola. Italian Dementia National Plan Working Group. “The Italian Dementia National Plan: Commentary,”. Annali dell'Istituto Superiore di Sanita. 2015;Vol. 51(No. 4):261–264. doi: 10.4415/ANN_15_04_02. and the. pp. [DOI] [PubMed] [Google Scholar]
- eHealth Stakeholder Group on Implementing the Digital Agenda for Europe. Widespread Deployment of Telemedicine Services in Europe. March 12, 2014. http://ec.europa.eu/information_society/newsroom/cf/dae/document.cfm?doc_id=5167 European Commission, As of May 25, 2018:
- Engelborghs S., Niemantsverdriet E., Struyfs H., Blennow K., Brouns R., Comabella M., Dujmovic I., van der Flier W., Frölich L., Galimberti D., Gnanapavan S., Hemmer B., Hoff E., Hort J., Iacobaeus E., Ingelsson M., de Jong F. J., Jonsson M., Khalil M., Kuhle J., Lleó A., de Mendonça A., Molinuevo J. L., Nagels G., Paquet C., Pernetti L., Roks G., Rosa-Neto P., Scheltens P., Skårsgard C., Stomrud E., Tumani H., Visser P. J., Wallin A., Winblad B., Zetterberg H., Duits F. and Teunissen C. E. “Consensus Guidelines for Lumbar Puncture in Patients with Neurological Diseases,”. Alzheimer's and Dementia: Diagnosis, Assessment, and Disease Monitoring. 2017;Vol. 8:111–126. doi: 10.1016/j.dadm.2017.04.007. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Geriatric Medicine Society. “National Societies”. 2018. http://www.eugms.org/our-members/national-societies.html webpage, Genoa, Italy, As of May 25, 2018:
- European Multiple Sclerosis Platform. “MS Nurse Pro Launched in Germany”. 2018. http://www.emsp.org/news-messages/ms-nurse-pro-launched-in-germany/ press release, As of May 25, 2018:
- Eurostat. “Physicians by Medical Speciality”. 2017. http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=hlth_rs_spec&lang=en database, As of September 16, 2018:
- Eurostat. “Your Key to European Statistics”. 2018. http://ec.europa.eu/eurostat/data/database homepage, As of May 25, 2018:
- Folstein M. F., Folstein S. E. and McHugh P. R. “‘Mini–Mental State’: A Practical Method for Grading the Cognitive State of Patients for the Clinician,”. Journal of Psychiatric Research. 1975;Vol. 12(No. 3):189–198. doi: 10.1016/0022-3956(75)90026-6. pp. [DOI] [PubMed] [Google Scholar]
- Gesundheitsberichterstattung des Bundes. “Medizinisch-Technische Großgeräte”. 2018. http://www.gbe-bund.de/gbe10/I?I=160:28777873D database, As of May 25, 2018:
- Giedigkeit Tomke. Dresdner Neueste Nachrichten. March 15, 2018. http://www.dnn.de/Dresden/Lokales/Neues-Infusionszentrum-und-neue-Therapie-fuer-Multiple-Sklerose “Neues Infusionszentrum und Neue Therapie fur Multiple Sklerose,”. As of May 25, 2018:
- Global Coalition on Aging and Alzheimer's Disease International. Dementia Innovation Readiness Index. April 2017. https://www.globalcoalitiononaging.com/data/uploads/documents/gcoa-adi-dementia-index.pdf As of June 5, 2018:
- Herukka S. K., Simonsen A. H., Andreasen N., Baldeiras I., Bjerke M., Blennow K., Engelborghs S., Frisoni G. B., Gabryelewicz T., Galluzzi S., Handels R., Kramberger M. G., Kulczyńska A., Molinuevo J. L., Mroczko B., Nordberg A., Oliveira C. R., Otto M., Rinne J. O., Rot U., Saka E., Soininen H., Struyfs H., Suardi S., Visser P. J., Winblad B., Zetterberg H. and Waldmar G. “Recommendations for Cerebrospinal Fluid Alzheimer's Disease Biomarkers in the Diagnostic Evaluation of Mild Cognitive Impairment,”. Alzheimer's and Dementia. 2017;Vol. 13(No. 3):285–295. doi: 10.1016/j.jalz.2016.09.009. pp. [DOI] [PubMed] [Google Scholar]
- Honig L. S., Vellas B., Woodward M., Boada M., Bullock R., Borrie M., Hager K., Andreasen N., Scarpini E., Liu-Seifert H., Case M., Dean R. A., Hake A., Sundell K., Hoffmann V. Poole, Carlson C., Khanna R., Mintun M., DeMattos R., Selzer K. J. and Siemers E. “Trial of Solanezumab for Mild Dementia Due to Alzheimer's Disease,”. New England Journal of Medicine. 2018;Vol. 378(No. 4):321–330. doi: 10.1056/NEJMoa1705971. pp. [DOI] [PubMed] [Google Scholar]
- “Italy Launches Pioneering Project to Identify Alzheimer's Risk. Agence France-Presse; December 6, 2017. https://medicalxpress.com/news/2017-12-italy-alzheimer.html ”. As of May 25, 2018: [Google Scholar]
- Kassenarztliche Bundesvereinigung. “Deutschlandweite Projection 2030—Arztzahlentwicklung in Deutschland”. October 5, 2016. http://www.kbv.de/media/sp/2016_10_05_Projektion_2030_Arztzahlentwicklung.pdf press briefing slides, As of May 25, 2018:
- Krolak-Salmon P., Leperre-Desplanques A., Maillet A., Moutet C., Vanacore N., Confaloni A., Lacorte E., Pucchio A. Di, Bacigalupo I., Rejdak K., Papuc E., Zaluska W., Mehrabian S., Spassov V., Raycheva M., Traykov L., Gronnestad B., Kristiansen K. Midtbo, Selbaeck G., Fiandra T. Di, Knauf-Hubel D., Politis A., Petroulia I., Mougias A., Georges J. and Diaz A. Report on the Benefits and the Risks of Dementia Diagnosis, Act on Dementia, Work Package 4: Diagnosis and Post-Diagnosis Supports for Dementia. 2017. http://www.actondementia.eu/sites/default/files/2018-02/Work%20package%204%20-%20Report%20on%20the%20Benefits%20and%20Risks%20of%20%20%20%20%20Dementia%20Diagnosis.compressed.pdf As of May 25, 2018:
- Leperre Desplanques Armelle, Brooker Dawn, Gove Dianne. and Krolak-Salmon Pierre. “P10. Alzheimer Cooperative Valuation in Europe (ALCOVE)”. 2013. https://www.alzheimer-europe.org/Conferences/Previous-conferences/2013-St-Julian-s/Detailed-programme-abstracts-and-presentations/P10.-Alzheimer-Cooperative-Valuation-in-Europe-ALCOVE Alzheimer Europe 2013 conference proceedings, St. Julian's, Malta, As of May 25, 2018:
- Liu J. L., Hlavka J. P., Hillestad R. and Mattke S. Assessing the Preparedness of the U.S. Health Care System Infrastructure for an Alzheimer's Treatment. Santa Monica, Calif.: RAND Corporation, RR-2272-BIOG; 2017. https://www.rand.org/pubs/research_reports/RR2272.html As of August 21, 2018: [Google Scholar]
- Ministero della Salute. “Alzheimer, il Progetto Interceptor: Un Modello di Screening della Popolazione a Rischio”. December 6, 2017. http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=3211 press release, As of May 25, 2018:
- Murphy M. P. “Amyloid-Beta Solubility in the Treatment of Alzheimer's Disease,”. New England Journal of Medicine. 2018;Vol. 378(No. 4):391–392. doi: 10.1056/NEJMe1714638. pp. [DOI] [PubMed] [Google Scholar]
- National Cancer Research Institute PET Core Lab. “PET Facilities”. 2018. http://www.ncri-pet.org.uk/pet_facilities.php webpage, As of May 25, 2018:
- Olsson B., Lautner R., Andreasson U., Öhrfelt A., Portelius E., Bjerke M., Hölttä M., Rosén C., Olsson C., Strobel G., Wu E., Dakin K., Petzold M., Blennow K. and Zetterberg H. “CSF and Blood Biomarkers for the Diagnosis of Alzheimer's Disease: A Systematic Review and Meta-Analysis,”. Lancet Neurology. 2016;Vol. 15(No. 7):673–684. doi: 10.1016/S1474-4422(16)00070-3. pp. [DOI] [PubMed] [Google Scholar]
- Ono T., Lafortune G. and Schoenstein M. OECD Health Working Papers. Vol. 62. Paris: OECD Publishing; 2013. “Health Workforce Planning in OECD Countries: A Review of 26 Projection Models from 18 Countries,”.https://www.oecd-ilibrary.org/social-issues-migration-health/health-workforce-planning-in-oecd-countries_5k44t787zcwb-en As of May 25, 2018: [Google Scholar]
- Organisation for Economic Co-operation and Development. Health at a Glance 2017: OECD Indicators. 2017a. http://www.oecd.org/health/health-systems/health-at-a-glance-19991312.htm As of August 9, 2018:
- Organisation for Economic Co-operation and Development. “Health Care Resources”. 2017b. http://stats.oecd.org/Index.aspx?DataSetCode=HEALTH_REAC database, OECD. Stat, As of May 25, 2018:
- Palmqvist S., Zetterberg H., Mattsson N., Johansson P., Minthon L., Blennow K., Olsson M., Hansson O. Swedish BioFINDER Study Group. “Detailed Comparison of Amyloid PET and CSF Biomarkers for Identifying Early Alzheimer Disease,”. Neurology. 2015;Vol. 85(No. 14):1240–1249. doi: 10.1212/WNL.0000000000001991. and the. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R., Lopez O., Armstrong M. J., Getchius T. S. D., Ganguli M., Gloss D., Gronseth G. S., Marson D., Pringsheim T., Day G. S., Sager M., Stevens J. and Rae-Grant A. “Practice Guideline Update Summary: Mild Cognitive Impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology,”. Neurology. 2018;Vol. 90(No. 3):126–135. doi: 10.1212/WNL.0000000000004826. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portet F., Ousset P. J., Visser P. J., Frisoni G. B., Nobili F., Scheltens P., Vellas B. and Touchon J. “Mild Cognitive Impairment (MCI) in Medical Practice: A Critical Review of the Concept and New Diagnostic Procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease,”. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;Vol. 77(No. 6):714–718. doi: 10.1136/jnnp.2005.085332. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Wirno A., Guerchet M., Ali G., Wu Y. and Prina M. World Alzheimer Report 2015: The Global Impact of Dementia, An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer's Disease International; 2015. https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf As of May 25, 2018: [Google Scholar]
- Royal College of Psychiatrists. Census 2017: Workforce Figures for Consultant and Specialty Doctor Psychiatrists. 2017. https://www.rcpsych.ac.uk/pdf/RCPsych_workforce_census_report_2017.pdf As of May 25, 2018:
- Sachsisches Krankenhaus Großschweidnitz. “Neurologische Ambulanzen”. 2018. https://www.skh-grossschweidnitz.sachsen.de/medizinische_einrichtungen/ambulanzen/neurologische_ambulanzen/ webpage, As of May 25, 2018:
- Tralongo P., Ferrau F., Borsellino N., Verderame F., Caruso M., Giffrida D., Butera A. and Gebbia V. “Cancer Patient-Centered Home Care: A New Model for Health Care in Oncology,”. Therapeutics and Clinical Risk Management. 2011;Vol. 7:387–392. doi: 10.2147/TCRM.S22119. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Library of Medicine. “A Study of Lanabecestat (LY3314814) in Early Alzheimer's Disease Dementia”. National Institutes of Health, U.S. Department of Health and Human Services; 2018a. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02972658 As of May 25, 2018: [Google Scholar]
- U.S. National Library of Medicine. “A Study of LY3202626 on Disease Progression in Participants with Mild Alzheimer's Disease Dementia (NAVIATE-AD)”. National Institutes of Health, U.S. Department of Health and Human Services; 2018b. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02791191 As of May 25, 2018: [Google Scholar]
- U.S. National Library of Medicine. A Study of CAD106 and CNP520 Versus Placebo in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer's Disease (Generation S1) National Institutes of Health, U.S. Department of Health and Human Services; 2018c. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02565511 As of May 25, 2018: [Google Scholar]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. 2017. http://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/ As of July 20, 2018:
- World Health Organization. Towards a Dementia Plan: A WHO Guide. 2018. http://www.who.int/mental_health/neurology/dementia/policy_guidance/en/ As of July 20, 2018:


