Abstract
Background and Aim:
Necrotizing soft-tissue infections (NSTIs) are common in the Indian subcontinent and are associated with high morbidity and mortality. The aim of this paper was to correlate clinical factors and Acute Physiology Health and Chronic Health Evaluation (APACHE) II score with mortality following NSTI.
Methodology:
Patients presenting to our tertiary-care center between November 1, 2014, and December 1, 2016, with NSTI and between the age of 15 and 90 years were included and entered into a prospectively maintained database. Fifty random patients were selected from the database and were divided according to the survival outcome into two groups: Group 1-survivors and Group 2-nonsurvivors. The two groups were compared for clinical factors and APACHE II score to identify the variable which correlated with the survival.
Results:
Mean age of the study cohort (n = 50, 44 males) was 50.8 ± 17.1 years. Fournier's gangrene was the most common manifestation (64%), followed by lower limb (14%). Infection was leading cause (34%) followed by trauma (16%) and prior surgery (14%). There were 16 in-hospital deaths (32%). Two groups were similar regarding age and sex. At presentation, nonsurvival group had significantly higher body surface area involvement (P = 0.001), anemia (P = 0.023), metabolic acidosis (P < 0.0001), serum creatinine (P = 0.007), and mean APACHE II score (P < 0.001). There was no difference between time from presentation to the first debridement.
Conclusions:
We found that APACHE II is a significant predictor of mortality. Early diagnosis and prompt aggressive treatment is the only way to improve outcome. Further studies with larger sample size are warranted.
Keywords: Acute Physiology Health and Chronic Health Evaluation II score, Fournier's gangrene, mortality, necrotizing fasciitis, soft-tissue infections
INTRODUCTION
Necrotizing soft-tissue infections (NSTIs) are infections of the subcutaneous tissue and fascia with variable involvement of the overlying skin and underlying muscle. They can be triggered by minor trauma and in some cases, may be idiopathic. These infections tend to spread rapidly causing large amounts of tissue destruction over a short period. The toxins released by the bacteria cause severe systemic toxicity which may lead to death unless prompt intervention is done.
NSTI can affect any region in the body, but most commonly involve extremities, perineum, and truncal areas.[1] Patients present with local signs of infection; however, severe pain disproportionate to local findings and associated with systemic toxic manifestations.[2] The disease usually starts in the superficial fascia and spreads into the subcutaneous fat, nerves, arteries, veins, and deep fascia. The causative bacteria may be aerobic, anaerobic, or mixed flora, although group A Streptococcus alone has the potential to lead to this infection. The organisms reach the subcutaneous tissue by growing and forming extensions from a contiguous infection or trauma to the area, including surgery.
Various studies have reported mortality rates up to 76% following NSTI, depending on several factors such as demographics of patients, clinical presentation, time of intervention, and prognostic scores, etc.[3,4,5,6,7,8,9,10,11,12,13,14,15] Over the years, studies have provided new insights into the etiology, pathogenesis, diagnosis, and treatment modalities for NSTI. Although the presentation of the disease has not changed significantly, increased awareness, early diagnosis of the condition, and prompt surgical intervention with broad-spectrum antibiotics have shown improving trends in the outcome.
Some identified poor prognostic factors include increasing age, number of organ failures at the time of admission, delay in presentation and treatment, diabetes mellitus, immunocompromised status as in associated malignancy, alcoholism, etc. HIV infection is a well-known predisposing factor; however, its role as poor prognostic factor has not yet been delineated.
Acute Physiology Health and Chronic Health Evaluation (APACHE) II score is well known scoring system for predicting severity of disease and the risk regarding mortality. It is based on modifiable factors such as clinical and biochemical parameters, nonmodifiable factors such as age.
This study was conducted to study the clinical presentation, treatment outcomes, factors affecting morbidity and mortality, and role of APACHE II score in predicting morbidity and mortality in patient with NSTI in a tertiary care hospital of India. A descriptive analysis of a cohort of 50 patients of NSTI was carried out. The various factors were statistically analyzed, and their individual and combined utility in prediction of mortality were studied. The APACHE II score was calculated for all the patients based on physiological and biochemical parameters at the time of admission, and its role in the prediction of outcome was evaluated.
METHODOLOGY
Patient recruitment
This randomized, prospective comparative study was carried out at a tertiary referral center of North-West India. Prior approval was obtained from the Institutional Review Board and Institutional Ethics Committee. All patients between the age of 15 and 90 years presenting at the outpatient department between November 1, 2014, and December 31, 2016, with NSTIs irrespective of the etiology were included in the study and were entered into a prospectively maintained database. Patients outside the age group, those with a history of previous surgical intervention for NSTI elsewhere and seeking further management at our hospital or patients admitted for other indications and who developed NSTI during the course of their treatment were excluded from the study. The prospective database was extracted onto a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA) and patients were deidentified and assigned a unique identification number. Using a random number generator, 50 random patients from the pooled data were selected who formed the study cohort [Figure 1].
Figure 1.
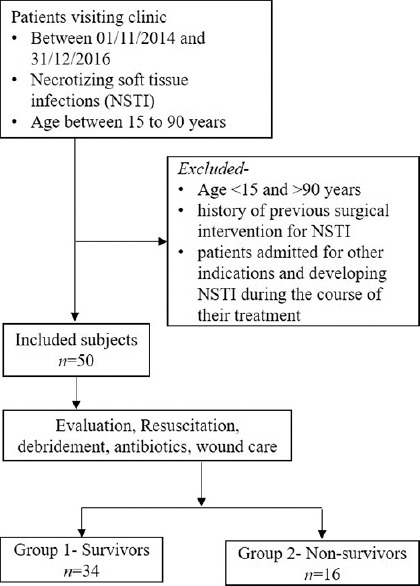
Flowchart depicting study protocol with inclusion and exclusion of patients and division of patients into groups and subgroups
Informed consent (written and verbal) was obtained from each patient for inclusion into the study. Patients were made aware through the information sheets and preliminary interviews that the choice to consent or otherwise would have no bearing on the treatment offered. The patients were subjected to a detailed evaluation by the first author.
Diagnosis
NSTI was suspected when patient had rapidly spreading infections of subcutaneous tissue and fascia with variable involvement of overlying skin and underlying muscle. The diagnosis was confirmed in the presence of fever, soft-tissue involvement with significant pain (out of proportion to skin findings), signs of toxicity, crepitus, and rapid progression of clinical manifestations along with positive pus cultures and characteristic findings on soft-tissue X-rays. The diagnosis was further established at the time of surgical exploration.
Data collection
A standardized form was used for data collection [Appendix 1]. Patient evaluation included demographic data, detailed history, general and systemic examinations followed by laboratory evaluation. Routine blood and urine examinations were done along with radiographic screening of the chest and X-ray of the affected limb. APACHE II score was calculated on the basis of the clinical parameters [Appendix 2].
Patient management
Patients were managed according to the standard hospital protocols. Patients were resuscitated and stabilized followed by prompt surgical debridement and complete clearance of the necrotic tissue. Repeat assessments of the wound were done at 24 and 48 h with repeat debridement, if necessary. At the time of admission and during debridement, wound swabs were sent for culture and sensitivity testing. Patients were initially started on empirical broad-spectrum antibiotic therapy which was modified according to the sensitivity report and patients' renal status.
Following debridement, all patients received daily dressings with povidone iodine, sodium hypochlorite solutions (Dakins solution), hydrogen peroxide, and natural unprocessed honey. Patients also received hyperbaric oxygen therapy. Patients who survived were discharged and followed up in the clinic.
Statistical analysis
Patients were divided into two groups on the basis of survival during the hospital stay. Group 1 included patients who survived, while Group 2 included patients who expired while undergoing treatment. Patient inclusion flowchart is shown in Figure 1. Author conducting statistical analysis was blinded of the identity of the included patients. All statistical analyses were done using Statistical Package for Social Sciences (SPSS), version 22.0 (IBM Corporation, Armonk, NY, USA). The normalcy of continuous data was checked using Kolmogorov–Smirnov test. Continuous variables are reported as mean with standard deviation. Qualitative or categorical variables were described as frequencies and proportions (%). Unpaired t-test was used to compare means between study groups. Chi-Square or Fisher's Exact test was used to compare categorical variables. All statistical tests were two sided and were performed at a significance level of α = 0.05 with 95% confidence interval.
RESULTS
Patient presentation
A total of 50 patients were included in the study according to the study protocol. The mean age of the included cohort was 50.8 ± 17.1 years (range 16 years to 89 years). Majority of the patients belonged to the age group of 51–60 years (26%) and 31–40 years (22%) [Figure 2]. Most patients were males (n = 44, 88%).
Figure 2.
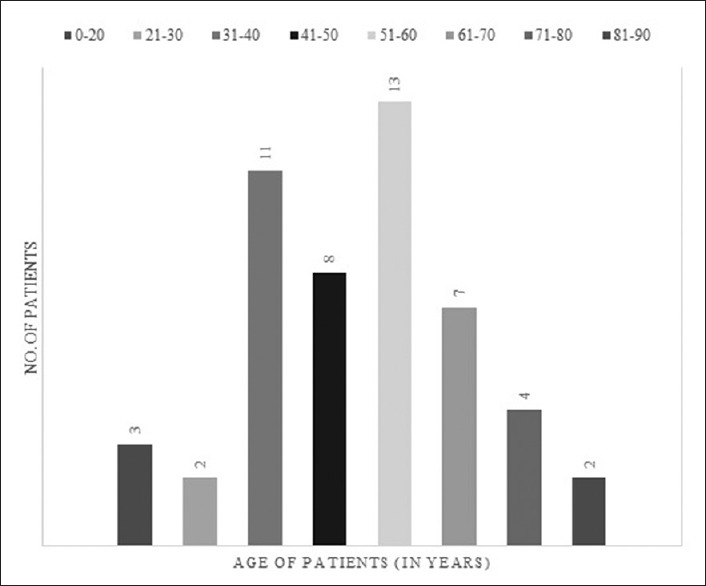
Segregation of patient cohort according to age group
Fournier's gangrene contributed to the majority of the cases (64%), followed by lower limb (14%) and gluteal region (8%) [Table 1]. An identifiable cause was found in 72% patients. Infections were leading cause seen in 17 (34%) patients. These included cutaneous, urogenital as well as perianal infections. Trauma (16%) and prior surgery (14%) were other common causes. Body surface area (BSA) involvement was calculated using Lund and Browder burn area chart. Two-third patients (n = 33, 66%) had ≤10% surface area involvement.
Table 1.
Characteristics of the study cohort
| Parameter | Total study cohort |
|---|---|
| Total included patients (n) | 50 |
| Mean age, years±SD (range) | 50.8±17.1 (16-89) |
| Sex, males:females | 44:6 |
| Areas involved, n (%) | |
| Fournier’s gangrene | 32 (64) |
| Lower limb | 7 (14) |
| Gluteal region | 4 (8) |
| Anterior abdominal wall | 3 (6) |
| Back | 3 (6) |
| Upper limb | 1 (2) |
| Mean percentage BSA involved | 9.9±5.8 |
| Etiology (%) | |
| Infection | 17 (34) |
| Trauma | 8 (16) |
| Previous surgery | 7 (14) |
| Injection site | 4 (8) |
| Idiopathic | 14 (28) |
| APACHE II score | |
| 0-5 | 1 |
| 6-10 | 14 |
| 11-15 | 16 |
| 16-20 | 9 |
| 21-25 | 5 |
| 26-30 | 5 |
| Mean±SD | 14.5±6.6 |
| Meantime: Admission to first debridement (days) | 5.5±3.5 |
| Mean hospital stay (days) | 13.0±10.4 |
| In-hospital death (%) | 16 (32) |
BSA: Body surface area, SD: Standard deviation, APACHE: Acute Physiology Health and Chronic Health Evaluation
Laboratory parameters
Mean hemoglobin level was 10.2 ± 2.5 g/dL with 23 (46%) patients diagnosed with anemia (defined as hemoglobin < 10 g/dL) at presentation. Mean white blood cell (WBC) count was 16,494 ± 8037 cells/mm3. Mean serum total protein and serum albumin levels were 4.9 ± 1.1 g/dL and 2.4 ± 0.8 g/dL, respectively. Acidosis on arterial blood analysis (pH < 7.3) was present in 16 (32%) patients.
Perioperative outcome
A total of 83 debridements were required on 50 study patients during their hospital stay (mean- 1.66). Nutritional supplementation in the form of total parenteral nutrition or intravenous albumin was required in 9 (18%) patients. Reconstructive surgical procedures were required in 17 (34%) patients and included skin grafting in 10 (20%), secondary suturing in 5 (10%), and testicular repositioning and below knee amputation each in 1 (2%) patient [Figure 3]. There was total of 16 in-hospital mortalities (32%).
Figure 3.
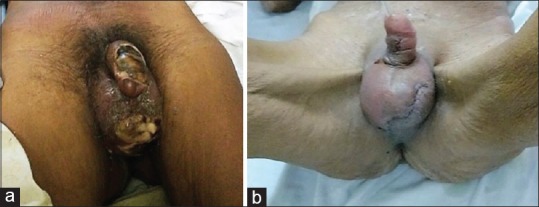
Case of Fournier's gangrene at presentation (a) and after debridement and secondary suturing (b)
Comparison between Group-1 (survivors) and Group-2 (nonsurvivors)
The two groups did not differ with respect to mean age and sex distribution [Table 2]. Most of the survivors had involvement of <10% of BSA, while the percentage of BSA involved in most of the nonsurvivors was between 11 and 20 (P = 0.014). Mean percentage BSA involvement was significantly higher in patients who did not survive (P = 0.001). Only 35% patients in Group-1 were anemic at presentation as compared to 69% anemic patients in Group-2 (P = 0.023). Mean hemoglobin level was significantly lower in Group-2 patients (9.0 vs. 10.7, P = 0.023) [Table 3]. There was a significantly higher incidence of metabolic acidosis at presentation in nonsurvivors (62.5%) as compared to survivors (2.9%) (P< 0.0001) with lower mean pH in nonsurvivor group. In addition, serum creatinine was significantly higher while serum albumin and urine output were lower in nonsurvivor group. The two groups did not differ significantly with respect to total WBC count at presentation, random blood glucose, total serum protein levels, SGOT, SGPT, serum alkaline phosphatase, and INR. There was no significant difference between groups with respect to mean time between onset of symptoms and admission, mean time between admission to first debridement, and mean hospital stay (mean time between admission and discharge or death). However, APACHE II score was significantly higher in patients who could not survive the disease (P< 0.001).
Table 2.
Comparison of demographic and clinical factors between survivors and nonsurvivors
| Group 1 (survivors) (n=34) | Group 2 (nonsurvivors) (n=16) | P | |
|---|---|---|---|
| Mean age (years) | 49.2±18.9 | 54.3±12.3 | 0.329 |
| Sex (male) (%) | 29 (85.3) | 15 (93.7) | 0.650 |
| Mean percentage of involvement (%) | 8.2±5.1 | 13.6±5.5 | 0.001 |
| Mean percentage BSA involved (%) | |||
| ≤10 | 27 (79.4) | 6 (37.5) | 0.014 |
| 11-20 | 6 (17.6) | 9 (56.3) | |
| >20 | 1 (2.9) | 1 (6.3) | |
| Meantime between the onset of symptoms and presentation (days) (%) | |||
| ≤3 | 8 (23.5) | 7 (43.8) | 0.228 |
| 4-7 | 19 (55.9) | 5 (31.3) | |
| >7 | 7 (20.6) | 4 (25) | |
| Mean time (admission to first debridement) (days) | 5.7±3.1 | 4.9±4.2 | 0.449 |
| Mean hospital stay (days) | 14.2±8.1 | 10.5±14.0 | 0.344 |
| Mean APACHE II score | 10.9±3.8 | 22.1±4.2 | <0.001 |
BSA: Body surface area, APACHE: Acute Physiology Health and Chronic Health Evaluation
Table 3.
Factors at presentation affecting mortality rate
| Group 1 (survivors) (n=34) | Group 2 (nonsurvivors) (n=16) | P | |
|---|---|---|---|
| Initial presentation | |||
| Age (years), n (%) | 0.025 | ||
| ≤60 | 29 (85.3) | 9 (56.3) | |
| >60 | 5 (14.7) | 7 (43.8) | |
| Gender (%) | 0.391 | ||
| Male | 29 (85.3) | 15 (93.8) | |
| Female | 5 (14.7) | 1 (6.3) | |
| Oliguria | 3 (8.8) | 7 (43.8) | 0.004 |
| Comorbidities (%) | |||
| Diabetes mellitus | 8 (23.5) | 7 (43.8) | 0.146 |
| Smoking | 10 (29.4) | 5 (31.3) | 0.895 |
| Alcohol | 11 (32.4) | 6 (37.5) | 0.72 |
| Immunosuppression | 2 (5.9) | 0 | NA* |
| Liver disease | 2 (5.9) | 1 (6.3) | 0.959 |
| Arterial insufficiency | 1 (2.9) | 2 (12.5) | 0.184 |
| Tuberculosis | 3 (8.8) | 1 (6.3) | 0.754 |
| >2 comorbidities | 9 (26.5) | 3 (18.8) | 0.551 |
| Laboratory parameters (mean) | |||
| Hemoglobin (g/dL) | 10.7±2.2 | 9.0±2.9 | 0.023 |
| Total WBC count (/mm3) | 15,800±7115 | 17.969±9809 | 0.437 |
| Random blood glucose | 158.9±98.2 | 266.1±303.9 | 0.187 |
| Creatinine | 1.5±0.7 | 2.0±0.6 | 0.007 |
| Protein | 5.3±0.9 | 5.0±0.8 | 0.314 |
| Albumin | 2.6±0.8 | 2.1±0.4 | 0.007 |
| SGOT | 45.3±31.9 | 72.7±63.4 | 0.065 |
| SGPT | 28.8±29.4 | 50.2±52.8 | 0.099 |
| Alkaline phosphatase | 94.6±64.9 | 130.6±49.2 | 0.102 |
| INR | 1.3±0.1 | 2.0±1.2 | 0.062 |
| Arterial blood pH | 7.4±0.1 | 7.3±0.1 | 0.001 |
NA: Not applicable as one of groups has zero value. WBC: White blood cell, SGOT: Serum glutamic oxaloacetic transaminase, SGPT: Serum glutamate-pyruvate transaminase
DISCUSSION
NSTI s, popularly known as “flesh-eating disease” not only endanger life of the patients but also lead to long-term morbidity and disability. Not only does it impact a patient's life physically but also psychologically and socioeconomically as well. Although comparatively rare in developed countries, its incidence in countries like India is high due to the presence of other diseases such as HIV, diabetes mellitus, and alcoholism providing a fertile ground for this life-threatening infection.
Streptococcus is the most common causative organism.[16] Most cases of NSTI caused by Group A Streptococcus are M protein types 1, 3, 12, or 28 and produce exotoxin A or B. The M protein impedes phagocytosis and along with the exotoxins, acts as super-antigens, which leads to secretion of cytokines and toxic shock-like syndrome. Staphylococcus aureus, Streptococcus pyogenes, and enterococci are the common Gram-positive aerobes known to cause NSTI. Escherichia coli is the most common Gram-negative enteric organism. Bacteroides species and peptostreptococcus are the most common anaerobes.[4,5,6]
NSTI initially induce localized inflammatory changes in the involved tissues. As the infection progresses, tissue necrosis occurs as a result of direct cell injury from bacterial toxins, significant inflammatory edema within a closed tissue compartment, thrombosis of blood vessels, and tissue ischemia. Ischemia, edema, and inflammation in a closed area lead to decreased oxygen tension promoting growth of obligate anaerobes such as Bacteroides fragilis and anaerobic metabolism in facultative aerobic organisms such as E. coli. Ischemic necrosis of the skin progresses into gangrene of the subcutaneous fat and dermis characterized by the formation of bullae, vesicles, and occasional ulceration. Bacterial metabolism produces hydrogen and nitrogen gas molecules which accumulate in subcutaneous space giving rise to classical crepitus on clinical examination.
A common example, Fournier's gangrene, is a localized form of NSTI involving the scrotum. It is broadly categorized into three groups depending on the source of infection-colorectal, cutaneous, and urogenital. The infection spreads along dartos fascia, tunica of scrotum and penis, Colles' fascia of the perineum, and may even invade as high as axilla. The glans penis, corpora spongiosa, and cavernosa, testes are usually preserved due to independent blood supply from the aorta. Testicular involvement, if reported, usually indicates a retroperitoneal or an intra-abdominal source of infection.
NSTI is associated with a wide range of mortality rates (6% to 76%).[3,4,5,6,7,8,9,10,11,12,13,14,15] The paucity of definite clinical features leading to low diagnostic accuracy and the polymicrobial nature of infection with rapid progression to fulminant sepsis are the leading causes of high-mortality rate. However, high index of suspicion, wider range of antibiotics, newer methods of dressing and wound care, and better intensive care facilities have all contributed to a decrease in mortality rate to 25% to 35% as reported in latest literatures.[15] Although the basics of management of NSTI are well defined, the survival of patients depends on factors outside treatment.[13,14] The current study analyzes the presenting factors and associated conditions which indicate a graver prognosis.
The mean age of presentation in current study was 50.8 years. Highest proportion of patients belonged to the 51–60 years age group followed closely by age group of 31–40 years. Age at presentation is an important criterion affecting the outcome of patients. Older patients have lesser ability to tolerate the stress caused by the generalized sepsis on the hemodynamics. The higher mortality in older age group can be explained by a decrease in immunity to fight illness, presence of comorbid conditions such as diabetes mellitus, IHD risk imposed by surgery and anesthesia and the response to treatment. The mean age was comparable to previous studies.[3,6,17,18,19,20]
The current study included 88% male patients. The high proportion of male patients can be attributed to the high incidence of Fournier's gangrene (64%) in this study with scrotum affected in all individuals. Lower limb (14%), gluteal region (8%), and abdominal wall (6%) were other common regions affected. Although NSTI of abdomen and perineum have received much attention because of the mortality rates as high as 76%,[19] involvement of extremities has been reported in approximately half of the studies.[21,22] NSTI that involve the vulva and perineum in women are seen to have a graver prognosis when compared with the involvement of other anatomical sites.[10,23]
An identifiable cause was found in 72% patients in the study. Infections were the leading cause, as seen in 34% patients. These included cutaneous, urogenital as well as perianal infections. Other causes included trauma (16%) and previous surgical procedure (14%) while no cause could be identified in 28% (labeled as idiopathic). Trauma was found to be the most common etiology in nongenital NSTI. McHenry et al.,[3] in their series of 65 patients found postoperative infections to be the most common cause, while other reports have identified trauma to be the leading cause.[19,24] Interestingly, most such reports come from western populations. The particular etiology was not demonstrated to have a significant impact on outcome by McHenry et al.[3]
Because of the limited experience for most clinicians, a high index of suspicion must be maintained to facilitate early diagnosis. Immunocompromised patients can pose a diagnostic challenge in this regard, as they may not manifest symptoms the same or as severely as do the immune-competent patients. In our study, two patients in were immunocompromised survivor group due to underlying AIDS, while none of the nonsurvivors had immunocompromised status. However, despite weakened immunity, both patients had routine clinical course with APACHE II scores of 12 and 8, respectively, and hospital stay of 6 and 4 days, respectively.
Several investigators have described the use of objective laboratory parameters for diagnosing NSTI and for predicting patient outcome. Chan et al.[25] were the first to demonstrate that the WBC count and serum sodium level were useful parameters in the diagnosis of NSTI using CART analysis. In another retrospective study, Wong and Wang[18] developed the Laboratory Risk Indicator for Necrotizing fasciitis scale using WBC, hemoglobin, sodium, glucose, serum creatinine, and C-reactive protein level using a multivariate regression model. In 1995, Laor et al.[6] developed an objective severity score named “Fournier gangrene severity index” using nine parameters (similar to APACHE II Score) to predict the severity of Fournier gangrene and probability of patient mortality, although there have been some conflicting reports about the efficacy of this scoring system.[8,26] Yaşar et al. compared the predictive accuracy of various scoring systems.[27] The predictive scores were found to be more strongly associated with nonsurvivors than survivors, with APACHE II and SAPS II found to be relatively superior in predicting mortality. This was further reflected in our study with survivors having significantly higher mean APACHE II scores.
Independent predictors have been described by authors to help identify patients with poorer prognosis and who would benefit from a more aggressive treatment approach. The presence of Clostridial infection has been found to be associated with poor outcome.[14] The most common isolated bacteria in our study was E. coli followed by Klebsiella [Table 4]. Our study highlights the difference in microbial flora in patients from the eastern and western hemisphere.
Table 4.
Bacteria recovered from the pus culture
| Organism isolated | Group 1 (survivors) (n=34*) | Group 2 (nonsurvivors) (n=16*) |
|---|---|---|
| Escherichia coli | 15 | 10 |
| Enterococcus | 7 | 2 |
| Klebsiella pneumoniae | 8 | 6 |
| Pseudomonas | 5 | 2 |
| Staphylococcus aureus | 4 | 4 |
| Streptococcus | 7 | 7 |
| Proteus | 1 | - |
| Candida albicans | 1 | - |
| Acinetobacter | 1 | - |
*One or more bacteria isolated from single patient
Fifteen (30%) patients had uncontrolled diabetes mellitus. Mean random blood glucose for the study cohort was 193.26 mg/dl. Hyperglycemia induced by NSTI in an otherwise controlled diabetic patient is not always fatal; however, the presence of uncontrolled diabetes with ketoacidosis is associated with fatal prognosis. It requires prompt treatment with insulin along with debridement for removal of septic focus.
Serum creatinine is a measure of RFTs and plays an important role due to the septic status of the patient at admission. These patients usually present with dehydration and anemia. Adequate hydration with monitoring of the urine output forms an important part of preoperative hemodynamic stabilization of the patient. The mean creatinine of the patients in our study was 0.6 mg/dL with maximum patients having values in the normal range of 1–2 mg/dL.
Even with optimal treatment, NSTIs portend significant morbidity and have mortality rates of 25%–35% in recent series.[15] Many studies have sought to determine the factors affecting mortality and morbidity in NSTI and patient-related factors have been found to be as important as early management in predicting survival.[13,14] We studied APACHE II score, which predict acute physiology health and chronic health status, to predict the outcome for the patients with this disease.
Yilmazlar et al. found that the APACHE II score of 13 or more correlated well with mortality.[13] Overall mortality rate in the study was 49%. In the present study, mean APACHE II score in survivors was 10.9 whereas in nonsurvivors, it was 22.1. All patients in nonsurvivor group had APACHE II score of 16 or more whereas only 3 patients among survivors had a score of 16 or more. Overall mortality rate was 32%. Shock (hypotension), diabetes mellitus, total WBC count, serum SGPT levels, serum creatinine, INR, and percentage of body area involved showed significant correlation with mortality. Anaya et al. in their study found a significant correlation of mortality with serum creatinine, TLC count, and heart disease.[14]
One of the weaknesses of the study was its small sample size. However, the study cohort was chosen randomly to avoid any selection bias. In addition, the two groups differed in the number of patients. However, since the sample cohort was chosen randomly, the two groups were considered representative of the overall patient population. Further, the study only assesses the in-hospital mortality. The lack of long-term follow-up is an inherent weakness of the study design. A multiphase study with larger sample size and a long-term follow-up is desirable to get better insight into the outcomes following NSTIs. In addition, the study was conducted in the western part of India and thus the results may not be representative of patients from other parts of the world. The strength of the study stems from the random nature of the patient selection from a prospectively collected pool of patients.
CONCLUSIONS
APACHE II score forms a simple and powerful tool as significant predictor of morbidity and mortality. Early diagnosis and prompt aggressive debridement, broad-spectrum antibiotics with supportive care remains a time-tested treatment of choice and is the only way to improve outcome. Further studies with larger sample size are warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Appendix 1: Pretest pro forma questionnaire
| Name of co-morbidities | Presence of co-morbidities | |
|---|---|---|
| Comorbidities | ||
| Diabetes mellitus | Yes/no | |
| Hypertension | Yes/no | |
| Immunocompromised | Yes/no | For example, HIV, HBV, malignancy |
| Addictions | Smoking, alcoholism, IV drug abuse | |
| Vascular insufficiency | Yes/no | Delays healing and increases causation |
| Other comorbidities | Yes/no | For example, tuberculosis, ALD |
| Source of infection | ||
| Trauma | Yes/no | Overt or trivial |
| Intramuscular/subcutaneous injections | Yes/no | Seeding of organisms |
| Infections: Cutaneous, urethral, perianal | Yes/no | e.g., dermatitis of scrotal skin, perianal abscess etc. |
| Idiopathic | Yes/no | Source not established |
| Other | Yes/no | e.g., previous surgery |
| Clinical criterion | ||
| Pain, swelling, redness | Yes/no | Inflammation |
| Discharge | Yes/no | Purulent/foul smelling |
| Crepitus | Yes/no | Gas formation in tissues |
| Oliguria | Yes/no | <1 ml/kg/day |
| Mental obtundation | Yes/no | Disoriented in time/space/person (recent onset) |
| Hypotension | Yes/no | Blood pressure <100 systolic |
| Laboratory data (all data is also analyzed as continuous variables) | ||
| Anemia | Yes/no | Hemoglobin <10 g/dl |
| Hyperglycemia | Yes/no | >200 mg/dl |
| Creatinine | Yes/no | >2 mg/dl |
| Hypoproteinemia | Yes/no | <6 g/dl |
| Hypoalbuminemia | Yes/no | <3 g/dl |
| Acidosis | Yes/no | pH <7.3 |
| Raised liver function | Yes/no | |
| Electrolytes (Na/K) | Continuous variable | |
| APACHE II score | Acute physiology + chronic health + age points | |
| Surgical treatment | ||
| Number of debridements | Discreet variable | |
| Colostomy | Yes/no | |
| Suprapubic cystostomy | Yes/no | |
| Orchidectomy | Yes/no | |
| Amputation | Yes/no | |
| Reconstructive surgery | Yes/no | Rehabilitation marker |
APACHE: Acute Physiology Health and Chronic Health Evaluation, HIV: Human immune-deficiency virus, HBV: Hepatitis B virus, ALD: Acute liver disease
Appendix 2: Acute Physiology Health and Chronic Health Evaluation II scoring format of physical and chemical investigated parameters
| Serial number | Parameters | +4 | +3 | +2 | +1 | 0 | +1 | +2 | +3 | +4 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Rectal temperature (°C) | >41 | 39-40.9 | - | 36-38.4 | 36-38.9 | 34-35.9 | 32-33.9 | 30-31.9 | <29 | |||
| 2. | Mean arterial pressure (mmHg) | >160 | 130-159 | 110-129 | - | 70-109 | - | 50-69 | - | <49 | |||
| 3. | Heart rate (bpm) | >180 | 140-179 | 110-139 | - | 70-109 | - | 55-69 | 40-54 | <39 | |||
| 4. | Respiratory rate (bpm) | >50 | 35-49 | - | 25-34 | 12-24 | 10-11 | 6-9 | - | <5 | |||
| 5. | Oxygen delivery (ml/min) | >500 | 350-499 | 200-349 | - | <200 | - | - | - | - | |||
| 6. | PO2 (mmHg) | - | - | - | - | >70 | 61-70 | - | 55-60 | <55 | |||
| 7. | Arterial pH | >7.7 | 7.6-7.69 | - | 7.5-7.59 | 7.3-7.49 | - | 7.25-7.3 | 7.15-7.2 | <7.15 | |||
| 8. | Serum sodium (mmol/L) | >180 | 160-179 | 155-159 | 150-154 | -130-149 | - | 120-139 | 111-119 | <110 | |||
| 9. | Serum potassium (mmol/L) | >7 | 6-6.9 | - | 3.5-5.4 | 3-3.4 | 2.5-2.9 | - | <2.5 | ||||
| 10. | Hematocrit (%) | >60 | - | 50-50.9 | 46-49.9 | 30-45.9 | - | 20-29.9 | - | <20 | |||
| 11. | Serum creatinine (mg/dl) | >3.5 | 2.0-3.4 | 1.5-1.9 | - | 0.6-1.4 | - | <0.6 | - | - | |||
| 12. | White cell count (103ml) | >40 | - | 20-39.9 | 15-19.9 | 3-14.9 | - | 1-2.9 | - | <1 | |||
| Age points | 0 | 2 | 3 | 5 | 6 | ||||||||
| Age (years) | <44 | 45-54 | 55-64 | 65-74 | >75 | ||||||||
| History of severe organ insufficiency points | Nonoperative patients | Emergency postoperative patients | |||||||||||
| Points | 5 | 5 | |||||||||||
REFERENCES
- 1.Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ. 2005;330:830–3. doi: 10.1136/bmj.330.7495.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eke N. Fournier's gangrene: A review of 1726 cases. Br J Surg. 2000;87:718–28. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 3.McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–63. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kossmann T, Simmen HP, Battaglia H, Brülhart KB, Trentz O. Necrotizing soft tissue infection of the extremities. Helv Chir Acta. 1994;60:509–11. [PubMed] [Google Scholar]
- 5.Kuo CF, Wang WS, Lee CM, Liu CP, Tseng HK. Fournier's gangrene: Ten-year experience in a medical center in Northern Taiwan. J Microbiol Immunol Infect. 2007;40:500–6. [PubMed] [Google Scholar]
- 6.Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier's gangrene. J Urol. 1995;154:89–92. [PubMed] [Google Scholar]
- 7.Ledingham IM, Tehrani MA. Diagnosis, clinical course and treatment of acute dermal gangrene. Br J Surg. 1975;62:364–72. doi: 10.1002/bjs.1800620510. [DOI] [PubMed] [Google Scholar]
- 8.Lin E, Yang S, Chiu AW, Chow YC, Chen M, Lin WC, et al. Is Fournier's gangrene severity index useful for predicting outcome of Fournier's gangrene? Urol Int. 2005;75:119–22. doi: 10.1159/000087164. [DOI] [PubMed] [Google Scholar]
- 9.Majeski J, Majeski E. Necrotizing fasciitis: Improved survival with early recognition by tissue biopsy and aggressive surgical treatment. South Med J. 1997;90:1065–8. doi: 10.1097/00007611-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Majeski JA, Alexander JW. Early diagnosis, nutritional support, and immediate extensive debridement improve survival in necrotizing fasciitis. Am J Surg. 1983;145:784–7. doi: 10.1016/0002-9610(83)90140-x. [DOI] [PubMed] [Google Scholar]
- 11.Meleney FL. Hemolytic Streptococcus gangrene. Arch Surg. 1924;9:317–64. [Google Scholar]
- 12.Bronder CS, Cowey A, Hill J. Delayed stoma formation in Fournier's gangrene. Colorectal Dis. 2004;6:518–20. doi: 10.1111/j.1463-1318.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 13.Yilmazlar T, Ozturk E, Alsoy A, Ozguc H. Necrotizing soft tissue infections: APACHE II score, dissemination, and survival. World J Surg. 2007;31:1858–62. doi: 10.1007/s00268-007-9132-1. [DOI] [PubMed] [Google Scholar]
- 14.Anaya DA, McMahon K, Nathens AB, Sullivan SR, Foy H, Bulger E, et al. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg. 2005;140:151–7. doi: 10.1001/archsurg.140.2.151. [DOI] [PubMed] [Google Scholar]
- 15.Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51:344–62. doi: 10.1067/j.cpsurg.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barzilai A, Zaaroor M, Toledano C. Necrotizing fasciitis: Early awareness and principles of treatment. Isr J Med Sci. 1985;21:127–32. [PubMed] [Google Scholar]
- 17.Manni JJ. A case of idiopathic scrotal gangrene (Fournier) with perineal extension. Ann Trop Med Parasitol. 1983;77:599–603. doi: 10.1080/00034983.1983.11811759. [DOI] [PubMed] [Google Scholar]
- 18.Wong CH, Wang YS. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis. 2005;18:101–6. doi: 10.1097/01.qco.0000160896.74492.ea. [DOI] [PubMed] [Google Scholar]
- 19.Liu YM, Chi CY, Ho MW, Chen CM, Liao WC, Ho CM, et al. Microbiology and factors affecting mortality in necrotizing fasciitis. J Microbiol Immunol Infect. 2005;38:430–5. [PubMed] [Google Scholar]
- 20.Golger A, Ching S, Goldsmith CH, Pennie RA, Bain JR. Mortality in patients with necrotizing fasciitis. Plast Reconstr Surg. 2007;119:1803–7. doi: 10.1097/01.prs.0000259040.71478.27. [DOI] [PubMed] [Google Scholar]
- 21.Bair MJ, Chi H, Wang WS, Hsiao YC, Chiang RA, Chang KY, et al. Necrotizing fasciitis in Southeast Taiwan: Clinical features, microbiology, and prognosis. Int J Infect Dis. 2009;13:255–60. doi: 10.1016/j.ijid.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Chen JL, Fullerton KE, Flynn NM. Necrotizing fasciitis associated with injection drug use. Clin Infect Dis. 2001;33:6–15. doi: 10.1086/320874. [DOI] [PubMed] [Google Scholar]
- 23.Fisher JR, Conway MJ, Takeshita RT, Sandoval MR. Necrotizing fasciitis. Importance of roentgenographic studies for soft-tissue gas. JAMA. 1979;241:803–6. doi: 10.1001/jama.241.8.803. [DOI] [PubMed] [Google Scholar]
- 24.Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med. 2008;26:170–5. doi: 10.1016/j.ajem.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Chan T, Yaghoubian A, Rosing D, Kaji A, de Virgilio C. Low sensitivity of physical examination findings in necrotizing soft tissue infection is improved with laboratory values: A prospective study. Am J Surg. 2008;196:926–30. doi: 10.1016/j.amjsurg.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Tuncel A, Aydin O, Tekdogan U, Nalcacioglu V, Capar Y, Atan A, et al. Fournier's gangrene: Three years of experience with 20 patients and validity of the Fournier's gangrene severity index score. Eur Urol. 2006;50:838–43. doi: 10.1016/j.eururo.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Yaşar NF, Uylaş MU, Badak B, Bilge U, Öner S, İhtiyar E, et al. Can we predict mortality in patients with necrotizing fasciitis using conventional scoring systems? Ulus Travma Acil Cerrahi Derg. 2017;23:383–8. doi: 10.5505/tjtes.2016.19940. [DOI] [PubMed] [Google Scholar]


