Abstract
Sodium butyrate is a histone deacetylase inhibitor that affects various types of brain damages. To investigate the effects of sodium butyrate on hippocampal dysfunction that occurs after whole-brain irradiation in animal models and the effect of sodium butyrate on radiation exposure-induced cognitive impairments, adult C57BL/6 mice were intraperitoneally treated with 0.6 g/kg sodium butyrate before exposure to 10 Gy cranial irradiation. Cognitive impairment in adult C57BL/6 mice was evaluated via an object recognition test 30 days after irradiation. We also detected the expression levels of neurogenic cell markers (doublecortin) and phosphorylated cAMP response element binding protein/brain-derived neurotrophic factor. Radiation-exposed mice had decreased cognitive function and hippocampal doublecortin and phosphorylated cAMP response element binding protein/brain-derived neurotrophic factor expression. Sodium butyrate pretreatment reversed these changes. These findings suggest that sodium butyrate can improve radiation-induced cognitive dysfunction through inhibiting the decrease in hippocampal phosphorylated cAMP response element binding protein/brain-derived neurotrophic factor expression. The study procedures were approved by the Institutional Animal Care and Use Committee of Korea Institute of Radiological Medical Sciences (approval No. KIRAMS16-0002) on December 30, 2016.
Keywords: sodium butyrate, radioprotector, ionizing radiation, hippocampal damage, cAMP response element binding, brain-derived neurotrophic factor, histone deacetylase inhibitor, neurogenesis
Chinese Library Classification No. R453; R363; R741
Introduction
Ionizing radiation-induced brain injury may involve the loss of neural precursor cells and alter hippocampal neurogenesis (Kim et al., 2013; Son et al., 2015b). The hippocampus, which is a structure of the limbic system, has been investigated in many studies on learning and memory (Kempermann et al., 1997). Cranial irradiation may result in prolonged reduction of hippocampal neurogenesis and may also be related to cognitive impairment (Kim et al., 2013; Son et al., 2015b). Cognitive impairment is a major side effect of cranial radiation exposure. In fact, previous reports have studied radiation-induced cognitive impairment in patients with brain tumors after radiation therapy (Crossen et al., 1994; van’t Spijker et al., 1997; Johannesen et al., 2003). A single radiation dose of 10 Gy has also been reported to cause memory impairment in animal models (Son et al., 2014, 2015a). In addition, multiple studies have demonstrated radiation-induced memory dysfunction based on behavioral test performance (Kim et al., 2008; Son et al., 2014, 2015a) and have shown that radiation exposure induces chronic cognitive impairment in mice, possibly via decreased hippocampal neurogenesis (Son et al., 2014, 2015a). Suppressed neurogenesis is one cause of hippocampus-related cognitive impairment (Lazarov and Hollands, 2016). Radiation can suppress neurogenesis via various mechanisms, and such a direct oxidative stress by reactive oxygen species is a major pathophysiological mechanism of radiation-induced normal tissue injury (Lee et al., 2012).
Histone deacetylase (HDAC) inhibitors were reported to induce antidepressant-like effects by increasing histone acetylation of specific gene promoters (Covington et al., 2009). Moreover, HDAC inhibitors were found to act as antidepressants by increasing cAMP response element binding protein (CREB) (Lin et al., 2012). Furthermore, sodium butyrate (SB), which is an HDAC inhibitor that can cross the blood-brain barrier and affect epigenetic machinery in the brain (Minamiyama et al., 2004), has been shown to exert antidepressant effects when administered intraperitoneally (Schroeder et al., 2007). These results suggest that SB could be protective against hippocampal dysfunction. Although accumulating evidence suggests that HDAC inhibitors may affect hippocampal function (Davie, 2003), few studies have attempted to characterize the molecular mechanisms of HDAC inhibitors in terms of their antidepressant effects in the hippocampus. Although altered phosphorylated cAMP response element binding protein (pCREB)/brain-derived neurotrophic factor (BDNF) expression is known to play an important role in hippocampal function, the relationship between epigenetic histone modification and pCREB/BDNF expression has not been elucidated in radiation-induced hippocampal dysfunction. Thus, we aimed to investigate the possible radioprotective effect of SB in the hippocampus. To accomplish this objective, we established C57BL/6 mouse models of chronic radiation injury (Son et al., 2014, 2015a) and treated them with SB to investigate hippocampal neurogenesis and detect pCREB/BDNF expression 30 days after radiation exposure.
Materials and Methods
Animals
Male C57BL/6 mice (6 weeks old), weighing within 21.8 ± 3.1 g, were purchased from Central Lab. Animal Inc., Seoul, Korea and included in this study. The mice were used after 1 week of quarantine to allow acclimatization. The animals were housed at 23 ± 2°C in 50 ± 5% humidity with the air exchanged 13–18 times per hour. Study procedures were approved by the Institutional Animal Care and Use Committee of Korea Institute of Radiological Medical Sciences (approval No. KIRAMS16-0002) on December 30, 2016. The animals were maintained according to the internationally accepted principles for laboratory animal use and care as found in the NIH guidelines (USA). The mice were randomly divided into four groups (n = 10 per group): sham, SB, irradiation, and irradiation + SB treatment.
Radiation and SB treatment
Animals were anesthetized by intraperitoneal injection of tiletamine/zolazepam (Zoletil 50®; Virak Korea; Seoul, Korea) 80 mg/kg and exposed to 10 Gy whole-brain irradiation (dose rate of 3.81 Gy/min) using six MV photon rays (ELEKTA; Stockholm, Sweden), with a 1.5 cm surface bolus. The dose and rate of radiation were based on a previous report and were considered optimal for inducing chronic radiation brain injury (Son et al., 2014, 2015a). Radiation was applied to the midline of the head, which was placed in the center of a mouse holder and aligned to the center of the beam line. The radiation source was 1 m from the skin.
Sham-irradiated mice were anesthetized and immobilized for the same period of time without radiation. SB (Sigma Aldrich, Carlsbad, CA, USA) was dissolved in physiological normal saline and intraperitoneally administered to the mice 30 minutes prior to radiation exposure at a dose of 0.6 g/kg. The concentration of SB and its dosage were based on a previous report and were considered optimal for radioprotection (Han et al., 2014). Control mice received the same dosage of vehicle in a similar manner. The mice were subjected to behavioral testing and sacrificed 30 days after irradiation.
Object recognition test
Behavioral dysfunction after cranial irradiation (10 Gy) was measured using an object recognition memory test (n = 10 mice/group). The training and testing procedures of the objective recognition test were performed as previously described (Kim et al., 2008; Yang et al., 2014). Briefly, during training, two differently shaped objects were presented to each mouse for 15 minutes. Twenty-four hours after training, another set of objects (one old object, and one new object) (cube and cylinder shaped) was presented to the trained mouse. The interaction of the mouse with each object, including approaches and sniffing, was scored. The rate of preference was defined as the ‘number of interactions with each object’ divided by the ‘total number of interactions with both objects’. Any preference for searching for a novel object was measured by individual discrimination ratios. The individual discrimination was defined as the ‘number of interactions with a new object’ divided by the ‘total number of interactions with both objects’.
Immunohistochemistry assay
For tissue analysis, mice from each group (irradiated and control) were treated as follows: the brains of the mice (n = 6/group) were removed and divided at the midline. The left hemisphere was fixed in 10% formalin solution for immunohistochemistry, and the hippocampus was removed from the right hemisphere and immediately stored at –70°C for western blot analysis.
Brains were sectioned and stained as previously described (Kim et al., 2008). The brain sections were incubated in normal goat serum (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA, USA) for 60 minutes to block nonspecific binding and then in a 1:100 dilution of rabbit polyclonal anti-doublecortin (DCX; neurogenic cell markers; Cell Signaling Technology, Beverly, MA, USA) antibody or a 1:100 dilution of rabbit monoclonal anti-pCREB (87G3; Cell Signaling Technology) antibody at 4°C overnight. The sections were then reacted with biotinylated goat anti-rabbit IgG (Vectastain Elite ABC kit). Immunolabeling was performed using the avidin biotin peroxidase complex (Vectastain Elite ABC kit), and the peroxidase reaction was developed using a diaminobenzidine substrate kit (DAB Substrate Kit SK-4100; Vector Laboratories).
The numbers of immature progenitor cells (positive for DCX) and pCREB-positive cells in the hippocampus were counted by an observer blinded to sample identity (Kim et al., 2010; Yang et al., 2010). The number of immunopositive cells within the subgranular zone (SGZ) of the supra- and infra-pyramidal blades of the dentate gyrus (DG) (two sections/mouse) was averaged and expressed as the mean ± standard error (SE) for each group.
Western blot analysis
Western blotting procedures were performed as previously described (Son et al., 2014). The blots were incubated overnight at 4°C with rabbit anti-pCREB antibody (1:1,000; Cell Signaling Technology) and BDNF (1:500; Abcam, Cambridge, UK). After the membranes were extensively washed with PBS-T and incubated with horseradish peroxidase-conjugated anti-rabbit antibodies (1:10,000; Thermo Fisher Scientific, Rockford, IL, USA), signals were visualized using a chemiluminescence kit (SuperSignal West Pico; Thermo Fisher Scientific). The membranes were stripped and re-probed with an anti-β-actin antibody (1:20,000; Sigma-Aldrich) for normalization. The bands were quantified as optical density ratio of pCREB and BDNF to beta-actin using Scion Image Beta 4.0.2 for Windows XP software (Scion, Frederick, ME, USA).
Statistical analysis
Statistical analysis was performed with SPSS version 18 (SPSS, Chicago, IL, USA) via one-way analysis of variance followed by Student-Newman-Keuls post hoc test for multiple comparisons. The results are reported as the mean ± SE. P value < 0.05 was considered statistically significant.
Results
SB improves radiation-induced memory deficits in the object recognition task
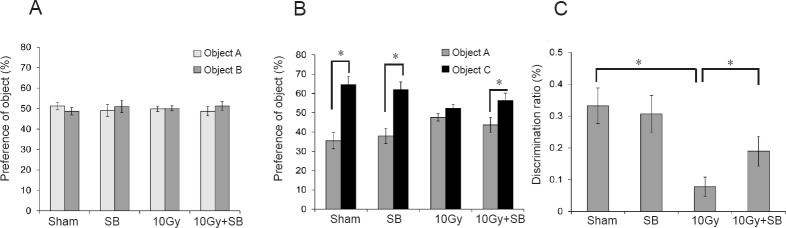
Using the hippocampus-dependent object recognition task, we found that the control and irradiated mice presented the same preferences for both objects during training (Figure 1A). During the testing phase, which occurred 24 hours after training, preference for the novel object was 64.6 ± 4.19% (P < 0.05, old object vs. novel object) in the sham group, 52.4 ± 1.95% in the irradiated mice (P = 0.11, old object vs. novel object), and 56.35 ± 3.83% (P < 0.05, old object vs. novel object) in the SB-treated irradiated mice. These results indicate that the irradiated mice showed impaired memory and that SB treatment attenuated the radiation-induced memory deficit (Figure 1B). In addition, the difference in discrimination ratios between irradiation and irradiation + SB treatment groups was significant (Figure 1C; P < 0.05).
Figure 1.
Beneficial effects of sodium butyrate (SB) on radiation-induced cognitive impairment after 30 days of radiation exposure.
(A) Sham and irradiated mice treated with vehicle or sodium butyrate exhibited no differences in their preference for the two objects (cube (object A) and pyramid shape (object B)) during training in the object recognition test. (B) During the test phase, the vehicle-treated irradiation group (10 Gy group) exhibited reduced preference for a novel object (cylinder shape (object C)) compared to the vehicle-treated sham group (sham group), and injection of sodium butyrate attenuated the radiation-induced cognitive impairment. (C) The discrimination ratio was markedly lower in the 10 Gy group, and SB treatment inhibited the decrease of discrimination ratio by radiation exposure after 30 days. The data are shown as the mean ± SE (n = 10/group). *P < 0.05 (one-way analysis of variance followed by Student-Newman-Keuls post hoc test).
SB attenuates radiation-induced impairment of hippocampal neurogenesis
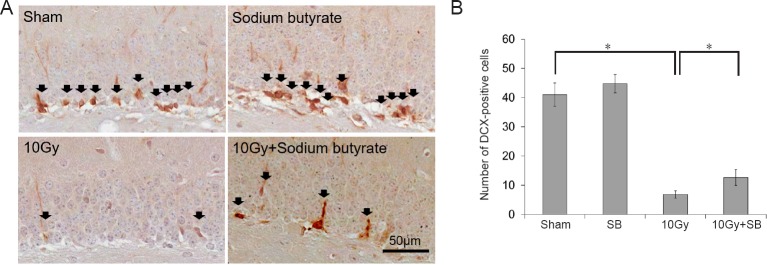
Immunohistochemically, immature progenitor cells were counted as the number of DCX-positive cells in the granular cell layer of the DG (Figure 2A). Cells expressing DCX were found in the DG of mice in the sham group (Figure 2B), and SB treatment did not alter the number of DCX-expressing cells (P < 0.05; Figure 2B). The number of DCX-positive cells in the hippocampus significantly declined 30 days after 10 Gy irradiation compared to the number in the sham group (P < 0.05; Figure 2B). However, SB treatment prior to irradiation greatly preserved the number of DCX-positive cells in the DG (P < 0.05; Figure 2B).
Figure 2.
Beneficial effects of sodium butyrate (SB) on radiation-induced decreases of doublecortin (DCX)-positive cells in the subgranular zone (SGZ) of the supra- and infra-pyramidal blades of the dentate gyrus (DG) of the hippocampus after 30 days radaition exposure.
(A) DCX immunoreactivity (arrows) in the DG of the hippocampus. Scale bar: 50 μm. (B) Quantification of DCX-immunoreactive cells. SB attenuated radiation-induced decrease in neurogenesis. The data are shown as the mean ± SE (n = 6/group). *P < 0.05 (one-way analysis of variance followed by Student-Newman-Keuls post hoc test).
SB attenuates the radiation-induced decrease of pCREB in the hippocampus
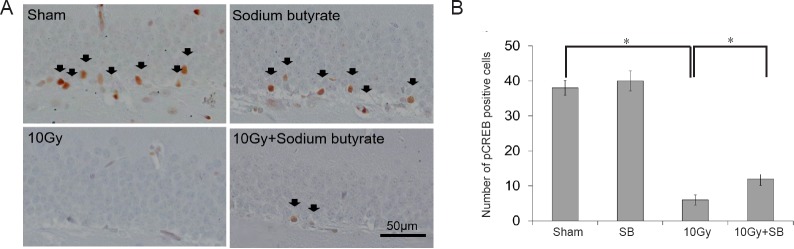
Immunohistochemically, constitutively pCREB-positive cells were observed in the DG of mice in the sham group (Figure 3B), with most positive cells located near the SGZ (Figure 3A). The number of pCREB-positive cells in the hippocampus significantly declined 30 days after 10 Gy irradiation (P < 0.05; Figure 3B). SB treatment alone did not change the number of pCREB-positive cells (P < 0.05; Figure 3B). However, SB treatment significantly increased the number of pCREB-positive cells in mice irradiated with 10 Gy (P < 0.05; Figure 3B).
Figure 3.
Beneficial effects of sodium butyrate (SB) on radiation-induced decreases in the number of phosphorylated cAMP response element binding protein (pCREB)-positive cells in the subgranular zone (SGZ) of the supra- and infra-pyramidal blades of the dentate gyrus (DG) of the hippocampus.
(A) pCREB immunoreactivity (arrows) in the DG of the hippocampus. Scale bar: 50 μm. (B) Quantification of pCREB-immunoreactive cells. SB attenuated radiation-induced decrease in pCREB-immunoreactive cells. The data are shown as the mean ± SE (n = 6/group). *P < 0.05 (one-way analysis of variance followed by Student-Newman-Keuls post hoc test).
SB attenuates radiation-induced decreases in pCREB/BDNF in the hippocampus
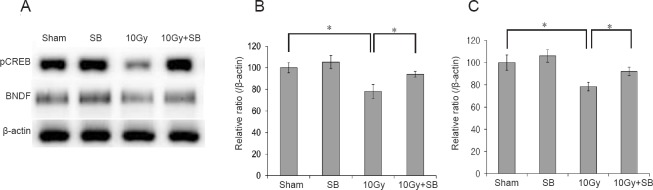
pCREB and BDNF modulate neurogenesis in the hippocampus. Therefore, we used western blot analysis to explore whether pCREB and BDNF were involved in the radiation-induced decrease in hippocampal neurogenesis. Radiation exposure decreased hippocampal pCREB protein levels, and this decrease was attenuated by SB treatment (P < 0.05; Figure 4A and B). pCREB, BDNF levels significantly decreased after irradiation, but BDNF levels were higher in SB-treated mice than in radiation-exposed mice (Figure 4A and C).
Figure 4.
Beneficial effects of sodium butyrate (SB) on radiation-induced decreases in phosphorylated cAMP response element binding protein (pCREB)/brain-derived neurotrophic factor (BDNF) expression.
(A) Western blots of pCREB and BDNF in hippocampal tissue. (B and C) The irradiated mice exhibited significantly decreased pCREB and BDNF expression in the hippocampus. Exposure to SB attenuated the radiation-induced decrease in pCREB/BDNF expression in the hippocampus. The data are shown as the mean ± SE (n = 6/group). *P < 0.05 (one-way analysis of variance followed by Student-Newman-Keuls post hoc test).
Discussion
In this study, we revealed that SB treatment attenuated radiation-induced hippocampal dysfunction and the associated behavioral deficits. These effects were suggested to be related to inhibition of radiation-induced decrease in immature neuronal cells and pCREB/BDNF expression in the hippocampus.
Cranial radiation therapy is known to damage hippocampal neurogenesis, which induces memory deficits (Raber et al., 2004); and thus, interventions are required to protect endogenous hippocampal neurogenesis against radiation-induced damage. It has been reported that 10 Gy of whole-brain irradiation induces hippocampal neurogenesis and consequent behavioral abnormalities, including cognitive impairment (Son et al., 2014, 2015a).
Neurogenesis in the adult brain is regulated in both a positive and negative manner by environmental, endocrine, and pharmacological stimuli. The cAMP cascade, including CREB, plays an important role in hippocampal neurogenesis in adults (Nakagawa et al., 2002). In addition, pCREB directly regulates the expression of BDNF, which is involved in neurogenesis of the hippocampus and memory processes (Dinel et al., 2011). Altered BDNF levels have been shown to affect the proliferation of hippocampal precursor cells and can lead to disruptions in memory formation (Adachi et al., 2008). Radiation exposure has been implicated in reducing the expression levels of pCREB and BDNF in the hippocampus. pCREB activation can protect against radiation-induced hippocampal dysfunction (Kim et al., 2010; Son et al., 2015a). Decreases in pCREB and BDNF in the mouse hippocampus are associated with memory defects after radiation (Son et al., 2014, 2015a). Therefore, downregulation of CREB/BDNF signaling may be associated with hippocampus-dependent memory impairment after radiation exposure. In this study, we confirmed that radiation decreased neurogenesis and pCREB levels in the hippocampus and led to cognitive impairment.
Cognitive impairment is reported to occur in up to 50–90% of adult patients with brain tumors who receive radiotherapy and survive > 6 months post-irradiation (Johannesen et al., 2003; Meyers and Brown, 2006). Previous clinical studies suggest that several drugs can prevent/ameliorate irradiation-induced cognitive impairment when they are applied at various times. However, an effective drug that has been tested in clinical trials is not currently available. Therefore, SB may be a potential therapeutic agent or radioprotector for the brain. Finally, it should be noted that previous studies used 1.2 g/kg SB to attenuate hippocampal dysfunction (Schroeder et al., 2007). However, we demonstrated here that 0.6 g/kg SB was sufficient to improve cranial irradiation-induced cognitive impairments. These results suggest that SB treatments with radiation therapy may improve the protective effect of SB against radiation-induced memory impairment.
In this study, we confirmed that SB treatment prevented radiation-induced learning disability in mice by inhibiting decreases in pCREB/BDNF in the hippocampus. However, further studies are needed to elucidate the mechanism of the radioprotective effect of SB. This study provides evidence that SB may confer an additional therapeutic effect by restoring pCREB/BDNF in the irradiated hippocampus.
Additional file: Open peer review reports 1 (106.3KB, pdf) and 2 (102.6KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that there is no conflict of interest.
Financial support: This work was supported by the Nuclear Research and Development Program (NRF-2012M2A2A7012377, NRF-2015M2B2B1068627 and NRF-2015R1C1A2A01053041) of the National Research Foundation of Korea (NRF) funded by the Korean government Ministry of Science, ICT & Future Planning.
Institutional review board statement: The study procedures were approved by the Institutional Animal Care and Use Committee of Korea Institute of Radiological Medical Sciences (approval No. KIRAMS16-0002) on December 30, 2016.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Christopher J. Andrews, University of Queensland, School of Medicine, Australia; Li Xiao, The Nippon Dental University, Japan.
Funding: This work was supported by the Nuclear Research and Development Program (NRF-2012M2A2A7012377, NRF-2015M2B2B1068627 and NRF-2015R1C1A2A01053041) of the National Research Foundation of Korea (NRF) funded by the Korean Government Ministry of Science, ICT & Future Planning.
P-Reviewers: Andrews CJ, Xiao L; C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covington HE, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 4.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 5.Dinel AL, André C, Aubert A, Ferreira G, Layé S, Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 2011;6:e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han A, Sung YB, Chung SY, Kwon MS. Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology. 2014;81:292–302. doi: 10.1016/j.neuropharm.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Johannesen TB, Lien HH, Hole KH, Lote K. Radiological and clinical assessment of long-term brain tumor survivors after radiotherapy. Radiother Oncol. 2003;69:169–176. doi: 10.1016/s0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 8.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JS, Lee HJ, Kim JC, Kang SS, Bae CS, Shin T, Jin JK, Kim SH, Wang H, Moon C. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J Radiat Res. 2008;49:517–526. doi: 10.1269/jrr.08020. [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Yang M, Cho J, Kim SH, Kim JC, Shin T, Moon C. Promotion of cAMP responsive element-binding protein activity ameliorates radiation-induced suppression of hippocampal neurogenesis in adult mice. Toxicol Res. 2010;26:177–183. doi: 10.5487/TR.2010.26.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JS, Yang M, Kim SH, Shin T, Moon C. Neurobiological toxicity of radiation in hippocampal cells. Histol Histopathol. 2013;28:301–310. doi: 10.14670/HH-28.301. [DOI] [PubMed] [Google Scholar]
- 12.Lazarov O, Hollands C. Hippocampal neurogenesis: learning to remember. Prog Neurobiol. 2016;138(140):1–18. doi: 10.1016/j.pneurobio.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YW, Cho HJ, Lee WH, Sonntag WE. Whole brain radiation-induced cognitive impairment: pathophysiological mechanisms and therapeutic targets. Biomol Ther. 2012;20:357–370. doi: 10.4062/biomolther.2012.20.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin H, Geng X, Dang W, Wu B, Dai Z, Li Y, Yang Y, Zhang H, Shi J. Molecular mechanisms associated with the antidepressant effects of the class I histone deacetylase inhibitor MS-275 in the rat ventrolateral orbital cortex. Brain Res. 2012;1447:119–125. doi: 10.1016/j.brainres.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 15.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 16.Minamiyama M, Katsuno M, Adachi H, Waza M, Sang C, Kobayashi Y, Tanaka F, Doyu M, Inukai A, Sobue G. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2004;13:1183–1192. doi: 10.1093/hmg/ddh131. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Son Y, Yang M, Kang S, Lee S, Kim J, Kim J, Park S, Kim JS, Jo SK, Jung U, Shin T, Kim SH, Wang H, Moon C. Cranial irradiation regulates CREB-BDNF signaling and variant BDNF transcript levels in the mouse hippocampus. Neurobiol Learn Mem. 2015a;121:12–19. doi: 10.1016/j.nlm.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Son Y, Yang M, Kim JS, Kim J, Kim SH, Kim JC, Shin T, Wang H, Jo SK, Jung U, Moon C. Hippocampal dysfunction during the chronic phase following a single exposure to cranial irradiation. Exp Neurol. 2014;254:134–144. doi: 10.1016/j.expneurol.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Son Y, Yang M, Wang H, Moon C. Hippocampal dysfunctions caused by cranial irradiation: a review of the experimental evidence. Brain Behav Immun. 2015b;45:287–296. doi: 10.1016/j.bbi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 23.van’t Spijker A, Trijsburg RW, Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: a meta-analytical review of 58 studies after 1980. Psychosom Med. 1997;59:280–293. doi: 10.1097/00006842-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, Jung U, Shin T, Wang H, Moon C. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav Immun. 2014;36:147–155. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, Kim JC, Wang H, Shin T, Moon C. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93:487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.