Keywords: nerve regeneration, Alzheimer's disease, natural plant drug, curcuminoids, miRNAs, amyloid precursor protein, amyloid-β, 3‘-untranslated region, Luciferase assays, neurons, neural regeneration
Abstract
Curcumin exerts a neuroprotective effect on Alzheimer’s disease; however, it is not known whether microRNAs are involved in this protective effect. This study was conducted using swAPP695-HEK293 cells as an Alzheimer’s disease cell model. swAPP695-HEK293 cells were treated with 0, 0.5, 1, 2, 5, and 10 μM curcumin for 24 hours. The changes in miR-15b-5p, miR-19a-3p, miR-195-5p, miR-101-3p, miR-216b-5p, miR-16-5p and miR-185-5p expression were assessed by real-time quantitative polymerase chain reaction. The mRNA and protein levels of amyloid precursor protein, amyloid-β40 and amyloid-β42 were evaluated by quantitative real-time polymerase chain reaction, western blot assays and enzyme-linked immunosorbent assays. swAPP695-HEK293 cells were transfected with miR-15b-5p mimic, or treated with 1 μM curcumin 24 hours before miR-15b-5p inhibitor transfection. The effects of curcumin on amyloid precursor protein, amyloid-β40 and amyloid-β42 levels were evaluated by western blot assays and enzyme-linked immunosorbent assay. Luciferase assays were used to analyze the interaction between miR-15b-5p and the 3′-untranslated region of amyloid precursor protein. The results show that amyloid precursor protein and amyloid-β expression were enhanced in swAPP695-HEK293 cells compared with HEK293 parental cells. Curcumin suppressed the expression of amyloid precursor protein and amyloid-β and up-regulated the expression of miR-15b-5p in swAPP695-HEK293 cells. In addition, we found a negative association of miR-15b-5p expression with amyloid precursor protein and amyloid-β levels in the curcumin-treated cells. Luciferase assays revealed that miR-15b-5p impaired the luciferase activity of the plasmid harboring the 3′-untranslated region of amyloid precursor protein. These findings indicate that curcumin down-regulates the expression of amyloid precursor protein and amyloid-β in swAPP695-HEK293 cells, which was partially mediated by miR-15b-5p via targeting of the 3′-untranslated region of amyloid precursor protein.
Chinese Library Classification No. R453; R741
Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disease. With an aging population, the prevalence of AD has increased year by year and it has become the fourth ranked killer of humans behind cardiovascular disease, cancer, and stroke. The central pathological changes of AD are currently considered to be extracellular amyloid-β (Aβ) atherosclerotic plaque deposition, intracellular neurofibrillary tangles, and lack of synaptic connections in the cerebral cortex and hippocampus (Alzheimer’s Association, 2018).
Although the etiology of AD is not yet clear, the widely accepted Aβ cascade hypothesis states that AD is a pathological syndrome that affects the stability of Aβ aggregation by directly or indirectly altering expression of the transmembrane glycoprotein, amyloid precursor protein (APP), the enzymatic cleavage of APP (which produces Aβ), and abnormal deposition/clearance of Aβ (Nhan et al., 2015; Lin et al., 2018). Therefore, AD may potentially be treated by reducing the production and aggregation of Aβ and enhancing the clearance of Aβ. Aβ is a fragment cleaved from APP via different enzymes: α-secretase, β-secretase and γ-secretase. Aβ exists as two forms, Aβ40 (~90%) and Aβ42 (which is less than 10%). Although the amount of Aβ42 is relatively small, it is believed to be more hydrophobic and highly aggregated than Aβ40 and it forms oligomers, fibrils, and deposits as atheromatous plaques, which cause damage to neurons and are considered to be at the pathological core of AD (Nhan et al., 2015).
Curcumin is a mixture of three monomers (curcumin, demethoxycurcumin, and bis-demethoxycurcumin). Curcumin is a natural plant drug and plays a protective role against AD (Goozee et al., 2016) by affecting Aβ metabolism (Cox et al., 2018; Chen et al., 2018). Comparison of the three monomeric components on anti-inflammatory effects (Somparn et al., 2007) and the inhibition of tumor necrosis factor-activated TNF-κB activity (Sandur et al., 2007) showed that curcumin was the most effective, suggesting that curcumin exerts a major pharmacological activity compared with the other two compounds (Sandur et al., 2007; Somparn et al., 2007).
MicroRNAs (miRNAs) are a class of evolutionarily conserved small RNA of ~22 nucleotides that recognize the 3′-untranslated region (3′-UTR) of target mRNAs. They interfere with mRNA stability and inhibit protein expression at the post-transcriptional level (Rupaimoole and Slack, 2017; Wang and Wang, 2018). miRNAs are abundant in brain tissue and can specifically affect neuronal growth and synapse formation. The expression levels of miRNAs in the brain of AD patients are remarkably different from those of healthy subjects of the same age (Rockenstein et al., 2001; Austin et al., 2009; Jiang et al., 2010). miRNAs have been identified as biological markers and as therapeutic targets for early diagnosis of AD (Galimberti et al., 2014; Liu et al., 2014; Bekris and Leverenz, 2015). It is noteworthy that curcumin can regulate genes by regulating the expression of multiple miRNAs (Ma et al., 2014; Toden et al., 2015; Ye et al., 2015; Momtazi et al., 2016), indicating a potential association between curcumin, AD and miRNAs. However, miRNAs involved in the protective effect of curcumin remain to be investigated.
This study investigated whether curcumin monomer has an effect on the expression of APP and Aβ. Further, we investigated whether the effect of curcumin on APP and Aβ is associated with miRNAs.
Materials and Methods
Cell treatment
Human embryonic kidney (HEK293) cells stably transfected with amyloid precursor protein bearing the Swedish mutation (swAPP695) (named swAPP695-HEK293) were provided by the Department of Biochemistry, Faculty of Medicine, University of Hong Kong, China.
To determine the effect of curcumin on the expression level of APP and Aβ, 1 × 105/mL cells were treated with curcumin (Sigma, St. Louis, MO, USA) dissolved in dimethyl sulfoxide at different concentrations (0, 0.5, 1, 2, 5, 10 μM) and incubated for 24 hours. The effect of curcumin (1 μM) on the expression of miR-15b-5p, miR-19a-3p, miR-195-5p, miR-101-3p, miR-216b-5p, miR-16-5p and miR-185-5p was evaluated by quantitative real-time polymerase chain reaction (qRT-PCR) at 24 hours post curcumin treatment. To explore the effect of miR-15b-5p on APP and Aβ expression, cells were transfected with miR-15b-5p mimics (GenePharma, Suzhou, China) or pre-treated with curcumin (1 μM) 24 hours before transfection with miR-15b-5p inhibitors (GenePharma, Suzhou, China).
qRT-PCR
Total RNA was isolated from cells using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and the extracted RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Am MiScript II RT Kit (Qiagen, Hilden, Germany) was used for RNA reverse transcription assays following the manufacturer’s protocol. The cDNAs were amplified using an ABI 7500 Real Time PCR system (Thermo Fisher Scientific) with an miScript SYBR Green PCR Kit (Qiagen) and miRNA or mRNA specific primers. The expression levels of miR-15b-5p and APP were calculated using the 2–ΔΔCt method (Livak and Schmittgen, 2001).
Western blot assays
Cells were lysed in RIPA lysis solution plus containing 2 μM PMSF on ice. Cell lysate was collected using a cell scraper and centrifuged at 13,780 × g for 5 minutes. The isolated proteins were quantified using a bicinchoninic acid assay (Thermo-Fisher Scientific) and separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, with up to 40 μg protein in each lane. Proteins were then transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA) and then incubated with rabbit anti-APP primary antibody (diluted 1:20,000, cat# ab32136; Abcam, Cambridge, MA, USA) or rabbit anti-GAPDH primary antibody (diluted 1:4000, Cat# 25778; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. Membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (diluted 1:5000, Cat# 111-035-003; Jackson, West Grove, PA, USA) at room temperature for 45 minutes. The bands were visualized using western blot enhanced chemiluminescence substrate (Bio-Rad, Hercules, CA, USA). Relative expression among groups was quantified by ImageJ software. GAPDH was used as a reference control.
Enzyme-linked immunosorbent assay
The amounts of Aβ40 and Aβ42 in cell supernatant were measured using a human Aβ ELISA kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Immunohistochemistry
Cells on slides were washed three times with phosphate buffered saline. Non-specific binding sites were blocked with blocking solution containing 1% bovine serum albumin and 0.1% saponin in phosphate buffered saline for 1 hour at room temperature. Subsequently, slides were incubated at 4°C overnight with APP rabbit monoclonal primary antibody (diluted 1:200, Cat# ab32136; Abcam) in blocking solution, washed with blocking buffer and then incubated at 37°C for 1 hour with a goat anti-rabbit IgG secondary antibody (Jackson) diluted 1:200 in blocking solution. Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO, USA). Glass coverslips were mounted onto slides using fluorescence mounting medium (Dako, Santa Clara, CA, USA), and analyzed using a ZEISS LSM 510 confocal microscope (ZEISS, Jena, Germany) and ImageJ software 1.48 v (NIH, Bethesda, MD, USA).
Luciferase experiments
Fragments of 245 bp containing a putative wild-type miR-15b-5p biding site (5′-CAG ATT GCT GCT-3′) or a mutant binding site (5′-CAT TTG CGG-3′) of the 3′-UTR of APP were synthesized (Sangon, Shanghai, China) and cloned into pmirGLO (Promega, WI, USA), resulting in APP 3′-UTR wild-type (WT) and APP 3′-UTR mutant (MT) vectors.
To confirm the interaction between miR-15b-5p and the APP 3′-UTR WT plasmid, pmirGLO/APP 3′-UTR WT and pmirGLO/APP 3′-UTR MT were transfected with miR-15b-5p mimics or miR-15b-5p mimics normal control (NC) into swAPP695-HEK 293 cells, resulting in six groups as follows. (1) pmirGLO + miR-15b-5p mimics NC; (2) pmirGLO + miR-15b-5p minics; (3) APP 3′-UTR WT + miR-15b-5p minics NC; (4) APP 3′ UTR WT + miR-15b-5p minics; (5) APP 3′-UTR MT + miR-15b-5p minics NC; (6) APP 3′ UTR MT + miR-15b-5p mimics. Luciferase assays were performed 48 hours after transfection to determine relative luciferase activity using a Dual-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions. Renilla luciferase activity was used for normalization.
Statistical analysis
All data are presented as the mean ± SD and were analyzed using SPSS 19.0 software (SPSS, Chicago, IL, USA). Two-sample t-test was used for analysis of two independent samples. Difference among more than three groups was compared using one-way analysis of variance. The mean between two groups with significant differences was analyzed using Fisher’s least significant difference method. A value of P < 0.05 was considered statistically significant.
Results
Ectopic expression of APP695 enhances the expression of APP and Aβ in HEK-293 cells
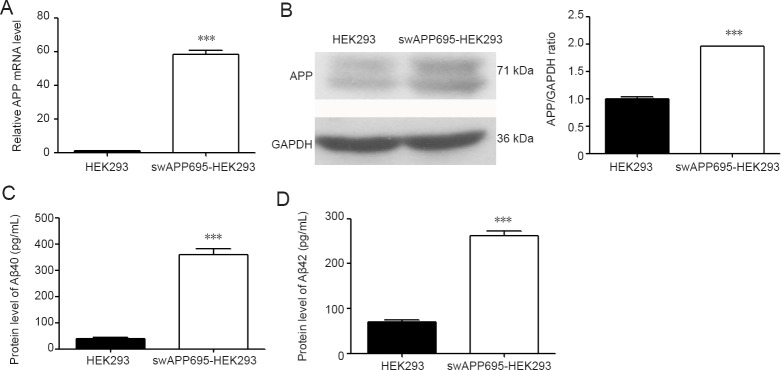
APP695 is an isoform of APP that is exclusively expressed in neurons (Xiao et al., 2015). The swAPP695-HEK293 cell line was used to study the effect of ectopic expression of APP695 in HEK-293 cells. As shown in Figure 1, qRT-PCR confirmed that the level of APP mRNA in swAPP695-HEK293 cells was 50-fold higher than that of normal HEK293 cells (P < 0.001; Figure 1A). Western blot assays also showed significantly higher levels of APP in swAPP695-HEK293 cells (P < 0.001; Figure 1B). ELISA revealed that both Aβ40 and Aβ42 were elevated in swAPP695-HEK293 cell supernatant (P < 0.001; Figure 1C & D).
Figure 1.
Expression of APP and Aβ in swAPP695-HEK293 and normal HEK293 cells.
(A) Real-time quantitative polymerase chain reaction of APP expression in each cell line. (B) Western blot assay of APP protein in each cell line. The levels Aβ40 (C) and Aβ42 (D) in the culture supernatant of each cell line were assessed by enzyme linked immunosorbent assay. Data are expressed as the mean ± SD. ***P < 0.001, vs. HEK293 (two-sample t-test). APP: Amyloid precursor protein; Aβ: amyloid-β.
Curcumin decreases the expression of APP and Aβ in swAPP695-HEK293 cells and up-regulates the expression of miR-15b-5p
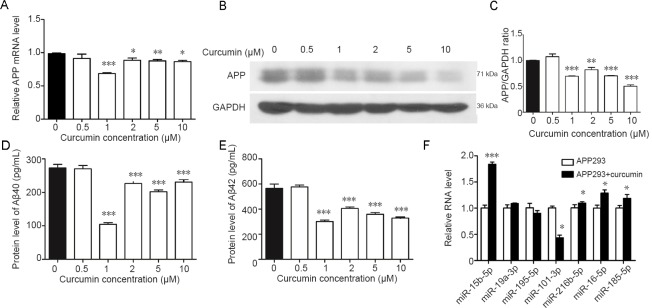
To evaluate the effect of curcumin on the expression of APP and Aβ, qRT-PCR and western blot assays were performed to detect the expression of APP and Aβ in response to 0, 0.5, 1, 2, 5 and 10 μM curcumin treatment. As shown in Figure 2, APP mRNA and protein levels were affected by curcumin treatment at concentrations of 1, 2, 5 and 10 μM, with a concentration of 1 μM giving the greatest suppression (Figure 2A, B & C). A similar trend was observed with the levels of Aβ40 and Aβ42 in the cell supernatant. The most effective concentration was 1 μM, although 2, 5 and 10 μM also caused significant suppression compared with untreated cells (0 μM) (Figure 2D & E). Based on these observations, curcumin treatment at 1 μM was used for subsequent experiments.
Figure 2.
Expression of APP and Aβ in swAPP695-HEK293 cells is affected by curcumin.
(A–C) The mRNA (A) and protein (B & C) levels of APP in cells treated with curcumin at different concentrations (0, 0.5, 1, 2, 5 and 10 μM). (D, E) Relative expression levels of Aβ40 (D) and Aβ42 (F) in cells treated with curcumin at different concentrations. (F) Relative expression of seven miRNAs (miR-15b-5p, miR-19a-3p, miR-195-5p, miR-101-3p, miR-216b-5p, miR-16-5p, and miR-185-5p) was assessed in swAPP695-HEK293 cells treated with curcumin (1 μM). Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, vs. 0 μM (one-way analysis of variance followed by Fisher’s least significant difference test). APP293: swAPP695-HEK293 cells; APP: amyloid precursor protein; Aβ: amyloid-β.
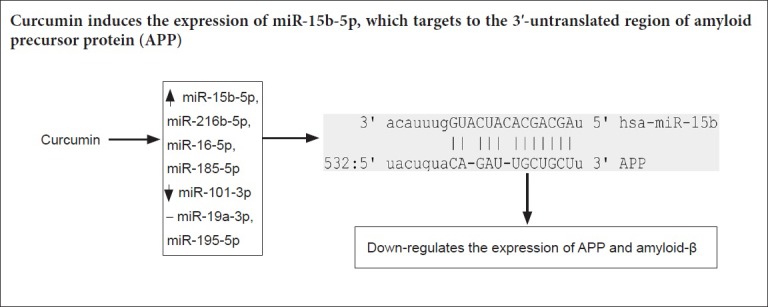
To further explore whether the effect of curcumin on the expression of APP and Aβ is associated with miRNAs, candidate miRNAs targeting APP were predicted using an online tool and the expression of seven candidate miRNA, including miR-15b-5p, miR-19a-3p, miR-195-5p, miR-101-3p, miR-216b-5p, miR-16-5p and miR-185-5p, was detected. As shown in Figure 2E, miR-15b-5p, miR-216b-5p, miR-16-5p and miR-185-5p were overexpressed when cells were treated with curcumin, while miR-101-3p expression was down-regulated. No significant change was observed in the expression of miR-19a-3p and miR-195-5p. Of the differentially expressed miRNAs, miR-15b-5p expression was the most up-regulated (Figure 2F).
The level of miR-15b-5p is negatively associated with the expression of APP and Aβ in curcumin-treated cells
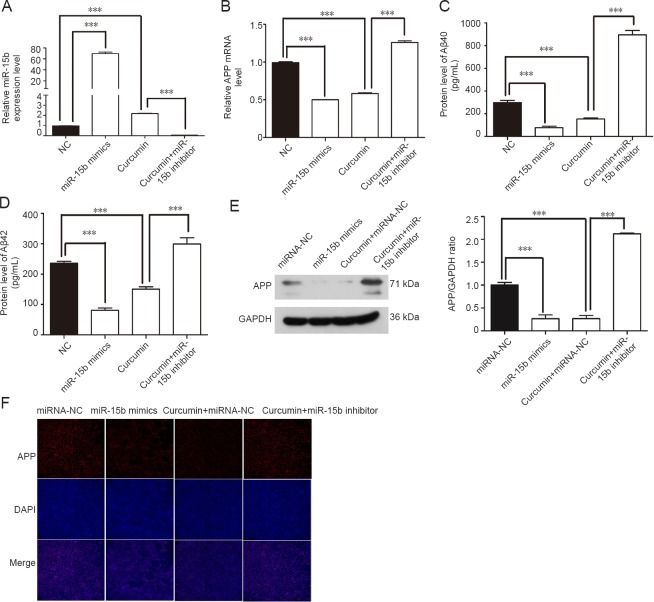
qRT-PCR confirmed that the miR-15b-5p mimic noticeably enhanced the expression of miR-15b-5p in swAPP695-HEK293 cells, while the miR-15b-5p inhibitor sharply suppressed its expression, even in cells treated with curcumin that showed overexpression of miR-15b-5p (Figure 3A). In contrast to the curcumin-induced upregulation of miR-15b-5p expression, curcumin down-regulated APP mRNA and Aβ40 and Aβ42 levels compared with untreated cells. Similar to curcumin treatment, overexpression of miR-15b-5p by the miR-15b-5p mimic remarkably reduced the levels of APP mRNA, Aβ40 and Aβ42. However, transfection of the miR-15b-5p inhibitor restored this overexpression (Figure 3B–D). Western blot assays showed that the miR-15b-5p mimic reduced APP protein levels, while the miR-15b-5p inhibitor enhanced APP protein levels (Figure 3E). Immunofluorescence staining showed similar results; miR-15b-5p mimic reduced APP protein levels and miR-15b-5p inhibitor enhanced APP protein levels (Figure 3F).
Figure 3.
Expression of APP and Aβ in swAPP695-HEK293 cells is affected by miR-15b-5p and curcumin treatment.
Expression of miR-15b-5p (A), APP mRNA (B), and APP protein (E) in normal, curcumin treated, miR-15b-5p mimic and miR-15b-5p inhibitor transfected cells. Levels of Aβ40 (C) and Aβ42 (D) in cell culture supernatant of each group were assessed by enzyme linked immunosorbent assay. (F) APP protein expression in each group was assessed by immunofluorescence. Original magnification: 200×. Data are expressed as the mean ± SD. ***P < 0.001 (one-way analysis of variance followed by Fisher’s least significant difference test). APP: Amyloid precursor protein; Aβ: amyloid-β; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; DAPI: 4′,6-diamidino-2-phenylindole; NC: normal control.
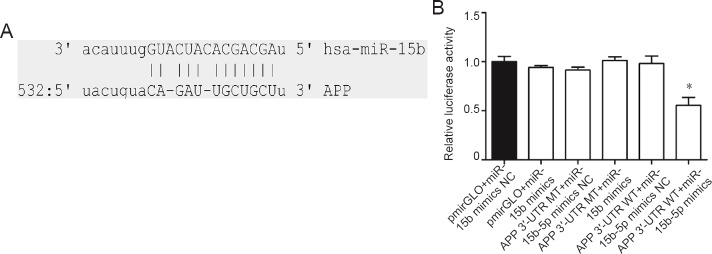
miR-15b-5p decreases the luciferase activity of the luciferase plasmid harboring the 3′-UTR of APP
Bioinformatic analysis predicted a binding site for the miR-15b-5p seed region within the 3′-UTR of APP (Figure 4A). To validate the regulatory relationship between miR-15b-5p and APP, WT and MT 3′-UTRs of APP were cloned into the luciferase reporter plasmid and cotransfected with miR-15b-5p mimic to determine whether the luciferase activity was affected by miR-15b-5p overexpression. As expected, only cells transfected with WT 3′-UTR of APP (APP 3′ UTR WT + miR-15b-5p mimics) and pmirGLO + miR-15b-5p mimic showed decreased luciferase activity (Figure 4B). In vehicle control cells (pmirGLO + miR-15b-5p mimics NC), negative control cells (pmirGLO + miR-15b-5p mimics, APP 3′-UTR WT + miR-15b-5p mimics NC) and cells cotransfected with APP 3′-UTR MT and miR-15b-5p mimics or miR-15b-5p mimics NC, the luciferase activity was not obviously changed (Figure 4B).
Figure 4.
Relative luciferase activity in sw-APP795-HEK293 cells co-transfected with pmirGLO/APP 3′-UTR WT, pmirGLO/APP 3′-UTR MT and miR-15b-5p mimics or miR-15b-5p mimics NC.
(A) Schematic diagram shows the putative biding site between miR-15b-5p and the 3′-UTR of APP, as predicted by an online tool (mirna.org). (B) Relative luciferase activity in each group. Data are expressed as the mean ± SD. *P < 0.05, vs. APP 3′-UTR WT + miR-15b-5p mimics NC (one-way analysis of variance followed by Fisher’s least significant difference test). APP: Amyloid precursor protein; UTR: untranslated region; WT: wild-type; MT: mutant; NC: normal control.
Discussion
The functions of miRNAs that mediate the effects of curcumin mixture on anti-tumorigenesis and anti-inflammation have been reported (Boyanapalli and Kong, 2015; Ye et al., 2015; Jiao et al., 2017). Our previous study found that curcumin is the most effective active ingredient in curcumin mixture at inhibiting APP and Aβ levels (Liu et al., 2010). However, the effect of curcumin on APP and Aβ remains to be confirmed. In particular it is not known whether the mechanism underlying this effect is associated with the regulation of miRNAs. This study used swAPP695-HEK293 cells, a cell line that stably overexpresses APP, to clarify whether curcumin intervention inhibits the expression of APP and Aβ and enhances the expression of several miRNAs. We showed that overexpression of miR-15b-5p noticeably inhibited the expression of APP and Aβ, while inhibition of miR-15b-5p promoted the expression of APP and Aβ. Bioinformatic prediction revealed a complementary site between the seed sequence of miR-15b-5p and the 3′-UTR of APP and luciferase assays confirmed this interaction.
Aβ is produced by digestion of APP. APP gene mutation can cause early-onset AD, amyloid pathological changes, and induce neuronal apoptosis (Wu et al., 2015). The expression of APP in the vasculature of AD patients is noticeably increased (Austin et al., 2009). Down-regulation of APP shows a beneficial effect in the content and clearance of Aβ (Jiang et al., 2010). In rats transfected with the APP gene, the deposition of Aβ atherosclerotic plaques can be detected in the early brain; and pathological changes characteristic of AD patients and corresponding clinical manifestations can be simulated (Rockenstein et al., 2001), indicating that APP is involved in the pathogenesis of AD. Our study found that curcumin can inhibit expression levels of APP and Aβ, which supports the inhibitory effect of curcumin on APP and Aβ, suggesting potential clinical value of curcumin in alleviating symptoms in AD patients.
miRNAs function as gene regulators by binding to the 3′-UTR of target genes, resulting in mRNA degradation or translational inhibition. Accumulated evidence shows that miRNAs regulate AD progression (Lukiw and Alexandrov, 2012). miRNAs are also a therapeutic targets of curcumin (Momtazi et al., 2016). In the present study, miR-15b-5p, miR-216b-5p, miR-16-5p, miR-185-5p and miR-101-3p were differentially expressed following curcumin treatment, indicating their potential involvement in curcumin therapy. Among these miRNAs, miR-15b-5p, miR-16-5p (Zhang et al., 2015), miR-185-5p (Sun et al., 2017), and miR-101-3p (Chang et al., 2016) are associated with cancer and nervous system degeneration (Lugli et al., 2015; Wan et al., 2015). miR-15b-5p produced the greatest response to curcumin intervention; therefore, we focused on its relationship with APP in subsequent analysis. However, this does not mean that other miRNAs are not involved in curcumin-induced effects. A previous study revealed that miR-15b-5p was differentially expressed in the preclinical phase of AD and could be used as an early circulating plasma biomarker for AD (Wan et al., 2015). Down-regulation of miR-15b-5p was confirmed by singleplex qPCR in AD patients compared with age- and sex-matched normal controls (Kumar et al., 2013). Despite this, the function of miR-15b-5p regarding the etiology of AD is still unclear. Our current study revealed that miR-15b-5p interacts with the 3′-UTR of APP and contributed to the curcumin-induced decrease in the level of APP and Aβ. Previously, hsa-miR-106, hsa-miR-153 and hsa-miR-101 were shown to target APP (Patel et al., 2008; Long and Lahiri, 2011; Long et al., 2012; Galimberti et al., 2014). Our study provides new evidence to show that miR-15b-5p is involved in AD, possibly through targeting the 3′-UTR of APP.
In summary, curcumin suppressed the expression of APP and Aβ in swAPP695-HEK293 cells. An underlying mechanism for these effects might be that curcumin induces miR-15b-5p expression, which targets the 3′-UTR of APP to decrease expression of APP and Aβ. We suggest that miR-15b-5p is a downstream target of curcumin and that miR-15b-5p might act as a biological marker for early diagnosis of AD and as a therapeutic target; however, the effect of curcumin on APP and Aβ remains to be investigated. In the future, we will investigate whether the protective effect of curcumin against AD involves reducing extracellular deposition of Aβ in neurons.
Additional file: Open peer review report 1 (83.9KB, pdf) .
Acknowledgments:
We are much grateful to Professor You-Qiang Song from the University of Hong Kong, who kindly provided with swAPP695- HEK293 cells.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Financial support: This study was supported by the Science and Technology Planning Project of Guangdong Province of China, No. 2016A020226022 (to HYL); the Medical and Health Technology Project of Guangzhou of China, No. 20161A011068 (to HYL); the Guangzhou Science Technology and Innovation Commission, China, No. 201704020043 (to QCG). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Ivan Fernandez-Vega, Hospital Universitario Central de Asturias, Spain.
Funding: This study was supported by the Science and Technology Planning Project of Guangdong Province of China, No. 2016A020226022 (to HYL); the Medical and Health Technology Project of Guangzhou of China, No. 20161A011068 (to HYL); the Guangzhou Science Technology and Innovation Commission of China, No.201704020043 (to QCG).
P-Reviewer: Fernandez-Vega I; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Allen J, Frenchman B, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14:367–429. [Google Scholar]
- 2.Austin SA, Sens MA, Combs CK. Amyloid precursor protein mediates a tyrosine kinase-dependent activation response in endothelial cells. J Neurosci. 2009;29:14451–14462. doi: 10.1523/JNEUROSCI.3107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekris LM, Leverenz JB. The biomarker and therapeutic potential of miRNA in Alzheimer’s disease. Neurodegener Dis Manag. 2015;5:61–74. doi: 10.2217/nmt.14.52. [DOI] [PubMed] [Google Scholar]
- 4.Boyanapalli SS, Kong AT. “Curcumin, the King of Spices”: epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Curr Pharmacol Rep. 2015;1:129–139. doi: 10.1007/s40495-015-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L, Yuan Y, Li C, Guo T, Qi H, Xiao Y, Dong X, Liu Z, Liu Q. Upregulation of SNHG6 regulates ZEB1 expression by competitively binding miR-101-3p and interacting with UPF1 in hepatocellular carcinoma. Cancer Lett. 2016;383:183–194. doi: 10.1016/j.canlet.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Du ZY, Zheng X, Li DL, Zhou RP, Zhang K. Use of?curcumin?in diagnosis, prevention, and treatment of Alzheimer's disease. Neural Regen Res. 2018;13:742–752. doi: 10.4103/1673-5374.230303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox SJ, Rodriguez Camargo DC, Lee YH, Reif B, Ivanova M, Ramamoorthy A. Amyloid aggregation of hIAPP, Aβ, and calcitonin altered by a curcumin derivative. Biophys J. 2018;114:226a. [Google Scholar]
- 8.Galimberti D, Villa C, Fenoglio C, Serpente M, Ghezzi L, Cioffi SM, Arighi A, Fumagalli G, Scarpini E. Circulating miRNAs as potential biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2014;42:1261–1267. doi: 10.3233/JAD-140756. [DOI] [PubMed] [Google Scholar]
- 9.Goozee KG, Shah TM, Sohrabi HR, Rainey-Smith SR, Brown B, Verdile G, Martins RN. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115:449–465. doi: 10.1017/S0007114515004687. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A, Ginsberg SD, Cataldo AM, Mathews PM, Nixon RA. Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci U S A. 2010;107:1630–1635. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiao DM, Yan L, Wang LS, Hu HZ, Tang XL, Chen J, Wang J, Li Y, Chen QY. Exploration of inhibitory mechanisms of curcumin in lung cancer metastasis using a miRNA-transcription factor-target gene network. PLoS One. 2017;12:e0172470. doi: 10.1371/journal.pone.0172470. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Kumar P, Dezso Z, MacKenzie C, Oestreicher J, Agoulnik S, Byrne M, Bernier F, Yanagimachi M, Aoshima K, Oda Y. Circulating miRNA biomarkers for Alzheimer’s disease. PLoS One. 2013;8:e69807. doi: 10.1371/journal.pone.0069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, Yang LK, Zhu J, Wang YH, Dong JR, Chen T, Wang D, Xu XM, Sun SB, Zhang L. Deep brain stimulation for the treatment of moderate-to-severe Alzheimer’s disease: Study protocol for a prospective self-controlled trial. Clin Trials Degener Dis. 2018;3:66–70. [Google Scholar]
- 14.Liu CG, Wang JL, Li L, Wang PC. MicroRNA-384 regulates both amyloid precursor protein and beta-secretase expression and is a potential biomarker for Alzheimer’s disease. Int J Mol Med. 2014;34:160–166. doi: 10.3892/ijmm.2014.1780. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Li Z, Qiu D, Gu Q, Lei Q, Mao L. The inhibitory effects of different curcuminoids on beta-amyloid protein, beta-amyloid precursor protein and beta-site amyloid precursor protein cleaving enzyme 1 in swAPP HEK293 cells. Neurosci Lett. 2010;485:83–88. doi: 10.1016/j.neulet.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Long JM, Lahiri DK. MicroRNA-101 downregulates Alzheimer’s amyloid-beta precursor protein levels in human cell cultures and is differentially expressed. Biochem Biophys Res Commun. 2011;404:889–895. doi: 10.1016/j.bbrc.2010.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long JM, Ray B, Lahiri DK. MicroRNA-153 physiologically inhibits expression of amyloid-beta precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem. 2012;287:31298–31310. doi: 10.1074/jbc.M112.366336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lugli G, Cohen AM, Bennett DA, Shah RC, Fields CJ, Hernandez AG, Smalheiser NR. Plasma exosomal miRNAs in persons with and without Alzheimer disease: altered expression and prospects for biomarkers. PLoS One. 2015;10:e0139233. doi: 10.1371/journal.pone.0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukiw WJ, Alexandrov PN. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol Neurobiol. 2012;46:11–19. doi: 10.1007/s12035-012-8234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Fang B, Zeng F, Pang H, Zhang J, Shi Y, Wu X, Cheng L, Ma C, Xia J, Wang Z. Curcumin inhibits cell growth and invasion through up-regulation of miR-7 in pancreatic cancer cells. Toxicol Lett. 2014;231:82–91. doi: 10.1016/j.toxlet.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Momtazi AA, Derosa G, Maffioli P, Banach M, Sahebkar A. Role of microRNAs in the therapeutic effects of curcumin in non-cancer diseases. Mol Diagn Ther. 2016;20:335–345. doi: 10.1007/s40291-016-0202-7. [DOI] [PubMed] [Google Scholar]
- 23.Nhan HS, Chiang K, Koo EH. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol. 2015;129:1–19. doi: 10.1007/s00401-014-1347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel N, Hoang D, Miller N, Ansaloni S, Huang Q, Rogers JT, Lee JC, Saunders AJ. MicroRNAs can regulate human APP levels. Mol Neurodegener. 2008;3:10. doi: 10.1186/1750-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1-42) J Neurosci Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- 26.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 27.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 28.Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull. 2007;30:74–78. doi: 10.1248/bpb.30.74. [DOI] [PubMed] [Google Scholar]
- 29.Sun CC, Zhang L, Li G, Li SJ, Chen ZL, Fu YF, Gong FY, Bai T, Zhang DY, Wu QM, Li DJ. The lncRNA PDIA3P interacts with miR-185-5p to modulate oral squamous cell carcinoma progression by targeting Cyclin D2. Mol Ther Nucleic Acids. 2017;9:100–110. doi: 10.1016/j.omtn.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toden S, Okugawa Y, Buhrmann C, Nattamai D, Anguiano E, Baldwin N, Shakibaei M, Boland CR, Goel A. Novel evidence for curcumin and boswellic acid-induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev Res (Phila) 2015;8:431–443. doi: 10.1158/1940-6207.CAPR-14-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan Y, Liu Y, Wang X, Wu J, Liu K, Zhou J, Liu L, Zhang C. Identification of differential microRNAs in cerebrospinal fluid and serum of patients with major depressive disorder. PLoS One. 2015;10:e0121975. doi: 10.1371/journal.pone.0121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XH, Wang TL. MicroRNAs of microglia: Wrestling with central nervous system disease. Neural Regen Res. 2018;13:2067–2072. doi: 10.4103/1673-5374.241444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Deng Y, Zhang S, Luo Y, Cai F, Zhang Z, Zhou W, Li T, Song W. Amyloid-beta precursor protein facilitates the regulator of calcineurin 1-mediated apoptosis by downregulating proteasome subunit alpha type-5 and proteasome subunit beta type-7. Neurobiol Aging. 2015;36:169–177. doi: 10.1016/j.neurobiolaging.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Q, Yan P, Ma X, Liu H, Perez R, Zhu A, Gonzales E, Tripoli DL, Czerniewski L, Ballabio A, Cirrito JR, Diwan A, Lee J-M. Neuronal-targeted TFEB accelerates lysosomal degradation of APP, reducing Aβ generation and amyloid plaque pathogenesis. J Neurosci. 2015;35:12137–12151. doi: 10.1523/JNEUROSCI.0705-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye M, Zhang J, Zhang J, Miao Q, Yao L, Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357:196–205. doi: 10.1016/j.canlet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Song Y, Zhang C, Zhi X, Fu H, Ma Y, Chen Y, Pan F, Wang K, Ni J, Jin W, He X, Su H, Cui D. Circulating MiR-16-5p and MiR-19b-3p as two novel potential biomarkers to indicate progression of gastric cancer. Theranostics. 2015;5:733–745. doi: 10.7150/thno.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.