Stroke is a leading cause of mortality and severe neurological disability worldwide; however, curative therapeutic options are limited. Although recanalization therapy using endovascular thrombectomy has demonstrated great benefits in the functional outcome of patients with acute ischemic stroke, the rate of excellent clinical outcomes remains limited. Therefore, the current recanalization therapeutic approach may require adjunctive therapy to improve outcomes.
Perampanel, a noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor antagonist, has demonstrated broad-spectrum anti-seizure activity when administered orally in preclinical animal models, including models of tonic–clonic generalized seizures, absence/myoclonic seizures, and temporal lobe epilepsy (Hanada et al., 2011). The efficacy, safety, and tolerability of perampanel for patients with epilepsy with various seizure types including partial-onset seizures with or without secondary generalization have been reproduced in clinical practice (Tsai et al., 2018). Recently, it has been reported that perampanel exerts a neuroprotective effect against brain injury in experimental models, such as cerebral ischemia, traumatic brain injury (TBI), and intraventricular hemorrhage (Dohare et al., 2016; Chen et al., 2017; Nakajima et al., 2018). In this paper, we briefly discuss the protective effects of perampanel against experimental cerebral ischemia.
Pharmacological characteristics of perampanel: Glutamate is involved in a variety of functions in the central nervous system, such as learning, memory, anxiety, neuronal development, perception of pain as well as immune functions. Excessive release of glutamate can lead to excitotoxicity in neuronal cells via the activation of N-methyl-D-aspartic acid (NMDA), 2-amino-3[3hydroxy-5-methyl-4-isoxazolyl] propionic acid (AMPA), and kainic acid glutamate receptors, which leads to an influx of calcium. This phenomenon is considered to be involved in numerous neurological and psychiatric diseases, including stroke and epilepsy. Based on the prominent role of glutamate in ischemic damage, the strategy for development of neuroprotective drugs can be focused on the blockade of glutamate receptors. The neuroprotective effects of NMDA receptor blockers in animal stroke models were outstanding. However, NMDA antagonists caused psychoactive effects, including schizophrenia-like symptoms, cognitive impairment, and cardiovascular side effects, in clinical trials for stroke and TBI (Meldrum and Rogawski, 2007).
Although alternations in intracellular calcium levels are a proximal event for AMPA receptor activation, several downstream signaling molecules, such as protein kinases, apoptotic factors, and pro-inflammatory cytokines, have been found to be involved in the AMPA receptor-mediated neuronal injury. AMPA receptor antagonists, such as talampanel, YM872, EGIS-8332, ZK 200775, and GYKI53405, have been demonstrated to attenuate brain damage after focal or global cerebral ischemia (Hanada et al., 2011). However, these compounds exhibit many short-comings, such as poor solubility, a short half-life, and precipitation in the kidney, which limit their clinical utility. The competitive AMPA/kainate antagonist, ZK 200775, was tested in patients with acute ischemic stroke, although the study was prematurely terminated due to significant sedative effects (Walters et al., 2005). Talampanel has demonstrated efficacy in patients with refractory partial-onset seizures, although with associated adverse events, including ataxia and dizziness, occurring around the peak plasma concentration. Pharmacokinetic studies of talampanel revealed a short terminal half-life (5.6 hours), which led the investigators to adopt a three-times-daily dosing regimen in efficacy studies. This regimen caused repeated plasma peaks of talampanel, which may have contributed to the adverse event profile. Hence, AMPA antagonists that do not interact with kainate and NMDA receptors and exhibit a longer half-life may be potentially beneficial in reducing the incidence and severity of adverse events. Perampanel inhibits AMPA-induced increases in intracellular Ca2+, with no obvious effects against kainate- and NMDA-induced Ca2+ responses, and has a much longer terminal half-life (approximately 105 hours) in human, as well as good bioavailability. Thus, perampanel may have advantages, since a less-frequent dosing regimen could be possible, resulting in a smoother drug concentration profile and potentially improved tolerability and fewer adverse events (Hanada et al., 2011). Perampanel has already been approved in over 60 countries as an adjunctive therapy for the treatment of partial seizures, with or without secondary generalization.
Protective mechanism of perampanel against experimental stroke: Epileptogenesis after brain injury stems from multifaceted pathways and signaling systems in the brain, including loss of neurovascular/blood-brain barrier integrity, increased release of neurotransmitters, reactive oxygen species, and inflammation (Tanaka and Ihara, 2017). The pathological mechanisms leading to such damage are similar to those involved in cerebral ischemia. On the basis of similarity of the cascade of synaptic and intracellular events exhibited by epilepsy and stroke, antiepileptic drugs have been tested as possible neuroprotective agents in animal models of stroke. In particular, oxidative stress enhances excitatory amino acid release and expression of specific genes, leading to lipid peroxidation and DNA oxidation, and subsequently resulting in neuronal apoptosis. Additionally, post-ischemic inflammatory events, such as microglial activation and neutrophil infiltration, play a vital role in the progression of brain edema and secondary brain damage via the production of nitric oxide and various proteolytic enzymes. On the other hand, the phosphatidylinositol-3-kinase (PI3K)/Akt pathway is a major pathway in cell survival. It upregulates anti-apoptotic proteins and down-regulates pro-apoptotic proteins. Phosphorylated Akt directly influences glycogen synthase kinase-3b, Bcl-2-associated apoptosis promoter/Bcl-2, caspase-9, IκB kinase, and Forkhead-related transcription factor 1. Therefore, strategies that involve suppressing oxidative and inflammatory injury of brain cells as well as upregulating the PI3K/Akt pathway could have a marked impact in improving the outcomes of cerebral ischemia.
Recently, we reported that post-ischemic single intraperitoneal administration of perampanel (1.5 mg/kg) reduced brain edema and infarct volumes and improved motor function after transient focal cerebral ischemia in rats. Immunological analysis showed that perampanel inhibited ionized calcium-binding adapter molecule 1-positive microglial activation, expression of pro-inflammatory cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α), and oxidative stress-related molecules, including 4-hydroxynonenal and 8-hydroxy-20-deoxyguanosine. Moreover, perampanel suppressed neurodegeneration via downregulation of Bcl-2 associated X protein and upregulation of Bcl-extra-large, with Akt activation (Nakajima et al., 2018). Similarly, oral administration of perampanel (10 mg/kg) reduced brain edema and infarct volume, and improved motor function, accompanied by regulated expression of inflammatory cytokines, including IL-1β, TNF-α, IL-10, and transforming growth factor-β (TGF-β), and inhibited nitric oxide generation via nitric oxide synthase pathways (Niu et al., 2018). Perampanel administration (5 mg/kg) reduced contusion volume and brain edema via inhibition of oxidative stress through upregulation of antioxidant enzymatic activity, including that of catalase, superoxide dismutase, and glutathione transferase, in a TBI model (Chen et al., 2017). Another in vitro study, using primary murine brain endothelial cells, showed that perampanel exerts a protective effect on blood-brain barrier permeability after oxygen glucose deprivation. Interestingly, the protective effect might be mediated by preservation of claudin-5 protein expression, but does not involve glutamate receptor expression and intracellular Ca2+ regulation (Lv et al., 2016). Moreover, perampanel reduced apoptosis of oligodendrocyte precursor cells, accompanied with reduction of microglia infiltration and production of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, and enhanced oligodendrocyte precursor cell maturation. Additionally, it promoted myelination in a rabbit model of intraventricular hemorrhage-induced white matter injury (Dohare et al., 2016).
Cognitive impairment is common after stroke and affects the patients’ quality of life substantially, placing a burden on caregivers as well as on society. A recent study showed that cognitive impairment after stroke is a strong predictor of long-term functional outcome and mortality, with added predictive value over established predictors. Perampanel (8 mg/kg) attenuated the long-term memory impairment in the Y-maze test as well as hippocampus neuronal loss in an in vivo pilocarpine rat model of status epilepticus (Mohammad et al., 2018). It has also been reported that perampanel improved spatial working memory at 30 days after ischemia, based on the Y-maze test, which was accompanied by suppression of ischemia-induced hippocampal CA1 neuronal loss and cortical thinning (Nakajima et al., 2018). Moreover, in a previous study using a rat cortical impact model for TBI, oral perampanel administration (5 mg/kg) improved spatial working memory in the Morris water-maze test (Chen et al., 2017). Furthermore, perampanel had no detrimental effect on behavioral tests, including tests for mood, global cognitive scores, and memory, in clinical practice (Meador et al., 2016). Taken together, these results suggested that anti-inflammation, anti-oxidation, anti-apoptosis, and upregulation of the PI3K/Akt pathway of perampanel, may lead to improvement of motor dysfunction and cognitive impairment after cerebral ischemia (Figure 1).
Figure 1.
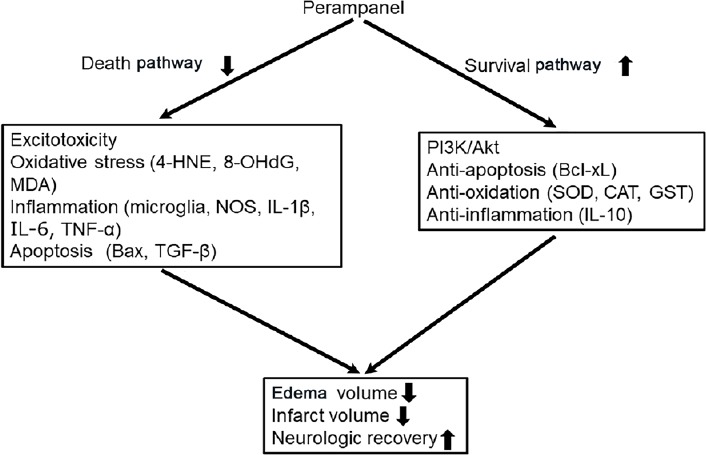
Suggested mechanism of perampanel-mediated neuroprotection in the treatment of cerebral ischemia.
As a potent noncompetitive antagonist of the AMPA receptor, perampanel ameliorates secondary ischemic brain damage. This reportedly involves several signaling pathways and multiple cellular and molecular changes. 4-HNE: 4-Hydroxynonenal; 8-OHdG: 8-hydroxy-20-deoxyguanosine; MDA: malondialdehyde; NOS: nitric oxide synthase; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; TGF-β: transforming growth factor-β: PI3K: phosphatidylinositol-3-kinase; Bcl-xL: Bcl-extra-large; SOD: superoxide dismutase; CAT: catalase; GST: glutathione transferase; IL-10: interleukin-10; AMPA: 2-amino-3[3hydroxy-5-methyl-4-isoxazolyl] propionic acid.
However, we speculate that a single administration of perampanel might be able to ameliorate ischemic brain injury in the very early stage after stroke. Some anti-epileptic drugs are shown to be promising tools for counteracting experimentally induced brain ischemia, whereas most of the putative neuroprotective agents previously tested in clinical trials showed either limited efficacy or relevant side effects. Further systemic studies such as dose response, multiple administration tests, appropriate administration route, and elucidation of a detailed protective mechanism will be needed before it can be clinically applied in patients with stroke.
Prospects: Perampanel may not only promote neuroprotection, with the current recanalization therapy, in the acute phase, but may also protect cognitive function in the chronic phase of an experimental ischemic stroke model. Since perampanel has been used in human clinical therapy, it has proven reliability and safety; thus, perampanel may have potential as an effective treatment for human stroke. To translate these findings into clinical practice, further studies are needed to investigate the optimal administration regimen, including dose, and administration timing, route, and times.
Additional file: Open peer review report 1 (99.1KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Giacinto Bagetta, University of Calabria, Italy.
P-Reviewer: Bagetta G; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Chen T, Dai SH, Jiang ZQ, Luo P, Jiang XF, Fei Z, Gui SB, Qi YL. The AMPAR antagonist perampanel attenuates traumatic brain injury through anti-oxidative and anti-inflammatory activity. Cell Mol Neurobiol. 2017;37:43–52. doi: 10.1007/s10571-016-0341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dohare P, Zia MT, Ahmed E, Ahmed A, Yadala V, Schober AL, Ortega JA, Kayton R, Ungvari Z, Mongin AA, Ballabh P. AMPA-kainate receptor inhibition promotes neurologic recovery in premature rabbits with intraventricular hemorrhage. J Neurosci. 2016;36:3363–3377. doi: 10.1523/JNEUROSCI.4329-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanada T, Hashizume Y, Tokuhara N, Takenaka O, Kohmura N, Ogasawara A, Hatakeyama S, Ohgoh M, Ueno M, Nishizawa Y. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52:1331–1340. doi: 10.1111/j.1528-1167.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 4.Lv JM, Guo XM, Chen B, Lei Q, Pan YJ, Yang Q. The noncompetitive AMPAR antagonist perampanel abrogates brain endothelial cell permeability in response to ischemia: involvement of claudin-5. Cell Mol Neurobiol. 2016;36:745–753. doi: 10.1007/s10571-015-0257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meador KJ, Yang H, Pina-Garza JE, Laurenza A, Kumar D, Wesnes KA. Cognitive effects of adjunctive perampanel for partial-onset seizures: A randomized trial. Epilepsia. 2016;57:243–251. doi: 10.1111/epi.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4:18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammad H, Sekar S, Wei Z, Moien-Afshari F, Taghibiglou C. Perampanel but not amantadine prevents behavioral alterations and epileptogenesis in pilocarpine rat model of status epilepticus. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1230-6. doi: 10.1007/s12035-018-1230-6. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima M, Suda S, Sowa K, Sakamoto Y, Nito C, Nishiyama Y, Aoki J, Ueda M, Yokobori S, Yamada M, Yokota H, Okada T, Kimura K. AMPA receptor antagonist perampanel ameliorates post-stroke functional and cognitive impairments. Neuroscience. 2018;386:256–264. doi: 10.1016/j.neuroscience.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 9.Niu HX, Wang JZ, Wang DL, Miao JJ, Li H, Liu ZG, Yuan X, Liu W, Zhou JR. The orally active noncompetitive AMPAR antagonist perampanel attenuates focal cerebral ischemia injury in rats. Cell Mol Neurobiol. 2018;38:459–466. doi: 10.1007/s10571-017-0489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T, Ihara M. Post-stroke epilepsy. Neurochem Int. 2017;107:219–228. doi: 10.1016/j.neuint.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Tsai JJ, Wu T, Leung H, Desudchit T, Tiamkao S, Lim KS, Dash A. Perampanel, an AMPA receptor antagonist: From clinical research to practice in clinical settings. Acta Neurol Scand. 2018;137:378–391. doi: 10.1111/ane.12879. [DOI] [PubMed] [Google Scholar]
- 12.Walters MR, Kaste M, Lees KR, Diener HC, Hommel M, De Keyser J, Steiner H, Versavel M. The AMPA antagonist ZK 200775 in patients with acute ischaemic stroke: a double-blind, multicentre, placebo-controlled safety and tolerability study. Cerebrovasc Dis. 2005;20:304–309. doi: 10.1159/000087929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


