Various isoforms of laminins are present in the basement membranes complexed with other structural proteins. In the central nervous system (CNS), they are primarily localized to the basement membranes of blood vessels and reactive astrocytes. Limited amounts of various laminin isoforms are also associated with neuronal cell bodies and axons, which are referred to as “neuronal” laminins (Yamamoto et al., 1988). They exist in a soluble form, free from other basement membrane proteins. Neurons either produce these soluble laminins or acquire them from astroglial cells. Laminin stimulates neuritogenesis and confers neuroprotection in vitro, but their exact role of these neuronal soluble laminins in the CNS is yet unknown. Laminins bind to various cell-surface receptors including integrins, dystroglycan, and the nonintegrin type 67 KDa laminin receptor (67LR). In neurons, it is well known that 67LR internalizes prion proteins and various bacteria and viruses (Nelson et al., 2008), but the signaling mechanisms by which 67LR mediates neuroprotection, particularly by soluble laminin, is not clearly known. Recently, we have found that soluble laminin (laminin-1 isoform) as well as its YIGSR pentapeptide corresponding to the 67LR-binding sequence present in the β1-chain of laminin, which can induce internalization of 67LR (Gopalakrishna et al., 2018). This endocytosis is dependent on adenylyl cyclase, protein kinase A, and the exchange protein directly activated by cyclic adenosine monophosphate (cAMP) (Epac). The internalized endosomes comprising adenylyl cyclase and other signaling enzymes, continue to generate signals such as cAMP for a sustained period of time which may contribute to neuroprotection. Considering that β-amyloid peptide (Aβ) is internalized through 67LR (Da Costa Dias et al., 2014), we postulate that laminin, by binding to and internalizing 67LR, inhibits entry of Aβ into neurons and thereby decreases Aβ-induced neurotoxicity. Thus, laminin-related agents that induce 67LR internalization could have therapeutic potential against Alzheimer’s disease (AD) and various other neurodegenerative diseases.
Internalization of 67LR by soluble laminin and role of cAMP, protein kinase A, and Epac: Our recent studies have shown that treatment of Neuroscreen-1 cells and its parent PC12 cells with laminin-1 for a short period of time induced an internalization of cell-surface associated 67LR (Gopalakrishna et al., 2018). 67LR-binding laminin peptide YIGSR also induced internalization of cell-surface associated 67LR (Figure 1), whereas integrin-binding peptide GRGDS did not induce internalization of 67LR. Cell-permeable analogs of cAMP also induced a decrease in cell-surface expression of 67LR and induced its internalization, an effect mimicked by agents that elevate intracellular cAMP, such as forskolin (which directly activates adenylyl cyclase and thus elevates intracellular cAMP) and rolipram (which inhibits cyclic nucleotide phosphodiesterase thus inhibits breakdown of cAMP). Consistent with this possible role of cAMP, laminin or YIGSR failed to induce internalization of 67LR in PC12 cells deficient in protein kinase A (PKA), suggesting the role of this kinase in the internalization. Another downstream effector of cAMP, Epac, is also involved in 67LR internalization further supporting the role of cAMP in this process.
Figure 1.
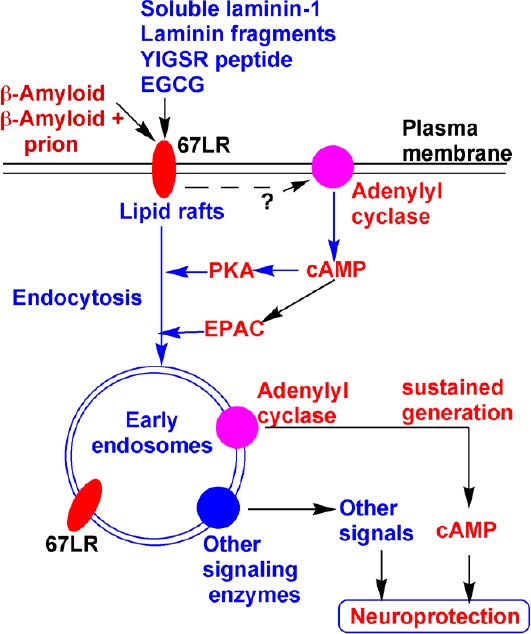
Schematic presentation of possible neuroprotective action of laminin, its proteolytic fragments, YIGSR peptide, and epigallocatechin-3-gallate (EGCG) via binding to 67 kDa laminin receptor (67LR) in opposition to β-amyloid peptide (Aβ).
Initial binding of laminin-1 and YIGSR to 67LR leads to activation of adenylyl cyclase by an unknown mechanism. The resulting transient elevation of cyclic adenosine monophosphate (cAMP), via activation of its effectors protein kinase A (PKA) and Epac, induces internalization of lipid raft-associated 67LR. Along with 67LR, other lipid raft-associated signaling enzymes, such as adenylyl cyclase, are also internalized. Early endosomes with activated receptor complexes, adenylyl cyclase, and other signaling enzymes could serve as signalosomes and induce a sustained elevation of cAMP and other signals. In addition, it is possible that the early endosomes may recruit cell-survival enzymes and serve as signaling platforms. Thus, the internalization of 67LR may confer neuroprotection. Similarly, EGCG also binds to 67LR and induces cell signaling for neuroprotection. Both Aβ and Aβ + prion complex bind to 67LR and their internalization causes toxicity. It is possible that both laminin and EGCG may block the binding of Aβ to 67LR and prevent neuronal toxicity induced by Aβ.
Colocalization of 67-LR and other lipid raft-associated enzymes to early endosomes: Since 67LR and adenylyl cyclase are localized to lipid rafts, endocytosis of 67LR by laminin and related agents could promote co-internalization of the some of the lipid raft-associated signaling enzymes as well. Our study in fact, showed that 67LR and adenylyl cyclase colocalize to early endosomes, suggesting that these early endosomes may serve as “signalosomes” in neurons (Gopalakrishna et al., 2018). In this scenario. endosome-associated adenylyl cyclase may contribute to a sustained generation of cAMP, an important neuroprotective signal. Furthermore, these endosomes may recruit additional cell-survival enzymes such as PI3 kinase, Akt, and PKC isoenzymes thereby serving as robust signaling platforms for neuroprotection. Internalization of cell-surface receptors has multiple consequences: the signal may be terminated; the receptor may help in cargo delivery; receptor recycling may occur; and the receptor may be degraded by lysosomes. In addition, early endosomes also function as signalosomes, causing a sustained elevation of signals; this is shown in the case of parathyroid hormone, which causes internalization of its own receptor along with adenylyl cyclase to early endosomes, producing a sustained elevation of cAMP (Vilardaga et al., 2014).
Signaling associated with early endosomes may be very important, especially in neurons. Internalization of nerve growth factor and brain-derived neurotrophic factor, along with the appropriate receptors and signaling complexes, have been shown to play an important role in further propagating the signal (Sorkin and von Zastrow, 2009). These signalosomes are retrogradely transported from the distal axons to the soma to promote transcriptional regulation. Previous studies showed that the laminin produced by some neurons is taken up by other neurons and is retrogradely transported (Yamamoto et al., 1988). This suggests that the internalization of laminin and its intracellular transport may have a role in neuronal regulation. While these internalizations were observed with laminin-1, whether other laminin isoforms also show this type of 67LR internalization and cell signaling remains to be determined.
Significance of 67LR endocytosis to neuronal survival against neurotrophin deprivation: A deprivation of neurotrophins leads to neuronal cell death. Currently, various studies are being conducted to determine the efficacy of neurotrophins for treating various neurodegenerative diseases. Cell death induced by serum deprivation of PC12 cells is frequently used as a model for identifying neuroprotective agents and elucidating their mechanisms. Using this model, we found the functional significance of the laminin and its peptide YIGSR for neuroprotection. Laminin, YIGSR, dibutyryl cAMP, and forskolin, all of which elevate intracellular cAMP, protected these cells from cell death induced by serum deprivation. However, these agents protected wild-type PC12 cells having PKA, they failed to protect PKA-deficient PC12 cells. Both adenylyl cyclase inhibitor (SQ 22536) and Epac inhibitor (ESI-09) inhibited YIGSR-induced protection of Neuroscreen-1 cells from cell death induced by serum withdrawal. The conditions that induced endocytosis of 67LR protected cells from death, whereas the conditions that did not induce the internalization of 67LR did not protect cells from death. Thus, the internalization of 67LR is important for laminin-mediated protection against cell death. The 67LR-blocking antibody (MLuC5) suppressed neuroprotective effects of YIGSR peptide, suggesting the role of this receptor in mediating neuroprotective action of this laminin peptide. Certainly, additional studies, particularly in vivo, are warranted to further assess the functional role of 67LR in neuroprotection.
Implication of 67LR internalization for protection against neurodegenerative diseases such as AD: Recent studies have shown the role of 67LR in eliciting neurotoxicity caused by Aβ, which is considered to play a crucial role in AD pathogenesis. Aβ binds to 67LR either directly or indirectly through an initial association with prions that subsequently bind to this receptor (Da Costa Dias et al., 2014). This leads to internalization of 67LR and Aβ-mediated neurotoxicity. Although the exact site to which Aβ binds in the 67LR sequence is not known, it is known that prions and YIGSR bind to the “peptide G” sequence present within the 67LR. Therefore, laminin and YIGSR peptide could compete with Aβ or prion-Aβ complex for 67LR and prevent their binding. In addition, the internalization of 67LR caused by YIGSR may decrease the presence of 67LR on the cell surface for the internalization of Aβ. Alternatively, the neuroprotective signaling induced by laminin and YIGSR may protect from neurotoxicity induced by Aβ that enters the cell through 67LR-independent mechanisms (Jarosz-Griffiths et al., 2016). For example, Aβ signaling decreases the phosphorylation of cAMP response element-binding protein (CREB), whereas laminin and other agents elevate cAMP, which could enhance the phosphorylation of CREB and thereby provide a counteractive mechanism to overcome the toxicity induced by Aβ. Previous studies have shown that laminin inhibits neuronal cell death by preventing fibril formation and interaction of Aβ with cell membranes (Drouet et al., 1999). It is also possible that a direct binding of laminin to cell-surface 67LR may be protective against Aβ toxicity. Green tea polyphenols, such as epigallocatechin-3-gallate (EGCG), have been shown to be neuroprotective in various neuronal diseases such as AD, Parkinson’s disease, and stroke (Weinreb et al., 2004). Interestingly, EGCG binds with high affinity to 67LR, induces internalization of this receptor, elicits neuroprotective signaling and potentiates the action of neurotrophins (Tachibana et al., 2004; Gundimeda et al., 2014). Since the EGCG-binding site on 67LR is in close proximity to the laminin-binding site, it is possible that EGCG may counteract Aβ toxicity by mechanism(s) described above for laminin.
There is an accumulating evidence that cerebrovascular injury/dysfunction represents a major mechanism underlying neurodegeneration. Under this setting, vascular basement membrane components are targeted for degradation by proteases such as metalloproteases and cathepsins resulting in the release of soluble proteolytic fragments (Lee et al., 2011). It is worth investigating whether the proteolytic fragments derived from laminin diffuse into the brain parenchyma and promote 67LR-mediated signaling in neurons as a protective response.
While 67LR is considered a culprit for cancer metastasis and internalization of pathogenic prions, and certain bacteria and viruses (Nelson et al., 2008), it is also a receptor for neuroprotective agents such as laminin, its peptides, and EGCG (Gundimeda et al., 2014; Gopalakrishna et al., 2018). Its internalization by “pathogenic agents” could lead to adverse events, but its internalization by “good agents” could lead to neuroprotection. Thus, future understanding of the bidirectional role of this unique receptor may help develop novel drugs for neuroprotection against AD, stroke and other neurodegenerative conditions.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Liu XL
References
- 1.Da Costa Dias B, Jovanovic K, Gonsalves D, Moodley K, Reusch U, Knackmuss S, Weinberg MS, Little M, Weiss SF. The 37kDa/67kDa laminin receptor acts as a receptor for Abeta42 internalization. Sci Rep. 2014;4:5556. doi: 10.1038/srep05556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drouet B, Pincon-Raymond M, Chambaz J, Pillot T. Laminin 1 attenuates beta-amyloid peptide Abeta(1-40) neurotoxicity of cultured fetal rat cortical neurons. J Neurochem. 1999;73:742–749. doi: 10.1046/j.1471-4159.1999.0730742.x. [DOI] [PubMed] [Google Scholar]
- 3.Gopalakrishna R, Gundimeda U, Zhou S, Bui H, Davis A, McNeill T, Mack W. Laminin-1 induces endocytosis of 67KDa laminin receptor and protects Neuroscreen-1 cells against death induced by serum withdrawal. Biochem Biophys Res Commun. 2018;495:230–237. doi: 10.1016/j.bbrc.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Gundimeda U, McNeill TH, Fan TK, Deng R, Rayudu D, Chen Z, Cadenas E, Gopalakrishna R. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: role of 67-kDa laminin receptor and hydrogen peroxide. Biochem Biophys Res Commun. 2014;445:218–224. doi: 10.1016/j.bbrc.2014.01.166. [DOI] [PubMed] [Google Scholar]
- 5.Jarosz-Griffiths HH, Noble E, Rushworth JV, Hooper NM. Amyloid-beta receptors: the good, the bad, and the prion Protein. J Biol Chem. 2016;291:3174–3183. doi: 10.1074/jbc.R115.702704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, Shaw C, Fidanboylu M, Orr AW, Ogunshola O, Fertala A, Thomas SA, Bix GJ. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005–3023. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson J, McFerran NV, Pivato G, Chambers E, Doherty C, Steele D, Timson DJ. The 67 kDa laminin receptor: structure, function and role in disease. Biosci Rep. 2008;28:33–48. doi: 10.1042/BSR20070004. [DOI] [PubMed] [Google Scholar]
- 8.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 10.Vilardaga JP, Jean-Alphonse FG, Gardella TJ. Endosomal generation of cAMP in GPCR signaling. Nat Chem Biol. 2014;10:700–706. doi: 10.1038/nchembio.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinreb O, Mandel S, Amit T, Youdim MBH. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J Nutr Biochem. 2004;15:506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Iwasaki Y, Yamamoto H, Konno H, Isemura M. Intraneuronal laminin-like molecule in the central nervous system: demonstration of its unique differential distribution. J Neurol Sci. 1988;84:1–13. doi: 10.1016/0022-510x(88)90169-4. [DOI] [PubMed] [Google Scholar]


