Abstract
Background:
Polypropylene mesh is widely used for surgical treatment of pelvic organ prolapse and stress urinary incontinence. While these surgeries demonstrate favorable functional and anatomical outcomes, their use has been limited by complications, the two most common being exposure and pain. Growing evidence suggests T lymphocytes play a critical role in regulating the host response to biomaterials.
Objectives:
To define and characterize the T cell response and correlate the response to collagen deposition in fibrotic capsules in mesh tissue complexes removed for the complications of pain versus exposure.
Study Design:
Patients who were scheduled to undergo a surgical excision of mesh for pain or exposure at Magee-Women’s Hospital were offered enrollment. Forty- two mesh-vagina tissue complexes were removed for the primary complaint of exposure (n=24) versus pain (n=18). Twenty-one patients agreed to have an additional vaginal biopsy away from the site of mesh and served as control tissue. T cells were examined via immunofluorescent labeling for cell surface markers CD4+ (T helper), CD8+ (cytotoxic) and foxp3 (T regulatory cell). Frozen sections were stained with H&E for gross morphology and picrosirius red for collagen fiber analysis. Interrupted sodium-dodecyl sulfate gel electrophoresis was used to quantify the content of collagens type I and III, and the collagen III/I ratio. Growth factors TGF-β and CTGF implicated in the development of fibrosis were measured via enzyme-linked immunosorbent assays. Data were analyzed using Student’s t-tests, mixed effects linear regression, and Spearman’s correlation coefficients.
Results:
Demographic data were not different between groups except for BMI, which was 31.7 for the exposure group and 28.2 for pain (P=0.04). Tissue complexes demonstrated a marked, but highly localized foreign body response. We consistently observed a teardrop shaped fibroma encapsulating mesh fibers in both pain and exposure groups, with the T cells localized within the tip of this configuration away from the mesh-tissue interface. All three T cell populations were significantly increased relative to control - CD4+ Thelper (P<0.001), foxp3+ Treg (P<0.001) and CD8+ cytotoxic T cell (P=0.034) in the exposure group. In the pain group, only Thelper (P<0.001) and Treg cells (P<0.001) were increased, with cytotoxic T cells (P=0.520) not different from control. Picrosirius red staining showed a greater area of green (thin) fibers in the exposure group (P=0.025) and red (thick) fibers in the pain group (P<0.001). The ratio of area green/(yellow + orange + red) representing thin vs. thick fibers was significantly greater in the exposure group (P=0.005). Analysis of collagen showed that collagen type I was increased by 35% in samples with mesh complications (exposure and pain) when compared to controls (P=0.043). Strong correlations between the pro-fibrosis cytokine TGF-β and collagen type I and III were found in patients with pain (r≥0.833; P=0.01) but not exposure (P>0.7).
Conclusion:
T cells appear to play a critical role in the long-term host response to mesh and may be a central pathway leading to complications. The complexity of this response warrants further investigation and has the potential to broaden our understanding of mesh biology and clinical outcomes.
Keywords: T cells, fibrosis, collagen, polypropylene mesh
INTRODUCTION
Polypropylene mesh is widely used for surgical treatment of pelvic organ prolapse and stress urinary incontinence. While these surgeries demonstrate favorable functional and anatomical outcomes, their use has been limited by complications1–6 - most commonly mesh exposure through the vaginal epithelium and pain7. The mechanism of the host-tissue response as it relates to these complications has not been well delineated.
All biomaterials, including polypropylene mesh when implanted in vivo, elicit highly orchestrated cellular and tissue responses that include inflammation and healing of the surgical wound as well as a foreign body reaction to the biomaterial resulting in its fibrous encapsulation effectively separating it from surrounding tissues.8–10 While in some patients the capsule is comprised of a thin layer of collagen and myofibroblasts, in others, it is thought to become pathologic with excessive fibrous tissue deposition. This has been proposed to result in contraction of the mesh causing it to pull on adjacent tissues and trigger pain.11 Mesh exposure is thought to occur when stiffness mismatches between the mesh and the underlying tissue initiate a maladaptive remodeling response characterized by tissue degradation and atrophy.11–13 Even though macrophages represent the mainstay of the foreign body response, studies of polypropylene meshes implanted into primates showed that CD3+ T cells were nearly as prevalent as macrophages in the inflammatory infiltrate surrounding mesh fibers.10
Growing evidence points towards T lymphocytes playing a critical role in regulating the host response to biomaterials including macrophage fusion and the extent of fibrosis through their interactions with macrophages and fibroblasts via cytokine and chemokine signaling.14–21 Characterization of the T cell response to polypropylene prolapse mesh and the role of T cells in complications, however, remains unclear. The purpose of this study was twofold: 1) to define and characterize the fibrotic capsule in polypropylene mesh tissue complexes removed for the separate complications of pain versus mesh exposure and 2) to compare the T cell response in patients with pain – a presumably fibrotic response vs exposure – a response purportedly associated with degradation.11 To further characterize the host response, we examined two predominant cytokines involved in mediating the transition to pathologic fibrosis - TGF-β1 and CTGF.9,22 We hypothesized that patients with pain would have increased fibrosis as measured by thicker collagen fibers in the fibrous capsule as compared to patients with mesh exposure and increased CD4+ Tcells due to their purported role in tissue fibrosis. In patients with exposure, we anticipated observing a less developed capsule with thinner collagen fibers associated with increased cytotoxic T cells indicative of tissue destruction.
MATERIALS AND METHODS
Patient acquisition
Patients undergoing surgical excision of mesh as part of a larger study (Magee Mesh Biorepository IRB# 10090194) were offered enrollment. For inclusion in the current study, mesh had to be removed for the primary indication of exposure or pain. Mesh exposure was defined as at least 2 mm of mesh visible through the vaginal epithelium; pain was defined as mesh being removed for the primary complaint of pain (with palpation, ambulation or intercourse) without evidence of exposure. Patients were excluded from the study if they had acute infection (fever, worsening pain and purulent drainage in the area of mesh) or erosion into the bowel or bladder. Patients were also excluded if they were unable to provide informed consent, were undergoing chronic immunosuppressive therapy or had an autoimmune disorder. After consent was obtained, baseline demographic data abstracted from the electronic medical record included age, race/ethnicity, body mass index (BMI), gravidy, parity, hormone use, menopausal status and smoking status (Table 1). Menopausal status was defined as premenopausal (regular menstrual periods within the last 12 months) and postmenopausal (no menstrual periods within the last 12 months.) Hormone use was defined as current use of systemic estrogen with or without progesterone or vaginal estrogen for > 3 months. Smoking was defined as current smoker (yes/no).
Table 1.
Descriptive statistics of study population.
| Variables | Mesh Exposure (n=24) | Pain (n=18) | P value |
|---|---|---|---|
| Age, years a | 52.92 ± 12.38 | 49.00 ± 11.88 | 0.31 |
| Body mass index, (kg/m2) a | 31.74 ± 5.90 | 28.22 ± 4.39 | 0.040 |
| Parity b | 2 (2, 3) | 2 (2, 3) | 0.30 |
| Time implanted, months a | 53.63 ± 37.70 | 48.39 ± 37.16 | 0.39 |
| Menopausal status c | 0.75 | ||
| Premenopausal | 8 (33) | 7 (39) | |
| Postmenopausal | 16 (67) | 11 (61) | |
| Smoking c | 0.58 | ||
| Nonsmoker | 8 (33) | 9 (50) | |
| Smoker | 7 (29) | 4 (22) | |
| Former Smoker | 9 (38) | 5 (28) | |
| Race/ethnicity c | -- | ||
| White | 24 (100) | 18 (100) | |
| Hormone usage c | 0.53 | ||
| Yes | 10 (42) | 10 (56) | |
| No | 14 (58) | 8 (44) | |
values given as mean ± standard deviation; P-value from Student’s t-test
values given as median (25 percentile, 75 percentile); P-value from Mann-Whitney U test
values given as count of patient numbers (frequencies); P-value from Fisher’s exact test
On the day of surgery, the excised mesh-tissue complex was placed in a sterile specimen container, immediately placed on ice and prepared for analysis. Patients were given the option of undergoing an additional full thickness vaginal biopsy from an uninvolved area (no mesh) on the anterior or posterior wall to serve as control tissue. The control specimen were also immediately placed on ice and sent for analysis.
Tissue extract acquisition and histologic preparation
After deep freezing, a portion was extracted in high salt extraction buffer for biochemical assays.11 Additional pieces were embedded into O.C.T. compound (Tissue-Tek; Sakura Finetek USA Inc, Torrance, CA), flash frozen in liquid nitrogen, sectioned (7μm), and stored at −80° C.
Immunofluorescent labeling of T cells
Tissue sections were quadruple-labeled for CD4+ (T helper 1, Santa Cruz Biotechnology, Dallas, Tx,) CD8+ (cytotoxic, Abcam, Cambridge, MA), foxp3 (regulatory T cell or Treg, Abcam, Cambridge, MA) and nuclear marker 4’, 6-diamidino-2-phenylindole, as described,11 and imaged with a Nikon ECLIPSE 90i upright microscope. 6 ×200 images were acquired over 2 locations within the tissue as described above. For each image, two trained technicians blinded to the complication group counted the number of total cells and the number of cells that expressed CD4, CD8 and foxp3, to define the T helper, cytotoxic T cell and T regulatory cell population, respectively.
Cytokine determination
Protein concentrations were quantified using DC protein assay (Bio-Rad, Hercules, CA). Quantification of cytokines TGF-B and CTGF was performed with the use of commercially available enzyme-linked immunosorbent assay kits. (R&D Systems, Minneapolis, MN, and MyBioSource, San Diego, CA, respectively). All samples were run in duplicate or triplicate with 40-ug total protein per sample per assay. A patient sample that had been characterized previously for analyte amounts served as an internal control.
Fibrotic capsule quantification
Frozen sections were stained with Masson’s trichrome and hematoxylin & eosin for gross tissue morphology, and picrosirius red for collagen fiber thickness.10–12,23–24 The picrosirius red images were taken using a Nikon ECLIPSE 90i upright microscope (Nikon USA, Melville, NY) using a polarized light setting. Six images were acquired over 2 locations of mesh-tissue interface (200x, three images were taken in each location) where the fibrotic capsule could be easily located. Nikon elements software was used to apply custom threshold color filters to quantify areas of red, orange, yellow and green in close proximity to mesh fibers, consistent with thickness of collagen fibers with red indicating thicker and green indicating thinner fibers. The same color thresholds were used for all samples. The ratio of green/(yellow + orange + red) was calculated to present the ratio of thin/thick fibers.
Collagen and collagen type III/I ratio
Interrupted sodium-dodecyl sulfate gel electrophoresis (SDS-PAGE) was used to quantify the contents of mature collagen type I and type III and their ratios.25 Briefly, the salt insoluble tissue pellets after protein extraction were digested with pepsin and freeze-dried. Samples were diluted in 2% SDS at 2mg/ml and isolated on 6% gels by interrupted SDS-PAGE. Purified collagen type I and III (Abcam, Cambridge, MA) were also run on the gels as internal controls and protein standards (pre-stained SDS-PAGE Standards High Range, Bio-Rad) was used to indicate molecular weight. Semi-quantification of collagen bands was performed by densitometric scanning of protein bands corresponding to α1(I) and α1(III) chains on an imaging densitometer (Bio-Rad Laboratories, Hercules, CA). The values were normalized to purified collagen type I α1(I) to minimized interexperimental errors. The relative collagen subtype III/I ratio was determined as α1(III) x2/ α1(I) x3.
Statistics and sample size calculation
Sample size calculation was based on demonstrating a difference in T cell populations as compared to control. Because of the limited amount of sample, T cell assays were prioritized and sample sizes were reduced on remaining assays based on sample availability (i.e. some samples were exhausted precluding our ability to perform all assays on them). Power analysis showed that 14 women would have 80% power to detect at least a 20% difference in number of T cells between mesh explant and control tissues based on a paired Student’s t-test evaluated at the 2-sided 0.05 significance level. Statistical analysis was performed with STATA software (version 14.2; StataCorp, College Station, TX) and statistical tests were evaluated at the 2-sided 0.05 significance level. Differences in demographics between participants with mesh exposure or pain were evaluated using Student’s t-, Mann-Whitney U, and Fisher’s exact tests, where appropriate. Comparison of thick vs thin fibers after picrosirius red staining was made using Student’s t-tests. Spearman’s ‘s correlation coefficient (r) was used to evaluate the relationship of T cell populations, cytokines, fiber thickness, and collagen ratios with each other and with demographics and time since mesh implantation. Linear regression was used to assess the relationship between T cell populations and time since implantation. Mixed effects linear models were used to evaluate differences in T cell populations, cytokines, and collagen subtypes measured in control, exposure, and pain tissue samples.
RESULTS
Demographic data
Mesh-vagina tissue complexes were excised from 42 women - 24 were excised for exposure and 18 for pain. Twenty-one patients agreed to have an additional biopsy from vagina away from the site of mesh implantation and served as no mesh controls. There were no differences in patient age, parity, menopausal status, race/ethnicity, smoking history, hormone use and time of mesh implantation. (Table 1, P>0.05). BMI was greater in the mesh exposure group compared to pain (31.7 vs. 28.2, P=0.04). The meshes that were excised are shown in Table 2. Length of time of mesh implantation varied from 1 – 144 months.
Table 2.
Excised mesh brand and type categorized by mesh complications. Of the 42 patients enrolled in this study, mesh brand information was not able to be determined for 1 of the patients in exposure group.
| Mesh Device | Removal because of exposure (n=24) | Removal because of pain (n=18) |
|---|---|---|
| AMS Monarc TOT | 4 | 3 |
| AMS Miniarc | 2 | |
| Bard Ajust Single Incision Sling | 2 | |
| Bard Pelvilace | 1 | |
| Boston Scientific Obtryx TOT | 1 | |
| Boston Scientific Solyx Single Incision Sling | 2 | 1 |
| Boston Scientific Prefyx | 1 | |
| Boston Scientific Lynx TVT | 1 | |
| Caldera Desara Sling System | 1 | |
| Coloplast Restorelle Y mesh | 1 | |
| Gynecare TVT Secur | 2 | 2 |
| Gynecare TVT | 2 | 1 |
| Gynecare TVT Exact | 1 | |
| Gynecare TVT Abbrevo | 1 | |
| TOT (hand cut prolene mesh) | 1 | |
| TOT unspecified | 4 | 4 |
| TVT unspecified | 1 | 1 |
| Original medical records not available | 2 |
Immunofluorescent labeling
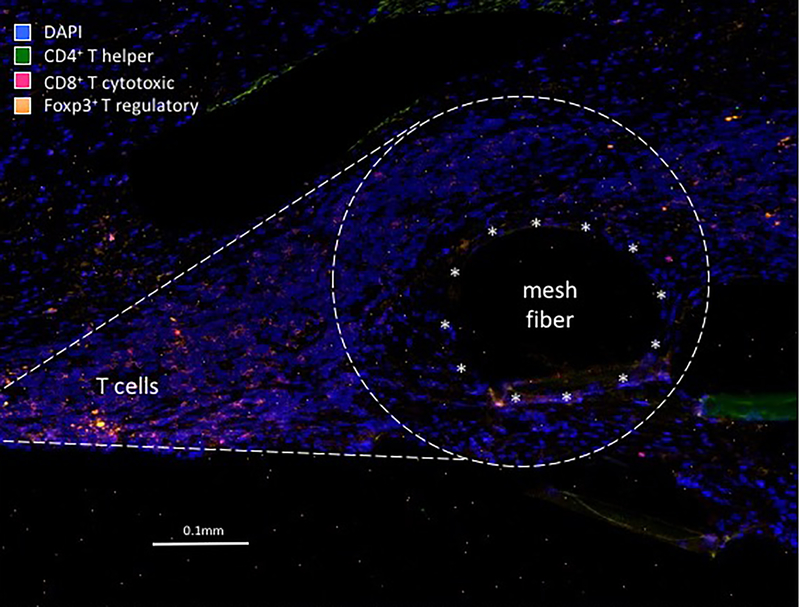
Mesh-tissue complexes demonstrated a marked, but highly localized foreign body response.11 We consistently observed a teardrop shaped cellular response including the mesh capsule around each mesh fiber in both pain and exposure groups. Within the teardrop, the T cell population and macrophages were spatially distributed at distinct sites. While the T cells localized to the “cap” of the teardrop away from the mesh-tissue interface, the macrophages were limited to the area immediately surrounding mesh fibers at the base of the teardrop (Figure 1).
Figure 1.
Immunoflourescent micrograph demonstrating the typical T cell response to a mesh fiber in women with mesh complications. Unlike what is typically seen with the foreign body response in which macrophages immediately surround the mesh fiber (typical position marked by asterisks), T cells are observed at a distinct location away from the fiber. Image taken at 200x magnification.
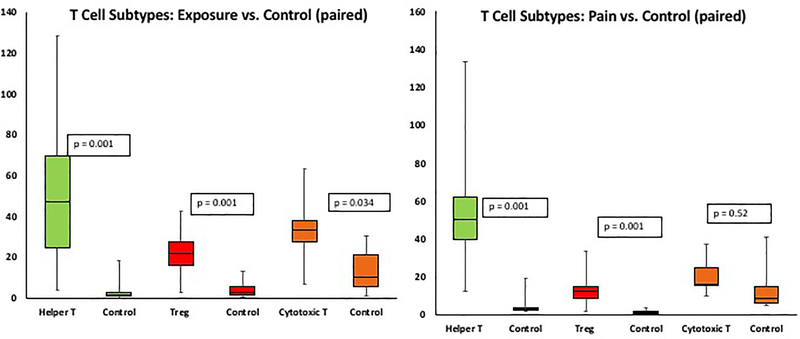
All three T cell populations were elevated in the exposure group as compared to control tissues - CD4+ Thelper (Th, P<0.001), foxp3+ Treg (P<0.001) and CD8+ cytotoxic T cell (P=0.034). In the pain group, however, only Th (P<0.001) and Treg cells (P<0.001) were increased with cytotoxic T cells (P=0.52) not different from control. A direct comparison of the pain and exposure groups showed more CD8+ cytotoxic T cells in the exposure group (P=0.032). Interestingly, a comparison of control tissue not containing mesh in women with pain vs exposure showed that women with pain had a higher number of Tregs (4.02 ± 3.95 vs 1.48 ± 1.27, P=0.042). In all mesh explants, CD4+ Th cells decreased with time after implantation (β= −0.44, P=0.025) where β is the mean change in the count of CD4+ Th cells per month. In other words, for each month of implantation Th cells decreased by 0.44 whereas the quantity of Tregs and cytotoxic T cells remained the same (P>0.9).
Biochemical endpoints
TGF-β decreased with increasing age (P=0.001). TGF-β was higher in mesh-vagina explants compared to control tissue but was not significantly different between exposure and pain groups (P<0.001, P=0.37, respectively). While CTGF was higher in mesh-vagina explants compared to control tissue (P<0.001) and not significantly different between pain and exposure (P=0.11), it was moderately to highly correlated with all T cell subtypes (Th r=0.639, P<0.001; cytotoxic T r=0.651, p<.001; Treg r= 0.644, P<0.001).
Picrosirius red staining
Characterization of the fibrous capsule via picrosirius red demonstrated a greater area of green (thin) fibers in the exposure group (P=0.025) and red (thick) fibers in the pain group (P<0.001). The ratio of green/(yellow + orange + red) was greater in the exposure group (P=0.005) as compared to the pain group indicating thinner fibers. There was a moderate positive correlation between length of mesh implantation and the area of orange (thick) fibers (r=.487, P=0.03), the area of yellow (thick) fibers (r=.460, P=0.041), as well as total capsule collagen and length of implantation (r=.497, P=0.026), supporting collagen deposition and maturation of the fibrous capsule over time. We did not find a correlation between fiber type (green, yellow, orange, red) and a specific population of T cell. We did find a positive correlation between the number of cells within the capsule and the ratio of (green/orange+yellow+red) supporting thinner fibers within the capsule with an increased cellular response (r=.524, P=0.037).
Collagen and collagen ratios
Analysis of collagen subtypes showed that collagen type I increased by 35% in samples with mesh complications (exposure and pain) when compared to controls (P=0.043) while collagen type III was not significantly different (P=0.478). In defining the relationship between TGF-β and collagen, we observed strong correlations of TGF- β with collagen I, collagen III, and the collagen III/I ratio in patients with pain (I: r=0.833, P=0.01; III r=0.833, P=0.01; III/I ratio: r=0.857, P=0.007) but not exposure (P>0.65).
COMMENTS
In mesh-tissue complexes removed for the complications of pain vs exposure, a highly specific host response is observed that is characterized by a fibrous capsule with a dense cellular infiltrate that includes macrophages and T cells at spatially distinct sites indicating disparate roles for these two cell populations. The most important findings in this study were that T cells, typically a transient population associated with the adaptive immune response remain elevated as part of the host response in tissues of women with complications relative to control tissue not containing mesh years after mesh implantation. No clear distinction was found in the T cell populations in tissue mesh complexes from women with pain vs exposure except that CD8+ cytotoxic T cells were higher in women with exposure. The finding of more Treg cells in the control tissue of women with mesh removed for pain deserves further investigation and we are currently studying differences in T cell immunity in patients with and without mesh complications. The fibrous capsule in women with pain had thicker more densely packed collagen fibers with a greater increase of collagen type I than women with exposure. In both the pain and exposure groups, the profibrotic cytokine CTGF was moderately to highly correlated with the three different populations of T cells.
We consistently observed a tear drop shaped fibroma encapsulating mesh fibers in both pain and exposure groups, with the T cells localized at the “cap” away from the mesh-tissue interface. The shape is frequently observed in both animal models and in women from whom mesh is excised for complications. It requires that a mesh fiber is cut on the cross section and therefore is not always observed. This distribution of T cells is different from the localization of macrophages, which is immediately abutting the mesh fibers.11 The shape of the response may represent micromotion of a stiff material (mesh) against a softer material (vagina) resulting in repetitive injury followed by an adaptive immune response with Th cells and cytotoxic T cells participating in the initial host response to the injury and Treg cells involved in resolution of inflammation and repair. Alternatively, the T cells may be responding to cytokines and chemokines released by macrophages at the fiber surface and the cell distribution represents a gradient of these signals.
In a previous study, we demonstrated a positive correlation between M2 (pro-remodeling) macrophages and the profibrotic cytokine IL-10 in women with pain.11 Our findings in the present study of thicker or more mature collagen fibers with a higher proportion of collagen type I in the pain group supports a progressive fibrosis as a potential mechanism contributing to pain. Likewise, we observed a positive correlation between thicker collagen fibers and length of mesh implantation suggesting maturation of capsule collagen over time. Finally, we showed a strong correlation between TGF-β and collagen type I in patients with pain but not exposure. Together the data suggest that a mechanism of pain is increased mature collagen deposition leading to increased stiffness of the tissue and decreased mobility causing it to pull on adjacent structures leading to pain.
TGF-β and CTGF were increased in the mesh-tissue complexes compared to controls even years following mesh implantation, providing further evidence of an on going chronic host response. While TGF-β is considered a central mediator of fibrosis, similar to IL-10 it also acts as an anti-inflammatory cytokine that mediates the transition from a proinflammatory response to a proremodeling tissue healing response. However, TGF-β may trigger chronic fibrosis when acting in synergy with other pro-fibrotic cytokines, such as CTGF.20 Our finding that the amount of TGF-β correlated with the collagen deposition (both I and III) in patients with pain provides a supportive evidence for the critical role of TGF-β in chronic fibrosis.
Our finding that CD8+ cytotoxic T cells are increased in women with exposure supports our hypothesis that exposure represents a degradative response. In a previous study,11 MMP-9 was found to be increased in patients with exposure relative to those with pain and matched controls. Whether induced by tissue micro-injury or in response to cytokine/chemokine signals, the adaptive immune response triggered following mesh implantation brings Th cells, foxp3+ Treg cells and CD8+ cytotoxic T cells into the region. Depending on the ongoing stimulus, our data suggest that Th and Treg favor a fibrotic response with collagen deposition. On the other hand, a higher cytotoxic T cell CD8+ population as seen in women with exposure appears to trigger degradation and eventually an exposure. Cytotoxic CD8+ T cells, among others, have the ability to produce MMP-9 upon stimulation,26 suggesting that mesh exposure is a potentially T cell–derived event with MMP-9 playing a pivotal role. It is our general impression that the response to mesh in most women is simply the default foreign body response that occurs after implantation of any device (breast implant, insulin pump, pacemaker etc). What is not clear is the reason why some individuals develop an overzealous foreign body response to implanted materials. Evolving data suggest that small perturbations in the host response to mesh may change the balance from normal healing to profibrosis to degradation. Future studies will determine which specific host factors direct this response.
A major limitation of the current study is that it was not possible to include a control group of mesh-tissue complexes that were obtained from women who underwent mesh implantation without a complication. As such, the current study does not assess the inflammatory response to prolapse mesh in women with a good outcome and focuses only on the inflammatory response in the setting of complications. We have analyzed the host response to mesh in a primate model in a prior study and found that the host response to a mesh with a stable geometry (no pore collapse) and adequate pore size (> 1mm) to preclude bridging fibrosis, is typical of the default foreign body response and is limited to the area immediately around the mesh fiber.10 Our control biopsies were comprised of vaginal tissue uninvolved with mesh yet were still obtained from women with a mesh complication. Thus, while this controls for individual variation in the immune response, it is possible that cell immunity is different in women with complications relative to those who do not experience a complication. Our strict criteria to include only patients with pain (absence of exposure) in the pain group and only exposure (absence of pain) in the exposure group limited our sample size as these complications often occur together. Due to the limited size of some of the excised meshes, we were not able to perform all of the assays on all of the samples. Thus, a lack of difference in some of the results, specifically collagen subtypes, may be due to an insufficient sample size. Finally, we employed all methods possible to quantitate our data as objectively as possible, but the methods are still dependent on tissue staining and cell labeling techniques and therefore, remain semi-quantitative.
In conclusion, T cells appear to play a critical role in the long-term host response to mesh and may be a central pathway leading to mesh complications. The complexity of the T cell response as it relates to normal and abnormal host response to mesh warrants further investigation and has the potential to broaden our understanding of mesh biology and clinical outcomes.
Figure 2.
Box and whisker plots demonstrating T helper (Th), T regulatory (Treg) and cytotoxic T cells (CTL) were greater in exposure group compared to paired control tissue (left), whereas in the pain group only T helper (Th) and T regulatory (Treg) cells were greater compared to paired controls (right).
Table 3.
Comparisons of growth factors and collagen measurements in vaginal mesh-tissue complexes removed for mesh exposure or pain.
| Tissue type | P-values b | |||||
|---|---|---|---|---|---|---|
| Control | Exposure | Pain | E vs. C | P vs. C | P vs. E | |
| T cell population | n=21 | n=20 | n=17 | |||
| CD4+ Thelper | 3.81 ± 5.30 | 52.75 ± 36.69 | 58.47 ± 56.02 | <0.001 | <0.001 | 0.722 |
| foxp3+ Treg | 3.17 ± 3.49 | 21.87 ± 17.18 | 14.62 ± 9.18 | <0.001 | <0.001 | 0.081 |
| CD8+ cytotoxic | 18.84 ± 27.83 | 37.38 ± 24.75 | 23.33 ± 15.04 | 0.034 | 0.520 | 0.032 |
| Growth factors | n=11 | n=15 | n=10 | |||
| TGF-β (μg/mg) | 2.18 ± 1.57 | 4.15 ± 1.89 | 4.67 ± 2.06 | 0.008 | 0.004 | 0.540 |
| CTGF (μg/mg) | 1.88 ± 1.16 | 6.51 ± 3.63 | 4.14 ± 2.93 | <0.001 | 0.008 | 0.060 |
| Collagen | n=12 | n=16 | n=11 | |||
| thin/thick fibers | - | 3.14 ± 1.09d | 1.72 ± 0.91d | - | - | 0.005 c |
| collagen I a | 0.91 ± 0.41 | 1.15 ± 0.48 | 1.33 ± 0.64 | 0.120 | 0.057 | 0.392 |
| collagen III a | 0.27 ± 0.18 | 0.28 ± 0.17 | 0.39 ± 0.32 | 0.920 | 0.256 | 0.251 |
| III/I ratio a | 0.19 ± 0.06 | 0.15 ± 0.06 | 0.18 ± 0.11 | 0.081 | 0.818 | 0.303 |
Values represent mean ± standard deviation
values normalized to internal controls
P-values from mixed effects linear regression
P-value from Student’s t-test
n=10; E: exposure; C: control; P: pain.
AJOG at a glance:
To define the T cell response to implanted urogynecologic meshes removed from women with complications and to correlate the T cell response with collagen deposition in the fibrotic capsule surrounding mesh fibers.
T cells remain elevated as part of the host response in tissue of women with complications compared to control tissue for years after implantation. Mesh-tissue complexes removed for exposure had more CD8+ cytotoxic T cells than those removed for pain. T cells have a spatially distinct distribution from macrophages indicating disparate roles for these two cell populations.
The literature examining the T cell response in women with vaginal mesh complications is scant. These findings suggest T cells may play a critical role in the long-term host response and lay the groundwork for further investigation.
ACKNOWLEDGEMENT
Funding from R01 HD061811 (P.A.M)
This work was supported by National Institutes of Health awards R01 HD061811. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding source had no involvement in the study design, collection of data, analysis of data, interpretation of data, writing of the report, or the decision to submit for publication.
These findings were presented at the American Urogynecologic Society (AUGS), Providence, RI, October 3–7, 2017.
Footnotes
DISCLOSURE: no disclosure
CONDENSATION
T cells play a critical role in the fibrotic response to mesh in women undergoing vaginal mesh excision for complications.
REFERENCES
- 1.Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME. Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol. 2011;117:242–50. [DOI] [PubMed] [Google Scholar]
- 2.Nieminen K, Hiltunen R, Takala T et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol. 2010;203:235 e1–8. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891–8. [DOI] [PubMed] [Google Scholar]
- 4.Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C. Nordic Transvaginal Mesh Group. Anterior Colporrhaphy versus Transvaginal Mesh for Pelvic-Organ Prolapse. N Engl J Med. 2011;364:1826–36. [DOI] [PubMed] [Google Scholar]
- 5.Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair: a systematic review. Obstet Gynecol. 2009;113:367–73. [DOI] [PubMed] [Google Scholar]
- 6.Feiner B, Jelovsek JE, Maher C. Efficacy and safety of transvaginal mesh kits in the treatment of prolapse of the vaginal apex: a systematic review. BJOG 2009;116:15–24. [DOI] [PubMed] [Google Scholar]
- 7.FS C Update on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse. In: FDA, editor. July 2011.
- 8.Anderson JM, Rodriguez A, Chang DT. Foreign Body Reaction to Biomaterials. Semin Immunol. 2008;20:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junge K, Binnebösel M, von Trotha KT et al. Mesh Biocompatibility: effects of cellular inflammation and tissue remodeling. Langenbecks Arch Surg. 2012; 397:255–70. [DOI] [PubMed] [Google Scholar]
- 10.Brown BN, Mani D, Nolfi AL, Liang R, Abramowitch S, Moalli PA. Characterization of the host inflammatory response following implantation of prolapse mesh in rhesus macaque. Am J Obstet Gynecol. 2015;213:668.e1–668.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolfi AL, Brown BN, Liang R, et al. Host response to synthetic mesh in women with mesh complications. Am J Obstet Gynecol 2016;215:206.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang R, Abramowitch S, Knight K, et al. Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG . 2013;120:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feola A, Abramowitch S, Jallah Z, et al. Deterioration in biomechanical properties of the vagina following implantation of a high stiffness prolapse mesh. BJOG. 2013;120:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017. April 15;53:13–28. [DOI] [PubMed] [Google Scholar]
- 15.Brodbeck WG, Macewan M, Colton E, Meyerson H, Anderson JM. Lymphocytes and the foreign body response: lymphocyte enhancement of macrophage adhesion and fusion. J Biomed Mater Res A. 2005;74:222–9. [DOI] [PubMed] [Google Scholar]
- 16.Chang DT, Jones JA, Meyerson H, et al. Lymphocyte/Macrophage Interactions: Biomaterial Surface-Dependent Cytokine, Chemokine, and Matrix Protein Production. J Biomed Mater Res A. 2008;87:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez A, MacEwan M, Colton E, Meyerson H, Anderson JM. Evaluation of Clinical Biomaterial Surface Effects on T Lymphocyte Activation. J Biomed Mater Res A. 2010;92:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez A, MacEwan SR, Meyerson H, Kirk JT, Anderson JM. The Foreign Body Reaction in T cell deficient mice. J Biomed Mater Res A. 2009;90:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wick G, Grundtman C, Mayerl C et al. The Immunology of Fibrosis. Annu Rev. Immunol. 2013;31:107–35. [DOI] [PubMed] [Google Scholar]
- 20.Wick G, Backovic A, Rabensteiner E et al. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 2010; 31(3): 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host Responses in Tissue Repair and Fibrosis. Annu Rev Pathol. 2013;8:241–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Review Immunol. 2015;15:271–82. [DOI] [PubMed] [Google Scholar]
- 23.Borges LF, Gutierres PS, Marana HR, Taboga SR. Picrosirius-polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron. 2007;38:580–3. [DOI] [PubMed] [Google Scholar]
- 24.Pierard GE. Sirius Red Polarization Method is Useful to Visualize the Organization of Connective Tissues but not the Molecular Composition of their Fibrous Polymers. Matrix. 1989;9:68–71. [DOI] [PubMed] [Google Scholar]
- 25.Sykes B, Puddle B, Francis M, Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun 1976;72:1472–80. [DOI] [PubMed] [Google Scholar]
- 26.Corry DB, Kiss A, Song LZ et al. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 2004;June 18:995–7. [DOI] [PMC free article] [PubMed] [Google Scholar]