Summary
Macrophages are central for the immune control of intracellular microbes. Heme oxygenase 1 (HO-1, hmox) is the first and rate limiting enzyme in the breakdown of heme originating from degraded senescent erythrocytes and heme-proteins, yielding equal amounts of iron, carbon monoxide and biliverdin. HO-1 is strongly up-regulated in macrophages in response to inflammatory signals, including bacterial endotoxin. In view of the essential role of iron for the growth and proliferation of intracellular bacteria along with known effects of the metal on innate immune function, we examined whether HO-1 plays a role in the control of infection with the intracellular bacterium Salmonella Typhimurium. We studied the course of infection in stably-transfected murine macrophages (RAW264.7) bearing a tetracycline-inducible plasmid producing hmox shRNA and in primary HO-1 knockout macrophages. While uptake of bacteria into macrophages was not affected, a significantly reduced survival of intracellular Salmonella was observed upon hmox knockdown or pharmacological hmox inhibition, which was independent of Nramp1 functionality. This could be traced to limitation of iron availability for intramacrophage bacteria along with enhanced stimulation of innate immune effector pathways, including the formation of reactive oxygen and nitrogen species and increased TNF-α expression. Mechanistically, these latter effects result from intracellular iron limitation with subsequent activation of NF-κB and further inos, tnfa and p47phox transcription along with reduced formation of the anti-inflammatory and radical scavenging molecules, CO and biliverdin as a consequence of HO-1 silencing.
Taken together our data provide novel evidence that the infection-driven induction of HO-1 exerts detrimental effects in the early control of Salmonella infection, whereas hmox inhibition can favourably modulate anti-bacterial immune effector pathways of macrophages and promote bacterial elimination.
Introduction
Macrophages play a central role in innate immune responses towards intracellular pathogens such as Mycobacteria and Salmonella. Through the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), macrophages eliminate pathogens (MacMicking et al., 1997; Mastroeni et al., 2000; Shiloh and Nathan, 2000; Vazquez-Torres et al., 2000; Bogdan, 2001; Weiss and Schaible, 2015). High output formation of nitric oxide (NO) is due to induction of inducible NO-synthase (iNOS or NOS2, inos) by cytokines and bacterial products, and the formation of ROS is mediated by NADPH-oxidase (PHOX or NOX, phox), consisting of five subunits. Phox generates bactericidal ROS such as oxygen radicals, H2O2 or hydroxyl radicals (Nathan and Shiloh, 2000; Bogdan, 2001; Weiss and Schaible, 2015). While iron can aggregate formation of ROS by the Fenton reaction (Rosen et al., 1995) it also plays a decisive role in host-pathogen interactions (Schaible and Kaufmann, 2004; Ganz, 2009; Nairz et al., 2010; Cassat and Skaar, 2013; Nairz et al., 2014). On one hand, iron is an essential nutrient for many microbes, which have developed multiple pathways to acquire this metal from their environment. Sufficient availability of iron is linked to microbial growth and pathogenicity, whereas iron limitation negatively impacts their proliferation (Kaplan, 2002; Olakanmi et al., 2002; Frawley et al., 2013). On the other hand, iron plays an essential role in the proliferation and differentiation of immune cells and exerts direct effects on innate immune function (Nairz et al., 2014). The latter can be attributed to a negative regulatory effect of iron on interferon-gamma (IFN-γ) -inducible immune effector pathways in macrophages, including iNOS and tumour necrosis factor alpha (TNF-α) formation (Oexle et al., 2003; Nairz et al., 2014). Consequently, competitive interactions between macrophage iron homeostasis and microbial iron acquisition systems form a central frontline that defines the course of infection (Schaible and Kaufmann, 2004; Weinberg, 2009; Nairz et al., 2010).
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a facultative intracellular Gram-negative bacterium that can cause fatal septicemia. Salmonella pathogenicity depends on the organism’s ability to invade macrophages, thus taking advantage of these cells as a habitat for multiplication and spreading within the host (Leung and Finlay, 1991; Andrews-Polymenis et al., 2010; Lahiri et al., 2010). The intracellular replication of Salmonella is highly dependent on a sufficient iron supply (Nairz et al., 2007; Andrews-Polymenis et al., 2010), while a restricted availability of iron in macrophages is associated with impaired proliferation of intracellular bacteria within these cells (Schaible and Kaufmann, 2004; Nairz et al., 2009).
Iron homeostasis within macrophages is regulated at multiple steps and significantly modified upon infection and inflammation (Nairz et al., 2014). Macrophages play a crucial role for body iron homeostasis as they take up and degrade senescent erythrocytes and re-utilize iron, which accounts for approximately 90% of the daily needs of the metal, mainly used for erythropoiesis (Hentze et al., 2010; Pantopoulos et al., 2012). The enzyme heme oxygenase 1 (HO-1, hmox) is of central importance in this process because it degrades heme to yield equal amounts of iron, carbon monoxide and biliverdin (Tenhunen et al., 1968; Ryter and Tyrrell, 2000). Apart from erythrocyte phagocytosis, macrophages can acquire transferrin bound iron via transferrin-receptor 1 (TfR1)-mediated endocytosis or ferrous iron via the divalent metal transporter 1 (Dmt1, Slc11a2). Importantly there is only one well-characterized iron export pathway, involving the transmembrane protein ferroportin (Fpn1, Slc40a1), which transports ferrous iron to the extracellular space where it is oxidized and incorporated into apo-transferrin (Tf) (Hentze et al., 2010; Pantopoulos et al., 2012). The expression of this crucial mediator of iron homeostasis is significantly affected during infection by inflammatory cytokines and their secondary products, both at the transcriptional and the post-transcriptional level (Mulero and Brock, 1999; Ludwiczek et al., 2003). However, the activation of regulatory networks may differ depending on the cellular localization of a microbe (Drakesmith and Prentice, 2012; Fang and Weiss, 2014; Nairz et al., 2014). The metabolic response to extracellular pathogens is considered to cause iron retention in macrophages. This is largely exerted by inflammation-triggered formation of hepcidin and subsequent degradation of Fpn1 on the cell surface, resulting in blockade of macrophage iron egress (Nemeth et al., 2004; Theurl et al., 2008; Ganz, 2009; Armitage et al., 2011; Arezes et al., 2015). In contrast, macrophage challenge with intracellular bacteria, such as Salmonella, induces the transcription of fpn1, resulting in stimulation of iron export and reduction of intracellular iron concentrations (Nairz et al., 2007; Van Zandt et al., 2008; Ward and Kaplan, 2012; Nairz et al., 2013; Nairz et al., 2015).
Immune modulatory functions have been observed for the three degradation products of heme catabolism, namely iron, carbon monoxide and biliverdin (reviewed by (Ryter et al., 2006; Soares and Weiss, 2015; Wegiel et al., 2015)), thereby linking putative HO-1 cytoprotective properties to the modulation of intracellular iron levels (Ferris et al., 1999). Furthermore, although HO-1 has been implicated in tolerance of malaria and treated bacterial infections with amelioration of tissue damage (Larsen et al., 2010; Gozzelino et al., 2012), the role of HO-1 for the control of intracellular bacterial infections has not been clearly established. We hypothesize, that HO-1 may affect iron availability for intracellular pathogens, thereby influencing the course of infection. Our results provide novel evidence for a direct involvement of HO-1 in host interactions with the intracellular bacterium S. Typhimurium.
Results
Intramacrophage bacterial replication is augmented by heme oxygenase 1
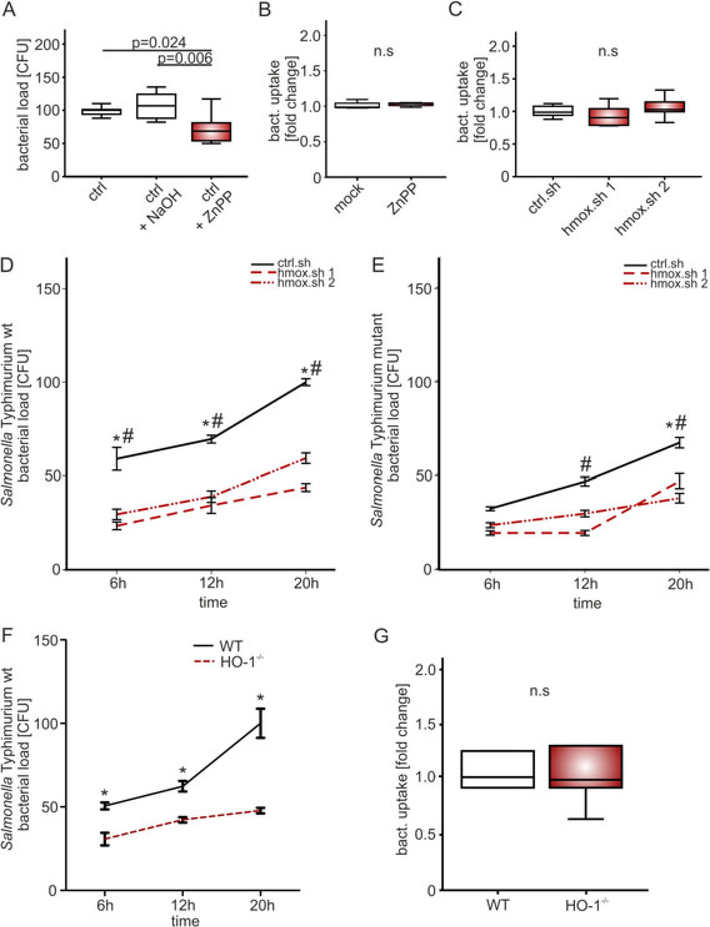
We first studied the impact of reduced heme oxygenase activity on intracellular bacterial counts in RAW264.7 cells infected with S. Typhimurium at a multiplicity of infection (MOI) of 10. The number of Salmonella colony forming units (CFUs) recovered from macrophages after 24 h of infection was significantly reduced following pre-treatment with the HO-1 Inhibitor zinc (II) protoporphyrin IX (ZnPP, 10 μM) as compared to the solvent-treated control cells (Fig. 1A). To rule out off-target effects of ZnPP we generated RAW264.7 cells stably expressing doxycycline inducible hmox or luciferase control shRNA (RAW.sh; hmox.sh; ctrl.sh). Doxycycline induction caused an overall reduction of hmox mRNA expression of 80 to 90%. Infection of these cells with S. Typhimurium resulted in significant and time-dependent decrease of bacterial numbers within macrophages as compared to macrophages stable transfected with control shRNA expressing luciferase (Fig. 1D). To verify these findings in primary cells, we studied bone marrow derived macrophages (BMDM) obtained from macrophage specific HO-1 knockout mice (LysM-Cre+/+HO-1fl/fl and control LysM-Cre−/−HO-1fl/fl) (Fig. 1F). Following infection with S. Typhimurium we observed significantly better control of intracellular bacterial numbers in macrophage specific HO-1 knockout cells (BMDM) compared with control cells, which confirmed the phenotype observed in RAW.sh cells. This observation could not be attributed to differences in Salmonella uptake or containment because intramacrophage bacterial numbers were the same in hmox knockdown and control macrophages as well as in primary wild type and HO-1 knockout bone marrow derived macrophages at 1 h after infection (Fig. 1B, C and G).
Fig. 1. Heme oxygenase gene knockdown reduces the survival of intracellular Salmonella Typhimurium.
(A) RAW 264.7, (B-E) RAW.sh macrophages (ctrl.sh, hmox.sh 1 and 2) and (F-G) BMDM from LysM-Cre+/+ HO-1fl/fl (HO-1−/−) and LysM-Cre−/− HO-1fl/fl (wt) mice were infected with (A-D, F-G) Salmonella enterica serovar Typhimurium (S. Tm) wild-type (wt) or (E) an isogenic Salmonella Typhimurium mutant (ΔentCΔsitΔfeo) strain at a MOI of 10 for the indicated period of time.
(A) Bacterial burden (CFU) was determined 24 h post-infection following treatment with 10 μM of HO-1 Inhibitor ZnPP or solvent (NaOH, PBS). (B-C, G) Bacterial uptake relative to mock/control (fold change) was measured one hr post infection to determine phagocytosis. (A-C, G) Data from three independent experiments performed in triplicate are shown (n = 9). Boxes depict lower quartile, mean and upper quartile; whiskers show maximum and minimum range. P-values are reported as determined by statistical analysis using ANOVA and Bonferroni correction. (D-F) Bacterial burden (CFU) was determined as mean ± SEM of at least three independent experiments performed in triplicate (n = 9) and normalized (%) relative to control. Whiskers show maximum and minimum range. P-values are reported as determined by statistical analysis using ANOVA and Bonferroni’s correction. * P < 0.001 for crtl.sh versus hmox.sh 1 or control (wt) BMDM versus HO-1−/− cells; # P < 0.001 for ctrl.sh versus hmox.sh 2 macrophages.
Based on the fact that hmox activity increases the intracellular concentration of molecular iron and because Salmonella are highly dependent on a sufficient supply of iron, we studied the effects of hmox silencing on infection with a Salmonella strain (Fig. 1E) that is deficient in enterobactin synthesis, sitABCD- and feo-mediated iron uptake (S. mt; ΔentCΔsitΔfeo) (Crouch et al., 2008). Although, the differences in bacterial counts between ctrl.sh and hmox.sh macrophages were less prominent than during infection with wt Salmonella, intramacrophage numbers of mutant Salmonella were still significantly lower upon hmox silencing than in wt macrophages. This indicated that bacterial iron restriction contributes to impaired bacterial proliferation upon hmox knockdown but is not exclusively responsible for the observed protective effect.
Effects of hmox silencing on macrophage iron homeostasis and bacterial iron acquisition
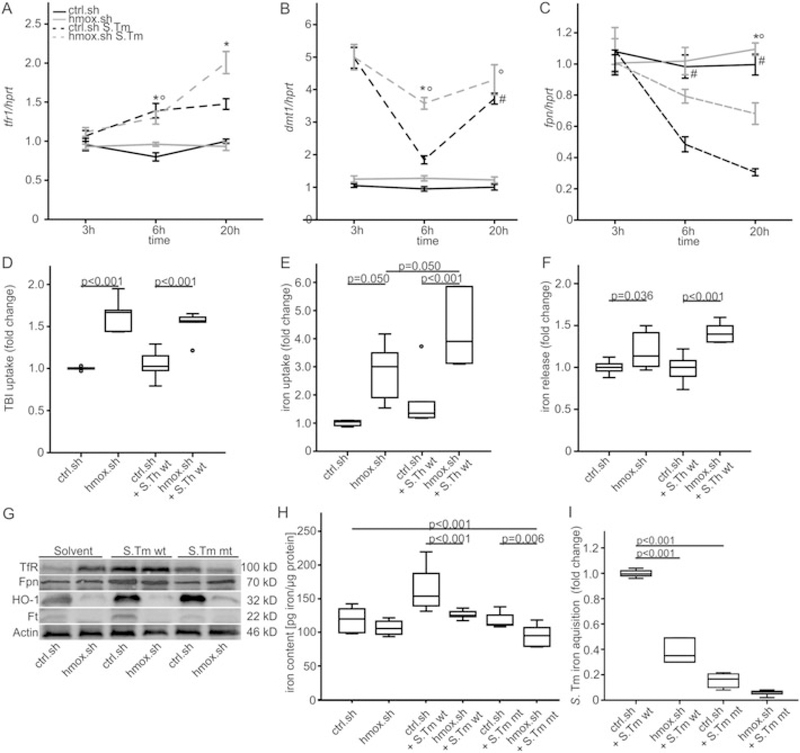
We subsequently studied the putative changes in macrophage iron homeostasis and expression of central iron transport genes in the absence/presence of hmox and in response to S. Typhimurium infection. The iron acquisition genes, transferrin receptor 1 (TfR1, tfr1) and divalent metal transporter 1 (Dmt1, dmt1), showed no difference in expression between uninfected hmox.sh and ctrl.sh macrophages. However, we observed a significant increase in tfr1 and dmt1 expression following infection with S. Typhimurium that was significantly higher in hmox. sh than in ctrl.sh cells (Fig. 2A and B). These changes were paralleled by a significant increase in transferrin- bound iron (TBI) and non-transferrin bound iron (NTBI) uptake by uninfected and infected hmox.sh cells as compared to ctrl.sh. (Fig. 2D and E). Increased cellular iron export (Fig. 2C and F) was observed in Salmonella infected hmox.sh macrophages 20 h post-infection, although cytoplasmic ferroportin (fpn1) mRNA levels were reduced at this time. Importantly, the enhanced iron export corresponded to increased Fpn1 protein levels in macrophages infected with Salmonella, as determined by Western Blot analysis (Fig. 2 G). In addition, the increased TfR1 and reduced ferritin protein levels in hmox knockdown macrophages at baseline and upon Salmonella infection are consistent with reduced intracellular iron concentrations as a consequence of increased iron export (Fig. 2G).
Fig. 2. Effects of hmox on iron acquisition and iron release in Salmonella infected macrophages.
(A-B, D-E) Iron import. Ctrl.sh cells (black line) and hmox knockdown macrophages (hmox.sh; grey line) were infected with Salmonella Typhimurium wild-type (S. Tm wt; dashed line) strain at a MOI of 10 for the indicated period of time.
(A) tfr1 and (B) dmt1 levels were determined by qRT-PCR. Values were normalized to the housekeeping gene hprt, and fold-changes relative to the untreated control (20 h) are shown. (D, E) The uptake of (D) TBI (transferrin loaded with 59Fe) and (E) iron (59Fe) for 4 h following an 18 h infection period was determined as described in Materials and methods. (C, F) Iron export. Macrophages were treated exactly as described above. (C) fpn1 levels by qRT-PCR and (F) iron (59Fe) release 20 h post infection were determined. (A-C) Data from at least three independent experiments are shown (ctrl.sh n =10; hmox.sh n = 9) and expressed as mean ± SEM. P-values are reported as determined by post hoc with Bonferroni correction. *# ° P < 0.001 (* ctrl.sh vs. hmox.sh infected with S. Tm; # ctrl.sh vs. ctrl.sh S.Tm; ° hmox.sh vs. hmox.sh S.Tm). (D-F) Data were compared and are depicted of three independent experiments (D, E) n = 6, (F) n = 12 ctrl.sh, n = 8 hmox.sh. P-values are reported as determined by post hoc analysis with Bonferroni’s correction following ANOVA. (G) Western blot analysis of whole cell lysates using specific antibodies to TfR1, Fpn1, heme oxygenase (HO-1), Ferritin (Ft) and the control β-Actin. One of three representative western blot experiments is shown. (H) Cellular iron content in phagocytes was measured by atomic absorption spectrometry. Macrophages were infected with Salmonella wt or mt (ΔentCΔsitΔfeo) for 48 h or left untreated. Results were normalized to protein content. Data from three independent experiments are shown (n = 8 untreated and S. Tm mt infected, n = 10 infected with S. Tm wt). (I) Bacterial iron acquisition within macrophages was determined after loading of infected macrophages with 59Fe. Data were normalized for Salmonella numbers determined by CFU count. Three independent experiments were carried out in duplicate (n = 6). Data were compared by ANOVA using Bonferroni’s correction (P-values). Values (boxes) are depicted as lower quartile, median and upper quartile with maximum and minimum range.
To gain further insights into the dynamics of cellular iron trafficking, we determined total intracellular iron concentrations via atomic absorption spectrometry (Fig. 2H). We found that hmox.sh phagocytes exhibited significantly lower iron contents following infection compared to infected control cells, which was also true when the infection was performed with a Salmonella mutant strain deficient in iron acquisition (Fig. 2H). We then assessed the effects of hmox knockdown on iron incorporation by engulfed bacteria. Remarkably, the high iron flux observed in hmox knockdown cells resulted in a significant reduction in iron acquisition by intracellular Salmonella as compared to Salmonella in ctrl.sh macrophages. The Salmonella triple mutant strain acquired significantly less iron than wt Salmonella (Fig. 2I).
Furthermore we wanted to examine whether or not the expression of the phagolysosomal protein Nramp1 (Slc11a1) is critical for the observed hmox mediated effects. Nramp1 expression confers resistance to a number of intracellular pathogens and mediates iron efflux into the cytoplasm along with the amelioration of a number of antimicrobial functions (Cellier et al., 2007; Fritsche et al., 2012). To study this issue we used RAW 264.7 macrophages which are stably transfected with a functional or non-functional Nramp1. As described in the literature the intracellular multiplication of Salmonella was significantly lower in RAW cells expressing the functional Nramp1 than in macrophages lacking Nramp1 activity (Cellier et al, 2007; Fritsche et al, 2012). However, blockade of HO-1 activity by ZnPP resulted in comparable reductions of bacterial numbers in both cell types suggesting that the growth promoting effect of HO-1 towards intramacrophage Salmonella is independent of Nramp1 functionality (Figure S2 B).
Impact of HO-1 on macrophage effector functions
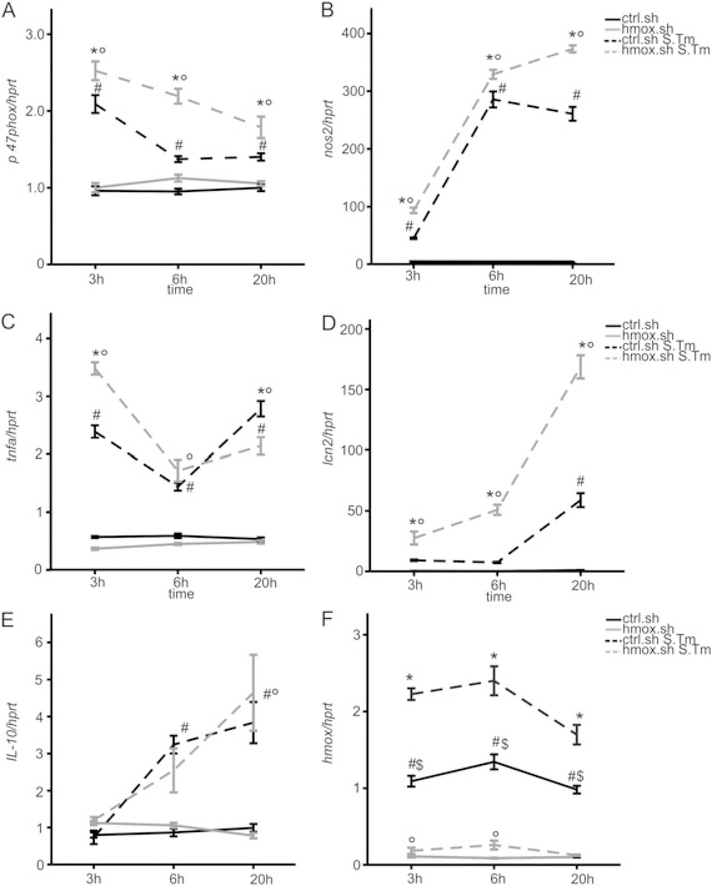
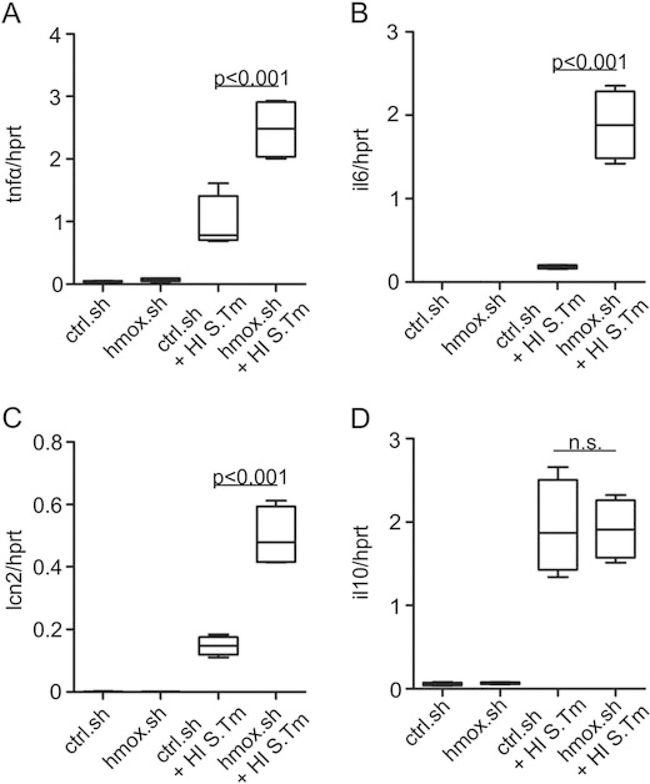
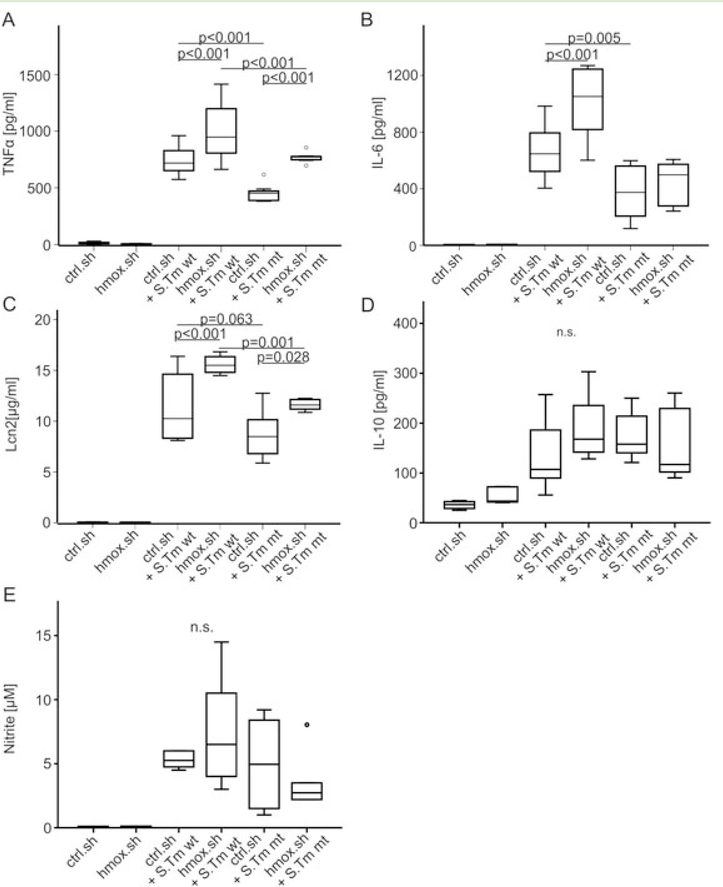
We then studied whether hmox knockdown impacts the efficacy of macrophage immune effector functions in the course of Salmonella infection, either via the observed alterations in macrophage iron transport pathways or by other mechanisms. We found that silencing of hmox was paralleled by a significant time-dependent induction of p47 phagocyte oxidase (p47phoX) and inducible nitric oxide synthase (nos2) mRNA expression as compared to infected control macrophages (Fig. 3A and B). The expression of p47phox declined over time, whereas nos2 expression progressively increased, reflecting the varying importance of these two radical forming genes at early and later stages of infection respectively (Mastroeni et al., 2000). However, at all time points, both genes were significantly higher expressed in hmox.sh than in control. sh cells (Fig. 3A and B). Furthermore, tumour necrosis factor alpha (tnfa) and interleukin 10 (il10) mRNA expression increased in both infected cell types. However, whereas tnfa formation was significantly higher in hmox.sh macrophages at early time points of infection and then declined, no significant differences in il10 expression were observed between infected hmox.sh and ctrl.sh macrophages (Fig. 3D and E). Importantly, upon infection with Salmonella, hmox knockdown macrophages expressed significantly higher levels of the anti-microbial siderophore scavenging peptide lipocalin 2 (lcn2). Of note, in comparison to control macrophages an increased expression of tnfa, il6 and lcn2 was also seen in hmox knockdown cells upon treatment with either heat-inactivated Salmonella (Fig. 4) or lipopolysaccharide (data not shown), pointing to a general and early immune de-activating effect of hmox in inflammatory macrophages.
Fig. 3. Effects of hmox gene knockdown on p47phox, nos2, tnfa, II-10 and lcn2 transcript expression.
Ctrl.sh (black line) and hmox.sh (grey line) macrophages infected with Salmonella (black or grey dashed line) were subjected to qRT-PCR at indicated time points. Expression of genes of interest was normalized to hprt expression and fold-change relative to untreated control is shown. Data from three independent experiments (n = 10 ctrl.sh; n = 8 hmox. sh) are shown. Error bars represent means ± SEM, values are reported as determined by ANOVA using Bonferroni’s correction. * $ P < 0.001 for hmox.sh versus ctrl.sh, both infected with S. Tm (*) or both uninfected ($); ° P < 0.001 for hmox.sh uninfected versus infected with S. Tm; # P < 0.001 for comparison of ctrl.sh and ctrl.sh infected with S. Tm.
Fig. 4. Early response to Salmonella is improved in hmox knockdown macrophages.
Ctrl.sh and hmox.sh macrophages supplemented with an identical number of heat-inactivated Salmonella (HI S. Tm; as a defined inflammatory stimulus not compromised by bacterial number) as described in Fig. 3 were subjected to qRT-PCR. Gene expression of (A) tnfa, (B) il6, (C) lcn2 and (D) il10 was determined 6 h post-infection and normalized to hprt expression levels. (A-D) Fold changes relative to untreated controls are shown. Data from three independent experiments are shown. Error bars represent means ± SEM; values are reported as determined by ANOVA using Bonferroni’s correction.
Notably, hmox1 knockdown in the cells was not compensated by an increase of hmox2 expression (Supplemental Figure S3 B).
In accordance with the transcriptional analyses, the concentrations of TNF-α, IL-6 and Lcn2 were significantly increased in culture supernatants of hmox.sh cells infected with Salmonella at 20 h post infection as compared to infected control.sh cells (Fig. 5A–C), whereas IL-10 concentrations did not differ between ctrl. sh and hmox.sh macrophages (Figs 5D and 4).
Fig. 5. Productionofpro-inflammatory mediators, Lcn2, IL-10 and nitrate during Salmonella infection following hmox gene knockdown.
(A-E) Ctrl.sh and hmox.sh cells were infected with Salmonella wt (S. Tm wt) or mutant ΔentCΔsitΔfeo (S. Tm mt) for 20 h before culture supernatants were analyzed for formation of (A) TNFα, (B) IL-6, (C) Lcn2, (D) IL-10 and (E) nitrate. (A-E) Boxes depict lower quartile, mean and upper quartile; whiskers show minimum and maximum range. Data are shown as mean ± SEM from at least three independent experiments. P-values are reported as determined by ANOVA using Bonferroni’s correction.
We then studied whether hmox knockdown macrophages exhibit better pathogen control because of increased reactive oxygen (ROS) and RNS production, as both p47phox and inducible nos2 expression were significantly increased in such infected cells.
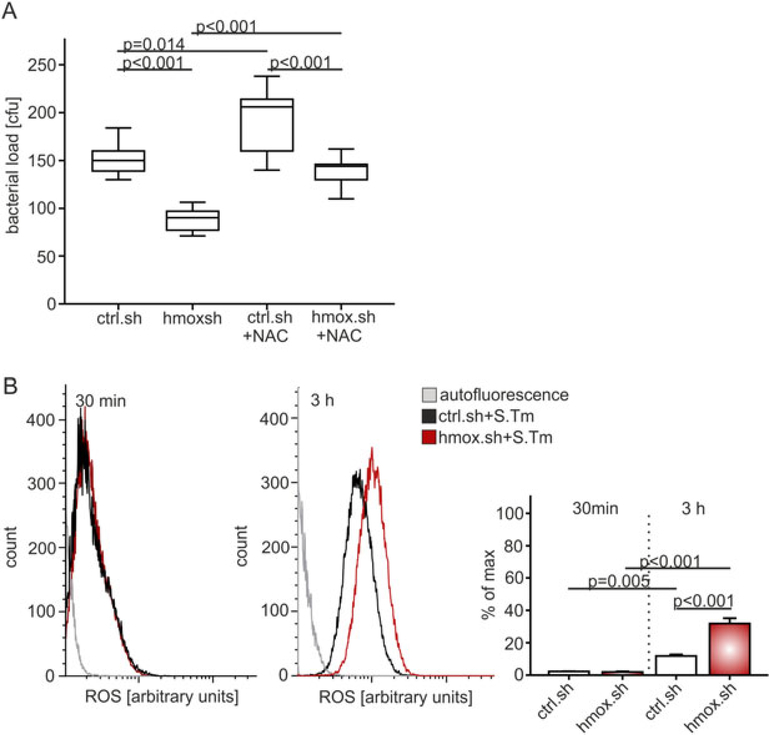
Salmonella-infected hmox.sh and control.sh macrophages were supplemented with the membrane-permeable compound N-acetyl-L-cysteine (NAC), known to quench ROS (Victor et al., 2003). NAC treatment significantly augmented the bacterial load of hmox.sh and control.sh macrophages to a comparable level (Fig. 6A). This suggested that the effects of hmox on macrophage antimicrobial activity are dependent on oxidative stress. To further assess ROS production, cells were assayed 30 min and 3 h post infection for oxyradical generation. Salmonella-infected hmox.sh cells produced significantly more ROS in comparison to the analogously treated crtl.sh macrophages (Fig. 6B). Notably, the production of RNS, measured via the concentration of the stable NO end-product nitrite in culture supernatants, was also increased in hmox.sh macrophages (Fig. 5E). To examine the possibility that higher ROS and cytokine production or heme accumulation may cause increased cell death in hmox deficient cells, we performed measurements of lactate dehydrogenase activity as a surrogate for cytotoxicity. Of note, we found no significant differences between hmox deficient (hmox.sh) and control cells with or without Salmonella infection (Supplementary Figure S3 A)
Fig. 6. Increased ROS production by hmox knockdown macrophages reduces their bacterial burden.
(A) Macrophages (hmox.sh versus ctrl.sh) were infected with Salmonella Typhimurium wt (S. Tm) and thereafter treated with the radical scavenger N-acetyl-L-cysteine (NAC) for 20 h. Boxes are depicted as lower quartile, median and upper quartile with minimum and maximum range. Data of at least three independent experiments are shown (n = 10). P-values are reported as determined by ANOVA using Bonferroni’s correction.
(B) ROS levels were measured via FACS analysis in ctrl.sh and hmox.sh cells infected with Salmonella at 30 min and 3 h post infection. The histograms represent the average ROS levels measured by CellROX™ fluorescence. Data of three independent experiments performed in triplicate are shown. P-values are reported as determined by ANOVA using Bonferroni’s correction.
Improved Salmonella control by hmox macrophages involves NF-κB activation
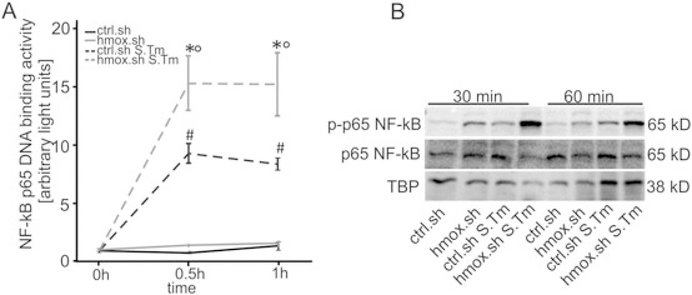
NF-κB is an important regulator of inflammatory responses, including the production of TNF-α. Moreover, it is linked to hmox gene regulation. Quantification of the specific DNA binding activity to the NF-κB subunit p65 revealed significantly increased p65 binding activity in the hmox knockdown cells infected with Salmonella Typhimurium compared to infected ctrl.sh cells (Fig. 7A). Western blot experiment data demonstrated that hmox.sh macrophages exhibit higher phosphorylation of the NF-κB p65 subunit in comparison to crtl.sh macrophages (Fig. 7B).
Fig. 7. Knockdown of hmox in macrophages show increased NF-κB activation.
RAW.sh macrophage-like cells were infected with Salmonella for the indicated periods of time. NF-κB DNA binding activity in nuclear protein extracts was evaluated by means of a specific p-65 NF-κB chemo-luminescence transcription factor assay. Data from three independent experiments are shown and expressed as arbitrary light units. Error bars are depicted as means ± SEM and were compared by ANOVA with Bonferroni’s correction. Asterisks indicate statistically significant differences between Salmonella infected control and hmox knockdown macrophages: * p < 0.001 for comparison of ctrl.sh versus hmox.sh infected with S. Tm; # p < 0.001 ctrl.sh versus ctrl.sh S. Tm;°p < 0.001 between hmox.sh and hmox.sh S. Tm.
(B) Protein extracts from parallel experiments described in (A) were used for evaluation of phosphorylated (p-) p65 NF-κB by means of Western blot analysis as described in Material and methods. Antibodies to p65 NF-κB and TATA-binding protein (as loading control) were used. One of three representative Western blot experiments is shown.
Impact of heme catabolites on bacterial survival and immune regulation
Induction of HO-1 and the degradation products of heme catabolism were shown to exert potent cytoprotective effects and homeostatic actions in preclinical models of inflammatory disorders such as sepsis, colitis and ischaemia-reperfusion injury (Otterbein et al., 2000; Yet et al., 2001; Hegazi et al., 2005; Chung et al., 2008). We questioned whether the lack of one of these products from the HO-1 catalysis might possibly be responsible for the improved bacterial killing found in the hmox knockdown macrophages. Therefore, we exposed Salmonella infected cells to iron (ferric ammonium citrate (FAC) as iron source), the CO-releasing agent CORM-2 or bilirubin and examined the bacterial load along with ROS production after this treatment (Supplemental Figure S1). Iron supplementation significantly increased the bacterial load in both, hmox knockdown and ctrl.sh macrophages thereby increasing the intramacrophage bacterial numbers in hmox.sh phagocytes to that observed in control cells. Moreover, CORM-2 treatment significantly reduced bacterial numbers in both cells types (Figure S1 A), but the differences between control and hmox knockdown cells remained evident. Addition of bilirubin did not change bacterial numbers in ctrl.sh cells but increased those in hmox.sh macrophages resulting in an insignificant change of bacterial numbers (Figure S1 A). ROS levels were significantly higher in hmox knockdown than in control cells, which was not significantly altered in the presence of CORM-2 or bilirubin (Fig. S1 B).
Discussion
This study provides evidence that hmox gene knockdown results in improved control of infection with intracellular bacteria. These can be attributed to impaired bacterial survival, mainly as a consequence of iron limitation for intramacrophage microbes along with stimulation of antimicrobial immune effector pathways including radical formation.
Specifically, our data show that hmox depletion causes an induction of Fpn1-mediated iron efflux (Fig. 2C, E) which limits the availability of iron for Salmonella. The efficacy of this Fpn1-mediated pathway to restrict intracellular bacteria growth has been shown in different in vitro bacterial models employing Mycobacteria, Chlamydia, Listeria and Salmonella (Chlosta et al., 2006; Nairz et al., 2007; Paradkar et al., 2008; Van Zandt et al., 2008; Bellmann-Weiler et al., 2010; Velayudhan et al., 2014; Haschka et al., 2015). The increase in Fpn1 expression may be linked to enhanced NO formation as described recently (Nairz et al., 2013) whereas a reduced expression of hepcidin in hmox.sh cells may contribute to Fpn1 stabilization on the cell surface.
In addition, Salmonella cannot utilize heme and thus requires the hmox breakdown product iron as a growth factor (Andrews-Polymenis et al., 2010). Therefore, hmox knockdown limits bacterial iron availability. Further, bacterial iron limitation in hmox-silenced macrophages is further promoted via increased production of Lcn2 which binds and neutralizes bacterial siderophores (Flo et al., 2004; Berger et al., 2006). Moreover, Lcn2 mediates shuttling of iron across cell membranes (Nairz et al., 2009; Liu et al., 2014; Nairz et al., 2015) which is most likely linked to its capacity to bind a mammalian siderophore (Bao et al., 2010; Liu et al., 2014).
Reduction in intracellular iron concentrations has been shown to promote the production of pro-inflammatory molecules such as TNF-α, IL-6, iNOS (nos2) or CCL-20 by activated macrophages (Weiss et al., 1994; Melillo et al., 1997; Ludwiczek et al., 2003; Oexle et al., 2003; Varesio et al., 2010), and these pathways are of importance for the control of intracellular pathogens like Salmonella (Andrews- Polymenis et al., 2010; Weiss and Schaible, 2015).
Our results, demonstrating increased p47phox expression and ROS formation, are in line with a previous observation reporting enhanced ROS formation in HO-1 knock out cells (Orozco et al., 2007). The oxidative burst is a central defence mechanism against intracellular pathogens, including Salmonella (Mastroeni et al., 2000; Vazquez-Torres et al., 2000). Thus, increased ROS and RNS production resulting from hmox knockdown enhances immune control of Salmonella. In addition, TNF-α produced in larger quantities as a result of hmox silencing further contributes to RNS and ROS production because of the co-activation of inos and p47phox expression (Ables et al., 2001). This leads to the question regarding the mechanism underlying increased expression of these immune response genes.
We detected increased NF-κB activity in association with improved anti-microbial immune responses in hmox.sh macrophages, which is consistent with NF-kB as a central regulator of inflammatory responses (Tak and Firestein, 2001). Intracellular free or labile iron has been observed to play an important role in stress signalling by NF-κB and is also associated with the expression of HO-1 (Varesio et al., 2010; Evstatiev and Gasche, 2012).
However, hmox knockdown macrophages have low iron content and reduced iron pools at the same time expressing significantly higher levels of NF-κB than the corresponding control cells. It might be speculated that labile iron molecules (Wessling-Resnick, 2010) which become abundant in hmox knockdown macrophages before being exported from these cells may activate NF-κB via Fenton chemistry mediated radical production. Alternatively, NF-κB activation may be a consequence of an increased oxidative burst, which is a regulator of NF-κB activation in inflammatory macrophages (Karin and Greten, 2005; Gloire et al., 2006; DiDonato et al., 2012). This is consistent with our findings of increased NADPH oxidase (p47phox) expression, the molecular source of ROS in response to LPS treatment (Gloire et al., 2006). In addition, the reduced abundance of CO and bilirubin, two end-products of the HO-1 pathway, further contributes to increased or prolonged oxidative stress, as these molecules have radical scavenging capacities and anti-inflammatory activities (Gozzelino et al., 2010).
Moreover, consistent with our observation that NAC treatment results in increased bacterial numbers and a reduction of TNF-α formation in hmox knockdown macrophages during Salmonella infection, NAC can prevent LPS-induced NF-κB activation and the production of inflammatory cytokines (Gloire et al., 2006). These observations suggest that NF-κB is activated because of a lack of HO-1 derived anti-inflammatory molecules (CO, bilirubin) and is consistent with previous findings showing that the CO releasing compound, CORM-2, inhibits the DNA binding activity of NF-κB resulting in reduced TNFα production (Sawle et al., 2005; Megias et al., 2007; Motterlini et al., 2012). Moreover, regulation of iron metabolism by HO-1 inhibits NF-κB activation via a mechanism that targets specifically the phosphorylation of its RelA/p65 subunit (Seldon et al., 2007).
Of note, CO was found to promote maturation of myeloid cells needed to sense and kill bacteria (Wegiel et al., 2014). Furthermore CO modulates the response of macrophages to bacterial LPS (Otterbein et al., 2000), thereby decreasing macrophage Toll-like receptor signalling with downstream activation of NF-κB (Wang et al., 2009) and promoting bacterial clearance together with HO-1 (Onyiah et al., 2013). Here we noted that the CO releasing agent CORM-2 reduced intramacrophage numbers of Salmonella Typhimurium in ctrl.sh and hmox knockdown cells. This is in a line with previously described bactericidal properties of CORMs against several pathogens including E. coli, Staphylococcus aureus and Pseudomonas aeruginosa (Desmard et al., 2009; Nobre et al., 2009; Tavares et al., 2011).
It is important to note, that HO-1 appears to have a dual role in infections. While our observations demonstrate that HO-1 exerts detrimental effects during the early control of intramacrophage bacteria like Salmonella, other investigators have found that HO-1 contributes to improved control of Salmonella replication within macrophages by exerting cytoprotective effects (Zaki et al., 2009) and prevents macrophages from programmed cell death during Myco-bacterium tuberculosis infection (Silva-Gomes et al., 2013). The dual role of HO-1 may thus become most evident in the course of persisting infections. Its downregulation appears to be beneficial for the immediate control of infection with intracellular pathogens, but in non-resolving infection HO-1 expression at later stages may be necessary to reduce inflammation driven tissue damage.
Up-regulation of HO-1 during malaria or sepsis was demonstrated in association with infection tolerance and limitation of tissue damage resulting in an improved survival (Gozzelino et al., 2010; Larsen et al., 2010). On the other side of the coin, HO-1 expression is associated with increased susceptibility to Salmonella bacteremia during malaria infection, and increased mortality from Salmonella infection in that setting (Cunnington et al., 2012). Furthermore, a recent study of Mycobacteria abscessus found that inhibition of HO-1, especially at the early stages of infection, limits intracellular bacterial numbers (Abdalla et al., 2015).
Our study adds another piece to the puzzle concerning the role of HO-1 in infection. Although HO-1 may be detrimental for the host and beneficial for the pathogen in the early stages of Salmonella infection, HO-1 subsequently promotes disease tolerance via cytoprotective effects and the prevention of macrophage apoptosis.
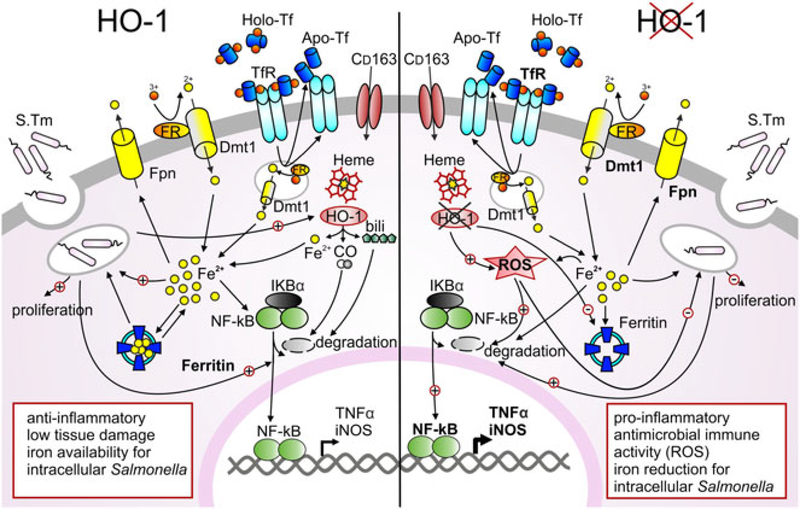
In summary (Fig. 8), the data presented here provide new mechanistic insights into the role of HO-1 in early Salmonella infection, emphasising its capacity to modulate anti-bacterial immune effector pathways and cellular iron homeostasis. The current study shows that hmox knockdown in macrophages limits bacterial replication by reducing intracellular iron pools and subsequent bacterial iron incorporation along with stimulation of anti-microbial immune effector mechanisms.
Fig. 8. Effects of heme oxygenase in regulating iron homeostasis and innate immune response of macrophages during Salmonella infection.
Macrophages play an important role in infected tissue. Within these cells heme bound iron is degraded via HO-1 to equal amounts of iron, carbon monoxide (CO) and biliverdin (bili). Ferric iron is acquired via transferrin receptor 1 (TfR1) mediated endocytosis of holoTf, reduced within the vesicle by a ferric reductase (FR) and released to the cytoplasmic pool via divalent metal transporter (Dmt1), while Tf and TfR are recycled to the surface. During infection, bacterial pathogens may employ mechanisms to increase the abundance of potential iron sources. Transitory iron accumulation in the cytosol may promote storage into ferritin, ferroportin (Fpn1)-mediated export and reduction of TfR1 expression, thereby limiting iron for the pathogen or lowering intracellular iron levels. Both LPS and iron stimulate NF-κB expression, resulting in an inflammatory response that includes expression of iNOS, TNFα and other cytokines. Macrophages with absent HO-1 function show an increased iron transport activity, strong iron utilization via TfR1 and Dmt1 and increased iron export via Fpn1. Cells may simultaneously restrict iron levels during Salmonella infection and increase production of reactive oxygen species (ROS). Restriction of iron and increased ROS production promote NF-κB mediated activation of a pro-inflammatory immune response that results in improved Salmonella control and may also result in tissue injury.
Of importance, our data allow us to speculate that during infection, cells with low HO-1 expression may be more resistant to infection with Salmonella than macrophages that strongly induce HO-1 as a consequence of iron recycling. This goes along with the observation that hemophagocytic macrophages, which express high levels of HO-1, provide a survival niche for Salmonella (Nix et al., 2007). Accordingly, infection with Salmonella leads to increased HO-1 expression in host cells, which may be seen as an attempt of the pathogen to weaken host-derived anti-microbial effector pathways and to secure a sufficient supply of iron.
Experimental procedures
Vectors for gene knockdown
Complementary shRNA oligonucleotides directed against either hmox1 or luciferase and containing BamHI and HindIII sticky ends (hmox1_1.sh 5′-GATCCCC-caagcagaacccagtctatTTCAAGAGAatagactgggttctgcttgTTTTTG-GAAA-3′ and 5′-AGCTTTTCCAAAAAcaagcagaacccagtctatTTCAAGAGAatagactgggttctgcttgGGG-3′ or hmox1_2.sh 5′-GATCCCCcaacagtggcagtgggaatTTCAAGAGAattcccactgccactgttgTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAcaacagtggcagtgggaat-TTCAAGAGAattcccactgccactgttgGGG-3′ (the sense target sequence is underlined; synthesized by MWG Ebersberg, Germany) were annealed and cloned into BglII-HindIII sites of the digested, dephosphorylated pENTR-THT-III. This GATEWAY-compatible vector contains a tetracycline-sensitive H1 promoter (THT) that controls the expression of the shRNA. The THT-shRNA cassette was recombined into the lentiviral RNAi destination vectors for conditional knockdown. The pGLTR-X-FP (encoding TetR-T2A-GFP) and pGLTR-X-S (encoding PuroR) destination vector for constitutive shRNA expression (Sigl et al., 2014) were used.
Generation of stable shRNA cell lines
Confluent 293 T cells were transiently transfected with the lentiviral expression vector pGLTR-X-FP (now including the sequence for hmox shRNA expression) in combination with psPAX and the VSV-G encoding vectors using Metafectene™ (Biontex, Martinsried, Germany). After 48 and 72 h viral supernatants were harvested, sterile filtered and supplemented with 4 μg/ml polybrene. Five×105 target cells (RAW264.7) were infected with 0.5 ml viral supernatant for 6 h. The newly designated RAW.sh cells were transfected with the lentiviral expression vector pGLTR-X-S together with the packaging vectors and Metafectene™ (Sigl et al., 2014). This allows dual selection for both GFP (via FACS sorting) and puromycin (Puro), before their knockdown efficiency was determined, and the cells were then used for subsequent experiments. Inducible shRNA transduction reached 82% (hmox.sh 2) and 94% (hmox.sh 1) hmox1 gene knockdown efficacy—after treatment of the RAW.sh macrophages with 1 μg/ml doxycycline 24–48 h prior to experiments as compared to the luciferase control (ctrl.sh). Apart from phenotype control, and unless stated differently, hmox.sh 1 was used for the hmox knockdown experiments (hereafter termed hmox.sh).
Cell culture
RAW 264.7 murine macrophage-like cells were maintained in complete DMEM containing 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (all purchased from Biochrom AG) at 37°C in humidified air containing 5% CO2. Cells were stimulated with 50 U/ml recombinant murine IFN-γ (muIFN- γ; purchased from R&D) and 10 ng/ml LPS (Escherichia coli 055:B5; obtained from Sigma); or 10 μM of the HO-1 inhibitor zinc (II) protoporphyrin IX (ZnPP; Sigma) dissolved in 0.1 N NaOH, for 24 h. Control samples were treated with PBS. Thereafter, supernatants were harvested and macrophages subjected to RNA preparation.
Atomic absorption spectrometry
Intracellular iron levels were determined by atomic absorption spectrometry as described elsewhere (Theurl et al., 2009).
Quantification of iron uptake and release by macrophages
To evaluate transferrin-bound iron (TBI) and non-transferrin-bound iron (NTBI) uptake or cellular release, RAW264.7 cells were infected with S. Typhimurium as detailed below and 59Fe-TBI or NTBI uptake and release were determined as described in Ludwiczek et al. (2003) and Nairz et al. (2007) using 10 μM of 59FeSO4 (Perkin Elmer®) plus 200 μM Trisodium-Citrate (Sigma) or 12.5 μg/ml 59Fe-bound Transferrin (Tf) respectively.
Bacterial iron acquisition
The iron uptake of intracellular Salmonella was assessed according to a modified protocol described in (Nairz et al., 2007). In brief, a standard in vitro Salmonella infection of RAW cells at a multiplicity of infection (MOI) of 10 was performed. One hour later, cells were washed 3 times with PBS plus gentamicin (25 μg/ml), then resuspended in serum-free uptake/release Buffer (DMEM; 2 mM L-glutamine; 25 mM Hepes; plus 1 μg/ml doxycycline and 10 μg/ml gentamycin). For the negative control cells were infected with heat-inactivated Salmonella. They are used as a defined inflammatory stimulus not compromised by bacterial number. Ten μM of 59FeSO4 (Perkin Elmer®) mixed with 400 μM Trisodium- Citrate (Sigma) were added. After incubation for 18 h under standard culture conditions cells were washed three times with NaCl 0.9% containing 50 μM FeSO4. Buffer was changed to washing buffer (0.01% SDS containing 1 mg/ml Pronase; Roche) and the cells were gently scraped from the dish. The cells were broken open by adding glas-beads (Lenz Laborglas GmbH) and vortexing the cell suspension at the highest setting for 1 min. An aliquot of the suspension was kept for assessment of total iron and bacterial load (CFU) by plating serial dilutions onto agar plates. One thousand U/ml DNAse I (Roche) was added to the remaining lysate for 10–20 min. Thereafter the supernatant was loaded onto 0.22 μm PDVF Filters (Milipore) and centrifuged 5 min at 10 000×g. The filter containing the trapped bacteria was washed 3–5 times with washing buffer, cut into pieces and placed into a γ-counter tube to assess the amount of Salmonella-associated 59Fe.
Isolation of bone marrow-derived macrophages (BMDM)
Tibias and femurs from 8 to 12 week old mice with macrophage specific deletions of HO-1 (LysM-Cre+/+HO-1fl/fl; HO−/−) and controls (LysM-Cre−/−HO-1fl/fl; wt) were collected in ice-cold PBS. The mice were kept under pathogen-free conditions at the Instituto Gulbenkian de Ciencia (Mamiya et al., 2008; Wegiel et al., 2014) or the KILM Research Labs of the Medical University of Vienna (Jais et al., 2014). Bones were sterilized with 70% ethanol and flushed with a 25-G needle using cold PBS containing 1% penicillin-streptomycin. Cells were seeded onto ∅ 10 cm dishes in DMEM supplemented with 10% FCS, 1% penicillin-streptomycin and 50 μg/ml macrophage colony-stimulating factor (M-CSF, purchased from Peprotech). Medium was changed every 2 days, and 6 days after preparation cells were harvested and seeded onto six-well plates at 0.5 × 106 cells per well. One day later cells were infected with Salmonella as described below.
Salmonella infection in vitro
RAW264.7 cells stable transfected with hmox1 shRNA or BMDM were used for in vitro infection assays generating comparable results. Prior to in vitro infection, macrophages were incubated in complete medium without antibiotics. Wild-type Salmonella enterica serovar Typhimurium (S. Typhimurium; S. Tm wt) strain ATCC14028 (resistant to tetracycline) or a Salmonella triple mutant derivative entC::aph sit::bla feo::Tn10 (Kanr Apr Tetr) were used for infection. The mutant strain, deficient in enterobactin synthesis, SitABCD- and Feo-mediated iron uptake (ΔentCΔsitΔfeo) was constructed and grown as previously described (Crouch et al., 2008). Salmonella were grown under sterile conditions in LB broth (Sigma) to late-logarithmic phase. Macrophages were infected with S. Typhimurium at a MOI of 10 and harvested as described (Nairz et al., 2009). For experiments employing heat-inactivated Salmonella, bacteria were suspended in complete DMEM, incubated at 70°C for 20 min and cooled to 37°C prior to use. For phagocytosis experiments, infections were terminated after 1 h by extensively washing the cells five times with PBS and subsequent lysis of the cells with Na-deoxycholate (Sigma). Please see Supporting Information for additional details regarding heme catabolism products.
RNA preparation, reverse transcription and polymerase chain reaction
Preparation of total RNA and quantification of mRNA expression by quantitative reverse transcription polymerase chain reaction (qRT-PCR) were performed exactly as described in (Nairz et al., 2009) using SsoFast™ qPCR Supermix (BioRad™). The primers and probes used for the experiments are shown in Supporting Information (Table S1).
Western blot analysis
Protein extracts were prepared with cytoplasmic lysis buffer (25mM Tris-HCl [pH7.4], 40mM KCl, and 1% Triton X-100) supplemented with 1 mg/ml aprotinin and 1 mg/ml leupeptin (all obtained from Sigma). Twenty micrograms of total protein was run on 10%−15% SDS-polyacrylamide gels, and Western blotting was performed exactly as described (Theurl et al., 2006) with a mouse anti-human TfR1 antibody (1:1000; Invitrogen), rabbit anti-human Ferritin (1:500; Sigma), rabbit anti-Fpn (1:400; self designed; Eurogentic), rabbit anti-HO-1 (1:1000; Enzo), rabbit anti-iNOS (1:1000; BD), rabbit anti-NFκB p65 or rabbit anti-phosphor NFκB p65 (both 1:1000; Cell Signalling). Blotting with either rabbit anti-TBP (1:1000; Cell Signalling) or rabbit anti-Actin antibody (1:1000; Sigma-Aldrich) was performed as a loading control.
Transcription factor assays
Nuclear protein extracts were prepared with the Nuclear and Cytoplasmic Extraction Reagent (Thermo Scientific). NF-kB p65-binding activity of nuclear extracts was assessed with a commercially available chemi-luminescent transcription factor assay kit in exact accordance with the manufacturer’s instructions (Thermo Scientific).
Detection of cytokines, nitrite and reactive oxygen species
Determination of cytokines in culture supernatants was performed with ELISA kits for TNF-α and Lcn2 (from R&D; Wiesbaden, Germany) and IL-6, IL-10 (from BD), following manufacturer’s instructions. Determination of nitrite, the stable oxidation product of NO, was carried out with the Griess-Ilosvay’s nitrite reagent (Merck) as described by Nairz et al. (2009). Detection of ROS was carried out after a standard infection experiment using CellROX®DeepRed Flow Cytometry Assay Kit (Life Technologies) according to the manufacturer’s instructions. In brief, CellROX®DeepRed was added at the concentration of 500 nM to the infected or induced cells and incubated for 30 min at 37°C. SYTOX Blue Dead Cell stain (1 mM) was then added and the cells analyzed by FACS sorting within the next 120 min. The following controls were included: Cells treated with N-acetyl-L-cysteine (NAC) or tert-butyl hydroperoxide (TBHP), and an unstained cell sample.
Statistical analysis
Statistical analysis was done with an SPSS statistical package. Significance was determined by analysis of variance (ANOVA) combined with Bonferroni’s correction. Unless otherwise specified, data are depicted as lower quartile, median and upper quartile (boxes) with minimum and maximum ranges. Individual values and means of log transformed values are depicted. Generally, P values less than 0.05 were considered significant in any test.
Supplementary Material
Fig. S1. Impact of heme catabolism products on bacterial burden and ROS production.
Fig. S2. Nramp1 functionality does not affect HO-1 mediated regulation of intracellular Salmonella replication.
Fig. S3. Effect of hmox1 knockdown on the cytotoxicity during Salmonella infection and hmox2 expression.
Table S1. Primers used for qRT-PCR. Annealing temperature was 60°C for all primer pairs.
Acknowledgement
The authors thank Sylvia Berger, Sabine Engl, Ursula Englmeier and Markus Seifert for excellent technical support. This work was supported by grants from the Austrian Research Fund (FWF) Project TRP-188 to G. Weiss, by NIH grant AI112640 to F. Fang, by the Fundaçêo Calouste Gulbenkian para a Ciência e Tecnologia (PTDC/SAU TOX/116627/2010, HMSP-ICT/0022/2010) to M. P. Soares and by the ‘Verein zur Förderung von Forschung und Weiterbildung in Infektiologie und Immunologie an der Medizinischen Universität Innsbruck’. The authors have no competing financial interests to declare.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this articleat the publisher’s web-site.
References
- Abdalla MY, Ahmad IM, Switzer B, and Britigan BE (2015) Induction of heme oxygenase-1 contributes to survival of Mycobacterium abscessus in human macrophages-like THP-1 cells. Redox Biol 4: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables GP, Takamatsu D, Noma H, El-Shazly S, Jin HK, Taniguchi T, et al. (2001) The roles of Nramp1 and Tnfa genes in nitric oxide production and their effect on the growth of Salmonella typhimurium in macrophages from Nramp1 congenic and tumor necrosis factor-alpha−/− mice. J Interferon cytokine Res: Off J Int Soc Interferon Cytokine Res 21: 53–62. [DOI] [PubMed] [Google Scholar]
- Andrews-Polymenis HL, Baumler AJ, McCormick BA, and Fang FC (2010) Taming the elephant: Salmonella biology, pathogenesis, and prevention. Infect Immun 78: 2356–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arezes J, Jung G, Gabayan V, Valore E, Ruchala P, Gulig PA, et al. (2015) Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe 17: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, et al. (2011) Hepcidin regulation by innate immune and infectious stimuli. Blood 118: 4129–4139. [DOI] [PubMed] [Google Scholar]
- Bao G, Clifton M, Hoette TM, Mori K, Deng SX, Qiu A, et al. (2010) Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol 6: 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann-Weiler R, Martinz V, Kurz K, Engl S, Feistritzer C, Fuchs D, et al. (2010) Divergent modulation of Chlamydia pneumoniae infection cycle in human monocytic and endothelial cells by iron, tryptophan availability and interferon gamma. Immunobiology 215: 842–848. [DOI] [PubMed] [Google Scholar]
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. (2006) Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A 103: 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2: 907–916. [DOI] [PubMed] [Google Scholar]
- Cassat JE, and Skaar EP (2013) Iron in infection and immunity. Cell Host Microbe 13: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier MF, Courville P, and Campion C (2007) Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infection/Institut Pasteur 9: 1662–1670. [DOI] [PubMed] [Google Scholar]
- Chlosta S, Fishman DS, Harrington L, Johnson EE, Knutson MD, Wessling-Resnick M, and Cherayil BJ (2006) The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun 74: 3065–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SW, Liu X, Macias AA, Baron RM, and Perrella MA (2008) Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest 118: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch ML, Castor M, Karlinsey JE, Kalhorn T, and Fang FC (2008) Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol 67: 971–983. [DOI] [PubMed] [Google Scholar]
- Cunnington AJ, de Souza JB, Walther M, and Riley EM (2012) Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med 18: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmard M, Davidge KS, Bouvet O, Morin D, Roux D, Foresti R, et al. (2009) A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. FASEB J: Off Pub Fed Am Soc Experiment Biol 23: 1023–1031. [DOI] [PubMed] [Google Scholar]
- DiDonato JA, Mercurio F, and Karin M (2012) NF-kappaB and the link between inflammation and cancer. Immunol Rev 246: 379–400. [DOI] [PubMed] [Google Scholar]
- Drakesmith H, and Prentice AM (2012) Hepcidin and the iron-infection axis. Science 338: 768–772. [DOI] [PubMed] [Google Scholar]
- Evstatiev R, and Gasche C (2012) Iron sensing and signalling. Gut 61: 933–952. [DOI] [PubMed] [Google Scholar]
- Fang FC, and Weiss G (2014) Iron ERRs with Salmonella. Cell Host Microbe 15: 515–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, et al. (1999) Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol 1: 152–157. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432: 917–921. [DOI] [PubMed] [Google Scholar]
- Frawley ER, Crouch ML, Bingham-Ramos LK, Robbins HF, Wang W, Wright GD, and Fang FC (2013) Iron and citrate export by a major facilitator superfamily pump regulates metabolism and stress resistance in Salmonella Typhimurium. Proc Natl Acad Sci U S A 110: 12054–12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche G, Nairz M, Libby SJ, Fang FC, and Weiss G (2012) Slc11a1 (Nramp1) impairs growth of Salmonella enterica serovar typhimurium in macrophages via stimulation of lipocalin-2 expression. J Leukoc Biol 92: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T (2009) Iron in innate immunity: starve the invaders. Curr Opin Immunol 21: 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, and Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72: 1493–1505. [DOI] [PubMed] [Google Scholar]
- Gozzelino R, Andrade BB, Larsen R, Luz NF, Vanoaica L, Seixas E, et al. (2012) Metabolic adaptation to tissue iron overload confers tolerance to malaria. Cell Host Microbe 12: 693–704. [DOI] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, and Soares MP (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323–354. [DOI] [PubMed] [Google Scholar]
- Haschka D, Nairz M, Demetz E, Wienerroither S, Decker T, and Weiss G (2015) Contrasting regulation of macrophage iron homeostasis in response to infection with Listeria monocytogenes depending on localization of bacteria. Metallomics: Integr Biometal Sci 7: 1036–1045. [DOI] [PubMed] [Google Scholar]
- Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, and Plevy SE (2005) Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med 202: 1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Galy B, and Camaschella C (2010) Two to tango: regulation of mammalian iron metabolism. Cell 142: 24–38. [DOI] [PubMed] [Google Scholar]
- Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, et al. (2014) Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell 158: 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J (2002) Mechanisms of cellular iron acquisition: another iron in the fire. Cell 111: 603–606. [DOI] [PubMed] [Google Scholar]
- Karin M, and Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5: 749–759. [DOI] [PubMed] [Google Scholar]
- Lahiri A, Lahiri A, Iyer N, Das P, and Chakravortty D (2010) Visiting the cell biology of Salmonella infection. Microbes Infection/Institut Pasteur 12: 809–818. [DOI] [PubMed] [Google Scholar]
- Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, et al. (2010) A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2: 51ra71. [DOI] [PubMed] [Google Scholar]
- Leung KY, and Finlay BB (1991) Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci U S A 88: 11470–11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Reba S, Chen WD, Porwal SK, Boom WH, Petersen RB, et al. (2014) Regulation of mammalian siderophore 2,5-DHBA in the innate immune response to infection. J Exp Med 211: 1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwiczek S, Aigner E, Theurl I, and Weiss G (2003) Cytokine-mediated regulation of iron transport in human monocytic cells. Blood 101: 4148–4154. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, and Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Katsuoka F, Hirayama A, Nakajima O, Kobayashi A, Maher JM, et al. (2008) Hepatocyte-specific deletion of heme oxygenase-1 disrupts redox homeostasis in basal and oxidative environments. Tohoku J Exp Med 216: 331–339. [DOI] [PubMed] [Google Scholar]
- Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, and Dougan G (2000) Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med 192: 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megias J, Busserolles J, and Alcaraz MJ (2007) The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells. Br J Pharmacol 150: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo G, Taylor LS, Brooks A, Musso T, Cox GW, and Varesio L (1997) Functional requirement of the hypoxia-responsive element in the activation of the inducible nitric oxide synthase promoter by the iron chelator desferrioxamine. J Biol Chem 272: 12236–12243. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Haas B,and Foresti R (2012) Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs). Med Gas Res 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulero V, and Brock JH (1999) Regulation of iron metabolism in murine J774 macrophages: role of nitric oxide-dependent and -independent pathways following activation with gamma interferon and lipopolysaccharide. Blood 94: 2383–2389. [PubMed] [Google Scholar]
- Nairz M, Ferring-Appel D, Casarrubea D, Sonnweber T, Viatte L, Schroll A, et al. (2015) Iron regulatory proteins mediate host resistance to Salmonella infection. Cell Host Microbe 18: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Haschka D, Demetz E, and Weiss G (2014) Iron at the interface of immunity and infection. Front Pharmacol 5: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, et al. (2013) Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med 210: 855–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Schroll A, Sonnweber T, and Weiss G (2010) The struggle for iron—a metal at the host-pathogen interface. Cell Microbiol 12: 1691–1702. [DOI] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, and Weiss G (2007) The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol 9: 2126–2140. [DOI] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Schroll A, Theurl M, Fritsche G, Lindner E, et al. (2009) Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood 114: 3642–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, and Shiloh MU (2000) Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97: 8841–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093. [DOI] [PubMed] [Google Scholar]
- Nix RN, Altschuler SE, Henson PM, and Detweiler CS (2007) Hemophagocytic macrophages harbor Salmonella enterica during persistent infection. PLoS Pathog 3: e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre LS, Al-Shahrour F, Dopazo J, and Saraiva LM (2009) Exploring the antimicrobial action of a carbon monoxide-releasing compound through whole-genome transcription profiling of Escherichia coli. Microbiology 155: 813–824. [DOI] [PubMed] [Google Scholar]
- Oexle H, Kaser A, Most J, Bellmann-Weiler R, Werner ER, Werner-Felmayer G, and Weiss G (2003) Pathways for the regulation of interferon-gamma-inducible genes by iron in human monocytic cells. J Leukoc Biol 74: 287–294. [DOI] [PubMed] [Google Scholar]
- Olakanmi O, Schlesinger LS, Ahmed A, and Britigan BE (2002) Intraphagosomal Mycobacterium tuberculosis acquires iron from both extracellular transferrin and intracellular iron pools. Impact of interferon-gamma and hemochromatosis. J Biol Chem 277: 49727–49734. [DOI] [PubMed] [Google Scholar]
- Onyiah JC, Sheikh SZ, Maharshak N, Steinbach EC, Russo SM, Kobayashi T, et al. (2013) Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology 144: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, et al. (2007) Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res 100: 1703–1711. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. (2000) Carbon monoxide has antiinflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428. [DOI] [PubMed] [Google Scholar]
- Pantopoulos K, Porwal SK, Tartakoff A, and Devireddy L (2012) Mechanisms of mammalian iron homeostasis. Biochemistry 51: 5705–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, and Ward DM (2008) Iron depletion limits intracellular bacterial growth in macrophages. Blood 112: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GM, Pou S, Ramos CL, Cohen MS, and Britigan BE (1995) Free radicals and phagocytic cells. Faseb J 9:200–209. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, and Choi AM (2006) Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650. [DOI] [PubMed] [Google Scholar]
- Ryter SW, and Tyrrell RM (2000) The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme Oxygenase has both pro- and Antioxidant Properties Free Radical Biol & med 28: 289–309. [DOI] [PubMed] [Google Scholar]
- Sawle P, Foresti R, Mann BE, Johnson TR, Green CJ, and Motterlini R (2005) Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br J Pharmacol 145: 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, and Kaufmann SH (2004) Iron and microbial infection. Nat Rev Microbiol 2: 946–953. [DOI] [PubMed] [Google Scholar]
- Seldon MP, Silva G, Pejanovic N, Larsen R, Gregoire IP, Filipe J, et al. (2007) Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-kappaB RelA phosphorylation at serine 276. J Immunol 179: 7840–7851. [DOI] [PubMed] [Google Scholar]
- Shiloh MU, and Nathan CF (2000) Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr Opin Microbiol 3: 35–42. [DOI] [PubMed] [Google Scholar]
- Sigl R, Ploner C, Shivalingaiah G, Kofler R, and Geley S (2014) Development of a multipurpose GATEWAY-based lentiviral tetracycline-regulated conditional RNAi system (GLTR). PLoS One 9: e97764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Gomes S, Appelberg R, Larsen R, Soares MP, and Gomes MS (2013) Heme catabolism by heme oxygenase-1 confers host resistance to Mycobacterium infection. Infect Immun 81: 2536–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MP, and Weiss G (2015) The iron age of host- microbe interactions. EMBO Rep 16: 1482–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, and Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares AF, Teixeira M, Romao CC, Seixas JD, Nobre LS, and Saraiva LM (2011) Reactive oxygen species mediate bactericidal killing elicited by carbon monoxidereleasing molecules. J Biol Chem 286: 26708–26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, and Schmid R (1968) The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 61: 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. (2009) Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood 113: 5277–5286. [DOI] [PubMed] [Google Scholar]
- Theurl I, Mattle V, Seifert M, Mariani M, Marth C, and Weiss G (2006) Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood 107: 4142–4148. [DOI] [PubMed] [Google Scholar]
- Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, et al. (2008) Autocrine formation of hepcidin induces iron retention in human monocytes. Blood 111: 2392–2399. [DOI] [PubMed] [Google Scholar]
- Van Zandt KE, Sow FB, Florence WC, Zwilling BS, Satoskar AR, Schlesinger LS, and Lafuse WP (2008) The iron export protein ferroportin 1 is differentially expressed in mouse macrophage populations and is present in the mycobacterial-containing phagosome. J Leukoc Biol 84: 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varesio L, Battaglia F, Raggi F, Ledda B, and Bosco MC (2010) Macrophage-inflammatory protein-3alpha/CCL-20 is transcriptionally induced by the iron chelator desferrioxamine in human mononuclear phagocytes through nuclear factor (NF)-kappaB. Mol Immunol 47: 685–693. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, and Fang FC (2000) Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med 192: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan J, Karlinsey JE, Frawley ER, Becker LA, Nartea M, and Fang FC (2014) Distinct roles of the Salmonella enterica serovar Typhimurium CyaY and YggX proteins in the biosynthesis and repair of iron-sulfur clusters. Infect Immun 82: 1390–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor VM, Rocha M, and De la Fuente M (2003) Regulation of macrophage function by the antioxidant N-acetylcysteine in mouse-oxidative stress by endotoxin. Int Immunopharmacol 3: 97–106. [DOI] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Nakahira K, Ryter SW, and Choi AM (2009) The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol 182: 3809–3818. [DOI] [PubMed] [Google Scholar]
- Ward DM, and Kaplan J (2012) Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta 1823: 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B, Hauser CJ, and Otterbein LE (2015) Heme as a danger molecule in pathogen recognition. Free Radic Biol Med 89: 651–661. [DOI] [PubMed] [Google Scholar]
- Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, et al. (2014) Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. JClin Invest 124: 4926–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED (2009) Iron availability and infection. Biochim Biophys Acta 1790: 600–605. [DOI] [PubMed] [Google Scholar]
- Weiss G, and Schaible UE (2015) Macrophage defense mechanisms against intracellular bacteria. Immunol Rev 264: 182–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Wachter H, and Hentze MW (1994) Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med 180: 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M (2010) Iron homeostasis and the inflammatory response. Annu Rev Nutr 30: 105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, et al. (2001) Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 89: 168–173. [DOI] [PubMed] [Google Scholar]
- Zaki MH, Fujii S, Okamoto T, Islam S, Khan S, Ahmed KA, et al. (2009) Cytoprotective function of heme oxygenase 1 induced by a nitrated cyclic nucleotide formed during murine salmonellosis. J Immunol 182: 3746–3756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Impact of heme catabolism products on bacterial burden and ROS production.
Fig. S2. Nramp1 functionality does not affect HO-1 mediated regulation of intracellular Salmonella replication.
Fig. S3. Effect of hmox1 knockdown on the cytotoxicity during Salmonella infection and hmox2 expression.
Table S1. Primers used for qRT-PCR. Annealing temperature was 60°C for all primer pairs.