Abstract
Purpose:
Little is known about the longitudinal patterns of buprenorphine adherence among pregnant women with opioid use disorder, especially when late initiation, nonadherence, or early discontinuation of buprenorphine during pregnancy may increase the risk of adverse outcomes. We aimed to identify distinct trajectories of buprenorphine use during pregnancy, and factors associated with these trajectories in Medicaid-enrolled pregnant women.
Methods:
A retrospective cohort study included 2361 Pennsylvania Medicaid enrollees aged 15 to 46 having buprenorphine therapy during pregnancy and a live birth between 2008 and 2015. We used group-based trajectory models to identify buprenorphine use patterns in the 40 weeks prior to delivery and 12 weeks postdelivery. Multivariable multinomial logistic regression models were used to identify factors associated with specific trajectories.
Results:
Six distinct trajectories were identified. Four groups initiated buprenorphine during the first trimester of the pregnancy (early initiators): 31.6% with persistently high adherence, 15.1% with moderate-to-high adherence, 10.5% with declining adherence, and 16.7% with early discontinuation. Two groups did not initiate buprenorphine until midsecond or third trimester (late initiators): 13.5% had moderate-to-high adherence and 12.6% had low-to-moderate adherence. Factors significantly associated with late initiation and discontinuation were younger age, non-white race, residents of rural counties, fewer outpatient visits, more frequent emergency department visits and hospitalizations, and lower buprenorphine daily dose.
Conclusions:
Six buprenorphine treatment trajectories during pregnancy were identified in this population-based Medicaid cohort, with 25% of women initiating buprenorphine late during pregnancy. Understanding trajectories of buprenorphine use and factors associated with discontinuation/nonadherence may guide integration of behavioral treatment with obstetrical/gynecological care to improve buprenorphine treatment during pregnancy.
Keywords: adherence, buprenorphine, group-based trajectory models, Medicaid, opioid use disorder, pharmacoepidemiology, pregnant, trajectory
1 ∣. INTRODUCTION
In the last 2 decades, opioid use disorder (OUD) and overdose deaths among reproductive-aged and pregnant women have increased dramatically in the United States.1 Pregnant women with OUD have an increased risk of adverse maternal, obstetric, and neonatal outcomes (eg, preterm birth, fetal growth restriction).2,3 Opioid agonist therapy has been shown to reduce illicit drug use and the risk of infection and improve adherence to prenatal care, maternal nutrition, and infant birth weight.4
Methadone has been the standard therapy for pregnant women with OUD for over 40 years. Emerging evidence supports buprenorphine (without naloxone) as an alternative first-line therapeutic option in pregnancy.5 A multicenter, randomized controlled trial demonstrated that buprenorphine-exposed infants had a shorter length of hospital stays, shorter treatment durations for neonatal abstinence syndrome (NAS), and a lower cumulative morphine dose to treat NAS compared to methadone-exposed newborns.5 Other advantages of buprenorphine over methadone for pregnant women include a lower risk of overdose and fewer drug interactions.6
Little evidence exists on longitudinal utilization and adherence patterns of buprenorphine therapy during the pregnancy and postpartum periods among pregnant women with OUD. Typical measures of adherence (eg, average over a year period) or simple criteria of discontinuation (eg, with a gap ≥30 days) do not capture all clinically relevant measures of buprenorphine use such as the timing and dynamic patterns of treatment initiation, nonadherence, and discontinuation during pregnancy. Group-based trajectory models with intuitive graphical results can leverage the dynamic nature of buprenorphine use and identify subgroups with similar change over time.7-9 In our prior work of 10 945 Pennsylvania Medicaid beneficiaries newly initiating buprenorphine,8 we successfully identified 6 distinct treatment trajectories during the first year of treatment using group-based trajectory models and examined their association with subsequent health outcomes. For example, refilling buprenorphine intermittently was associated with a 24% higher risk of emergency department (ED) visits. Yet, our prior work largely focused on nonpregnant beneficiaries and did not examine timing of initiation and discontinuation patterns of buprenorphine during pregnancy and postdelivery. Therefore, we aimed to identify distinct longitudinal utilization and adherence trajectories of buprenorphine treatment and associated factors among pregnant women with OUD in Pennsylvania Medicaid program. Given that substantial numbers of pregnant women with OUD are Medicaid eligible and Medicaid is the largest funder of substance use disorder treatment services,10,11 our findings may shed light on patterns of buprenorphine therapy among pregnant women with OUD and factors associated with late initiation, nonadherence, or early discontinuation for target interventions (eg, case management).
2 ∣. METHODS
2.1 ∣. Data sources
In this retrospective longitudinal analysis, we used Pennsylvania Medicaid administrative claims data of all fee-for-service and managed care enrollees from January 2008 through September 2015. Pennsylvania Medicaid ranks the fourth largest of 50 US states by expenditure and the seventh by enrollment (~3 million enrollees annually).12 The datasets capture eligibility and enrollment information, and all health care services reimbursed by Medicaid including outpatient, inpatient, and professional services as well as prescription drugs. This study of deidentified data was deemed human subjects exempt from review by the University of Pittsburgh Institutional Review Board.
2.2 ∣. Study cohort
Eligible cohort included women aged 15 to 46 years, receiving buprenorphine prescriptions during pregnancy either for sublingual buprenorphine or for buprenorphine/naloxone combination products in generic or proprietary formulations. We excluded women who were dually eligible for Medicare because their prescription drug utilization was not captured in the Medicaid data. We excluded patients who only used intravenous or transdermal buprenorphine formulations, which are approved by the US Food and Drug Administration for pain and not for OUD. Among the 2582 women who received ≥1 buprenorphine prescription, we excluded 221 women who also received methadone during pregnancy (168 switching from buprenorphine to methadone, 15 using methadone prior to buprenorphine, and 38 switching between methadone and buprenorphine for multiple times) to avoid misclassification of treatment switching as nonadherence or discontinuation of OUD treatment. Global reimbursement for methadone in Medicaid also imposes a challenge on measuring adherence to methadone in claims data.
We identified each patient's delivery dates and their birth outcomes, and excluded those who only had induced/spontaneous abortions (Appendix Figure S1). According to the US National Committee for Quality Assurance (NCQA)'s quality measures of prenatal and postpartum care, we used 280 days prior to the delivery date as the estimated conception date.13 Patients were required to have continuous Medicaid enrollment during the first trimester (ie, 176 to 280 days prior to delivery) and 43 days prior to delivery and 12 weeks after delivery to obtain information on predictor variables and allow for complete follow-up.13 The 12-week postdelivery period was chosen because pregnant women in Pennsylvania Medicaid, like many other states, have coverage until 12 weeks after delivery, and this window was considered as clinically reasonable for a postpartum visit (which is recommended at 6 weeks). We followed each patient's prescription buprenorphine fills from the estimated conception date through 12 weeks following the delivery date. When women (n = 407) had multiple pregnancy episodes with buprenorphine treatment during the study period, only the first episode was included in the analysis. The final analytic sample included 2361 women.
2.3 ∣. Outcome variable: trajectories of buprenorphine refill patterns
Our primary outcome of interest was membership in a distinct trajectory of buprenorphine use based on a longitudinal analysis of the days covered with buprenorphine during pregnancy and 12 weeks postdelivery.7-9,14 Our analyses proceeded in 2 steps.
First, based on dispensing date and days supplied, we created daily flags if the day was covered with buprenorphine starting from the estimated conception date to 12 weeks postdelivery, to establish buprenorphine treatment trajectories.15,16 When a dispensing occurred before the previous dispensing should have run out, utilization of the refill was assumed to begin the day after the end of the previous dispensing.
Second, group-based trajectory models can identify differential patterns of individual change over time and characterize subgroups more likely to follow certain trajectories.17,18 We estimated group-based trajectory models using longitudinal binary daily flags as the outcome variable (ie, covered vs not covered with buprenorphine), and the time variable was days since the estimated conception date (1-365). In each model, we used the most flexible functional form of time to allow the trajectories to emerge from the data. Output of trajectory models includes estimated probabilities of group membership for each individual, and estimated trajectory curves over time.17,18 We selected the final model based on a combination of (1) the Bayesian information criterion (BIC), wherein the largest value indicates the best-fitting model, (2) an estimated proportion of each trajectory group that was sufficiently large (>0.05), and (3) application of Nagin's criteria to assess final model adequacy.19 The Nagin's criteria of a well-performed trajectory model includes an average posterior probability ≥0.7 for all groups, odds correct classification ≥5.0 for all groups, estimated probability of membership in each group close to the proportion of sample assigned to each group, and narrow confidence intervals.19 These models were estimated using STATA 14.0 (Stata-Corp LP, College Station, TX) and the TRAJ macro (free download at http://www.andrew.cmu.edu/user/bjones).
2.4 ∣. Factors associated with different buprenorphine trajectories
After determining the most appropriate trajectory models, we compared the patient characteristics across identified trajectories with X2 test and analysis of variance, as appropriate. We then examined the association between the membership in a trajectory group and the factors that were significantly different across trajectories from univariate analyses using a multivariable multinomial logistic regression model. We reported adjusted odds ratios (ORs) with 95% confidence intervals (CI). Statistical significance was defined as 2-tailed P < .05.
Based on prior studies of buprenorphine therapy for OUD and medication adherence,8,20-31 we examined the following patient-level factors: sociodemographic factors including age, race/ethnicity (white vs non-white), type of health plan (fee-for-service or managed care), and eligibility category (pregnancy vs temporary assistance for the needy families [TANF] and other categories). Based on the classification from the National Center for Health Statistics 2010 Rural-Urban County Continuum, we also included an urbanicity measure of enrollees’ county of residence (metropolitan vs nonmetropolitan; https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/) because access to care may vary by urbanicity. Health status factors were measured during pregnancy and included diagnosis of OUD, other nonopioid drug use disorder, alcohol use disorder, mental health disorders, tobacco use, adverse pregnancy history (ie, preterm labor/delivery, other high-risk pregnancy), and history of prior methadone treatment any time between 2008 and prior to pregnancy (see supplement Table S1 for the detailed diagnosis and procedure codes).
Health service use factors included any inpatient utilization, number of emergency department (ED) visits, number of outpatient visits, number of other prescriptions with unique ingredients (as a proxy measure of disease and medication complexity), and number of unique prescribers for nonbuprenorphine prescriptions. We also calculated buprenorphine average daily dose (mg/day), maximum increase in buprenorphine dose (mg), number of unique buprenorphine prescribers, and average proportion of days covered (PDC) from estimated conception date to 12 weeks postdelivery. Finally, we measured whether patients received any behavioral counseling as proxy of quality of care for their OUD during the pregnancy.32 We also examined whether women received any drug urine tests during pregnancy although false positive results from urine drug testing raise concerns regarding negative consequences (eg, accusation of child abuse/neglect by state laws).33
2.5 ∣. Sensitivity and post hoc analyses
Women with OUD eligible for Medicaid due to pregnancy may have either no or a very short look-back period prior to the estimated conception date during which factors prior to pregnancy can be measured. This limited our ability to assess the prediction accuracy that was possible before observing any follow-up and to avoid factors such as changes in patient health status that may themselves be consequences of the use (or nonuse) of buprenorphine. Therefore, we conducted an additional sensitivity analysis excluding health service use factors from our final model to ensure the robustness of our findings. Results from the sensitivity analyses were similar to the main findings (supplemental Table S5). Furthermore, we were unable to examine the association between the identified trajectories and relevant clinical outcomes including prenatal care visits (unmeasurable due to being paid via global payment with delivery), neonatal outcomes (without linkage between maternal and infant records), and long-term maternal outcomes (loss of Medicaid eligibility after 12 weeks postdelivery prior to Medicaid expansion in 2015). We conducted a post hoc analysis to examine proportions of all-cause ED visits and hospitalization between 85 days and 1 year postdelivery across trajectories.
3 ∣. RESULTS
3.1 ∣. Trajectories of buprenorphine refill patterns
Among 2361 pregnant women filling buprenorphine prescriptions, the overall average PDC was 59% (SD 31%) from the estimated conception date through 12 weeks postdelivery, with considerable between-enrollee and within-enrollee variability. According to a combination of BIC value (BIC = −303 412.82), estimated group proportions, and Nagin's criteria, a model with 6 distinct trajectories for buprenorphine use performed optimally and was selected as the final model (supplement Tables S2 and S3).17
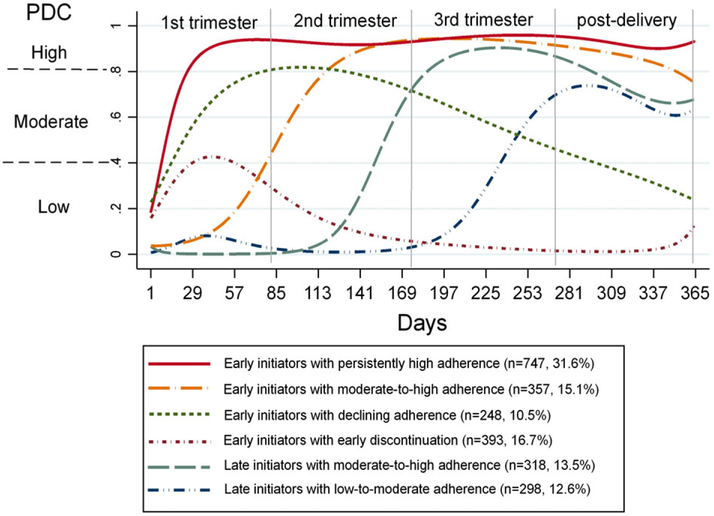
Figure 1 illustrates the predicted probability of each trajectory of days of covered with buprenorphine from the estimated conception date to 12 weeks postdelivery. Four of the 6 groups (73.6% of the cohort) initiated buprenorphine during the first trimester of pregnancy (ie, early initiators); however, there were distinct groups with respect to the adherence level. Specifically, one-third (n = 747, 31.6% of total cohort, 95% CI = 29.8%-33.5%) were early initiators with persistently high adherence, 15.1% (95% CI = 13.7%-16.6%) with moderate-to-high adherence, 10.5% (95% CI = 9.3%-11.8%) with declining adherence over time, and 16.7% (95% CI = 15.1%-18.1%) with early discontinuation of buprenorphine (ie, before third trimester). The remaining 2 trajectories did not initiate buprenorphine until midsecond trimester or third trimester (ie, late initiators) but with different adherence levels: 13.5% (95% CI = 12.1%-14.9%) having moderate-to-high adherence and 12.6% (95% CI = 11.2%-13.9%) having low-to-moderate adherence.
FIGURE 1.
Trajectories of buprenorphine use during pregnancy and 12 weeks postdelivery. PDC, proportion of days covered with buprenorphine [Colour figure can be viewed at wileyonlinelibrary.com]
The overall mean age was 27.8 (SD 4.6) years (Table 1). The majority of patients were white (95%) and in a managed care plan (88%), and resided in metropolitan counties (75%). Almost 90% of women were eligible for Medicaid through TANF or other eligibility groups, and the remaining were eligible through their pregnancy. Nearly 80% of women had at least 1 claim with a diagnosis of OUD in pregnancy. Comorbid nonopioid drug use disorders (72.8%), alcohol use disorder (4.6%), mental health disorders (40.4%), and tobacco use (68.0%) were also prevalent. Eighteen percent of women had an adverse pregnancy history. Two-thirds of women had received medication-assisted therapy prior to their pregnancy (buprenorphine = 55.9%, methadone = 2.5%, buprenorphine and methadone = 7.8%).
TABLE 1.
Characteristics of pregnant Medicaid enrollees filling buprenorphine prescriptions overall and by trajectory group: 2008 to 2015 (N = 2361)
| Trajectory Group |
|||||||
|---|---|---|---|---|---|---|---|
| Early Initiators with |
Late Initiators with |
||||||
| Characteristics | Overall (n = 2361) |
Persistently High Adherence (n = 747) |
Moderate-to-High Adherence (n = 357) |
Declining Adherence (n = 248) |
Early Discontinuation (n = 393) |
Moderate-to-High Adherence (n = 318) |
Low-to-Moderate Adherence (n = 298) |
| Sociodemographic | |||||||
| Age, mean (SD)† | 27.8 (4.6) | 28.5 (4.5) | 27.7 (4.8) | 27.9 (4.3) | 27.2 (4.8) | 27.6 (4.6) | 27.0 (4.3) |
| Race, %† | |||||||
| White | 94.5 | 96.3 | 95.8 | 92.3 | 89.1 | 97.5 | 94.3 |
| Non-white | 5.5 | 3.8 | 4.2 | 7.7 | 10.9 | 2.5 | 5.7 |
| Health plan type, %* | |||||||
| Fee-for-service | 11.9 | 10.4 | 10.6 | 9.3 | 11.7 | 14.2 | 17.5 |
| Managed care | 88.1 | 89.6 | 89.4 | 90.7 | 88.3 | 85.9 | 82.6 |
| Type of Medicaid eligibility, %† | |||||||
| Pregnancy | 10.1 | 6.6 | 13.5 | 4.8 | 9.9 | 16.7 | 12.8 |
| TANF and others | 89.9 | 93.4 | 86.6 | 95.2 | 90.1 | 83.3 | 87.3 |
| Resided county, %† | |||||||
| Metropolitan | 74.5 | 79.0 | 69.2 | 73.8 | 80.7 | 67.6 | 69.5 |
| Nonmetropolitan | 24.7 | 20.8 | 30.3 | 24.6 | 18.6 | 31.5 | 29.2 |
| Health status during pregnancy | |||||||
| OUD, %*** | 79.9 | 81.4 | 89.6 | 71.4 | 66.7 | 83.3 | 85.6 |
| Other nonopioid drug use disordersa, %† | 72.8 | 77.1 | 77.0 | 68.2 | 61.6 | 73.3 | 74.8 |
| Alcohol use disorders, %* | 4.6 | 4.8 | 3.6 | 4.0 | 2.3 | 7.9 | 5.4 |
| Mental health disordersb, % | 40.4 | 40.8 | 39.8 | 40.3 | 41.2 | 42.8 | 36.6 |
| Tobacco use, %** | 68.0 | 68.7 | 72.8 | 63.3 | 60.8 | 73.3 | 68.5 |
| Adverse pregnant historyc, % | 17.8 | 19.7 | 18.8 | 15.3 | 16.3 | 16.4 | 17.1 |
| Medication-assisted therapy history prior to pregnancy, %† | |||||||
| Buprenorphine | 55.9 | 80.3 | 43.4 | 69.8 | 56.0 | 26.1 | 29.9 |
| Methadone | 2.5 | 0.8 | 3.4 | 1.6 | 2.5 | 5.0 | 3.4 |
| Buprenorphine and methadone | 7.8 | 11.5 | 6.7 | 10.9 | 4.8 | 5.4 | 3.4 |
| None | 33.9 | 7.4 | 46.5 | 17.7 | 36.6 | 63.5 | 63.4 |
Abbreviations: OUD, opioid use disorders; TANF, temporary assistance for the needy families.
Comparison between 6 trajectory groups:
P < .05.
P < .01.
P < .001.
P < .0001.
Includes diagnosis of dependence on illicit substances other than opioids (cocaine, cannabis, amphetamine, hallucinogen, other sedatives and other substances) or a diagnosis of “antepartum drug dependence,” “maternal drug dependence at delivery,” or “postpartum drug dependence” (see supplemental Table S1).
Mental health disorders include major depression disorder, bipolar disorder, and schizophrenia (see supplemental Table S1).
Adverse pregnancy history includes diagnosis of history of preterm labor or delivery, or other high-risk pregnancy.
Table 1 shows a descriptive comparison of characteristics by buprenorphine trajectory. Compared to early initiators with persistently high adherence, late initiators regardless of their adherence level of buprenorphine were slightly younger (27.0-27.6 vs 28.5 years), more likely to be eligible for Medicaid through pregnancy (12.8%-16.7% vs 6.6%), less likely to live in metropolitan counties (67.6%-69.5% vs 79.0%), and had more frequent diagnoses of comorbid alcohol use disorders (5.4%-7.9% vs 4.8%). Compared to early initiators with persistently high adherence, early initiators with declining adherence or early discontinuation were less likely to be white (89.1%-92.3% vs 96.3%), have OUD diagnoses (66.7%-71.4% vs 81.4%), other nonopioid drug use disorders (61.6%-68.2% vs 77.1%), and tobacco use (60.8%-63.3% vs 68.7%). All of these differences were statistically significant (P < .01).
3.2 ∣. Patterns of health service use, buprenorphine therapy, and quality of care
In Table 2, compared to early initiators with persistently high adherence, late initiators or early initiators with declining adherence or early discontinuation had a higher hospitalization rate (9.1%-14.8% vs 6.7%, P < .001) during pregnancy. Early initiators with declining adherence or early discontinuation or late initiators with low-to-moderate adherence also had fewer outpatient visits (9.5-9.9 vs 11.3, P < .0001). Compared to early initiators with persistently high adherence, the other 5 trajectory groups, on average, used a lower buprenorphine daily dose (14.7-16.5 vs 17.6 mg/day, P < .0001). Overall, 30% to 40% of women received behavioral counseling, and 67% to 77% had a urine drug test during pregnancy.
TABLE 2.
Patterns of health service use, buprenorphine use, and quality of care during pregnancy of enrollees receiving buprenorphine therapy by trajectory group (N = 2361)
| Early Initiators with |
Late Initiators with |
|||||
|---|---|---|---|---|---|---|
| Persistently High Adherence (n = 747) |
Moderate-to-High Adherence (n = 357) |
Declining Adherence (n = 248) |
Early Discontinuation (n = 393) |
Moderate-to-High Adherence (n = 318) |
Low-to-Moderate Adherence (n = 298) |
|
| Health service use during pregnancy | ||||||
| Any hospitalizations, n (%)*** | 50 (6.7) | 30 (8.4) | 29 (11.7) | 50 (12.7) | 29 (9.1) | 44 (14.8) |
| No. of ED, mean (SD)** | 2.3 (3.3) | 3.0 (4.2) | 2.5 (3.9) | 3.3 (6.1) | 2.8 (4.8) | 3.1 (6.3) |
| No. of outpatient visits, mean (SD)† | 11.3 (8.7) | 12.3 (8.9) | 9.5 (8.5) | 9.5 (9.1) | 11.7 (9) | 9.9 (7.4) |
| Number of other prescriptionsa, mean (SD)* | 6.3 (4.2) | 6.7 (5.1) | 6.1 (4.3) | 6.6 (5.3) | 6.2 (4.7) | 5.6 (4.7) |
| Number of unique prescribers for other prescriptions, mean (SD) | 3.0 (1.9) | 3.2 (2.3) | 3.0 (2.0) | 3.0 (2.4) | 2.8 (2.1) | 2.8 (2.4) |
| Buprenorphine treatment patterns | ||||||
| Mean daily dose (mg/day) during first trimester, (SD)† | 17.4 (5.5) | 13.3 (7.5) | 16.6 (5.9) | 12.6 (8.5) | NAc | NAc |
| Mean daily dose (mg/day) during pregnancy, (SD)† | 17.6 (5.5) | 15.7 (5) | 16.5 (5.8) | 15.5 (6.2) | 15.3 (5.2) | 14.7 (5.5) |
| No. of unique buprenorphine prescribersb, mean (SD)† | 1.7 (1.0) | 1.8 (1.1) | 1.7 (1.1) | 1.2 (0.5) | 1.5 (0.8) | 1.3 (0.7) |
| PDC during the pregnancy and postdelivery, mean (SD)† | 0.92 (0.06) | 0.73 (0.09) | 0.59 (0.14) | 0.13 (0.10) | 0.50 (0.11) | 0.29 (0.12) |
| Quality of care during pregnancy | ||||||
| Any behavioral counseling, %* | 33.6 | 39.8 | 31.5 | 32.3 | 40.6 | 37.9 |
| Any urine drug test, % | 73.6 | 76.5 | 73.4 | 66.7 | 71.7 | 70.5 |
Abbreviations: NA, not available; PDC, proportion of days covered.
Comparison between 6 trajectory groups:
P < .05.
P < .01.
P < .001.
P < .0001.
With unique drug ingredients.
Six hundred thirteen unique prescribers who wrote at least 1 buprenorphine prescription to the women in our study cohort (21.4% of buprenorphine claims had missing prescriber information).
The average dose of buprenorphine during the first trimester was not applicable for women who were later initiators.
3.3 ∣. Factors associated with specific buprenorphine trajectories: multivariable multinomial logistic regression
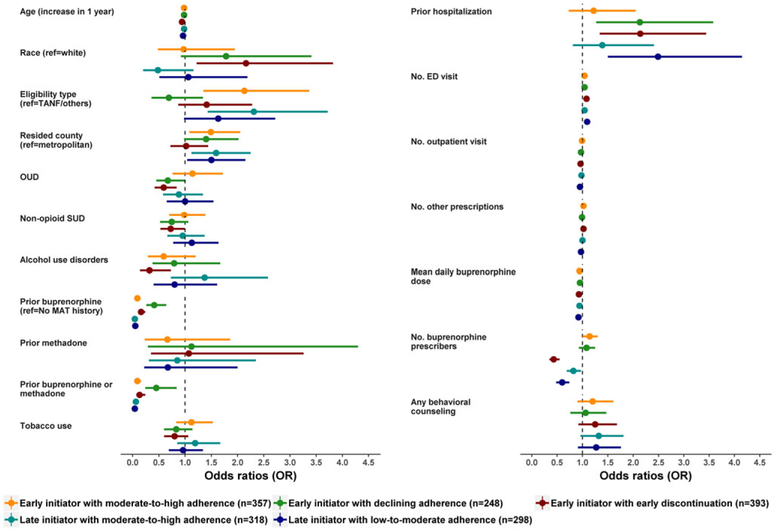
Figure 2 and supplement Table S4 show the factors associated with specific trajectories from the multivariable multinomial logistic regression model, using the early initiators with persistently high adherence trajectory group as the reference group compared with the other 5 groups individually. For example, compared to early initiators with persistently high adherence, significant factors that increase odds of being as early initiators with early discontinuation were younger age (OR = 0.94, 95% CI = 0.91-0.97), non-white race (OR = 2.16, 95% CI = 1.22-3.82), and having no diagnosis of OUD coded (OR = 0.59, 95% CI = 0.42-0.84). History of other substance use disorders were also associated with odds of being in the group of early initiators with early discontinuation (OR = 0.72, 95% CI = 0.53-0.99 for other nonopioid drug use disorders and OR = 0.32, 95% CI = 0.14-0.73 for alcohol use disorder). Higher hospitalizations during pregnancy were also associated more commonly in the group of early initiators with early discontinuation (OR = 2.14, 95% CI = 1.34-3.44). Women who lived in nonmetropolitan counties had a 50% to 59% higher odds to be late initiators (with moderate-to-high adherence: OR = 1.59, 95% CI = 1.12-2.25; with low-to-moderate adherence: OR = 1.50, 95% CI = 1.04-2.15).
FIGURE 2.
Factors associated with specific trajectories from multivariable multinomial logistic regression model (reference group: early initiators with persistently high adherence). MAT: medical assisted therapy; OUD: opioid use disorder; SUD: substance use disorders; TANF, temporary assistance for the needy families. [Colour figure can be viewed at wileyonlinelibrary.com]
Late initiators with low-to-moderate adherence also were more likely to have more frequent hospitalizations (OR = 2.49, 95% CI = 1.50-4.15). Women who had prior buprenorphine therapy prior to their pregnancy were less likely to have suboptimal adherence or premature discontinuation of buprenorphine therapy (varied from early initiators with declining adherence: OR = 0.41 [95% CI = 0.21-64] to late initiators with moderate-to-high adherence: OR = 0.04 [95% CI = 0.03-0.06]). Enrollees having higher buprenorphine daily dose during pregnancy were less likely to have poor adherence or discontinue early (varied from late initiators with low-to-moderate adherence: OR = 0.92 [95% CI = 0.90-0.95] to early initiators with declining adherence: OR = 0.95 [95% CI = 0.92-0.98]).
3.4 ∣. Post hoc analysis: proportions of all-cause ED visits and/or hospitalization across identified trajectories
As shown in Table 3, late initiators with low-to-moderate adherence (37.2 per 100 person years) and early initiators with early discontinuation (37.4 per 100 person years) appear to have higher risk of all-cause hospitalization/ED visits between 85 days and 1 year post-delivery compared to early initiators with persistently high adherence (26.9 per 100 person years) and other trajectory groups (28.5-29.5 per 100 person years; P < .0001).
TABLE 3.
Proportions of all-cause emergency department visits and/or hospitalization between 85 days and 1 year post-delivery across trajectories: a post hoc analysis
| Trajectory Groups (n)a | All-Cause Emergency Department Visits/ Hospitalization, per 100 Person Years* |
|
|---|---|---|
| Early initiators with | Persistently high adherence (n = 747) | 26.9 |
| Moderate-to-high adherence (n = 357) | 28.5 | |
| Declining adherence (n = 248) | 29.5 | |
| Early discontinuation (n = 393) | 37.4 | |
| Late initiators with | Moderate-to-high adherence (n = 318) | 28.6 |
| Low-to-moderate adherence (n = 298) | 37.2 |
Abbreviations: ED, emergency department; PDC, proportion of days covered.
Four groups initiated buprenorphine during the first trimester of the pregnancy (early initiators): 31.6% with persistently high adherence (ie, persistently having a high PDC >0.8), 15.1% with moderate-to-high adherence (ie, initiating with a moderate PDC between 0.4 and 0.8 and increasing to a high PDC >0.8), 10.5% with declining adherence (ie, initiating with a moderate PDC but declining overtime to a low PDC <0.4), and 16.7% with early discontinuation (ie, initiating with a low PDC and discontinued before the third trimester). Two groups did not initiate buprenorphine until midsecond or third trimester (late initiators): 13.5% had moderate-to-high adherence (initiating with a moderate PDC, increasing to a high PDC in the third trimester and then decreasing to a moderate PDC postdelivery), 12.6% had low-to-moderate adherence (initiating with a low PDC and increasing to a moderate PDC).
P < .0001 for the comparison across 6 groups.
4 ∣. DISCUSSION
Our study yielded 3 important insights into buprenorphine treatment among pregnant women with OUD in a large state Medicaid program. First, we identified 6 distinct trajectories of buprenorphine treatment throughout pregnancy and until 12 weeks postpartum. This variability in use patterns may arise from a variety of factors including barriers to obtaining OUD treatment among pregnant women such as lack of available buprenorphine treatment providers, patient nonadherence to treatment, and/or physician discontinuation of treatment. Second, despite nearly 75% of women initiating buprenorphine early (during the first trimester), over one-third of these early initiators had poor refill adherence or discontinued buprenorphine therapy early (ie, before third trimester). Third, we found marked differences across the trajectory groups in terms of characteristics that may be used by health systems for targeting interventions: for example, residence in rural, underserved areas and the presence of polysubstance use.
The major strength of our study is the use of a group-based trajectory model to examine longitudinal buprenorphine refill patterns over time, rather than using a single adherence measure (eg, average proportion of days covered during pregnancy) or an arbitrary cutpoint for discontinuation (eg, a gap in treatment for ≥30 days). Single adherence measures may not reflect different levels and patterns of engagement in treatment over time, which are influenced by different patient, provider, and access to care factors. For example, a patient consistently refilling early in treatment and then discontinuing could have the same proportion of days covered as a patient who refills intermittently throughout the entire pregnancy.7,9 Therefore, trajectory models provide the understanding of heterogeneity in buprenorphine therapy among pregnant women with OUD and may target different interventions to women with different barriers and needs.8
Two findings related to the timing of treatment initiation and discontinuation are worth noting. First, one-quarter of women were late initiators and did not initiate treatment until the second trimester (ie, 13.5% with moderate-to-high adherence and 12.6% with low-to-moderate adherence). Second, 16.7% of our cohort discontinued treatment before the third trimester (ie, early initiators with early discontinuation). Potential explanations both for late initiation and for early discontinuation of buprenorphine therapy include unplanned pregnancies and delayed prenatal care presentation,34 fear of being reported to the police based on state policies,35 disbelief in the efficacy of care, and a study population with a baseline high risk for nonadherence to medications.25,34,36-38 Given that premature discontinuation of buprenorphine may elevate the risk of relapse to opioid use and adverse obstetric and neonatal outcomes,3,4 Medicaid programs might consider investing in programs that offer woman-centered services tailored to patient needs and patient risk profiles to boost patient adherence and improve retention rates, especially among early initiators with early discontinuation and late initia-tors with low-to-moderate adherence.39,40
Several factors were identified that could be used to target patients at high risk of late initiation or early buprenorphine discontinuation including younger age, racial/ethnic minority groups, rural residency, lack of OUD diagnoses, nonopioid substance use disorder, and alcohol use disorder during pregnancy. In addition, late initiators (regardless of their adherence levels) and early initiators with early discontinuation had more inpatient visits and a lower mean buprenorphine daily dose during pregnancy. Given an increase in apparent clearance of buprenorphine during pregnancy, dose escalation of buprenorphine is suggested.41 Clinicians may consider split dosing in patients complaining of discomfort and craving in the afternoon/evening to prevent relapse.42,43 Approximately 40% of our cohort had comorbid mental health disorders. Psychosocial treatment is recommended, and several meta-analyses showed the safety of selective serotonin reuptake inhibitors (SSRIs) in pregnancy (except paroxetine with an increased risk of cardiac defects).44
Our study has several limitations. First, our study relied on administrative billing data that lacked laboratory results and sociobehavioral information. In the absence of data on timing of conception calculated from the last menstrual period, we applied the delivery date algorithm (ie, 280 days prior to the delivery date) that was used in the US NCQA's quality measures of prenatal and postpartum care.13 In a validation study of the conception date,45 applying the delivery date-based algorithm to examine prescription drug exposure in the first trimester for women without preterm births or pregnancy complications was accurate (sensitivity ~92% and specificity ~98%). Nevertheless, the sensitivity for the delivery date algorithm among women with preterm deliveries was low (66%).45 Women with preterm deliveries may be misclassified as late initiators, although the extent to which this would bias our estimates is unknown. Potential unmeasured confounders also cannot be ruled out from our observational study. Second, similar to all claims-based measures of adherence, it is unknown whether the dispensed drugs were actually consumed by the patients. Third, the US Food and Drug Administration labeled sublingual buprenorphine for treating OUD and not for pain. However, 20% of the study cohort had no OUD diagnosis recorded. It may be that physicians are either not billing Medicaid for OUD treatment while the pharmacy is concurrently billing Medicaid for the prescription or are providing buprenorphine for off-label indications. Fourth, although we were unable to study the association between these trajectories and relevant clinical outcomes including number of prenatal care visits, neonate outcomes, and long-term maternal outcomes, our post hoc analysis showed that early initiators with early discontinuation and late initiators with low-to-moderate adherence had higher proportions of all-cause hospitalization/ED visits between 85 days and 1 year postdelivery compared to other trajectory groups. However, prior to Medicaid expansion in 2015, over a quarter of pregnant women with OUD eligible for Medicaid due to pregnancy may have lost their Medicaid eligibility after 12 weeks postdelivery. Future studies using a prospective design or data that capture longer and more complete data post-delivery are thus warranted.44 Fifth, women completing withdrawal or detoxification therapy may be misclassified as nonadherent or as discontinuing buprenorphine. However, both the Substance Abuse and Mental Health Services Administration (SAMHSA) and American Congress of Obstetricians and Gynecologists (ACOG) do not recommend withdrawal or detoxification therapy due to an increased risk of relapse to illicit drugs, preterm labor, fetal distress, and pregnancy loss.33,44 Finally, findings derived from the Pennsylvania Medicaid population may not be generalizable to other Medicaid populations with a different demographic profile or programmatic features.46,47 Nevertheless, Pennsylvania is 1 of the largest Medicaid programs by expenditures and monthly enrollment. The features of demographics (except lower Hispanic population) and health care utilization in Pennsylvania Medicaid are similar to those seen in other state Medicaid programs.48
Pregnancy is a transformative state (both biologically and socially) that may provide a “window of opportunity” for OUD treatment and provide enormous potential for behavioral changes for this vulnerable population.49 Our study showed 6 unique trajectories of longitudinal buprenorphine refills among pregnant Medicaid-enrolled women in Pennsylvania. These trajectories and associated factors may be further used to develop more targeted interventions to improve care and health outcomes among pregnant women with OUD.
Supplementary Material
KEY POINTS.
Six buprenorphine treatment trajectories with different adherence levels were identified among pregnant Medicaid beneficiaries.
A quarter of pregnant women did not initiate buprenorphine until midsecond or third trimester.
Factors significantly associated with late initiation and discontinuation were younger age, non-white race, residents of rural counties, having fewer outpatient visits, more frequent emergency department visits and hospitalizations, and lower buprenorphine daily dose.
Understanding distinct buprenorphine trajectories and associated factors may better guide buprenorphine treatment, integrate behavioral treatment with obstetrical/gynecology care, and target interventions among pregnant women.
Acknowledgments
FUNDING SOURCE
This work was supported by an intergovernmental agreement between the Pennsylvania Department of Human Services, the University of Pittsburgh, the University of Arizona Health Sciences Career Development Award (Dr. Lo-Ciganic), the Building Interdisciplinary Research Careers in Women's Health Program (K12HD043441, Dr. Jarlenski), and the National Institute on Drug Abuse Career Development Award (K23DA038789, Dr. Krans). The contents represent the views of the authors only and not necessarily those of the US government.
Funding information
National Institute on Drug Abuse, Grant/Award Number: K23DA038789; Building Interdisciplinary Research Careers in Women's Health Program University of Pittsburgh, Grant/Award Number: K12HD043441; Pennsylvania Department of Human Services, University of Arizona Health Sciences; Career Development Award, University of Arizona
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest.
ROLE OF THE FUNDING SOURCE
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The Pennsylvania Department of Human Services reviewed and approved the manuscript prior to publication per the terms of the data use agreement.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration:2016. [Google Scholar]
- 2.The American College of Obstetricians and Gynecologists (ACOG). Committee Opinion No. 524: opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–1076. [DOI] [PubMed] [Google Scholar]
- 3.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121(6):1158–1165. [DOI] [PubMed] [Google Scholar]
- 4.Mozurkewich EL, Rayburn WF. Buprenorphine and methadone for opioid addiction during pregnancy. Obstet Gynecol Clin North Am. 2014;41(2):241–253. [DOI] [PubMed] [Google Scholar]
- 5.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saia KA, Schiff D, Wachman EM, et al. Caring for pregnant women with opioid use disorder in the USA: expanding and improving treatment. Curr Obstet Gynecol Rep. 2016;5(3):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin JM, Shrank WH, Pakes J, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789–796. [DOI] [PubMed] [Google Scholar]
- 8.Lo-Ciganic WH, Gellad WF, Gordon AJ, et al. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction. 2016;111(5):892–902. [DOI] [PubMed] [Google Scholar]
- 9.Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305(16):1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levit KR, Mark TL, Coffey RM, et al. Federal spending on behavioral health accelerated during recession as individuals lost employer insurance. Health Aff (Millwood). 2013;32(5):952–962. [DOI] [PubMed] [Google Scholar]
- 11.Levit KR, Stranges E, Coffey RM, et al. Current and future funding sources for specialty mental health and substance abuse treatment providers. Psychiatr Serv. 2013;64(6):512–519. [DOI] [PubMed] [Google Scholar]
- 12.Rustgi VK, Davis GL, Herrine SK, McCullough AJ, Friedman SL, Gores GJ. Future trends in hepatology: challenges and opportunities. Hepatology. 2008;48:655–661. [DOI] [PubMed] [Google Scholar]
- 13.US National Committee for Quality Assurance (NCQA): Prenatal and postpartum care. Available at http://www.ncqa.org/portals/0/PrenatalPostpartumCare.pdf. Accessed 12 June 2017.
- 14.Lo-Ciganic WH, Donohue JM, Jones BL, et al. Trajectories of diabetes medication adherence and hospitalization risk: a retrospective cohort study in a large state Medicaid program. J Gen Intern Med. 2016;31(9):1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nau DP. Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication Adherence: Pharmacy Quality Alliance; 2012. Available at http://www.pqaalliance.org/images/uploads/files/PQAPDCvsMPR.pdf. Accessed 13 June 2017.
- 16.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3–12. [DOI] [PubMed] [Google Scholar]
- 17.Nagin DS. Group-Based Modeling of Development Cambridge. MA: Harvard University Press; 2005. [Google Scholar]
- 18.Twisk J, Hoekstra T Classifying developmental trajectories over time should be done with great caution: a comparison between methods. J Clin Epidemiol. 2012;65(10):1078–1087. [DOI] [PubMed] [Google Scholar]
- 19.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6(1):109–138. [DOI] [PubMed] [Google Scholar]
- 20.Bosworth HB, Oddone EZ. A model of psychosocial and cultural antecedents of blood pressure control. J Natl Med Assoc. 2002;94(4):236–248. [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171(9):814–822. [DOI] [PubMed] [Google Scholar]
- 22.Fischer MA, Choudhry NK, Brill G, et al. Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124(1081):e1089–e1022. [DOI] [PubMed] [Google Scholar]
- 23.Gordon AJ, Haas GL, Luther JF, Hilton MT, Goldstein G. Personal, medical, and healthcare utilization among homeless veterans served by metropolitan and nonmetropolitan veteran facilities. Psychol Serv. 2010;7(2):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon AJ, Kavanagh G, Krumm M, et al. Facilitators and barriers in implementing buprenorphine in the veterans health administration. Psychol Addict Behav. 2011;25(2):215–224. [DOI] [PubMed] [Google Scholar]
- 25.Miotto K, Hillhouse M, Donovick R, et al. Comparison of buprenorphine treatment for opioid dependence in 3 settings. J Addict Med. 2012;6(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliva EM, Harris AHS, Trafton JA, Gordon AJ. Receipt of opioid agonist treatment in the veterans health administration: facility and patient factors. Drug Alcohol Depend. 2012;122(3):241–246. [DOI] [PubMed] [Google Scholar]
- 27.Parran TV, Adelman CA, Merkin B, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106(1):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein BD, Gordon AJ, Sorbero M, Dick AW, Schuster J, Farmer C. The impact of buprenorphine on treatment of opioid dependence in a Medicaid population: recent service utilization trends in the use of buprenorphine and methadone. Drug Alcohol Depend. 2012;123(1-3):72–78. [DOI] [PubMed] [Google Scholar]
- 29.Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20(11):1038–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroupe KT, Murray MD, Stump TE, Callahan CM Association between medication supplies and healthcare costs in older adults from an urban healthcare system. J Am Geriatr Soc. 2000;48(7):760–768. [DOI] [PubMed] [Google Scholar]
- 31.Stroupe KT, Teal EY, Tu W, Weiner M, Murray MD. Association of refill adherence and health care use among adults with hypertension in an urban health care system. Pharmacotherapy. 2006;26(6):779–789. [DOI] [PubMed] [Google Scholar]
- 32.Gordon AJ, Lo-Ciganic WH, Cochran G, et al. Patterns and quality of buprenorphine opioid agonist treatment in a large Medicaid program. J Addict Med. 2015;9(6):470–477. [DOI] [PubMed] [Google Scholar]
- 33.The American Congress of Obstetricians and Gynecologists (ACOG). Toolkit on state legislation: pregnant women & prescription drug abuse, dependence and addiction; 2014. Available at https://www.acog.org/-/media/Departments/Government-Relations-and-Out-reach/NASToolkit.pdf. Accessed 12 June 2017.
- 34.Schempf AH, Strobino DM. Drug use and limited prenatal care: an examination of responsible barriers. Am J Obstet Gynecol. 2009;200(412):e411–e410. [DOI] [PubMed] [Google Scholar]
- 35.Guttmacher Institute. State laws and policies. Substance abuse during pregnacy; 2017. Available at https://www.guttmacher.org/state-policy/explore/substance-abuse-during-pregnancy. Accessed April 2, 2017.
- 36.Clark RE, Baxter JD. Responses of state Medicaid programs to buprenorphine diversion: doing more harm than good? JAMA Intern Med. 2013;173(17):1571–1572. [DOI] [PubMed] [Google Scholar]
- 37.Khanna R, Pace PF, Mahabaleshwarkar R, Basak RS, Datar M, Banahan BF. Medication adherence among recipients with chronic diseases enrolled in a state Medicaid program. Popul Health Manag. 2012;15(5):253–260. [DOI] [PubMed] [Google Scholar]
- 38.Rinaldo SG, Rinaldo DW. Availability Without Accessibility? State Medicaid Coverage and Authorization Requirements for Opioid Dependence Medications Report prepared for the American Society of Addiction Medicine. San Francisco, CA: The Avis Group; 2013. Available at https://www.asam.org/docs/default-source/advocacy/aaam_implications-for-opioid-addiction-treatment_final Accessed 12 June 2017. [Google Scholar]
- 39.Campbell CI, Alexander JA. Health services for women in outpatient substance abuse treatment. Health Serv Res. 2005;40(3):781–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grella CE. From generic to gender-responsive treatment: changes in social policies, treatment services, and outcomes of women in substance abuse treatment. J Psychoactive Drugs. 2008;40(Suppl 5):327–343. [DOI] [PubMed] [Google Scholar]
- 41.Bastia JR, Chen H, Zhang H, et al. Dose-adjusted plasma concentrations of sublingual buprenorphine are lower during than after pregnancy. Am J Obstet Gynecol. 2017;216:64 e61–64 e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farid WO, Dunlop SA, Tait RJ, Hulse GK. The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: review of human and animal data. Curr Neuropharmacol. 2008;6(2):125–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Society of Addiction Medicine. National practice guideline for the use of medications in the treatment of addiction involving opioid use; 2015. Available at https://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-jam-article.pdf?sfvrsn=0. Accessed 12 June 2017.
- 44.Substance Abuse and Mental Health Services Administration (SAMHSA). Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. HHS Publication No. (SMA) 18-5054. Substance Abuse and Mental Health Services Administration: Rockville, MD; 2018. [Google Scholar]
- 45.Toh S, Mitchell AA, Werler MM, Hernandez-Diaz S. Sensitivity and specificity of computerized algorithms to classify gestational periods in the absence of information on date of conception. Am J Epidemiol. 2008;167(6):633–640. [DOI] [PubMed] [Google Scholar]
- 46.Center for Disease Control and Prevention (CDC). Health, United States 2012: With Special Feature on Emergency Care. Hyattsville, MD: Centers for Disease Control; 2013. Available at https://www.cdc.gov/nchs/data/hus/hus12.pdf Accessed 12 June 2017. [Google Scholar]
- 47.Stroupe KT, Teal EY, Weiner M, Gradus-Pizlo I, Brater DC, Murray MD. Health care and medication costs and use among older adults with heart failure. Am J Med. 2004;116(7):443–450. [DOI] [PubMed] [Google Scholar]
- 48.The Henry J Kaiser Family Foundation. Medicaid & CHIP Indicators. Available from: http://www.kff.org/state-category/medicaid-chip/.Accessed August 27, 2018.
- 49.Daley M, Argeriou M, McCarty D. Substance abuse treatment for pregnant women: a window of opportunity? Addict Behav. 1998;23(2): 239–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.