Abstract
Exercise is an evolutionary conserved survival function that nowadays has beneficial health effects. The increased metabolic activity of contracting skeletal muscle affects the biology of many organs involved in regulating muscle functions. The discovery of hormones and cytokines secreted by bone and skeletal muscle during exercise, has recently added experimental credence to the notion that a crosstalk exists between these organs. Bone through the hormone osteocalcin, promotes exercise capacity in the mouse. After binding to a G-coupled protein receptor, Gprc6a, osteocalcin increases nutrients uptake and catabolism in myofibers during exercise. The catabolic aspect of osteocalcin distinguishes it from insulin signaling. In addition, osteocalcin regulates the endocrine function of skeletal muscle because it enhances the expression of interleukin-6 (IL-6). IL-6 is produced and secreted by contracting skeletal muscle and exerts autocrine, paracrine and systemic effects. One of the systemic functions of IL-6 is to drive the generation of bioactive osteocalcin. Altogether, these studies have revealed a feed-forward loop between bone and skeletal muscle that are necessary and sufficient for optimum exercise capacity. This endocrine regulation of exercise biology, suggest novel and adapted strategies for the prevention or treatment of age related muscle loss.
Keywords: Exercise, Osteocalcin, IL-6
1. Introduction
The ability to exercise has been essential for the survival of most vertebrates and is in part responsible for the successful evolution of ancestral Homo sapiens [1]. Nowadays that sedentary habits have increased the risk of chronic and life-threatening diseases, exercise has been shown to have beneficial health consequences through mechanisms that are poorly understood [2-4]
Exercise requires that several organs become involved at the same time; hence, this represents a major challenge to whole-body homeostasis [5]. Indeed, the increased metabolic activity of contracting skeletal muscle requires changes in almost every tissue in order to maintain whole-body homeostasis. Not surprisingly, orchestrating such a complex process relies heavily on inter-organ crosstalk. In that respect, identifying secreted molecules that could mediate these inter-organ communications during exercise is a task of fundamental importance to understand the regulation of exercise capacity. The majority of the already described circulating factors secreted during exercise originate from skeletal muscle [6]. These molecules have been named myokines, by analogy to the adipokines that are produced by adipose tissue [7].
Yet, it has been assumed for decades, that a close functional relationship between skeletal muscle and bone may exist [8]. The fact that bone is an endocrine organ that regulates energy metabolism [9] has fastened the demonstration that bone-derived hormones are important regulators of muscle function and adaptation to exercise. Addressing this question is now more easily addressable because of the advances in mouse genetics. The fact that bone is one of the latest organs to appear during evolution explain why genes regulating bone function or expressed in bone cells are so highly conserved in terms of function between mice and humans [9-19]. This alone explains why the bone field more than many other fields could take so much advantage of mouse genetics.
The purpose of this review is to describe the endocrine regulation of skeletal muscle during exercise by bone. To this end, we will focus primarily on the functions of the bone-derived hormone osteocalcin and the myokine interleukin-6 (IL-6) and the feed forward loop between bone and skeletal muscle that promotes adaptation to exercise [20]. This work should contribute in the long run to achieve a better understanding of exercise biology which could suggest adapted treatment of some degenerative human conditions.
1.1. Physiology of exercise: focus on skeletal muscle metabolism
The physiology of exercise can be defined as the study of the integrated physiological responses that take place during exercise and that are caused by or are a response to the increased energy demand of contracting skeletal muscle. The modality, frequency, intensity or duration of exercise are all factors that determine these physiological responses [5].
Exercise requires the generation of force by skeletal muscle, a process that needs energy in the form of adenosine triphosphate (ATP) [21]. A remarkable characteristic of skeletal muscle is that it has the capacity to quickly modulate the rate of energy production in response to locomotion. Indeed, myofibers store only a small amount of ATP that is immediately used at the onset of muscle contraction. Hence, if exercise lasts beyond a few seconds, myofibers need to generate ATP. This occurs through 3 different metabolic pathways [22]. First, the ATP-phosphagen system in which phosphocreatine donates a phosphate to ADP to generate ATP. This anaerobic pathway is the fastest way to synthetize ATP. Second, anaerobic glycolysis, in which glucose-6-phosphate derived from skeletal muscle glycogen or blood glucose is transformed into pyruvate that is next reduced to lactate. This is the second-fastest way to synthetize ATP. And third, aerobic respiration, where the acetyl-CoA produced by the degradation of glucose (glycolysis) and fatty acids (FAs) (β-oxidation) enters the tricarboxylic acid (TCA) in the mitochondria, which is coupled to oxidative phosphorylation in the electron transport chain. This later pathway requires O2 and is the slowest way to produce ATP. The perturbation in skeletal muscle metabolism that represents the contraction of myofibers results in the activation of signaling networks that are needed in order to increase energy production.
Signaling molecules, including protein kinases (AMPK, 5′ AMP-activated protein kinase; CaMKII, Ca2+/calmodulin-dependent protein kinase II or JNK, c-Jun N-terminal kinases) and deacetylases (Sirtuins) are activated during exercise in a temporal manner and modulate downstream targets, including transcription factors [23-29]. These transcriptional regulators modulate the expression of genes involved in glucose and lipid catabolism. The list of the known transcription factors regulating energy metabolism in skeletal muscle during exercise includes, but is not limited to: cyclic AMP response element binding protein (CREB); myocyte enhancer factor 2 (MEF2); peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α); peroxisome proliferator-activated receptors (PPARs) or forkhead transcription factor, O box subfamily (FOXO1). The relative activation and contribution of each pathway is dependent on the intensity, duration and modality of exercise. That these pathways are regulated by redundant mechanisms indicates that the uptake and utilization of nutrients by contracting myofibers is absolutely essential for the ability to exercise. Yet a question of critical importance that is raised by these events is to identify the systemic cues responsible for the modulation of muscle energy metabolism during exercise.
2. Bone as an endocrine organ
The skeleton provides first and foremost the ability to stand, walk and run. In addition, the unexpected realization that bone is an endocrine organ and the growing number of physiological processes it regulates in this capacity have considerably broadened and enriched bone biology (Fig. 1) [30-32] This aspect of the skeleton biology raises two fundamental questions: Can we surmise which physiological functions should be influenced by bone? And beyond that a broader question is looming: why would the skeleton be an endocrine organ in the first place? A way to answer these related questions is to use as guides a cell biological unique characteristic of bone.
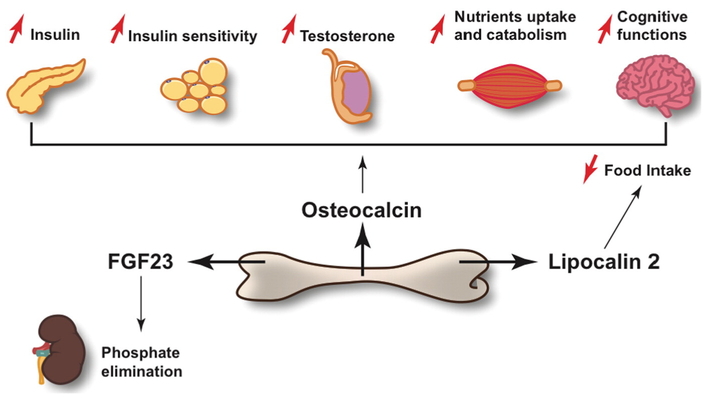
Fig. 1.
Schematic representation of the physiological functions affected by osteocalcin, lipocalin 2 and FGF23, the three known osteoblast-derived hormones. Osteocalcin stimulates insulin secretion in the pancreas, insulin sensitivity in adipose tissue, nutrients uptake and catabolism inmuscle and testosterone production in the testis. In β-cells, the Leydig cells of the testis and in myofibers, osteocalcin signals through Gprc6a. In addition, osteocalcin promotes monoamine neurotransmitter synthesis and cognitive functions in the brain. FGF23 acts in the kidney to favor phosphate elimination. FGFR1 and Klotho mediate FGF23 signal in the tubular cells of the kidney. Lipocalin 2 binds to the melanocortin 4 receptor in neurons of the hypothalamus to inhibit food intake.
Bone is the only tissue in the body that contains a cell type, the osteoclast, which has the function to actively destroy (resorb) the host tissue [33]. This function is part of the homeostatic process named bone (re)modeling [34], which is essential for growth, ambulation, repair of micro and macro damages and therefore has been central for the survival of bony vertebrates. Bone (re)modeling that is characterized by alternating phases of bone resorption by the osteoclasts and bone formation by the osteoblasts [34], occurs daily in multiple locations in one of the organs that covers the largest surface in the body of bony vertebrates. This implies that this binary process has to be energetically expensive. If this hypothesis is correct, a close link between bone and energy metabolism must exist. This notion is fully supported by clinical evidences. Indeed, any situation that results in a reduced food, i.e. energy, intake causes an arrest of longitudinal growth in children and a decrease in bone mass in adults [35,36]. Experiments designed to explore the regulation of energy metabolism by the skeleton, resulted in 2007 in the identification of osteocalcin as a hormone mediating in part the regulation of energy metabolism by bone [9]. We should mention here that osteocalcin is not the only bone-derived hormone [30,32].
2.1. The functions of osteocalcin
At the time Osteocalcin genes were inactivated in the mouse, osteocalcin was the only known osteoblast-specific secreted protein [37]. Surprisingly, Osteocalcin-deficient mice (Osteocalcin−/−) showed a striking but completely unexpected phenotype: without being obese, these mice had more visceral fat than control littermates. The systematic study of this curious phenotype allowed the genetic demonstration in mice and later in humans that osteocalcin regulates as a hormone several aspects of energy metabolism [9,38,39]. Accordingly, osteocalcin has structural features of a hormone: it is produced as a pro-molecule that is cleaved in osteoblasts before being secreted, is present in the circulation at a concentration in the ng/ml range and the levels of this molecule in blood in humans follow a circadian rhythm [37,40].
The strength of the demonstration that osteocalcin is a hormone and the definition of its functions were greatly enhanced by the availability of a loss-of-function model (Osteocalcin−/−) and a gain-of-function model (Esp−/−) of osteocalcin, each of them serving as an internal control and a mirror image of the other. This analysis revealed that osteocalcin favors the expression and secretion of insulin and β-cell proliferation and mass [41]. Moreover, Osteocalcin−/− mutant mice fed a normal diet are glucose intolerant and insulin resistant and show decreased energy expenditure, whereas the opposite is true in Esp−/− mice [9]. In addition, exogenous osteocalcin almost completely rescue the glucose intolerance developed in wild-type (WT) mice fed a high-fat diet thus indicating that osteocalcin is not only necessary but it is also sufficient to regulate at least one physiological process [42]. These studies, along with many subsequent ones, revealed that osteocalcin is biologically active when its gamma-carboxyglutamic acid residues are undercarboxylated. Later, molecular and genetic experiments established that bone resorption is the arm of bone (re)modeling responsible for osteocalcin decarboxylation and activation [43]. The receptor mediating osteocalcin signaling in pancreatic β-cells and other peripheral tissues is a GPCR called Gprc6a. Cell-specific gene deletion and genetic epistasis experiments have established that in vivo osteocalcin is the ligand of Gprc6a that is responsible for Gprc6a regulation of glucose homeostasis [14,41].
One question of fundamental importance, both in terms of evolutionary biology and of physiology, raised by the favorable influence of osteocalcin on glucose homeostasis is to know whether there is any difference between osteocalcin and Insulin functions. In that context and taking into account the fundamental role of skeletal muscle in the maintenance of normal whole-body glucose homeostasis, it became important to determine whether osteocalcin influences physiologically any aspect of muscle energy metabolism. One way to answer this question was the study of osteocalcin functions during exercise, a physiological situation characterized by an increase in muscle glucose uptake and a concomitant decrease in circulating insulin levels [20]. There was one more incentive to address this question: the circulating levels of bioactive osteocalcin after a single bout of endurance exercise double in mice and increase in humans. This increase in bioactive osteocalcin is due in part to an increase in bone resorption [20].
2.2. A role for osteocalcin in adaptation to exercise
Another reason to ask whether osteocalcin regulates adaptation to exercise arises from a common feature to all the known functions of osteocalcin: those are physiological processes that are affected early and severely during aging. This observation supports the idea that osteocalcin might regulate preferentially functions that decline overtime, such as for instance skeletal muscle function and the ability to exercise. What made this hypothesis so attractive is that circulating levels of bioactive osteocalcin decrease early and steeply during aging in mice, monkeys and humans of both genders and do not increase after exercise to the same extent in old mice as in young ones. In mice at least, this decrease in circulating bioactive osteocalcin occurs at the same time than the ability to perform exercise declines [20]. These observations were an incentive to test if the administration of exogenous osteocalcin to wild-type (WT) mice might increase their ability to run. A single injection of exogenous osteocalcin immediately before exercise or a chronic delivery of this hormone for one month not only increased the exercise capacity of young mice but also restored aerobic endurance in older mice to what is seen in young adult mice [20]. Of note, at the same time it increases muscle function in older mice, chronic delivery of osteocalcin also favors a gain in muscle mass [44]. The unexpected outcome of these experiments revealed that exogenous osteocalcin is not only necessary but is also sufficient to reverse the age-induced decline in exercise capacity and muscle mass observed in mice.
The fact that bone is a sensor of mechanical forces during exercise raised the question of whether osteocalcin might influence adaptation to exercise, at least in part, by signaling in skeletal muscle. The previous identification of a receptor for osteocalcin in peripheral organs (Gprc6a) provided the necessary tool to test rigorously and in vivo this hypothesis. Moreover, the fact that exogenous osteocalcin increases exercise capacity in aged WT but not Gprc6a-muscle specific deficient mice (Gprc6aMck−/−) supported the idea that osteocalcin signals in skeletal muscle to promote exercise.
The analysis of 3 month-old Osteocalcin−/− and Gprc6aMck−/− mice revealed that when forced to run on a treadmill at a constant speed and until exhausted, Osteocalcin−/− and Gprc6aMck−/− mice run 20% to 30% less than control littermates, a phenotype that worsens overtime [20]. How does osteocalcin regulate muscle functions in order to promote endurance? A first approach to answer this question relied on the use of indirect calorimetry to measure oxygen consumption during exercise in Gprc6aMck−/− mice. Consistent with the decrease in endurance, maximal oxygen consumption during exercise is significantly lower in Gprc6aMck−/− mice than in control littermates. Because mitochondria content in skeletal muscle is directly associated with aerobic capacity, the decrease in oxygen consumption during exercise observed in Gprc6aMck−/− mice raised the question of whether osteocalcin regulates mitochondria biogenesis and/or function. Multiple experimental evidences negative in nature and that will not be cited here because of space constraints, showed that the ability of osteocalcin signaling in myofibers to favor adaptation to exercise is not secondary to a measurable effect on skeletal muscle mitochondria number or function [20].
Instead, what was observed is that osteocalcin regulates the uptake and catabolism of nutrients in muscle during exercise. In that respect and this is a point of fundamental importance, osteocalcin is different from insulin that is an anabolic hormone. Glucose, which is the main nutrient used by skeletal muscle to generate energy at the onset of exercise, is stored in myofibers in the form of glycogen. However, the breakdown of skeletal muscle glycogen during exercise is lower in Gprc6aMck−/− and Osteocalcin−/− mice than in control ones thus inferring that osteocalcin favors glycogenolysis. Moreover, the uptake of extracellular glucose is decreased in skeletal muscle of Gprc6aMck−/− mice after running, but increases in WT mice receiving exogenous osteocalcin prior to exercise. Subsequent studies revealed that the accumulation of TCA cycle intermediates in skeletal muscle normally seen in WT mice after exercise [45] is blunted in Gprc6aMck−/− mice. This reflects in part a decrease in the entry of carbon originating from glucose into the TCA cycle. Genetic experiments performed in cell culture showed that the transcription factor CREB, a mediator of osteocalcin signaling in other cell types [14,41], is required for the increase in glucose utilization caused by osteocalcin in myofibers [20].
During a prolonged exercise when animals exhaust their reserve of glycogen, the uptake and catabolism of FAs progressively increase in skeletal muscle [46,47]. This was an incentive to test whether osteocalcin signaling in myofibers also affects these processes. A first approach to address this question relied on the measurement of skeletal muscle and plasma accumulation of acylcarnitines, which are a reliable indication of FAs utilization in cells [48]. In WT mice, exercise induces a significant increase in the accumulation of long- and medium-chain acylcarnitines in skeletal muscle. However, this increase does not occur nearly to the same extent in skeletal muscle of Gprc6aMck−/− mice. What occurs instead, is a significant rise in the plasma levels of some acylcarnitines in Gprc6aMck−/− mice when compared to control ones. Consistent with these observations, cell-based experiments showed that osteocalcin favor FAs oxidation in myofibers in an AMPK-dependent manner [20].
Altogether, these observations revealed that osteocalcin signaling in myofibers promotes the uptake and catabolism of glucose and FAs during exercise, this certainly explains at least in part the decreased exercise performance observed in Osteocalcin−/− and Gprc6aMck−/− mice. Importantly from a translational point of view, these functions of osteocalcin decline as mice age, but can be restored by the administration of exogenous osteocalcin to WT mice.
To determine whether osteocalcin has additional means to regulate exercise capacity besides its regulation of glucose and FAs catabolism, a first approach was to perform a transcriptomic analysis in muscles of control and Gprc6aMck−/− mice after a single bout of endurance exercise. Unexpectedly, the post-exercise rise of muscle Il6 expression and circulating Interleukin-6 (IL-6) were markedly attenuated in Gprc6aMck−/− or Osteocalcin−/− mice when compared to control mice [20]. IL-6 is the first myokine that was shown to be secreted into the circulation in response to muscle contraction and it acts in an autocrine, paracrine and endocrine fashion [49]. The functions of exercise-derived IL-6 are addressed with detail in the next section of this review. In addition, the regulation of IL-6 by osteocalcin raised the fundamental question of whether another function of this myokine could be to signal back to bone.
3. Skeletal muscle as an endocrine organ
Again, exercise is synonymous of an increase in skeletal muscle function, and for that myofibers need to increase the uptake and catabolism of nutrients. In addition, during resting periods skeletal muscle stores in the form of triglycerides and glycogen a substantial amount of energy. For that reason, skeletal muscle is a gatekeeper of energy homeostasis. The importance of this later function is illustrated by the variety of disorders that are secondary to perturbations on muscle energy metabolism, such as metabolic syndrome or Type 2 Diabetes [50]. Given the role of muscle on systemic energy homeostasis, one could expect that inter-organ communications signaling from muscle cells might be important for balancing metabolism across the body[51]. Still, an endocrine function of skeletal muscle was not obvious.
The first biologically active secreted muscle factor ever identified was myostatin (also known as growth and differentiation factor (GDF)-8), a muscle-specific member of the transforming growth factor β superfamily [52]. The main function of myostatin is to suppress skeletal muscle growth and Myostatin inactivation results in a massive muscle hyperthophy in sheep, cattle, mice and humans. In addition to the autocrine control of muscle growth, myostatin is secreted into the circulation and acts in distal tissues to modulate metabolism. Myostatin-deficient mice show reduced total and intramuscular body fat and improved insulin sensitivity, however and this an important aspect of its biology, myostatin does not improve muscle function [53]. More recently, myostatin was shown to directly modulate bone remodeling by stimulating the differentiation of osteoclasts [54].
However, many years before the discovery of myostatin and the endocrine functions of skeletal muscle, several laboratories already sought a circulating “exercise factor” that would be released in response to the increased energy demand of contracting skeletal muscle and would mediate some of the exercise-induced responses in distant organs. Ever since, it has been consistently demonstrated that skeletal muscle secretes a growing number of cytokines and other biologically active proteins (referred to as myokines) into the blood, mostly in response to contractile activity or sometimes pathological diets [55]. The list of identified myokines include myostatin, decorin, IL-15, IL-7, IL-8, BDNF, IGF-1, FGF21, FSTL-1, irisin, meteorin-like and IL-6 [49,56-64]. However, we still do not know all the exact mechanisms that trigger the synthesis and secretion of myokines during muscle contraction.
3.1. The unexpected functions of IL-6 during exercise
It has been known for decades that exercise induces changes in the immune system [65]. Yet, it was a study designed to understand the mechanisms undelaying exercise-induced changes in the distribution of lymphocyte subpopulations, that led to the discovery that exercise causes an increase in the circulating levels of many cytokines [7,66,67].
IL-6 was the first molecule found to be secreted into blood in response to muscle contraction [49,68]. Initially, it was observed that the circulating levels of this cytokine increase after exercise in humans and murine models in a manner that is proportional to the length of exercise and the amount of muscles involved, being running the kind of effort that induces a higher increase in blood IL-6 levels [57,66,69-71]. Additionally, it was demonstrated that IL-6 is expressed in cultured myotubes, murine myofibes and muscle satellite cells [72-75]. The realization that contracting skeletal muscle releases significant amounts of IL-6 into the circulation, and that this function is independent of muscle injury and tumor necrosis factor, raised the question of whether this cytokine could have a role in metabolism rather than in inflammation. Testing this idea revealed that muscle-derived IL-6 release during exercise is enhanced when muscle glycogen levels are low and that IL-6 has autocrine, paracrine and endocrine functions during exercise [6,70,74]. IL-6 promotes glucose uptake and FAs oxidation in skeletal muscle, increases glucose production in the liver and lipolysis in the white adipose tissue [70,76]. In skeletal muscle IL-6 signals through gp130Rβ/IL-6Rα homodimer, which leads to the activation of AMPK and the subsequent increase in glucose uptake and FAs oxidation [77]. Accordingly, Il6-deficient mice (Il6−/−) show decreased adaptation to exercise when compared to control mice [78]. Taken together, these data identify IL-6 as a pleiotropic cytokine with a key role in exercise metabolism.
Yet, an important question that remained to be addressed was to determine the nature of the mechanisms regulating IL-6 production in muscle during exercise. Skeletal muscle contraction has been established as the trigger leading to the increase in circulating IL-6. However, it is still uncertain if and how systemic clues might contribute to the regulation of IL-6 secretion by skeletal muscle in response to exercise. The observation that osteocalcin signals in myofibers and is necessary for the increase in Il6 expression in myofibers prompted us to explore further the crosstalk between bone and skeletal muscle. Moreover, previous experimental observations showing that IL-6 signals in bone cells [79] raised another important question: could IL-6 act in bone in a feed forward loop to increase the production of bioactive osteocalcin during exercise?
4. The cross-talk between bone and skeletal muscle during exercise
Asking whether IL-6 could act in bone to increase the production of bioactive osteocalcin was a relevant question because at the time it was demonstrated that exercise induces bone resorption and therefore the production of bioactive osteocalcin, the mechanisms underlying this function were unknown. The analyses of Il6−/− mice before and after a single bout of endurance exercise revealed that the increase in blood markers of bone resorption and in bioactive osteocalcin that is observed in WT mice is blunted in Il6−/− mice [20]. Furthermore, bone resorption after exercise was also attenuated in Gprc6aMck−/− mice, a model in which exercise-induced IL-6 circulating levels are low. Altogether, these experiments revealed the existence of a feed forward loop between bone (via osteocalcin) and muscle (via Il-6) that promotes adaptation to exercise through at least 3 different synergistic mechanisms (Fig. 2): first, osteocalcin increases nutrient uptake and catabolism in myofibers; second, osteocalcin triggers the increase in the skeletal muscle expression and secretion of IL-6. This in turn, might allow the generation of extra-muscular glucose and FAs [70,76]. Third, IL-6 increases the production of bioactive osteocalcin. This model does not exclude in any way the possibility that osteocalcin and IL-6 might have additional functions to modulate adaptation to exercise. We should emphasize here that these findings do not exclude the possibility that other molecules made in bone cells or myofibers contribute to this crosstalk.
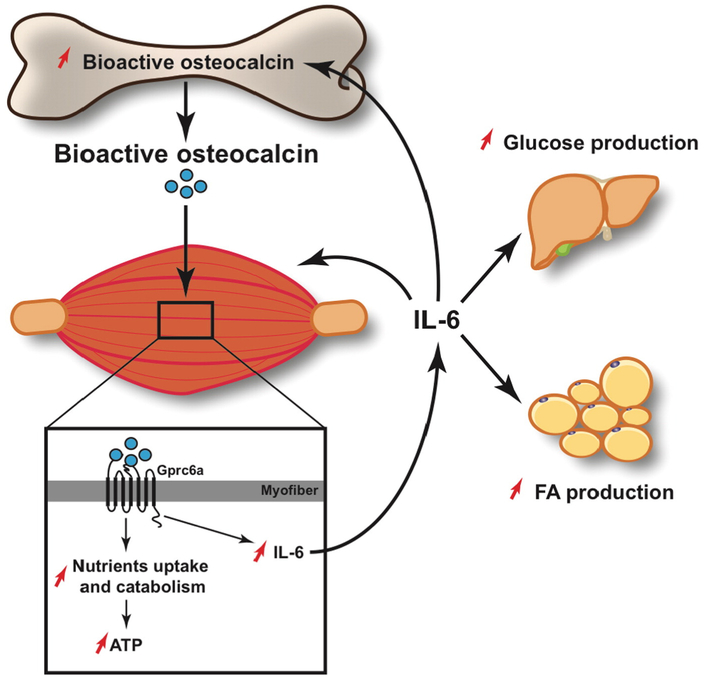
Fig. 2.
Schematic representation of the molecularmechanism of action of osteocalcin in skeletalmuscle during exercise. Circulating levels of undercarboxylated and bioactive osteocalcin increases during exercise. Osteocalcin signals in myofibers through Gprc6a, where it promotes the uptake and catabolism of nutrients. In addition, osteocalcin signaling in myofibers induces the expression of IL-6 and the increase in circulating levels of this myokine. IL-6 signals back to skeletal muscle where it favors glucose and FAs utilization. IL-6 also stimulates FAs production in the white adipose tissue and glucose production in the liver. Furthermore, IL-6 signals to bone to increase the production of bioactive osteocalcin.
Since both IL-6 and osteocalcin regulate similar aspects of skeletal muscle metabolism during exercise, e.g. the increase in glucose and FAs catabolism, an essential question was to determine whether osteocalcin was acting independently or not of IL-6 to promote glucose and FAs utilization in myofibers. A first approach was to study the effect of exogenous osteocalcin on glucose and FAs utilization in WT and Il6−/− cultured myofibers and myotubes. These experiments revealed that osteocalcin can induce these functions in a similar manner in control and Il6-defficient cells, which indicates that the presence of IL-6 is not required for the catabolic effects of exogenous osteocalcin on skeletal muscle cells [20]. While redundant regulation of skeletal muscle metabolism is probable, it becomes important to determine whether osteocalcin and IL-6 might have synergistic function in order to increase glucose and FAs uptake and utilization in skeletal muscle during exercise.
The regulation of bone resorption and bioactive osteocalcin production by IL-6 also raised the question of whether this myokine acts on bone cells to fulfill this function. Cell-based experiments revealed that adding exogenous IL-6 to cultured calvaria osteoblasts increases the expression of Rankl, a cytokine that favors osteoclasts differentiation, and decreases the one of Osteoprotegerin (Opg), a decoy receptor for Rankl and an inhibitor of bone resorption [80]. These evidences suggest that in vivo IL-6 might act during exercise on cells of the osteoblasts lineage to increase bone resorption and the production of bioactive osteocalcin. At this point, extensive genetic and molecular studies are required to fully understand the regulation of bioactive osteocalcin by IL-6 during exercise. While the fact that IL-6 regulates bone remodeling and physiology is clear, the role of this cytokine is still an enigma because it appears to be a double-edge sword [81]. This is another reason to pursue the investigation of how muscle-derived IL-6 regulates osteoblasts/osteoclast function during exercise and the physiological consequences of this regulation.
5. Conclusions
During the last decades, multiple metabolic pathways engaged in response to exercise have been characterized [5]. Yet, the identification of well-defined links between exercise-induced responses and specific health benefits remains elusive. In this regard, understanding the crosstalk between contracting skeletal muscle and the functions of other organs is essential. The realization that skeletal muscle and bone, the two organs responsible for locomotion, have endocrine functions might set up a whole new agenda of research in exercise physiology.
The identification of a feed-forward loop between bone, via osteocalcin, and skeletal muscle, via IL-6, that is necessary and sufficient to promote adaptation to exercise in young and older animals represents a significant advance in our understanding of how the musculoskeletal system controls a function that is essential for survival of all vertebrates. This crosstalk between bone and muscle that facilitates exercise performance in mice, appears blunted in older mice but can be restored by osteocalcin administration. We propose that one important consequence of this function of bone may be that osteocalcin could be a means to treat age-related decrease in muscle mass and function. Future studies are obviously needed to determine if exogenous osteocalcin could improve exercise capacity in individuals with exercise intolerance and what might be the potential therapeutic applications of this function. As importantly going forward, the cellular and molecular mechanisms underlying the regulation of bioactive osteocalcin by IL-6 during exercise remain to be completely elucidated. Likewise, we now need to test whether muscle-derived IL-6 exerts a positive influence on bone (re)modeling in untrained or trained animals.
The recent physiological, genetic and molecular characterization of a crosstalk between bone and muscle during exercise raises additional questions that need to be addressed. One of them is the strong possibility that other tissues and organs might be influenced by skeletal muscle and bone signaling to promote adaptation to exercise. Another one is the notion that hormones secreted by bone and skeletal muscle might play a role in mediating some of the health benefits of exercise.
References
- [1].Bramble DM, Lieberman DE, Endurance running and the evolution of Homo, Nature 432 (7015) (2004) 345–352. [DOI] [PubMed] [Google Scholar]
- [2].Bauer UE, Briss PA, Goodman RA, Bowman BA, Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA, Lancet 384 (9937) (2014) 45–52. [DOI] [PubMed] [Google Scholar]
- [3].Booth FW, Gordon SE, Carlson CJ, Hamilton MT, Waging war on modern chronic diseases: primary prevention through exercise biology, J. Appl. Physiol. 88 (2) (1985) 774–787 2000. [DOI] [PubMed] [Google Scholar]
- [4].Booth FW, Lees SJ, Physically active subjects should be the control group, Med. Sci. Sports Exerc. 38 (3) (2006) 405–406. [DOI] [PubMed] [Google Scholar]
- [5].Egan B, Zierath JR, Exercise metabolism and the molecular regulation of skeletal muscle adaptation, Cell Metab. 17 (2) (2013) 162–184. [DOI] [PubMed] [Google Scholar]
- [6].Pedersen BK, Febbraio MA, Muscles, exercise and obesity: skeletal muscle as a secretory organ, Nat. Rev. Endocrinol 8 (8) (2012) 457–465. [DOI] [PubMed] [Google Scholar]
- [7].Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B, Searching for the exercise factor: is IL-6 a candidate? J. Muscle Res. Cell Motil. 24 (2–3) (2003) 113–119. [DOI] [PubMed] [Google Scholar]
- [8].Novotny SA, Warren GL, Hamrick MW, Aging and the muscle-bone relationship, Physiology (Bethesda) 30 (1) (2015) 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G, Endocrine regulation of energy metabolism by the skeleton, Cell 130 (3) (2007) 456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Booth SL, Centi A, Smith SR, Gundberg C, The role of osteocalcin in human glucose metabolism: marker or mediator? Nat. Rev. Endocrinol. 9 (1) (2013) 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferron M, Hinoi E, Karsenty G, Ducy P, Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice, Proc. Natl. Acad. Sci. U. S. A. 105 (13) (2008) 5266–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Laize V, Martel P, Viegas CS, Price PA, Cancela ML, Evolution of matrix and bone gamma-carboxyglutamic acid proteins in vertebrates, J. Biol. Chem. 280 (29) (2005) 26659–26668. [DOI] [PubMed] [Google Scholar]
- [13].Oury F, Ferron M, Huizhen W, Confavreux C, Xu L, Lacombe J, Srinivas P, Chamouni A, Lugani F, Lejeune H, Kumar TR, Plotton I, Karsenty G,Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis, J Clin. Invest. 123 (6) (2013) 2421–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, Karsenty G, Endocrine regulation of male fertility by the skeleton, Cell 144 (5) (2011) 796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G, Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation, Cell 89 (5) (1997) 747–754. [DOI] [PubMed] [Google Scholar]
- [16].Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, Owen MJ, Mertelsmann R, Zabel BU, Olsen BR, Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia, Cell 89 (5) (1997) 773–779. [DOI] [PubMed] [Google Scholar]
- [17].Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ, Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development, Cell 89 (5) (1997) 765–771. [DOI] [PubMed] [Google Scholar]
- [18].Terry A Kilbey A. Vaillant F, Stewart M, Jenkins A, Cameron E, Neil JC, Conservation and expression of an alternative 3′ exon of Runx2 encoding a novel proline-rich C-terminal domain, Gene 336 (1) (2004) 115–125. [DOI] [PubMed] [Google Scholar]
- [19].Zheng Q, Zhou G, Morello R, Chen Y, Garcia-Rojas X, Lee B,Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo, J. Cell Biol. 162 (5) (2003) 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, Bacchetta J, Szulc P, Kitsis RN, de Cabo R, Friedman RA, Torsitano C, McGraw TE, Puchowicz M, Kurland I, Karsenty G, Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise, Cell Metab. 23 (6) (2016) 1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Podolsky RJ, Arata T, Force generating mechanisms in striated muscle, Adv. Exp. Med. Biol. 226 (1988) 319–330. [PubMed] [Google Scholar]
- [22].Brooks GA (Ed.), Exercise Physiology: Human Bioenergetics and Its Applications, third ed., xxi, Mayfield Pub, Mountain View, Calif., 2000. (851 p). [Google Scholar]
- [23].Canto C, Gerhart-Hines Z,Feige JN, Lagouge M, Noriega L,Milne JC, Elliott PJ, Puigserver P, Auwerx J, AMPK regulates energy expenditure by modulating NAD + metabolismand SIRT1 activity, Nature 458 (7241) (2009) 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ, Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle, J. Physiol. 588 (Pt 10) (2010) 1779–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Green HJ, Helyar R, Ball-Burnett M, Kowalchuk N, Symon S, Farrance B, Metabolic adaptations to training precede changes in muscle mitochondrial capacity, J. Appl. Physiol. 72 (2) (1985) 484–491 1992. [DOI] [PubMed] [Google Scholar]
- [26].Howlett RA, Parolin ML, Dyck DJ, Hultman E, Jones NL, Heigenhauser GJ, Spriet LL, Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs, Am. J. Phys. 275 (2 Pt 2) (1998) R418–R425. [DOI] [PubMed] [Google Scholar]
- [27].Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ, An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1alpha and activates mitochondrial biogenesis in human skeletal muscle, Am. J. Phys. Regul. Integr. Comp. Phys. 300 (6) (2011) R1303–R1310. [DOI] [PubMed] [Google Scholar]
- [28].Petersen AC, McKenna MJ, Medved I, Murphy KT, Brown MJ, Della Gatta P, Cameron-Smith D, Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle, Acta Physiol (Oxford) 204 (3) (2012) 382–392. [DOI] [PubMed] [Google Scholar]
- [29].Rose AJ, Kiens B, Richter EA, Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise, J. Physiol. 574 (Pt 3) (2006) 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fukumoto S, Shimizu Y, Fibroblast growth factor 23 as a phosphotropic hormone and beyond, J. Bone Miner. Metab. 29 (5) (2011) 507–514. [DOI] [PubMed] [Google Scholar]
- [31].Karsenty G, Olson EN, Bone and muscle endocrine functions: unexpected paradigms of inter-organ communication, Cell 164 (6) (2016) 1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J, Lanzano P, Deng L, Leibel RL, Rubin M, Nicholas T, Chung W, Zeltser LM, Williams KW, Pessin JE, Kousteni S, MC4R-dependent suppression of appetite by bone-derived lipocalin 2, Nature 543 (7645) (2017) 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Teitelbaum SL, Bone resorption by osteoclasts, Science 289 (5484) (2000) 1504–1508. [DOI] [PubMed] [Google Scholar]
- [34].Ducy P, Schinke T, Karsenty G, The osteoblast: a sophisticated fibroblast under central surveillance, Science 289 (5484) (2000) 1501–1504. [DOI] [PubMed] [Google Scholar]
- [35].Legroux-Gerot I, Vignau J, Collier F, Cortet B, Bone loss associated with anorexia nervosa, Joint Bone Spine 72 (6) (2005) 489–495. [DOI] [PubMed] [Google Scholar]
- [36].Misra M, Klibanski A, The neuroendocrine basis of anorexia nervosa and its impact on bone metabolism, Neuroendocrinology 93 (2) (2011) 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hauschka PV, Lian JB, Cole DE, Gundberg CM, Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone, Physiol. Rev. 69 (3) (1989) 990–1047. [DOI] [PubMed] [Google Scholar]
- [38].Motyl KJ, McCabe LR, Schwartz AV, Bone and glucose metabolism: a two-way street, Arch. Biochem. Biophys. 503 (1) (2010) 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, Bassali RW, Davis CL, Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function, J. Clin. Endocrinol. Metab. 96 (7) (2011) E1092–E1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF, Osteocalcin in human serum: a circadian rhythm, J. Clin. Endocrinol. Metab. 60 (4) (1985) 736–739. [DOI] [PubMed] [Google Scholar]
- [41].Wei J, Hanna T, Suda N, Karsenty G, Ducy P, Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a, Diabetes 63 (3) (2014) 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G, Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice, Bone 50 (2) (2012) 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G, Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism, Cell 142 (2) (2010) 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mera P, Laue K, Wei J, Berger JM, Karsenty G, Osteocalcin is necessary and sufficient to maintain muscle mass in older mice, Mol Metab 5 (10) (2016) 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gibala MJ, MacLean DA, Graham TE, Saltin B, Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise, Am. J. Phys. 275 (2 Pt 1) (1998) E235–E242. [DOI] [PubMed] [Google Scholar]
- [46].Hawley JA, Hargreaves M, Joyner MJ, Zierath JR, Integrative biology of exercise, Cell 159 (4) (2014) 738–749. [DOI] [PubMed] [Google Scholar]
- [47].Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM, Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency, J. Biol. Chem. 280 (39) (2005) 33588–33598. [DOI] [PubMed] [Google Scholar]
- [48].Overmyer KA, Evans CR, Qi NR, Minogue CE,Carson JJ, Chermside-Scabbo CJ, Koch LG, Britton SL, Pagliarini DJ, Coon JJ, Burant CF, Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation, Cell Metab. 21 (3) (2015) 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pedersen BK, Febbraio MA, Muscle as an endocrine organ: focus on muscle-derived interleukin-6, Physiol. Rev. 88 (4) (2008) 1379–1406. [DOI] [PubMed] [Google Scholar]
- [50].Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI, The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome, Proc. Natl. Acad. Sci. U. S.A.104 (31) (2007) 12587–12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Baskin KK, Winders BR, Olson EN, Muscle as a “mediator” of systemic metabolism, Cell Metab. 21 (2) (2015) 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McPherron AC, Lawler AM, Lee SJ, Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member, Nature 387 (6628) (1997) 83–90. [DOI] [PubMed] [Google Scholar]
- [53].McPherron AC, Lee SJ, Suppression of body fat accumulation in myostatin-defi- cientmice,J. Clin. Invest. 109 (5) (2002) 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, Beckmann D, Paruzel P, Bertrand J, Redlich K, Koers-Wunrau C, Stratis A, Korb-Pap A, Pap T, Myostatin is a direct regulator ofosteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice, Nat. Med. 21 (9) (2015) 1085–1090. [DOI] [PubMed] [Google Scholar]
- [55].Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP, Role of myokines in exercise and metabolism, J. Appl. Physiol. 103 (3) (1985) 1093–1098 2007. [DOI] [PubMed] [Google Scholar]
- [56].Kanzleiter T, Rath M, Gorgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schurmann A, Eckardt K, The myokine decorin is regulated by contraction and involved in muscle hypertrophy, Biochem. Biophys. Res. Commun. 450 (2) (2014) 1089–1094. [DOI] [PubMed] [Google Scholar]
- [57].Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK, Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition,J. Physiol. 584 (Pt 1) (2007) 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Akerstrom T, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK, Exercise induces interleukin-8 expression in human skeletal muscle, J. Physiol. 563 (Pt 2) (2005) 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [59].Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS, Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run,J. Appl. Physiol. 94 (5) (1985) 1917–1925 2003. [DOI] [PubMed] [Google Scholar]
- [60].Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, Akerstrom T, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Bruunsgaard H, Pedersen BK, Febbraio MA, Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase, Diabetologia 52 (7) (2009) 1409–1418. [DOI] [PubMed] [Google Scholar]
- [61].Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K, FGF21 is an Akt-regulated myokine, FEBS Lett. 582 (27) (2008) 3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K, Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism, J. Biol. Chem. 283 (47) (2008) 32802–32811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM, A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis, Nature 481 (7382) (2012) 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM, Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis, Cell 157 (6) (2014) 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pedersen BK, Hoffman-Goetz L, Exercise and the immune system: regulation, integration, and adaptation, Physiol. Rev. 80 (3) (2000) 1055–1081. [DOI] [PubMed] [Google Scholar]
- [66].Febbraio MA, Pedersen BK, Muscle-derived interleukin-6: mechanisms for activation and possiblebiological roles, FASEBJ.16 (11) (2002) 1335–1347. [DOI] [PubMed] [Google Scholar]
- [67].Febbraio MA, Pedersen BK, Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc. Sport Sci. Rev. 33 (3) (2005) 114–119. [DOI] [PubMed] [Google Scholar]
- [68].Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B, Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6, J. Physiol. 529 (Pt 1) (2000) 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fischer CP, Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 12 (2006) 6–33. [PubMed] [Google Scholar]
- [70].Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK, Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction, Diabetes 53 (7) (2004) 1643–1648. [DOI] [PubMed] [Google Scholar]
- [71].Ostrowski K, Schjerling P, Pedersen BK, Physical activity and plasma interleukin-6 in humans–effect of intensity of exercise, Eur. J. Appl. Physiol. 83 (6) (2000) 512–515. [DOI] [PubMed] [Google Scholar]
- [72].Bartoccioni E, Michaelis D, Hohlfeld R, Constitutive and cytokine-induced production of interleukin-6 by human myoblasts, Immunol. Lett. 42 (3) (1994) 135–138. [DOI] [PubMed] [Google Scholar]
- [73].De Rossi M, Bernasconi P, Baggi F, de Waal Malefyt R, Mantegazza R, Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation, Int. Immunol. 12 (9) (2000) 1329–1335. [DOI] [PubMed] [Google Scholar]
- [74].Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD, Transcriptional activation ofthe IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content, FASEB J. 15 (14) (2001) 2748–2750. [DOI] [PubMed] [Google Scholar]
- [75].Steenbergen RD, OudeEngberink VE, Kramer D, Schrijnemakers HF, Verheijen RH, Meijer CJ, Snijders PJ, Down-regulation of GATA-3 expression during human papillomavirus-mediated immortalization and cervical carcinogenesis, Am.J.Pathol. 160 (6) (2002) 1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK, Interleukin-6 stimulates lipolysis and fat oxidation in humans, J. Clin. Endocrinol. Metab. 88 (7) (2003) 3005–3010. [DOI] [PubMed] [Google Scholar]
- [77].Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA, Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase, Diabetes 55 (10) (2006) 2688–2697. [DOI] [PubMed] [Google Scholar]
- [78].Faldt J, Wernstedt I, Fitzgerald SM, Wallenius K, Bergstrom G, Jansson JO, Reduced exercise endurance in interleukin-6-deficient mice, Endocrinology 145 (6) (2004) 2680–2686. [DOI] [PubMed] [Google Scholar]
- [79].Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumaki K, Taga T, et al. , Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6, Proc. Natl. Acad. Sci. U. S. A. 90 (24) (1993) 11924–11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Teitelbaum SL, Ross FP, Genetic regulation of osteoclast development and function, Nat. Rev. Genet. 4 (8) (2003) 638–649. [DOI] [PubMed] [Google Scholar]
- [81].Blanchard F, Duplomb L, Baud’huin M, Brounais B, The dual role ofIL-6-type cytokines on bone remodeling and bone tumors, Cytokine Growth Factor Rev. 20 (1) (2009) 19–28. [DOI] [PubMed] [Google Scholar]