Abstract
Purpose
Previous epidemiological and cost studies of fungal meningitis have largely focused on single pathogens, leading to a poor understanding of the disease in general. We studied the largest and most diverse group of fungal meningitis patients to date, over the longest follow-up period, to examine the broad impact on resource utilization within the United States.
Methodology
The Truven Health Analytics MarketScan database was used to identify patients with a fungal meningitis diagnosis in the United States between 2000 and 2012. Patients with a primary diagnosis of cryptococcal, Coccidioides, Histoplasma, or Candida meningitis were included in the analysis. Data concerning healthcare resource utilization, prevalence and length of stay were collected for up to 5 years following the original diagnosis.
Results
Cryptococcal meningitis was the most prevalent type of fungal meningitis (70.1 % of cases over the duration of the study), followed by coccidioidomycosis (16.4 %), histoplasmosis (6.0 %) and candidiasis (7.6 %). Cryptococcal meningitis and candidiasis patients accrued the largest average charges ($103 236 and $103 803, respectively) and spent the most time in the hospital on average (70.6 and 79 days). Coccidioidomycosis and histoplasmosis patients also accrued substantial charges and time in the hospital ($82 439, 48.1 days; $78 609, 49.8 days, respectively).
Conclusion
Our study characterizes the largest longitudinal cohort of fungal meningitis in the United States. Importantly, the health economic impact and long-term morbidity from these infections are quantified and reviewed. The healthcare resource utilization of fungal meningitis patients in the United States is substantial.
Keywords: cryptococcal meningitis, candida meningitis, histoplasma meningitis, coccidioides meningitis, prevalence, healthcare resource utilization, infectious disease, fungal meningitis
Introduction
Fungal meningitis (FM) can manifest from the dissemination of any human fungal pathogen into the subarachnoid space (SAS), where the cerebrospinal fluid (CSF) resides. Usually this occurs secondary to a systemic infection, as the fungus breaches the blood–brain barrier (BBB) into the intrathecal space, either haematogeneously or locally with trauma and/or surgery [1]. The SAS and its contents are normally immunologically protected, having both functional and anatomic barriers to invasion by both foreign pathogens and native immune responses [2]. Major primary fungal pathogens, including C. neoformans/C. gattii, C. immitis/C. posadasii or H. capsulatum, can cause central nervous system (CNS) infections in both apparently immunocompetent and immunocompromised individuals, while opportunistic fungi such as Candida species penetrate the CNS secondary to an immunocompromised state or dysfunctional protective structures [1].
Cryptococcal meningitis (CrM)
Cryptococcal meningitis (CrM) is the most common cause of adult meningitis—particularly in regions with high HIV burden—and is a growing cause of concern around the world [3]. Caused by Cryptococcus neoformans or Cryptococcus gattii, CrM manifests when a cryptococcal infection disseminates to the CNS and is associated with an uncertain prognosis [1, 4, 5]. Cryptococcal infections were reported in fewer than 500 patients globally prior to the 1960s, but clinicians witnessed a dramatic rise during the early AIDS pandemic of the 1980s, with over 330 cases per year in the United States alone [6, 7]. As the HIV pandemic grew, CrM followed. HIV is the largest risk factor for CrM and is associated with up to 79 % of CrM cases. CrM accounts for up to 15–17 % of AIDS-related mortality, even in resource-available settings [3, 8–10]. Over the last decade, approximately 1 million cases were diagnosed on an annual basis worldwide, with approximately 700 000 deaths per year, up to 500 000 of which occurred in Sub-Saharan Africa alone [3, 5]. With the increasing availability of more widespread antiretroviral therapy (ART) for treatment of HIV infection, the burden of CrM has decreased modestly worldwide. In fact, the most recent estimates indicate around 200 000 new cases per year, with 120 000 deaths [11]. However, a sobering study showed that with ART availability in sub-Saharan Africa cases of cryptococcosis have levelled off at what is still a high rate [12]. Moreover, while the rate of undiagnosed HIV is decreasing in the United States, it is still the case that CrM is commonly diagnosed late in the disease process among socio-economically disadvantaged populations and those with less obvious risk factors, which leads to potentially worse outcomes [13]. HIV-negative and non-transplant CrM is a less common but significant problem, with a high rate of mortality (20–30 %) despite available therapy [10]. Of the HIV-uninfected patients, one large series identified 25 % of CrM patients as receiving steroid therapy, 24 % with chronic kidney, liver, or lung disease, 16 % with a malignancy and 15 % with solid-organ transplants [14]. However, when HIV infection is excluded, up to 30 % of CrM cases can occur in apparently immunocompetent individuals with no underlying disease [14], and they are commonly caused by C. gattii [1].
Despite the substantial emphasis on management of CrM, the literature is scant and incomplete regarding the overall economic costs of CrM, and single-centre studies have focused on the initial hospitalization, which may give a limited view of the economic impact [15]. An aim of this study was to provide a longitudinal view of the incidence and economic burden of CrM in the United States.
Coccidioides meningitis (CoM)
Coccidioides meningitis (CoM) is the result of disseminated coccidioidomycosis, caused by the sibling dimorphic fungi, Coccidioides immitis and Coccidioides posadasii, with geographical boundaries. The incidence of coccidioidomycosis within the United States has been increasing since 1998, and was last reported at 42.6 per 100 000 in 2011, with 66 % of cases occurring in Arizona and 31 % in California [16]. Approximately 1 to 3 % of coccidioidomycosis cases disseminate [16], and within the first few months after acute primary infection 33 to 50 % develop meningitis [1, 17]. Similar to other types of FM, it is likely that immunosuppressed individuals are at higher risk for CoM [16]. However, Bronnimann and colleagues linked only 2 % of CoM cases with immunosuppressive therapy or HIV infection prior to disease onset [18], indicating far less influence from immune suppression than other forms of FM and suggesting a potential genetic role in meningitis risk [1]. For instance, any individual may experience disseminated coccidioidomycosis, but the risk of dissemination seems to be higher in Filipinos, African Americans, diabetics and third trimester pregnancy individuals [16, 19–25]. CoM may result in severe and fatal complications if not identified and treated immediately [26]. It is uniformly fatal without any treatment [27], but current antifungal regimens cannot reliably cure CoM [1, 16, 28]. With the advent of antifungal treatment, the mortality rate has dropped to ~30 %, but morbidity remains unchanged [29].
Histoplasma meningitis (HM)
Histoplasma meningitis (HM) is a geographical fungal infection caused by the dimorphic fungus Histoplasma capsulatum and is endemic to certain areas within the United States, South America, Southeast Asia and Africa [30, 31]. Immunocompromised individuals are at risk for developing lethal progressive disseminated histoplasmosis [1, 30]. Specifically, HM occurs in AIDS patients, solid-organ transplant recipients, and patients taking corticosteroids or TNF-α antagonists [1, 32–37]. Wilson et al. reported a total of 4950 diagnosed cases of histoplasmosis in 1994, with an incidence of 19.02 per million of the US population, and 3681 patients diagnosed with histoplasmosis in 1996, with an incidence of 13.62 per million of the US population [38]. In a clinical review of H. capsulatum, Wheat et al. identified CNS manifestation in 10–20 % of disseminated histoplasmosis cases [37]. The mortality rate for those with CNS involvement was 20–40 %, with a relapse rate of 50 % in those who survive [39, 40].
Candida meningitis (CaM)
Candida meningitis (CaM) is a fungal infection of the CNS caused by several Candida species, including Candida albicans, Candida tropicalis, Candida lusitaniae and Candida parapsilosis, which are distributed worldwide and are encountered in patients globally [1]. Candida infection is known to disseminate to the CNS in immunocompromised patients or in the setting of anatomic abnormalities [1, 41]. Notably, CaM is most common in infants with an immature BBB, while C. albicans is the most common fungal isolate from both newborn and infant CSF [17]. Moreover, neonates who develop candidaemia are at greater risk for CNS dissemination of the disease than adults [42]. Neurosurgical procedures also breach the BBB and offer an opportunity for direct inoculation of the CNS with yeasts [1]. In fact, CaM is the most common fungal meningitis for patients with ventriculoperitoneal shunts or ventriculostomies and often occurs with concurrent post-surgical antimicrobial prophylaxis [43–45]. The mortality associated with untreated CaM ranges from 50–97 % [46]. After treatment, mortality decreases to 10–30 %, with percentages varying by risk group [1, 46].
Because infants are a particularly at-risk population, studies from other groups have identified the incidence of CaM in neonates as 0.4%, and 1.1 % in infants weighing <1500 g at birth. Importantly, the mortality rate in infants varies from 12–35 % [42]. Another study of premature infants identified a 44 % mortality rate from CaM, with 60 % combined mortality and developmental disabilities, versus 28 % in age-matched controls [47].
Fungal meningitis healthcare economics and resource utilization
Soaring healthcare costs and healthcare reforms have put an increasing emphasis on creating cost-effective therapies for acute, subacute and chronic conditions. For CNS infections, there are more than 72 000 hospitalizations for meningitis annually, arising from either fungal, viral, bacterial or parasitic origins [48]. This results in expenditures totalling $1.2 billion dollars annually in hospital costs for the US healthcare system [48]. Specifically, FM is a growing national concern for both healthcare costs and overall patient care. For instance, among the various pathogens that cause meningitis, the greatest overall mortality in the US was attributed to those individuals diagnosed with FM (9.1 %) [48]. Moreover, mortality rates of at least 50 % for FM have been reported in immunocompromised cohorts in certain regions and countries, such as Southeast Asia and Africa, even with treatment [49–54]. Although comparatively rare for CNS infections (7.3 % of meningitis-related hospitalizations) [48], management of FM is not only deadly but may be costly to the healthcare system because of its chronic sequelae producing long-term morbidity and thus utilization of healthcare resources.
While other studies have analysed the epidemiology [1, 32, 55–57], treatment options [1, 55, 58], pathogenesis [1] and costs [15, 38] of these FMs separately, there are no current comprehensive and conclusive analyses examining the prevalence, patient characteristics and US health economic burden over a longitudinal observation period. Therefore, the goal of this study was to provide the relative and overall prevalence of specific FMs and their subsequent 5-year healthcare resource utilization (HCRU) and economic impact on a national scale across the United States. This study combines the largest group of FM patients analysed to date with the longest follow-up interval for FM.
Methods
The Truven Health Analytics MarketScan inpatient and outpatient research database was utilized to retrospectively quantify the prevalence, economic impact and healthcare resource utilization due to FM in the United States between 2000 and 2012. Patients were identified by the primary International Classification of Diseases-9 Codes (ICD-9-CM) for all FM types, and then subdivided by type of FM, so that fungus-specific quantification and comparison could be performed (Table S1, available in the online version of this article).
Data were collected at first admission for FM, 1 year prior to initial FM diagnosis (baseline), and for up to 5 years following diagnosis. Patient characteristics, including year of diagnosis, age at initial diagnosis, sex, insurance status, discharge status, region, baseline annual costs, employment status, baseline annual length of stay (LoS), follow-up years, inpatient/outpatient status, mortality status and Charlson comorbidity index score (CCI), were collected for the entire cohort. Mortality was defined as the number of in-hospital deaths due to each type of FM. CCI is a validated measure for the long-term prognosis of patients due to comorbid diseases [59]. A score of 0 is awarded to patients with no comorbid disease, and a score of 6 is awarded for severe diseases such as malignant tumours. A patient’s CCI score is calculated as the sum of all individual comorbid diseases [60]. The CCI was classified as 1, 2 and 3+ in this study.
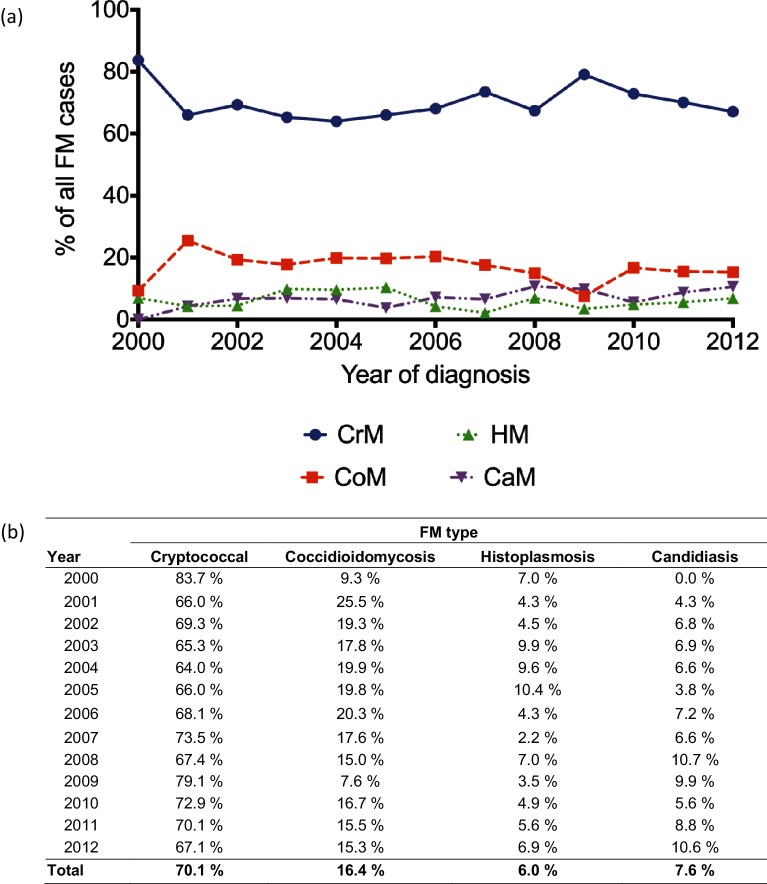
The primary outcomes of interest were prevalence, healthcare resource utilization (HCRU) and mortality. Prevalence was defined as the proportion of patients diagnosed with a specific fungus among all FM types. Fig. 1 denotes the number of patients diagnosed with each type of FM and the prevalence for each year between 2000 and 2012. HCRU comprised LoS and cost data. LoS was defined as the number of days a patient was hospitalized during their inpatient visits. Costs were calculated as the total inpatient and outpatient expenses to the hospital from the primary admission and up to 5 years after admission. Negative and extremely large value outliers were removed by excluding the highest and lowest 1.5 % of values to account for these outliers. It is important to note that monetary values reflect the costs in the years that data were collected and are not corrected for inflation over time.
Fig. 1.
Prevalence of fungal meningitis in the United States from 2000–2012. (a) Line charts and (b) corresponding values of prevalence of fungal meningitis in the United States between 2000 and 2012.
Statistical analysis
The patient characteristics were summarized by mean±standard deviation (SD) and range for continuous numerical variables or frequency for categorical variables. Annual expenditure and LoS for patients diagnosed with FM during the diagnosis year, and up to 5-year follow-up times, were summarized by their mean±SD values. The 95 % confidence interval of patient mortality at each follow-up period was calculated to determine the stability of the estimates. A 5-year Kaplan–Meier plot was then generated to visualize length of survival, measured in days from the date of initial diagnosis, and patients surviving beyond that follow-up period were censored. Due to sample size limitations within FM groups, a Kaplan–Meier plot was only generated for CrM. A longitudinal analysis was used to model the value of log(cost) in each 1-year interval using a generalized estimating equations (GEE) model to account for the correlation of the same patient’s cost in multiple years. The model included meningitis type, sex, age, employment status, insurance, prior Charlson score, region of country, meningitis diagnosis year and follow-up years after diagnosis as the independent covariates, among which sex, employment status, Charlson score, country region and insurance were evaluated as categorical variables. The GEE model assumed an exchangeable correlation structure for patients with multiple years of data. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and the figures were created using GraphPad Prism 7.0 c for Mac (GraphPad Software, La Jolla California, USA; www.graphpad.com).
Results
A total of 1927 patients with an FM diagnosis between 2000 and 2012 were identified in the Marketscan database. Of this cohort, 1351 patients (70.11 %) were diagnosed with CrM, 315 (16.35 %) were diagnosed with CoM, 146 (7.58 %) were diagnosed with CaM and 115 (5.97 %) were diagnosed with HM. Interestingly, the prevalence of each of the individual types of FM remained at consistent levels over the years of observation (Fig. 1).
Patient demographics
Cryptococcal meningitis (CrM)
The patient demographics are described in Table 1. Most patients diagnosed with CrM were middle-aged (M=48.5 years, SD=14.9) and male (66.2 %). The available regional data identifiers indicate that CrM patients are most frequently located in the Southern region of the United States (27.8 %), followed by the North Central (9.2 %), Northeast (7.3 %) and West (6.4 %) regions. Of note, the data from patients with Medicaid insurance did not include details of their geographical distribution. In fact, almost half (48.0 %) of those diagnosed with CrM were covered by Medicaid insurance, followed by 40.9 % with private insurance and 11.2 % with Medicare. Only a small percentage of patients had a full-/part-time job (12.3 %) or were retired/Medicare-eligible/disabled (10.4 %) at the time of diagnosis. The vast majority of those diagnosed with CrM had a CCI score of 3+ (74.5 %), with 8.3 % with a CCI of 2 and 6.7 % with a CCI of 1, while 10.6 % were given a CCI score of 0. The length of follow-up ranged between 0.0 to 13.0 years, with an average follow-up of 2.1 years (SD=2.4).
Table 1. Epidemiology of fungal meningitis in the United States, 2000–2012.
| Variable | Cryptococcal (N=1351) | Coccidioidomycosis (N=315) | Histoplasmosis (N=115) | Candidiasis (N=146) | All FM combined (N=1927) |
|---|---|---|---|---|---|
| Age at first diagnosis | |||||
| Mean (SD) | 48.5 (14.9) | 53.1 (15.9) | 56.9 (16.3) | 49.7 (17.7) | 49.8 (15.5) |
| Range | (18.0–96.0) | (18.0–91.0) | (20.0–86.0) | (18.0–91.0) | (18.0–96.0) |
| Sex | |||||
| Male | 894 (66.2 %) | 177 (56.2 %) | 62 (53.9 %) | 68 (46.6 %) | 1201 (62.3 %) |
| Female | 457 (33.8 %) | 138 (43.8 %) | 53 (46.1 %) | 78 (53.4 %) | 726 (37.7 %) |
| Insurance type | |||||
| Commercial Claims | 552 (40.9 %) | 215 (68.3 %) | 65 (56.5 %) | 78 (53.4 %) | 910 (47.2 %) |
| Medicaid | 648 (48.0 %) | 23 (7.3 %) | 17 (14.8 %) | 41 (28.1 %) | 729 (37.8 %) |
| Medicare | 151 (11.2 %) | 77 (24.4 %) | 33 (28.7 %) | 27 (18.5 %) | 288 (14.9 %) |
| Region | |||||
| Medicaid (unknown) | 648 (48.0 %) | 23 (7.3 %) | 17 (14.8 %) | 41 (28.1 %) | 729 (37.8 %) |
| Northeast | 99 (7.3 %) | 6 (1.9 %) | 7 (6.1 %) | 17 (11.6 %) | 129 (6.7 %) |
| North Central | 124 (9.2 %) | 32 (10.2 %) | 33 (28.7 %) | 25 (17.1 %) | 214 (11.1 %) |
| South | 376 (27.8 %) | 48 (15.2 %) | 50 (43.5 %) | 44 (30.1 %) | 518 (26.9 %) |
| West | 86 (6.4 %) | 203 (64.4 %) | 7 (6.1 %) | 14 (9.6 %) | 310 (16.1 %) |
| Unknown | 18 (1.3 %) | 3 (1.0 %) | 1 (0.9 %) | 5 (3.4 %) | 27 (1.4 %) |
| Employment status | |||||
| Full-time/part-time | 166 (12.3 %) | 81 (25.7 %) | 22 (19.1 %) | 28 (19.2 %) | 297 (15.4 %) |
| Retiree/Medicare Eligible/disabled | 140 (10.4 %) | 79 (25.1 %) | 29 (25.2 %) | 20 (13.7 %) | 268 (13.9 %) |
| Other | 397 (29.4 %) | 132 (41.9 %) | 47 (40.9 %) | 57 (39.0 %) | 633 (32.8 %) |
| Medicaid (unknown) | 648 (48.0 %) | 23 (7.3 %) | 17 (14.8 %) | 41 (28.1 %) | 729 (37.8 %) |
| Index Charlson comorbidity score | |||||
| 0 | 143 (10.6 %) | 93 (29.5 %) | 29 (25.2 %) | 26 (17.8 %) | 291 (15.1 %) |
| 1 | 90 (6.7 %) | 44 (14.0 %) | 19 (16.5 %) | 22 (15.1 %) | 175 (9.1 %) |
| 2 | 112 (8.3 %) | 59 (18.7 %) | 20 (17.4 %) | 26 (17.8 %) | 217 (11.3 %) |
| 3+ | 1006 (74.5 %) | 119 (37.8 %) | 47 (40.9 %) | 72 (49.3 %) | 1244 (64.6 %) |
| Follow-up years | |||||
| Mean (SD) | 2.1 (2.4) | 2.3 (2.5) | 2.6 (2.6) | 1.5 (1.7) | 2.1 (2.4) |
| Range | (0.0–13.0) | (0.0–11.9) | (0.0–10.9) | (0.0–7.7) | (0.0–13.0) |
| Inpatient death indicator | |||||
| No | 719 (86.1 %) | 154 (92.8 %) | 29 (96.7 %) | 85 (82.5 %) | 987 (87.0 %) |
| Yes | 116 (13.9 %) | 12 (7.2 %) | 1 (3.3 %) | 18 (17.5 %) | 147 (13.0 %) |
Coccidioides meningitis (CoM)
For those diagnosed with CoM between 2000 and 2012, the average age at initial diagnosis was 53.1 years of age (SD=15.9). A majority of the patients diagnosed with CoM were male (56.2 %). As expected, CoM diagnoses were most prevalent in the Western region of the United States (64.4 %), followed by the Southern (15.2 %), North Central (10.2 %) and Northeast (1.9 %) regions. A higher percentage of patients were privately insured (68.3 %), followed by those with Medicare (24.4 %) and then those with Medicaid (7.3 %). Patients diagnosed with CoM were mostly full-time/part-time employees (25.7 %) or retired/Medicare-eligible/disabled (25.1 %), as opposed to Medicaid-supported (7.3 %), in accordance with the insurance data. A large portion of the patients diagnosed with CoM had a CCI score of 3+ (37.8 %), with 18.7 % having a CCI of 2, 14.0 % having a CCI of 1 and 29.5 % having a CCI score of 0. The length of follow-up ranged between 0.0 to 11.9 years, with an average follow-up of 2.3 years (SD=2.5). Further details on the patient demographics are available in Table 1.
Histoplasmosis meningitis
The mean age of patients diagnosed with HM between 2000 and 2012 was 56.9 years (SD=16.3), with the majority of diagnosed patients being male (53.9 %). The available regional identifiers indicate that HM is most prevalent in the Southern region of the United States (43.5 %), with a smaller percentage of cases being found in the North Central (28.7 %), West (6.1 %) and Northeast regions (6.1 %). Most patients had commercial private insurance (56.5 %), followed by Medicare (28.7 %) and Medicaid (14.9 %). Patients with HM were mostly retired/Medicare-eligible/disabled (25.2 %), with the rest being either full-time/part-time employees (19.1 %) or supported by Medicaid (14.8 %). The majority of patients had a CCI score of 3+ (40.9 %), followed by a CCI score of 0 (25.2 %), then a score of 2 (17.4 %) or 1 (16.5 %). The length of follow-up ranged between 0.0 and 10.9 years, with an average follow-up of 2.6 years (SD=2.6) (Table 1).
Candida meningitis (CaM)
Patients diagnosed with CaM between 2000 and 2012 were, on average, 49.7 years of age (SD=17.7), while the majority were (53.4 %) female. CaM was most prevalent in the Southern region of the United States (30.1 %), followed by the North Central (17.1 %), Northeast (11.6 %) and Western regions (9.6 %). Private insurance was most common among CaM patients (53.4 %), with Medicaid (28.1 %) and Medicare (18.5 %) less prominent. The distribution of employment status for CaM patients was reported as Medicaid-supported (28.1 %), full- or part-time employees (19.2 %), retirees/Medicare-eligible/disabled (13.7 %), or other (39.0 %). The vast majority of CaM patients had a CCI score of 3+ (49.3 %), with 17.8 % being given a CCI score of 2, 15.1 % having a CCI of 1 and 17.8 % having a score of 0. The range of length of follow-up went from 0 to 7.7 years, with an average follow-up of 1.5 years (SD=1.7). Further demographic information is available in Table 1.
Cost, length of stay and mortality rates
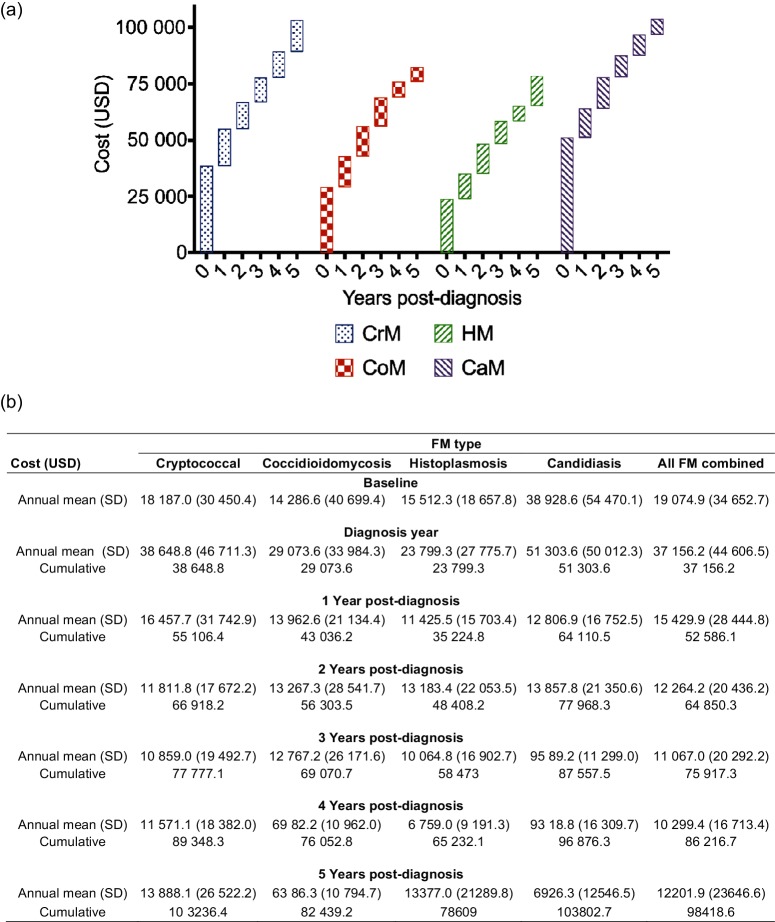
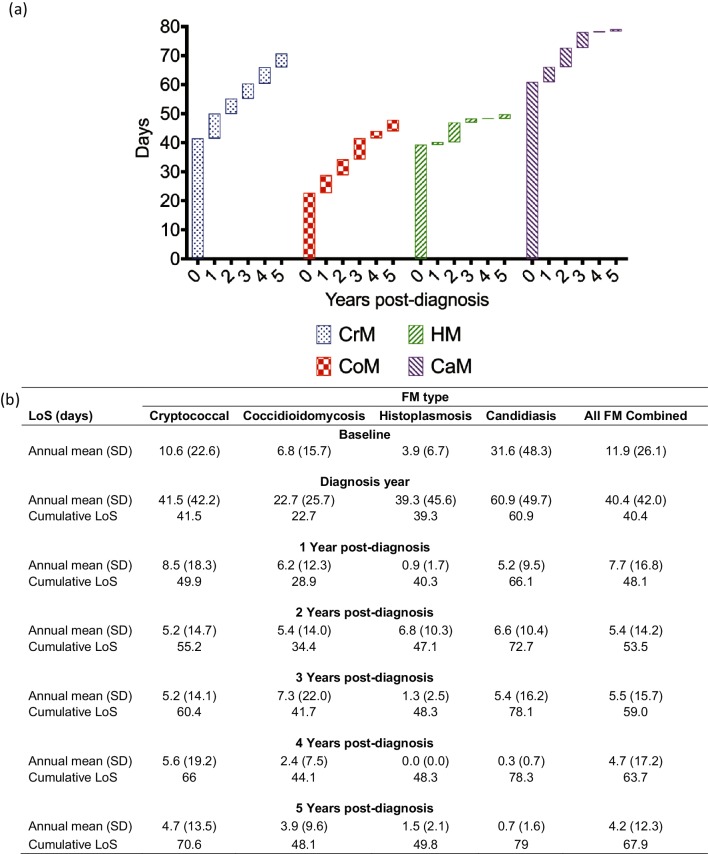
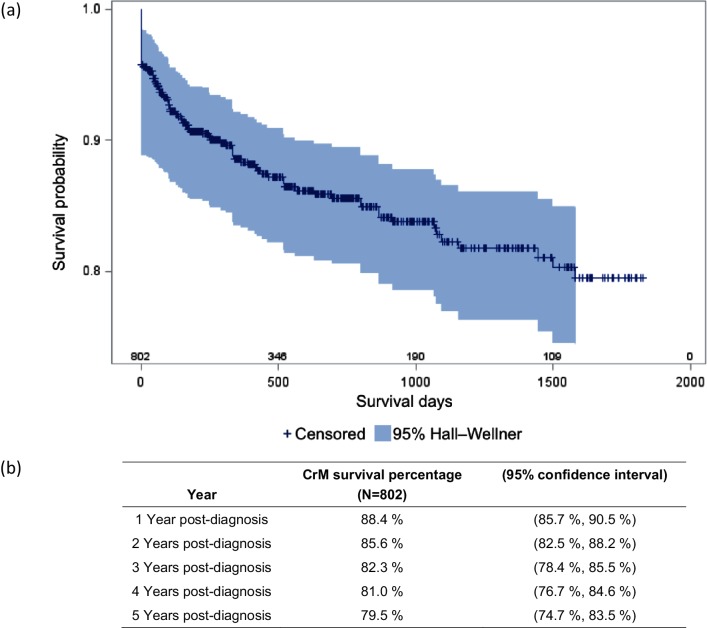
The Truven Marketscan Database offers a unique opportunity to study a large cohort of patients over an extended period of time. No previous studies have analysed a group as large as this cohort with as many fungi as feature in this study. Fig. 2 depicts the various costs associated with FM. Fig. 3 relates to LoS. Fig. 4 illustrates mortality.
Fig. 2.
Cost of fungal meningitis in the United States, 2000–2012. (a) Waterfall plot and (b) corresponding values of the annual and cumulative cost burden of fungal meningitis on the United States healthcare system between 2000 and 2012. Each like-patterned box in (a) represents a single year of the 6-year tracking period, and each height corresponds to the annual cost for that respective year. The stacking boxes yield the cumulative cost over that time period.
Fig. 3.
Length of stay (LoS) for fungal meningitis. (a) Waterfall plot and (b) corresponding values of the annual and cumulative LoS burden of fungal meningitis on the United States healthcare system between 2000 and 2012. Each like-patterned box in (a) represents a single year of the 6-year tracking period, and each height corresponds to the annual LoS for that respective year. The stacking boxes yield the cumulative LoS over that time period.
Fig. 4.
Kaplan–Meier curve for cryptococcal meningitis inpatient survival. (a) Kaplan–Meier curve and (b) corresponding values for cryptococcal meningitis inpatients.
Cryptococcus meningitis (CrM)
The average baseline cost for individual treatment, 1 year prior to CrM diagnosis, was $18 187 (Fig. 2). Fig. 2 also depicts the annual costs during the year of diagnosis and every year for the following 5 years of treatment. The average cost for patients during the year of initial diagnosis with CrM increased to $38 649, and then averaged $12 917 over the next 5 years. The cumulative total cost over 5 years was $103 236.
The average annual LoS of patients 1 year prior to diagnosis with CrM was 10.6 days, but notably, by 1 year after diagnosis, this number had quadrupled to 41.5 days (Fig. 3). Over the next 4 years, the average annual LoS decreased to 4.7 days per year. This corresponded to a cumulative average LoS of 70.6 days hospitalized at 5 years after the initial diagnosis year.
The Kaplan–Meier curve (Fig. 4) depicts the proportion of survival after each mortality event over a 5-year period. Patients that were lost to follow-up were censored and characterized as dashes on the Kaplan–Meier curve. The survival rate for CrM declined sharply from day 0 to day 500, and thereafter continued to decline at a decreasing rate over the 5-year observation period. Fig. 4 also offers a summary of the survival of CrM inpatients over a 5-year period, with 88.4 % of patients surviving at year one after diagnosis and 79.5 % surviving to year five.
Coccidioides meningitis (CoM)
On average, the baseline annual cost for a CoM patient 1 year prior to diagnosis was $14 287 (Fig. 2). During the year of diagnosis, the mean annual expenditure increased to $29 074, with a subsequent average of $10 673 per year through 5 years. Moreover, the cumulative total cost over 5 years was $82 439 (Fig. 2).
The baseline mean annual LoS was tracked for patients 1 year before (baseline) and up to 5 years after CoM diagnosis. The baseline LoS was 6.8 days (Fig. 3) and it peaked during the year of diagnosis (22.7 days), with a subsequent mean of 5.0 days annually over the next 5 years. This corresponded to a cumulative average LoS of 48.1 days at 5 years after the initial diagnosis year (Fig. 3).
Histoplasma meningitis (HM)
One year prior to diagnosis, the mean annual hospital cost for HM patients (baseline) was $15 512 (Fig. 2). During the year of diagnosis, the average annual cost increased to $23 799, with a subsequent average annual cost of $12 252 over the next 5 years post-diagnosis. The cumulative average annual cost over 5 years totalled $78 609 (Fig. 2).
The mean annual length of hospital stay 1 year prior to diagnosis (baseline) was approximately 3.9 days (Fig. 3). Average annual LoS peaked during the first diagnosis year (39.3 days), subsequently averaging 2.1 days annually over the next 5 years. This corresponded to a cumulative average LoS of 49.8 days at 5 years after the initial diagnosis year (Fig. 3).
Candida meningitis (CaM)
The baseline mean annual hospital cost for a patient 1 year prior to CaM diagnosis was $38 929 (Fig. 2). During the year of diagnosis, the mean annual costs increased to $51 304, with a subsequent average annual cost of $10 500 over the next 5 years. Fig. 2 represents the cumulative average annual cost over 5 years, totalling $103 803.
The baseline mean annual LoS 1 year prior to CaM diagnosis was 31.6 days (Fig. 3). The average annual LoS peaked during the first diagnosis year (60.9 days) and averaged 3.6 days annually over the next 5 years. This corresponded to a cumulative average LoS of 79.0 at 5 years after the initial diagnosis year (Fig. 3).
Annual cost based on multivariate longitudinal analysis
Table 2 presents a multivariate longitudinal analysis to model the value of log(cost) in each 1-year interval using a generalized estimating equations (GEE) model and assuming an exchangeable correlation structure to account for the correlation of the same patient’s costs in multiple years. This model includes meningitis type, sex, age, CCI score, insurance type, year of initial diagnosis, employment status, region and follow-up years as covariates.
Table 2. Multivariate regression on annual cost.
| Covariate | Cost ratio (95 % CI) | (95 % confidence interval) | P-value |
|---|---|---|---|
| Meningitis type | |||
| Histoplasmosis | 1.11 | (0.92, 1.34) | 0.271 |
| Coccidioidomycosis | 0.90 | (0.71, 1.14) | 0.379 |
| Candidiasis | 1.68 | (1.34, 2.10) | ***<.001 |
| Cryptococcal | Reference | ||
| Sex | |||
| Female | 1.20 | (1.04, 1.38) | *0.014 |
| Male | Reference | ||
| Age at first diagnosis | 1.00 | (0.99, 1.00) | 0.564 |
| Index Charlson comorbidity score | |||
| 3+ | 3.04 | (2.42, 3.80) | ***<.001 |
| 2 | 2.24 | (1.74, 2.88) | ***<.001 |
| 1 | 1.72 | (1.31, 2.27) | ***<.001 |
| 0 | Reference | ||
| Insurance type | |||
| MDCR | 0.89 | (0.70, 1.12) | 0.322 |
| MAID | 0.57 | (0.44, 0.74) | ***<.001 |
| CCAE | Reference | ||
| Year of initial diagnosis | 1.03 | (1.01, 1.06) | ***0.007 |
| Employment status | |||
| Other** | 1.06 | (0.88, 1.29) | 0.539 |
| Retiree/Medicare/Eligible/disabled | 1.00 | (0.80, 1.26) | 0.998 |
| Full-time/part-time | Reference | ||
| Region | |||
| Unknown | 1.09 | (0.56, 2.12) | 0.801 |
| West | 0.95 | (0.69, 1.30) | 0.747 |
| South | 0.94 | (0.76, 1.15) | 0.550 |
| North Central | 1.15 | (0.91, 1.45) | 0.252 |
| Northeast | Reference | ||
| Follow-up diagnosis year | 0.80 | (0.77, 0.84) | ***<.001 |
CCAE, Commercial Claims and Encounters; MDCR, Medicare; MAID, Medicaid.
*P<.05 (number of observations used in the regression: 6232).
**P<.01 (other includes: surviving spouse/dependent; other/unknown).
***P<.001.
For categorical variables, this analysis revealed that there was no significant difference in the annual cost for patients based on their age, employment status, or region. However, the annual cost for patients with a CCI score of 1 is expected to be 72 % higher than that for patients with a CCI score of 0 [P<0.001, CR 1.72, 95 % CI (1.31, 2.27)]. Similarly, the annual cost for patients with a CCI score of 2 and 3 is expected to be 124 % [P<0.001, CR 2.24, 95 % CI (1.74, 2.88)] and 204 % [P<0.001, CR 3.04, 95 % CI (2.42, 3.80)] higher than that for patients with a CCI score of 0, respectively. Moreover, compared to cryptococcal patients, candidiasis patients are expected to spend 68 % more annually for treatment [P<0.001, CR 1.68, 95 % CI (1.34, 2.10)]. Furthermore, when other covariates are held at a fixed value, each unit increase of follow-up year after initial diagnosis will be expected to have a 20 % decrease of annual cost [P=<0.001, CR 0.80, 95 % CI (0.77, 0.84)]. Further, each unit increase in year of initial diagnosis is expected to add a 3 % increase in cost [P=.007, CR 1.03, 95 % CI (1.91, 1.06)], which accounts for the annual cost of inflation. Additionally, female patients are expected to have a 20 % higher annual cost compared to male patients when other covariates are held at a fixed value [P=0.014, CR 1.20, 95 % CI (1.04, 1.38)]. On the other hand, the annual average cost for Medicaid patients is 43 % lower than that for privately insured patients [P<0.001, CR 0.57, 95 % CI (0.44, 0.74)].
Discussion
Susceptibility to FM is mediated by the complex but generally effective host defence system. For example, neurosurgical procedures offer an opportunity to bypass the natural structural barriers of the CNS, and are a well-characterized risk factor for FM [1, 61, 62]. Treatments or syndromes that weaken the immune system increase susceptibility to FM. Disorders and treatments such as HIV/AIDS, certain lymphoreticular malignancies, diabetes, iron chelation and corticosteroid therapies can weaken the host immune system and are risk factors for FM [1, 63]. Drugs and other treatments, such as certain chemotherapies, powerful antibacterial regimens, organ transplants and their immunosuppressants, immune biological modifiers, or high-dose corticosteroid treatments can contribute to FM pathogenesis [1, 64]. Moreover, there are five major factors that affect outcomes during FM infection: (1) underlying disease; (2) fungal burden at the CNS site; (3) development of the host inflammatory response; (4) increased intracranial pressure development and its control; and (5) specific symptoms at presentation of the infection (particularly level of consciousness or mental status) [1, 65, 66].
This study retrospectively analysed the records of 1927 adult patients with FM to determine variations in healthcare costs and outcomes that might be due to race, sex, or socioeconomic status. There were several significant findings from this study. For the 13 years of this study, the years 2000–2012, the relative proportion of each type of FM analysed remained relatively constant (Fig. 1). Certain diseases, including HIV infection and cancer, medications such as steroids or anti-TNF-alpha, and surgical procedures including organ transplant, weaken the immune response of patients and can make individuals susceptible to FM. The most commonly encountered FM is CrM, accounting for almost 70 % of all FM cases in this cohort. It is important to note that while CrM, CoM and HM are seen in middle-aged patients, the predominant patient population for CaM is strikingly different. Candida infection is mostly acquired in hospital settings and is generally observed in infants and children. Since our study only included patients above the age of 18 years, the prevalence of paediatric candidiasis is not accurately represented and the actual prevalence of CaM is undoubtedly higher than has been presented in this study. Despite this limitation, the study provides a unique perspective on the strikingly high costs of CaM management even in adults, a cost that is commonly ignored by other studies, which have largely focused on paediatric and infant cases of CaM.
Another common characteristic of FM patients is a high burden of co-morbid diseases, with >60 % of patients in this cohort recording a CCI score of 3 or higher. Furthermore, as indicated by the healthcare costs 1 year prior to diagnosis, these patients have generally received substantial recent healthcare resource utilization, which is likely due to management of prior underlying comorbid diseases. As described by the GEE model, the cost of treatment for an FM patient with a CCI score of 1 is expected to be 72 % higher than for those with a CCI score of 0. Similarly, these costs increase significantly with increasing CCI score, being 124 % higher for patients with a CCI score of 2 and 204 % higher for patients with CCI score of 3 as compared to patients with a CCI score of 0. This trend of increasing cost holds true for patients within individual FMs. This increase in healthcare costs with increasing co-morbidities reflects a general principle in health economics and highlights the complexity of the treatment of FM patients and its substantial burden on the healthcare economy within the United States.
A significantly larger number of patients diagnosed with CrM were unemployed and insured by Medicaid (48 % for CrM as compared to 28.1 % for CaM, 14.8 % for HM and 7.3 % for CoM), confirming that CrM is clearly a disease of the economically disadvantaged. Given that antiretroviral therapy (ART) is very effective in treating HIV and in extending the lives of those who otherwise would have previously died of AIDS within this time period, the consistently high numbers of CrM cases may be attributed to the growing number of HIV patients in marginalized populations, without access to ART, not diagnosed, or not taking ART [15, 55]. Holmquist et al. found that people living in the poorest communities were disproportionately more likely to be hospitalized with a meningitis-related diagnosis, and this is particularly the case for those infected with FM [48].
The geographical distribution clearly shows that the Southern region of the United States represents the most common location for three out of the four FM. From the endemic histoplasma and the partially endemic Cryptococcus through to the ubiquitous Candida, this area of the USA has the highest burden of FM. It is also important to note that while CoM is primarily observed in the geographically restricted environment of the west, the mobile society in which we live allows CoM to be found in all parts of the USA. Clinicians outside the endemic area of the west must still consider it in a FM differential diagnosis and a travel history will be important.
Among individual FM infections, CaM patients had the highest cost of healthcare at baseline, which continues to accumulate during the year of diagnosis and in the following years. For example, one of the major patient populations susceptible to adult CaM is neurosurgical patients. The expensive nature of these surgical procedures and morbidities may be a predominant reason for significantly higher baseline healthcare costs for CaM patients. CrM patients have the second highest cost of healthcare at baseline, reflecting the fact that many of these patients are immunocompromised (i.e. transplant recipients and those with other underlying diseases). It should be noted that, with the exception of diagnosis year, the baseline costs for all FM types were greater than the costs after the diagnosis year. We believe that this represents an escalation of care for patients before they develop FM. Importantly, with CrM, the cost of initial treatment in this cohort averages $38 648 in the first year alone. Moreover, because this study tracked the long-term costs of CrM, we were able to quantify the previously neglected cumulative costs of CrM, which total $103 236 over 5 years. Our interpretation of this novel finding is that CrM targets patients with severe underlying diseases, which require significant resources to manage, and/or we may have seriously underestimated the long-term morbidity and economic impact of the CNS infection.
Furthermore, healthcare cost estimates for all the FMs show that the average cumulative cost per patient over 5 years is approximately $100 000. Interestingly, our baseline estimates for cost and LoS were comparable to those in the statistical brief released by HCUP for 2006, which estimated $26 100 per year in costs and an average hospital stay of 14.7 days [56]. In a specific medical centre cost analysis at Duke University, we observed that the cost of initial hospitalization for CrM in 2004–5 was $24 821 [15]. On the other hand, researchers at the University of Minnesota estimated a much higher cost of $75 121, solely controlled by the changing costs of the most effective initial treatment [67]. This figure is based on cost estimation using 2015 US medication prices for the combination therapy of 2 weeks of intravenous (IV) amphotericin B and flucytosine. Much of this substantial increase, as seen in the analysis from University of Minnesota, was driven by the massive increase in flucytosine cost secondary to pricing issues and use of drug exclusivity. However, these medical centre estimates did not approach the costs associated with complications, relapses, or outpatient care once a patient has been discharged. We have provided additional data for these follow-up years, demonstrating the limitation of only analysing the initial treatment, as this grossly underestimates the overall scope of the total amount of healthcare resource utilization for FM. These costs and additional hospitalizations are under-reported in the literature and may be attributed to the multitude of comorbidities and underlying diseases that specifically afflict FM patients. However, these treatments and costs may also be ascribed to persistent FM complications, such as increased intracranial pressure requiring shunts, immune reconstitution inflammatory syndrome (IRIS) and persistent neurological deficits requiring intensive care.
It is important to note that long-term LoS and costs after 5 years for a presently incurable infection such as CoM are not included in our calculations for the aggregate economic impact of FM. Furthermore, the indirect costs of health-related loss of productivity and wages are substantial for both patients and employers and are not captured in this analysis. There have not been any recent breakthroughs in FM treatment, with no new pharmaceutical agents or interventional approaches having been developed. It is reasonable to hypothesize that improvements to the type or efficacy of FM treatment might improve the clinical outcomes and economic impact of the disease. The therapies that are currently available for treating FM provide pharmacological targeting of the yeast, requiring at least 2 weeks of initial high doses of antifungal drugs and then a maintenance therapy for months to follow. There is a desperate need for new therapeutic options that address the known pathophysiology of FM, including targeted reduction in fungal burden and related inflammatory molecules (for example, the inflammatory components of cytokine storm and the polysaccharide antigen for CrM) that are known to exacerbate the disease pathology. Also of significant importance is the length of time that is required for a therapy to control the pathogen and change the course of the disease. If the window of time required for treating FM were reduced this might significantly improve the outcomes and costs of meningitis for patients and healthcare providers.
Our study examined a large nationwide patient population, thereby reducing the variations that are present in single-institution studies. This study combined the largest group of FM patients studied to date with the longest follow-up interval ever analysed. MarketScan collects data from a variety of practices and insurers across the United States. Although it does carry the inherent limitations of a retrospective analysis, it does provide a broad view of infectious disease practices and cost-effectiveness data from across the United States. Unfortunately, this study was unable to stratify patients in terms of their complexity of care, types of interventions and the specific antifungal regimens used. Variations in coding may also have affected the data that were extracted from the Marketscan database, while the lack of detailed clinical information prevents us from analysing how the presence or absence of certain complications, comorbidities and treatment regimens independently and congruently affected both annual expenditures and LoS. It is important to note that the long-term HCRU outcomes here are limited in this sense. After the index FM diagnosis, every clinical encounter was included for HCRU calculations to broadly illustrate the resource utilization of FM patients over time as a whole. Despite these limitations, this study demonstrates the steady prevalence of FM over the past decade. In addition, it highlights the significant cost of FM, not only in the initial year of diagnosis, but also over the subsequent 5 years, due to the significant morbidity and costs that accompany the diagnosis and treatment of FM.
Supplementary Data
Funding information
The Duke Biostatistics Core's support of this project was made possible by grant number UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH. This study was also supported by grant numbers 1R41AI120751 01, 1R41AI2030401 and R01 AI73896 (J. R. P.) R01AI93257 (J. R. P.) from the NIAID, a component of NIH.
Conflicts of interest
J. R. P. conflicts: research grants, honoraria and consulting (Merck, Astellas, Pfizer, Viamet, F-2G, Vical, Cidara, Amplyx, Matinas, Scynexis and ARON). None of the other authors have any conflicts of interest to report.
Footnotes
Abbreviations: CaM, Candida meningitis; CoM, Coccidioides meningitis; CrM, cryptococcal meningitis; HM, Histoplasma meningitis.
One supplementary table is available with the online version of this article.
References
- 1.Gottfredsson M, Perfect JR. Fungal meningitis. Semin Neurol. 2000;20:307–322. doi: 10.1055/s-2000-9394. [DOI] [PubMed] [Google Scholar]
- 2.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 3.Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2017;13:13–24. doi: 10.1038/nrneurol.2016.167. [DOI] [PubMed] [Google Scholar]
- 4.Leal AL, Faganello J, Fuentefria AM, Boldo JT, Bassanesi MC, et al. Epidemiological profile of cryptococcal meningitis patients in Rio Grande do Sul, Brazil. Mycopathologia. 2008;166:71–75. doi: 10.1007/s11046-008-9123-2. [DOI] [PubMed] [Google Scholar]
- 5.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee U, Datta K, Majumdar T, Gupta K. Cryptococcosis in India: the awakening of a giant? Med Mycol. 2001;39:51–67. doi: 10.1080/mmy.39.1.51.67. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS–100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson PA, Bauer M, Leal MA, Evans SG, Holtom PD, et al. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1999;28:82–92. doi: 10.1086/515074. [DOI] [PubMed] [Google Scholar]
- 9.van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of allergy and infectious diseases mycoses study group and AIDS clinical trials group. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 10.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS One. 2013;8:e56269. doi: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman GD, Jeffrey Fessel W, Udaltsova NV, Hurley LB. Cryptococcosis: the 1981–2000 epidemic. Mycoses. 2005;48:122–125. doi: 10.1111/j.1439-0507.2004.01082.x. [DOI] [PubMed] [Google Scholar]
- 13.Mirza SA, Phelan M, Rimland D, Graviss E, Hamill R, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. Clin Infect Dis. 2003;36:789–794. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 14.Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 15.Dodds Ashley E, Drew R, Johnson M, Danna R, Dabrowski D, et al. Cost of invasive fungal infections in the era of new diagnostics and expanded treatment options. Pharmacotherapy. 2012;32:890–901. doi: 10.1002/j.1875-9114.2012.01124. [DOI] [PubMed] [Google Scholar]
- 16.Stockamp NW, Thompson GR. Coccidioidomycosis. Infect Dis Clin North Am. 2016;30:229–246. doi: 10.1016/j.idc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Bouza E, Dreyer JS, Hewitt WL, Meyer RD. Coccidioidal meningitis. An analysis of thirty-one cases and review of the literature. Medicine. 1981;60:139–172. [PubMed] [Google Scholar]
- 18.Bronnimann DA, Adam RD, Galgiani JN, Habib MP, Petersen EA, et al. Coccidioidomycosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1987;106:372–379. doi: 10.7326/0003-4819-106-3-372. [DOI] [PubMed] [Google Scholar]
- 19.Rouhani AA. Infectious disease/CDC update. Update on emerging infections: news from the centers for disease control and prevention. Ann Emerg Med. 2016;67:131–134. doi: 10.1016/j.annemergmed.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, et al. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev. 2013;26:505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41–57. doi: 10.1016/S0891-5520(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 22.Kirkland TN, Fierer J. Coccidioidomycosis: a reemerging infectious disease. Emerg Infect Dis. 1996;2:192–199. doi: 10.3201/eid0203.960305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blair JE. Coccidioidal meningitis: update on epidemiology, clinical features, diagnosis, and management. Curr Infect Dis Rep. 2009;11:289–295. doi: 10.1007/s11908-009-0043-1. [DOI] [PubMed] [Google Scholar]
- 24.Bercovitch RS, Catanzaro A, Schwartz BS, Pappagianis D, Watts DH, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363–368. doi: 10.1093/cid/cir410. [DOI] [PubMed] [Google Scholar]
- 25.Powell BL, Drutz DJ, Huppert M, Sun SH. Relationship of progesterone- and estradiol-binding proteins in Coccidioides immitis to coccidioidal dissemination in pregnancy. Infect Immun. 1983;40:478–485. doi: 10.1128/iai.40.2.478-485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmquist L, Russo CA, Elixhauser A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006. Meningitis-related hospitalizations in the United States, 2006. HCUP Statistical Brief #57, July 2008. [PubMed] [Google Scholar]
- 27.Vincent T, Galgiani JN, Huppert M, Salkin D. The natural history of coccidioidal meningitis: VA-Armed Forces cooperative studies, 1955-1958. Clin Infect Dis. 1993;16:247–254. doi: 10.1093/clind/16.2.247. [DOI] [PubMed] [Google Scholar]
- 28.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of Coccidioidomycosis. Clin Infect Dis. 2016;63:e112-146. doi: 10.1093/cid/ciw538. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis. 2006;42:103–107. doi: 10.1086/497596. [DOI] [PubMed] [Google Scholar]
- 30.Bradsher RW. Histoplasmosis and blastomycosis. Clin Infect Dis. 1996;22:S102–S111. doi: 10.1093/clinids/22.Supplement_2.S102. [DOI] [PubMed] [Google Scholar]
- 31.Schuster JE, Wushensky CA, di Pentima MC. Chronic primary central nervous system histoplasmosis in a healthy child with intermittent neurological manifestations. Pediatr Infect Dis J. 2013;32:794–796. doi: 10.1097/INF.0b013e31828d293e. [DOI] [PubMed] [Google Scholar]
- 32.Zarrin M, Mahmoudabadi AZ. Central nervous system fungal infections; a review article. Jundishapur J Microbiol. 2010;3:41–47. [Google Scholar]
- 33.Duncan RA, von Reyn CF, Alliegro GM, Toossi Z, Sugar AM, et al. Idiopathic CD4+ T-lymphocytopenia–four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393–398. doi: 10.1056/NEJM199302113280604. [DOI] [PubMed] [Google Scholar]
- 34.Weidenheim KM, Nelson SJ, Kure K, Harris C, Biempica L, et al. Unusual patterns of Histoplasma capsulatum meningitis and progressive multifocal leukoencephalopathy in a patient with the acquired immunodeficiency virus. Hum Pathol. 1992;23:581–586. doi: 10.1016/0046-8177(92)90137-r. [DOI] [PubMed] [Google Scholar]
- 35.Anaissie E, Fainstein V, Samo T, Bodey GP, Sarosi GA. Central nervous system histoplasmosis. An unappreciated complication of the acquired immunodeficiency syndrome. Am J Med. 1988;84:215–217. doi: 10.1016/0002-9343(88)90416-0. [DOI] [PubMed] [Google Scholar]
- 36.Wright SH, Czaja AJ, Katz RS, Soloway RD. Systemic mycosis complicating high dose corticosteroid treatment of chronic active liver disease. Am J Gastroenterol. 1980;74:428–432. [PubMed] [Google Scholar]
- 37.Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system. A clinical review. Medicine. 1990;69:244–260. doi: 10.1097/00005792-199007000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wilson LS, Reyes CM, Stolpman M, Speckman J, Allen K, et al. The direct cost and incidence of systemic fungal infections. Value Health. 2002;5:26–34. doi: 10.1046/j.1524-4733.2002.51108.x. [DOI] [PubMed] [Google Scholar]
- 39.Hariri OR, Minasian T, Quadri SA, Dyurgerova A, Farr S, et al. Histoplasmosis with deep CNS involvement: case presentation with discussion and literature review. J Neurol Surg Rep. 2015;76:e167-172. doi: 10.1055/s-0035-1554932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs. 2007;21:293–318. doi: 10.2165/00023210-200721040-00004. [DOI] [PubMed] [Google Scholar]
- 41.Chadwick DW, Hartley E, MacKinnon DM. Meningitis caused by Candida tropicalis. Arch Neurol. 1980;37:175–176. doi: 10.1001/archneur.1980.00500520073014. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez M, Moylett EH, Noyola DE, Baker CJ. Candidal meningitis in neonates: a 10-year review. Clin Infect Dis. 2000;31:458–463. doi: 10.1086/313973. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen MH, Yu VL. Meningitis caused by Candida species: an emerging problem in neurosurgical patients. Clin Infect Dis. 1995;21:323–327. doi: 10.1093/clinids/21.2.323. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Portocarrero J, Martín-Rabadán P, Saldaña CJ, Pérez-Cecilia E. Candida cerebrospinal fluid shunt infection. Report of two new cases and review of the literature. Diagn Microbiol Infect Dis. 1994;20:33–40. doi: 10.1016/0732-8893(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 45.Poon WS, Ng S, Wai S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: a randomised study. Acta Neurochir Suppl. 1998;71:146–148. doi: 10.1007/978-3-7091-6475-4_43. [DOI] [PubMed] [Google Scholar]
- 46.Henao N. Infections of the central nervous system by Candida. J Infect Dis Immun. 2011;3:79–84. [Google Scholar]
- 47.Lee BE, Cheung PY, Robinson JL, Evanochko C, Robertson CM. Comparative study of mortality and morbidity in premature infants (birth weight, < 1,250 g) with candidemia or candidal meningitis. Clin Infect Dis. 1998;27:559–565. doi: 10.1086/514712. [DOI] [PubMed] [Google Scholar]
- 48.Holmquist L. Meningitis-related hospitalizations in the United States, 2006: Statistical Brief #57. In: Russo C, Elixhaurser A, editors. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2008. (editors) [PubMed] [Google Scholar]
- 49.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 50.Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2004;72:99–118. doi: 10.1093/bmb/ldh043. [DOI] [PubMed] [Google Scholar]
- 51.Bratton EW, El Husseini N, Chastain CA, Lee MS, Poole C, et al. Approaches to antifungal therapies and their effectiveness among patients with cryptococcosis. Antimicrob Agents Chemother. 2013;57:2485–2495. doi: 10.1128/AAC.01800-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Twarog M, Thompson GR. Coccidioidomycosis: recent updates. Semin Respir Crit Care Med. 2015;36:746–755. doi: 10.1055/s-0035-1562900. [DOI] [PubMed] [Google Scholar]
- 54.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–132. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enoch DA, Ludlam HA, Brown NM. Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol. 2006;55:809–818. doi: 10.1099/jmm.0.46548-0. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC) Characteristics associated with HIV infection among heterosexuals in urban areas with high AIDS prevalence – 24 cities, United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2011;60:1045–1049. [PubMed] [Google Scholar]
- 57.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 58.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother. 2009;53:24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 60.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 61.Brenier-Pinchart MP, Leclercq P, Mallié M, Bettega G. Candida meningitis possibly resulting from a harpoon injury. Eur J Clin Microbiol Infect Dis. 1999;18:454–455. doi: 10.1007/s100960050319. [DOI] [PubMed] [Google Scholar]
- 62.Zunt JR. Infections of the central nervous system in the neurosurgical patient. Handb Clin Neurol. 2010;96:125–141. doi: 10.1016/S0072-9752(09)96009-2. [DOI] [PubMed] [Google Scholar]
- 63.Zunt JR. Central nervous system infection during immunosuppression. Neurol Clin. 2002;20:1–22. doi: 10.1016/S0733-8619(03)00070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobs CS, Etherton MR, Lyons JL. Fungal infections of the central nervous system. Curr Infect Dis Rep. 2014;16:449. doi: 10.1007/s11908-014-0449-2. [DOI] [PubMed] [Google Scholar]
- 65.Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974;80:176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 66.Dismukes WE, Cloud G, Gallis HA, Kerkering TM, Medoff G, et al. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N Engl J Med. 1987;317:334–341. doi: 10.1056/NEJM198708063170602. [DOI] [PubMed] [Google Scholar]
- 67.Merry M, Boulware DR. Cryptococcal meningitis treatment strategies affected by the explosive cost of flucytosine in the United States: a cost-effectiveness analysis. Clin Infect Dis. 2016;62:1564–1568. doi: 10.1093/cid/ciw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.