Abstract
Purpose
Potent extracellular toxins including alpha-haemolysin, Panton–Valentine leukocidin (PVL) and toxic-shock syndrome toxin 1 (TSST-1) significantly contribute to Staphylococcus aureus pathogenesis, thus, toxin suppression is a primary focus in treatment of staphylococcal disease. S. aureus maintains complex strategies to regulate toxin expression and previous data have demonstrated that subinhibitory concentrations of beta-lactam antibiotics can adversely increase S. aureus exotoxin production. The current study evaluates the effects of subinhibitory concentrations of tedizolid, a second-generation oxazolidinone derivative, on expression of staphylococcal exotoxins in both methicillin-resistant and methicillin-sensitive S. aureus.
Methodology
S. aureus exotoxin expression levels were compared at 12 and 24 h following treatment with tedizolid, linezolid, nafcillin or vehicle control.
Results
Our findings show that the level of antibiotic required to alter toxin production was strain-dependent and corresponds with the quantity of toxin produced, but both tedizolid and linezolid could effectively reduce expression of alpha-haemolysin, PVL and TSST-1 toxin at subinhibitory concentrations. In contrast, nafcillin showed less attenuation and, in some S. aureus strains, led to an increase in toxin expression. Tedizolid consistently inhibited toxin production at a lower overall drug concentration than comparator agents.
Conclusion
Together, our data support that tedizolid has the potential to improve outcomes of infection due to its superior ability to inhibit S. aureus growth and attenuate exotoxin production.
Keywords: Staphylococcus aureus, subinhibitory, antibiotic, bacterial, exotoxin
Introduction
Staphylococcus aureus is a prevalent human pathogen capable of causing a wide range of diseases. The versatility of S. aureus as a pathogen is attributable, in part, to an assortment of virulence factors. Of these, S. aureus secreted toxins such as alpha-haemolysin, Panton–Valentine leukocidin (PVL) and toxic-shock syndrome toxin 1 (TSST-1) can contribute to infection in general and necrotizing fasciitis, haemorrhagic necrotizing pneumonia and toxic-shock syndrome, specifically [1–3]. Staphylococcal exotoxins can exert local effects, causing cellular injury and tissue necrosis, and systemically, causing life-threatening inflammatory disorders. Toxin neutralization studies, highlighting reduced cytopathic effects and improved outcomes [4–12], confirm the fundamental role that staphylococcal toxins play in pathogenesis of life-threatening infections.
Expression of staphylococcal exotoxins is controlled in response to organism growth, density and environmental cues [13–15]. During antibiotic therapy, pathogens encounter a range of antibiotic concentrations and may be exposed to subinhibitory levels dependent upon multiple factors including resistance by the pathogen or antibiotic penetration into the environmental niche (e.g. biofilm or host tissue). Our laboratory and others have demonstrated that subinhibitory doses of beta-lactam antibiotics can adversely increase and prolong S. aureus toxin production [16–18]. Additional studies have shown phenotypic and genotypic changes that result from exposure to subinhibitory concentrations of different classes of antibiotics [19]. Together, these findings demonstrate the significant ability of antibiotics to modify bacterial metabolism, biofilm formation and toxin production that influence bacterial virulence, underscoring a potentially detrimental impact on disease outcome.
Tedizolid, a novel second-generation oxazolidinone approved for treatment of acute bacterial skin and skin structure infections (ABSSSI), demonstrates potent antimicrobial activity against S. aureus with reduced selection for antibiotic resistance [20–22]. While previous studies support that protein synthesis inhibitors, such as oxazolidinones, can suppress production of alpha-haemolysin, PVL and TSST-1 [17, 18, 23, 24], others have shown that subinhibitory concentrations of protein synthesis inhibitors trigger a stress response and paradoxically induce bacterial virulence [25–27]. The study described herein examines the effects of tedizolid on four pathogenic S. aureus strains to determine how subinhibitory exposure influences S. aureus exotoxin production.
Methods
Bacterial strains and culture
Strains of S. aureus used in the current study were as follows: S. aureus FPR3757 (an MRSA USA300 isolate [28]), S. aureus 1560 (an MRSA USA400 isolate [29]), S. aureus CDC 368–04 (an MRSA isolate that produces TSST-1 but not PVL), S. aureus 04–002 (an MSSA isolate from a patient with STSS [30]) and S. aureus ATCC 29213 (a reference strain for antibiotic susceptibility testing). All S. aureus strains were plated on blood agar medium (5 % sheep blood; Hardy Diagnostics, Santa Maria, CA) and cultured in cation-adjusted Mueller–Hinton II broth (Becton Dickinson, Franklin Lakes, NJ) for growth and analysis.
MIC determination
Antibiotics used in the current study were: tedizolid (Merck, Kenilworth, NJ), linezolid (Pfizer, New York, NY), and nafcillin (Bristol-Myers Squibb, New York, NY). Antibiotics were resuspended in Mueller–Hinton media (nafcillin, linezolid) or DMSO (tedizolid) prior to subsequent dilution in Mueller–Hinton media. DMSO (0.3 %) was included as a vehicle control for tedizolid. Susceptibility of S. aureus strains to individual antibiotics was determined using the broth microdilution method, in accordance with the Clinical and Laboratory Standards Institute guidelines. Briefly, isolated colonies were used to inoculate overnight cultures. The next day, S. aureus were sub-cultured and grown at 37 °C in 5 % CO2 with shaking (200 r.p.m.) to mid-log phase. Bacteria were harvested by centrifugation, washed in sterile saline and seeded in 96-well flat-bottom plates at 5×105 c.f.u. ml−1 in the presence of antibiotic. Plates were incubated at 37 °C in 5 % CO2 for 24 h at which point growth inhibition was determined.
Antibiotics’ effects on S. aureus growth and toxin production
To monitor S. aureus growth, isolated colonies were used to inoculate overnight cultures. Log-phase cultures (0.3–0.4 AU) were diluted to a concentration of 5×105 c.f.u. ml−1, at which time individual antibiotics were added (time=0 h). Cultures were maintained at 37 °C in 5 % CO2 with shaking (200 r.p.m.). Viable bacterial counts were determined at 3, 6, 9, 12 and 24 h following the introduction of antibiotics by dilution plating on blood agar plates. Samples were collected at 12 and 24 h for quantitation and subsequent analysis of the effects of antibiotics on toxin production.
PVL and TSST-1 toxin quantification
Supernatants from individual 10 ml cultures were filter-sterilized (0.22 µm) and stored at −80 °C for toxin protein assays. PVL (LukS) and TSST-1 were quantified from S. aureus culture supernatants by ELISA. For PVL, EIA/RIA plates were coated with 1 µg ml−1 anti-PVL mAb 1D9 (IBT Bioservices, Gaithersburg, MD) in 50 mM carbonate buffer (pH 9.6). Capture antibody was removed and plates were blocked with PBS+5 % skim milk overnight at 4 °C. LukS-PV standard (0.8–50 ng ml−1; IBT Bioservices) and diluted culture supernatants (1 : 200 to 1 : 800) were applied to ELISA plates for 2 h at 37 °C. Plates were washed with PBS-Tween 20 (0.05 %) and successively incubated with 0.25 µg ml−1 rabbit polyclonal anti-PVL (LukS) (IBT Bioservices) and HRP-linked anti-rabbit IgG (H+L) (Cell Signaling, Danvers, MA) and assays were developed with 1-step Ultra TMB (Life Technologies, Grand Island, NY). Recombinant LukF-PV (IBT Bioservices) was undetectable by these methods, indicating that our PVL immunoassay was specific to LukS-PV. For TSST-1, EIA/RIA plates were coated with 5 µg ml−1 anti-TSST-1 affinity purified sheep antisera (Toxin Technology, Sarasota, FL). Capture antisera was removed and plates were blocked in PBS+5 % skim milk overnight at 4 °C. TSST-1 standard (0.15–20 ng ml−1; Toxin Technology) and diluted culture supernatants (1 : 2000 to 1 : 8000) were applied to ELISA plates for 2 h at 37 °C. Plates were washed with PBS-Tween 20 (0.05 %) and incubated with a 1 : 1200 dilution of HRP-conjugated anti-TSST-1 sheep antisera (Toxin Technology) followed by development with 1-step Ultra TMB. Notably, S. aureus Protein A did not interfere with either immunoassay at concentrations <500 ng ml−1.
Alpha-haemolysin analysis
Alpha-haemolysin activity was assayed by a standard rabbit erythrocyte lysis assay, as described previously [18]. In brief, sterile-filtered culture supernatants were diluted twofold in DPBS in a microtitre plate and an equal volume of washed rabbit erythrocytes (2 % in DPBS) was added. Sterile deionized water was included as a 100 % haemolysis control. After incubation for 1 h at 37 °C, plates were centrifuged, supernatants were transferred to a new microtitre plate, and the absorbance was read at 550 nm. Activity (haemolytic units ml−1) was defined as the inverse of the dilution causing 50 % haemolysis, multiplied by 2.
Statistical analysis
For each experimental condition, a minimum of three independent biological replicates were collected and tested. The concentration of toxin was compared between antibiotic and control treatment for each antibiotic using a one-way ANOVA followed by Dunnett’s multiple comparisons test (GraphPad Prism 7.03; GraphPad Software, La Jolla, CA).
Results
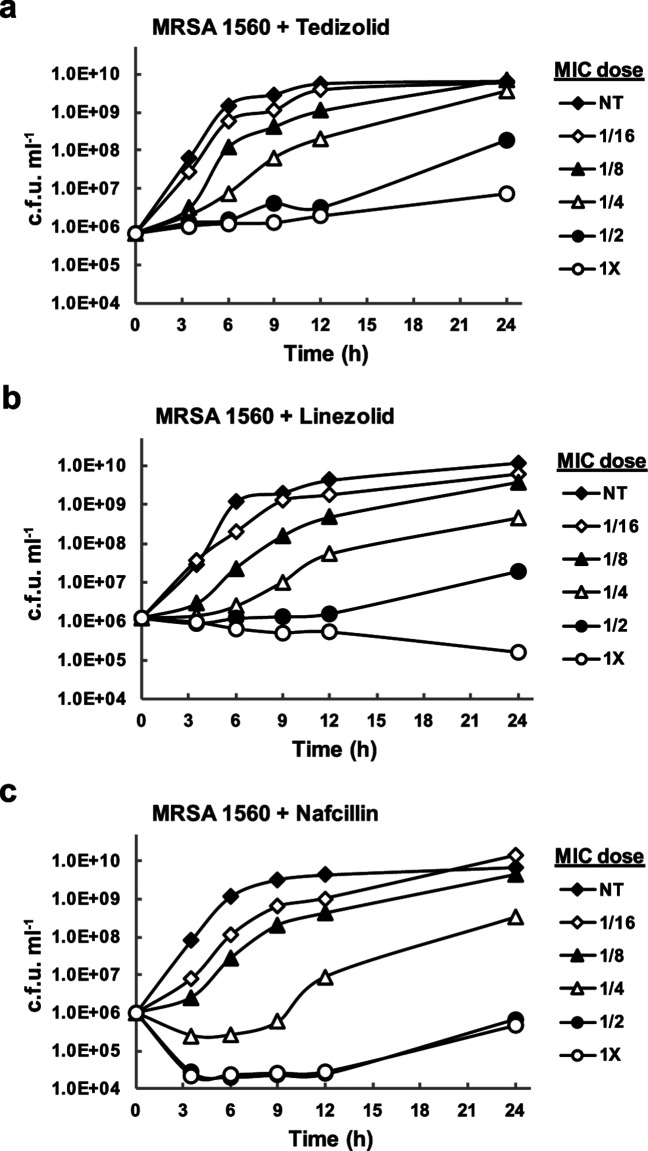
S. aureus growth was monitored following the addition of sub-MIC concentrations of antibiotics at the mid-logarithmic stage of growth. The MICs for antibiotics used in this study are provided in Table 1.Notably, tedizolid inhibited growth at concentrations four to eightfold lower than that of linezolid in each S. aureus isolate. Dose-dependent effects on growth were observed for each antibiotic tested (Figs 1, S1–S3, available in the online version of this article). While 1/16× MIC and 1/8× MIC concentrations of antibiotics resulted in a lag in exponential bacterial growth, cultures still grew to densities comparable to that of vehicle control by 24 h. Higher antibiotic doses, particularly 1/2× MIC and 1× MIC, had a detrimental effect on bacterial growth and impaired their ability to reach densities detected in the control treatment at 24 h (Fig. 1 and Table S1). Due to the impact of higher antibiotic concentrations on bacterial density, the 1/16× MIC, 1/8× MIC, 1/4× MIC and vehicle control treatments were selected for analysis of subinhibitory antibiotic effects on S. aureus toxin production. S. aureus cultures treated with subinhibitory antibiotics, or vehicle control, were evaluated at late-log/early stationary (12 h) and stationary (24 h) phases of growth for effects on toxin expression.
Table 1.
Characteristics of S. aureus strains used in this study
| MIC (µg ml−1)† | Toxin profile‡ | |||||
|---|---|---|---|---|---|---|
| S. aureus strain* | Nafcillin | Linezoild | Tedizolid | PVL | TSST-1 | HLA |
| FPR3757 (MRSA) | 6.3 | 2.0 | 0.5 | + | − | + |
| Strain 1560 (MRSA) | 12.5 | 4.0 | 0.5 | + | − | + |
| CDC 368-04 (MRSA) | 12.5 | 4.0 | 0.5 | − | + | + |
| 04-002 (MSSA) | 0.8 | 2.0 | 0.5 | − | + | + |
Fig. 1.
Effect of sub-MIC antibiotics on S. aureus growth. The growth of methicillin-resistant S. aureus strain 1560 was monitored over time in the presence of varying concentrations of antibiotic. Bacteria were grown in Mueller–Hinton II broth containing (a) tedizolid, (b) linezolid or (c) nafcillin at 1/16X, 1/8×, 1/4×, 1/2× or 1× the MIC. Culture samples were collected at 0, 3, 6, 9, 12 and 24 h for bacterial quantitation. NT, no treatment control.
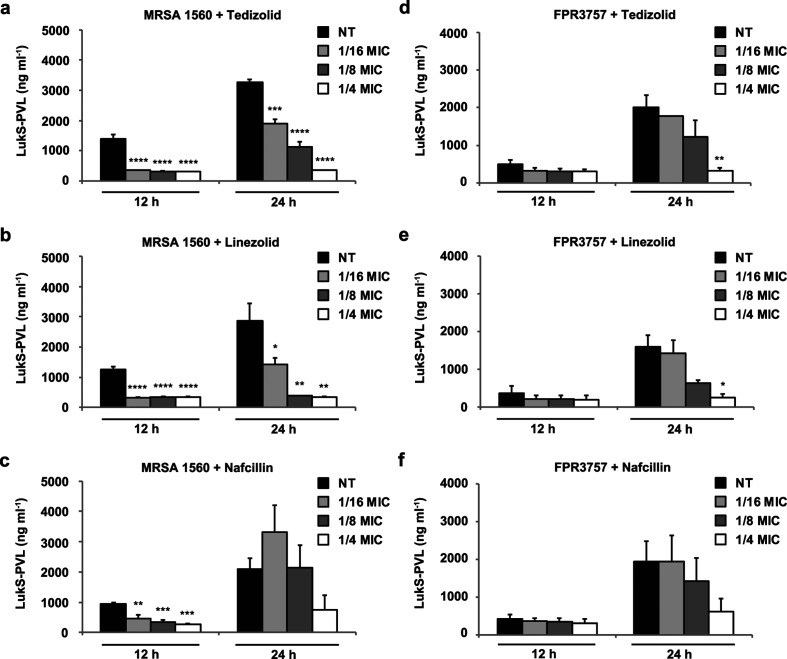
PVL, a bicomponent toxin encoded by staphylococcal bacteriophage, targets neutrophils, macrophages and monocytes [31, 32] and has been epidemiologically linked to severe staphylococcal infections [33, 34]. Recent studies have demonstrated the importance of PVL in the pathogenesis of S. aureus pneumonia and skin infection [35–37]. Consequently, we chose to assess levels of PVL toxin in response to subinhibitory antibiotic treatment. PVL expression was monitored by indirect ELISA of the LukS-PVL subunit (Fig. 2). Both tedizolid and linezolid suppressed expression of LukS-PVL in MRSA 1560 by 12 h at 1/16× the MIC (Fig. 2a, b). While 1/8× MIC, 1/16× MIC and 1/4× MIC showed similar effects at 12 h, dose-dependent suppression of LukS-PVL was more evident at 24 h, when toxin expression was elevated. In contrast to treatments with tedizolid or linezolid, nafcillin failed to show consistent attenuation of toxin expression (Fig. 2c). To confirm these findings, PVL expression was evaluated in a second S. aureus strain following antibiotic exposure (Fig. 2d–f). While S. aureus FPR3757 expressed slightly less LukS-PVL, compared to MRSA 1560, higher doses of oxazolidinone antibiotics (i.e. 1/8v× MIC) were required for reduced toxin expression in S. aureus FPR3757. Together, these findings demonstrate that subinhibitory concentrations of tedizolid and linezolid effectively inhibit PVL expression in multiple strains of S. aureus. While strain-specific dosage effects were observed, no adverse induction of PVL toxin was detected in response to subinhibitory levels of oxazolidinone antibiotics.
Fig. 2.
Effects of tedizolid and comparator antibiotics on S. aureus PVL toxin expression. S. aureus strains were cultured in the presence of 1/16× MIC, 1/8× MIC, 1/4× MIC or vehicle control for 12 and 24 h. Levels of LukS-PVL were quantified from filter-sterilized culture supernatants by ELISA. Each panel shows LukS-PVL expression levels following treatment with the indicated antibiotic in (a–c) MRSA 1560 and in (d–f) S. aureus FPR3757. Bars represent average toxin expression±standard error from three biological replicates. Asterisks denote statistical significance of (*) P<0.05, (**) P<0.01, (***) P<0.001 and (****) P<0.0001 compared to the no treatment (NT) vehicle control at each corresponding timepoint.
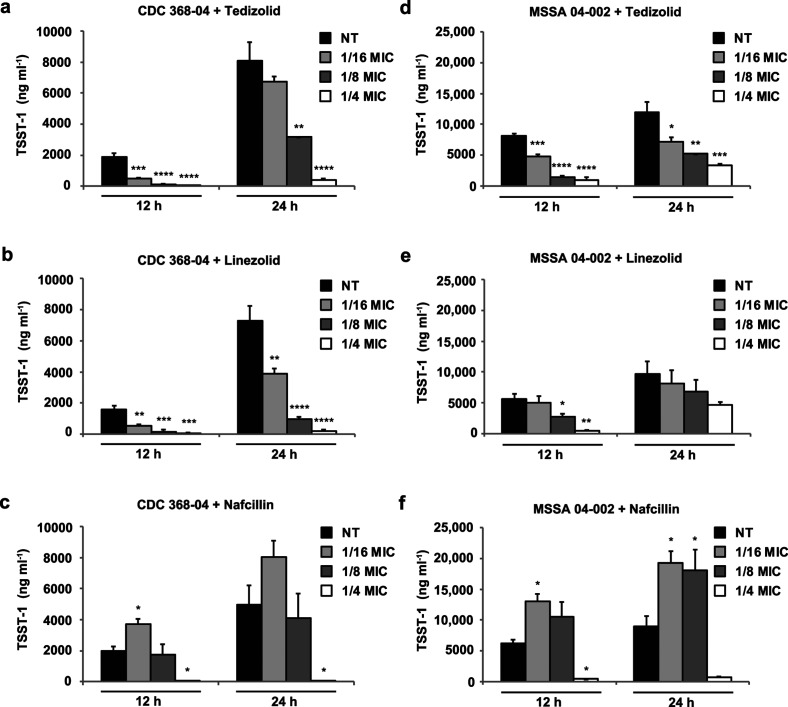
Next, levels of TSST-1 toxin were evaluated from S. aureus clinical isolates following exposure to antibiotic treatment (Fig. 3). Encoded by a mobile pathogenicity island, TSST-1 is expressed during post-exponential growth and stimulates the release of large amounts of cytokines causing severe inflammation with fever, widespread effects on the vascular system and shock [1]. Subinhibitory levels of both tedizolid and linezolid suppressed expression of TSST-1 in S. aureus CDC 368–04 at 12 and 24 h (Fig. 3a, b). In MSSA 04–002, a strain that produces elevated levels of TSST-1, dose-dependent effects were also evident following treatment with subinhibitory concentrations of tedizolid and linezolid (Fig. 3d, e). Interestingly, nafcillin exposure at 1/16× the MIC considerably increased TSST-1 toxin expression in both S. aureus strains (Fig. 3c, f). A 1/8× MIC nafcillin concentration also sustained increased toxin expression in MSSA 04–002, in comparison to vehicle control.
Fig. 3.
Effects of tedizolid and comparator antibiotics on S. aureus TSST-1 toxin expression. Levels of TSST-1 were quantified from culture supernatants by ELISA. Each panel shows TSST-1 expression levels following treatment with the indicated antibiotic in (a–c) CDC 368–04 and in (d–f) MSSA 04–002. Bars represent average toxin expression±standard error from three biological replicates. Asterisks denote statistical significance of (*) P<0.05, (**) P<0.01, (***) P<0.001 and (****) P<0.0001 compared to the no treatment (NT) vehicle control at each corresponding timepoint.
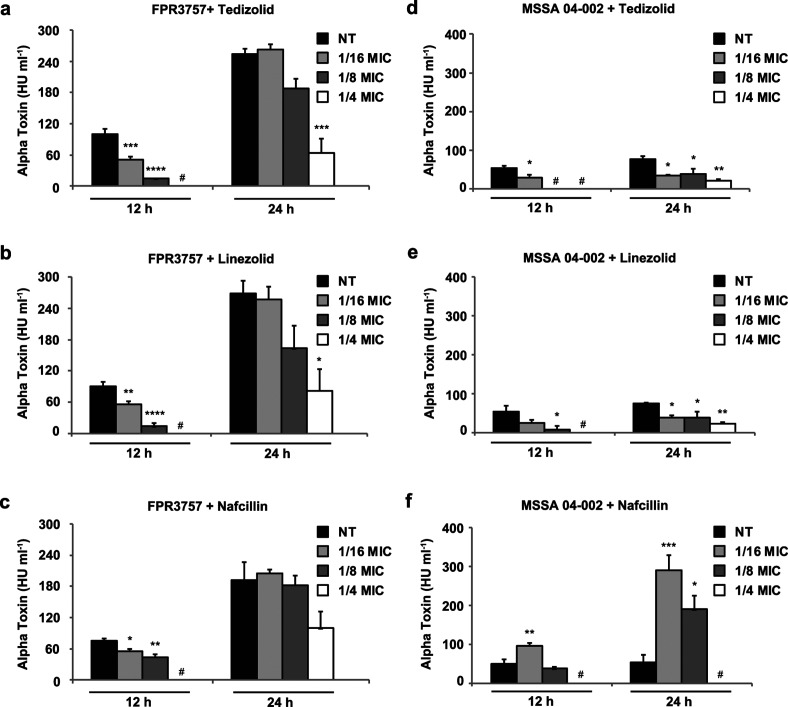
Lastly, alpha-haemolysin toxin activity was assayed from all four S. aureus strains in response to antibiotic treatment (Fig. 4). Part of the core genome, alpha-haemolysin encodes a pore-forming toxin that acts as one of the major virulence determinants in staphylococcal pathogenesis [38–40]. Consistent with the findings for PVL and TSST-1, alpha-haemolysin activity decreased in a dose-dependent manner upon treatment with subinhibitory levels of tedizolid and linezolid, particularly at 12 h (Fig. 4a–b, d–e). S. aureus FPR3757 represents a high alpha-haemolysin producer and antibiotic inhibition was most pronounced with lower toxin expression observed at 12 h. S. aureus 04–002 represents a low alpha-haemolysin producer and 1/16× MIC of tedizolid was sufficient to reduce alpha-haemolysin toxin activity at both 12 and 24 h. Notably, subinhibitory concentrations of nafcillin significantly increased haemolytic activity in MSSA 04–002 (Fig. 4f), but not S. aureus FPR3757 (Fig. 4c), MRSA 1560 or CDC 368–04 (data not shown). Taken together, our results provide evidence that subinhibitory concentrations of tedizolid and linezolid effectively reduce staphylococcal toxin expression while beta-lactam antibiotics, such as nafcillin, have less predictable effects and may adversely increase toxin expression.
Fig. 4.
Effects of tedizolid and comparator antibiotics on S. aureus alpha-haemolysin activity. Alpha-haemolysin activity was quantified from culture supernatants by rabbit erythrocyte lysis assay. Each panel shows alpha-haemolysin activity following treatment with the indicated antibiotic in (a–c) S. aureus FPR3757 and in (d–f) MSSA 04–002. Bars represent average toxin expression±standard error from three biological replicates. Asterisks denote statistical significance of (*) P<0.05, (**) P<0.01, (***) P<0.001 and (****) P<0.0001 compared to the no treatment (NT) vehicle control at each corresponding timepoint. A hash mark (#) indicates that no toxin was detected.
Discussion
Antibiotics are some of the most well-studied natural and synthetic compounds. With their prevalent usage, concerns have been raised regarding “off-target” bacterial responses. At high concentrations, antimicrobial agents lead to bactericidal or bacteriostatic effects, whereas exposure to lower concentrations of antibiotics can result in genetic and phenotypic variations that enable adaptation and alter bacterial pathogenicity [19, 41]. Previous studies documenting the influence of subinhibitory antibiotic exposure on biofilm formation, the bacterial stress response and toxin production in S. aureus [16–18, 26, 42] highlight adverse effects of antibiotics that underscore a potentially detrimental impact on disease outcome.
The contributions of staphylococcal toxins and other secreted factors to human disease are well-recognized. As such, protein-synthesis inhibitors are expected to have a distinct advantage in treating toxigenic S. aureus infections by attenuating toxin production and virulence factor expression. Studies in our laboratory have shown that clindamycin and linezolid inhibit S. aureus toxin production in vitro and improve the clinical response to staphylococcal toxic shock syndrome [30]. Intriguingly, others have shown that subinhibitory concentrations of protein synthesis inhibitors can induce bacterial virulence [25–27]. The current study demonstrates that tedizolid effectively inhibits S. aureus exotoxin production, thus, reducing the likelihood of an adverse S. aureus toxigenic response. While, we would expect expression of other virulence factors to be inhibited by subinhibitory concentrations of tedizolid as well, further analysis is required to assess the global impact of tedizolid on S. aureus.
Notably, toxin expression levels varied among individual S. aureus isolates and our findings demonstrate that subinhibitory concentrations of antibiotics affected toxin expression in a strain-dependent manner. For example, suppression of alpha-haemolysin in S. aureus FPR3757 requires higher levels of tedizolid than S. aureus 04–002. Similarly, low-dose nafcillin did not induce expression of alpha-haemolysin in S. aureus FPR3757, likely due to already high endogenous expression. Overall, the evaluation of a larger collection of S. aureus strains might better establish effects of tedizolid on toxin expression. While our understanding of mechanisms governing variable endogenous toxin expression among S. aureus isolates remains incomplete, subinhibitory concentrations of tedizolid and linezolid consistently suppressed toxin expression in different isolates and throughout different stages of S. aureus growth.
The current study demonstrates effective toxin suppression by tedizolid in vitro. While the effects of sub-MIC oxazolidinone, or beta-lactam, antibiotics have not been directly evaluated during experimental S. aureus infection, evidence from other infection models support that low-dose antibiotic effects are relevant during bacterial infection. Low-dose ciprofloxacin has been demonstrated to prime uropathogens for adherence and invasion and exacerbate chronic infection [43]. Similarly, chloramphenicol and erythromycin have been shown to modulate Acinetobacter baumannii capsule production providing increased resistance to killing during systemic disease [44]. Thus, conducting such in vivo studies for S. aureus in the future would validate the ability of tedizolid to inhibit toxin expression over a range of antibiotic concentrations.
Overall, the mechanism of action of antibiotics remains a prime factor in treating S. aureus and other toxin-related infections. Namely, protein synthesis inhibitors have proven superior to cell wall-active antibiotics for treatment of clostridial gas gangrene and group A streptococcus soft tissue infections using conventional antibiotic doses [45–47]. Linezolid has been shown to significantly reduce staphylococcal toxin expression in vivo [30, 48] and Le et al. recently demonstrated that inhibitory concentrations of tedizolid (AUC0-24; 14.9±1.6 µg·h ml–1) suppressed alpha-haemolysin and PVL production in a necrotizing pneumonia model, resulting in reduced bacterial burden and increased survival [49]. Our findings add convincing evidence that tedizolid can potently reduce staphylococcal toxin expression at subinhibitory concentrations, contrasting effects observed with beta-lactam antibiotics. As concerns grow regarding the emergence of clindamycin and linezolid resistance, tedizolid offers a compelling treatment alternative for ABSSSI to improve outcomes for S. aureus and other toxin-related infections.
Supplementary Data
Funding information
This material is based upon work supported by Merck (MISP 53441), the U.S. Department of Veterans Affairs Office of Research and Development Biomedical Laboratory Research Program, the National Institutes of Health Centers of Biomedical Research Excellence (P20GM109007), and by the National Institutes of Health IDeA Networks of Biomedical Research Excellence (P20GM103408).
Conflicts of interest
Funds from Merck Investigator-Initiated Grant 53 441 supported these studies and provided partial salary support for E.J.K. and D.D.B. This and other funding sources played no role in the design, execution, analysis or reporting of this research.
Footnotes
Abbreviations: ABSSSI, acute bacterial skin and skin structure infections; AUC, area under the curve; HLA, alpha-haemolysin; MIC, minimal inhibitory concentration; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; PVL, Panton-Valentine leucocidin; TSST-1, toxic-shock syndrome toxin 1.
One supplementary table and three supplementary figures are available with the online version of this article.
References
- 1.Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DY, et al. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev. 2013;26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins. 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 4.Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, et al. Novel structurally designed vaccine for S. aureus α-hemolysin: protection against bacteremia and pneumonia. PLoS One. 2012;7:e38567. doi: 10.1371/journal.pone.0038567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre PF, Thompson MR, Adinolfi LE, Gillis ZA, Parsonnet J. Neutralization of toxic shock syndrome toxin-1 by monoclonal antibodies in vitro and in vivo. Infect Immun. 1988;56:135–141. doi: 10.1128/iai.56.1.135-141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karauzum H, Adhikari RP, Sarwar J, Devi VS, Abaandou L, et al. Structurally designed attenuated subunit vaccines for S. aureus LukS-PV and LukF-PV confer protection in a mouse bacteremia model. PLoS One. 2013;8:e65384. doi: 10.1371/journal.pone.0065384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menzies BE, Kernodle DS. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun. 1996;64:1839–1841. doi: 10.1128/iai.64.5.1839-1841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocca CP, Brady RA, Burns DL. Role of antibodies in protection elicited by active vaccination with genetically inactivated alpha hemolysin in a mouse model of staphylococcus aureus skin and soft tissue infections. Clin Vaccine Immunol. 2014;21:622–627. doi: 10.1128/CVI.00051-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes-Robles T, Lubkin A, Alonzo F, Lacy DB, Torres VJ. Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis. EMBO Rep. 2016;17:428–440. doi: 10.15252/embr.201540994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampedro GR, Dedent AC, Becker RE, Berube BJ, Gebhardt MJ, et al. Targeting Staphylococcus aureus α-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J Infect Dis. 2014;210:1012–1018. doi: 10.1093/infdis/jiu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaulding AR, Lin YC, Merriman JA, Brosnahan AJ, Peterson ML, et al. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine. 2012;30:5099–5109. doi: 10.1016/j.vaccine.2012.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arvidson S, Tegmark K. Regulation of virulence determinants in Staphylococcus aureus. Int J Med Microbiol. 2001;291:159–170. doi: 10.1078/1438-4221-00112. [DOI] [PubMed] [Google Scholar]
- 14.Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong YQ. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40:1–9. doi: 10.1016/S0928-8244(03)00309-2. [DOI] [PubMed] [Google Scholar]
- 15.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 16.Kernodle DS, Mcgraw PA, Barg NL, Menzies BE, Voladri RK, et al. Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis. 1995;172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 17.Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, et al. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 1998;42:2817–2823. doi: 10.1128/AAC.42.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens DL, Ma Y, Salmi DB, Mcindoo E, Wallace RJ, et al. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis. 2007;195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 19.Davies J, Spiegelman GB, Yim G. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol. 2006;9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Ferrández O, Urbina O, Grau S. Critical role of tedizolid in the treatment of acute bacterial skin and skin structure infections. Drug Des Devel Ther. 2017;11:65–82. doi: 10.2147/DDDT.S84667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locke JB, Hilgers M, Shaw KJ. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700) Antimicrob Agents Chemother. 2009;53:5265–5274. doi: 10.1128/AAC.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke JB, Zurenko GE, Shaw KJ, Bartizal K. Tedizolid for the management of human infections: in vitro characteristics. Clin Infect Dis. 2014;58:S35–S42. doi: 10.1093/cid/cit616. [DOI] [PubMed] [Google Scholar]
- 23.Dumitrescu O, Badiou C, Bes M, Reverdy ME, Vandenesch F, et al. Effect of antibiotics, alone and in combination, on Panton-Valentine leukocidin production by a Staphylococcus aureus reference strain. Clin Microbiol Infect. 2008;14:384–388. doi: 10.1111/j.1469-0691.2007.01947.x. [DOI] [PubMed] [Google Scholar]
- 24.Herbert S, Barry P, Novick RP. Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus. Infect Immun. 2001;69:2996–3003. doi: 10.1128/IAI.69.5.2996-3003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joo HS, Chan JL, Cheung GY, Otto M. Subinhibitory concentrations of protein synthesis-inhibiting antibiotics promote increased expression of the agr virulence regulator and production of phenol-soluble modulin cytolysins in community-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:4942–4944. doi: 10.1128/AAC.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schilcher K, Andreoni F, Dengler Haunreiter V, Seidl K, Hasse B, et al. Modulation of Staphylococcus aureus Biofilm Matrix by Subinhibitory Concentrations of Clindamycin. Antimicrob Agents Chemother. 2016;60:5957–5967. doi: 10.1128/AAC.00463-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaki J, Synold T, Wong-Beringer A. Antivirulence potential of TR-700 and clindamycin on clinical isolates of Staphylococcus aureus producing phenol-soluble modulins. Antimicrob Agents Chemother. 2011;55:4432–4435. doi: 10.1128/AAC.00122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 29.Diep BA, Sensabaugh GF, Somboonna N, Somboona NS, Carleton HA, et al. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J Clin Microbiol. 2004;42:2080–2084. doi: 10.1128/JCM.42.5.2080-2084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens DL, Wallace RJ, Hamilton SM, Bryant AE. Successful treatment of staphylococcal toxic shock syndrome with linezolid: a case report and in vitro evaluation of the production of toxic shock syndrome toxin type 1 in the presence of antibiotics. Clin Infect Dis. 2006;42:729–730. doi: 10.1086/500265. [DOI] [PubMed] [Google Scholar]
- 31.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci USA. 2010;107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, et al. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diep BA, Gillet Y, Etienne J, Lina G, Vandenesch F. Panton-Valentine leucocidin and pneumonia. Lancet Infect Dis. 2013;13:566. doi: 10.1016/S1473-3099(13)70102-6. [DOI] [PubMed] [Google Scholar]
- 34.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 35.Prince A, Wang H, Kitur K, Parker D. Humanized mice exhibit increased susceptibility to Staphylococcus aureus Pneumonia. J Infect Dis. 2017;215:1386–1395. doi: 10.1093/infdis/jiw425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipinska U, Hermans K, Meulemans L, Dumitrescu O, Badiou C, et al. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS One. 2011;6:e22864. doi: 10.1371/journal.pone.0022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng CW, Biancotti JC, Berg BL, Gate D, Kolar SL, et al. Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Staphylococcus aureus Skin Infection. PLoS Pathog. 2015;11:e1005292. doi: 10.1371/journal.ppat.1005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel AH, Nowlan P, Weavers ED, Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55:3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laureti L, Matic I, Gutierrez A. Bacterial responses and genome instability induced by subinhibitory concentrations of antibiotics. Antibiotics. 2013;2:100–114. doi: 10.3390/antibiotics2010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan JB, Izano EA, Gopal P, Karwacki MT, Kim S, et al. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio. 2012;3:e00198. doi: 10.1128/mBio.00198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goneau LW, Hannan TJ, Macphee RA, Schwartz DJ, Macklaim JM, et al. Subinhibitory antibiotic therapy alters recurrent urinary tract infection pathogenesis through modulation of bacterial virulence and host immunity. MBio. 2015;6 doi: 10.1128/mBio.00356-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015;11:e1004691. doi: 10.1371/journal.ppat.1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens DL, Bryant AE, Hackett SP. Antibiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis. 1995;20:S154–S157. doi: 10.1093/clinids/20.Supplement_2.S154. [DOI] [PubMed] [Google Scholar]
- 46.Stevens DL, Gibbons AE, Bergstrom R, Winn V. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis. 1988;158:23–28. doi: 10.1093/infdis/158.1.23. [DOI] [PubMed] [Google Scholar]
- 47.Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18:1096–1100. doi: 10.1097/00006454-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Sharma-Kuinkel BK, Zhang Y, Yan Q, Ahn SH, Fowler VG. Host gene expression profiling and in vivo cytokine studies to characterize the role of linezolid and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA) murine sepsis model. PLoS One. 2013;8:e60463. doi: 10.1371/journal.pone.0060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le VTM, Le HN, Pinheiro MG, Hahn KJ, Dinh ML, et al. Effects of Tedizolid Phosphate on Survival Outcomes and Suppression of Production of Staphylococcal Toxins in a Rabbit Model of Methicillin-Resistant Staphylococcus aureus Necrotizing Pneumonia. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.02734-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.