Abstract
Background.
Significant progress has been made in reducing methicillin-resistant Staphylococcus aureus (MRSA) infections among hospitalized patients. However, the decreases in invasive MRSA infections among recently discharged patients have been less substantial. To inform prevention strategies, we assessed risk factors for invasive MRSA infection after acute-care hospitalizations.
Methods.
We conducted a prospective, matched case-control study. A case was defined as MRSA cultured from a normally sterile body site in a patient discharged from a hospital within the prior 12 weeks. Eligible case patients were identified from 15 hospitals across 6 US states. For each case patient, 2 controls were matched for hospital, month of discharge, and age group. Medical record reviews and telephone interviews were performed. Conditional logistic regression was used to identify independent risk factors for post-discharge invasive MRSA.
Results.
From 1 February 2011 through 31 March 2013, 194 case patients and 388 matched controls were enrolled. The median time between hospital discharge and positive culture was 23 days (range, 1–83 days). Factors independently associated with post-discharge MRSA infection included MRSA colonization (matched odds ratio [mOR], 7.71; 95% confidence interval [CI], 3.60–16.51), discharge to a nursing home (mOR, 2.65; 95% CI, 1.41–4.99), presence of a chronic wound during the post-discharge period (mOR, 4.41; 95% CI, 2.14–9.09), and discharge with a central venous catheter (mOR, 2.16; 95% CI, 1.13–4.99) or a different invasive device (mOR, 3.03; 95% CI, 1.24–7.39) in place.
Conclusions.
Prevention efforts should target patients with MRSA colonization or those with invasive devices or chronic wounds at hospital discharge. In addition, MRSA prevention efforts in nursing homes are warranted.
Keywords: epidemiology, methicillin-resistant Staphylococcus aureus, risk-factors, bacteremia
Methicillin-resistant Staphylococcus aureus (MRSA) infections remain a significant public health concern [1]. In 2011, an estimated 48 000 invasive MRSA infections occurred among individuals in the community with recent healthcare exposure in the United States; nearly 38 000 (79%) of those infections were in individuals hospitalized during the prior year [1]. Invasive MRSA infections can be difficult to treat, and nearly 25% of patients fail recommended therapy [2]. In 2011, an estimated 11 000 deaths were associated with invasive MRSA infections [1, 3].
Decades of work determining risk factors for hospital-onset MRSA infection have facilitated development of effective infection prevention guidance, which led to significant declines in hospital-onset invasive MRSA infections. [1, 4, 5]. However, nearly 80% of all invasive MRSA infections in 2011 occurred outside of the hospital setting, mostly among patients who had been recently hospitalized, with a large proportion of infections occurring within 12 weeks after discharge (ie, high-risk period) [1, 6]. Little is known regarding risk factors for invasive MRSA infections among individuals recently discharged from hospitals; such information is critical to developing novel interventions for the post-discharge setting.
We conducted a prospective matched-case control study to identify risk factors associated with invasive MRSA infections among recently discharged patients. Our goals were to enhance our understanding of the clinical and epidemiologic characteristics of these infected patients and advance our knowledge regarding the relative importance of various risk factors to inform the development of effective prevention strategies.
METHODS
Study Population
The Centers for Disease Control and Prevention (CDC) Active Bacterial Core Surveillance program has been conducting active population- and laboratory-based surveillance for invasive MRSA infections since 2004 [7]. We used this system to prospectively identify patients with invasive MRSA from February 2011 through March 2013 from 15 hospitals across 6 US states (California, Connecticut, Georgia, Minnesota, New York, and Tennessee).
A patient was considered an eligible case patient for the study if all of the following inclusion criteria were met: (1) MRSA was isolated from a normally sterile site (ie, blood, cerebrospinal fluid, pleural fluid, peritoneal fluid, joint/synovial fluid, or bone) after hospital discharge or within the first 3 days during a second hospital admission (ie, community-onset MRSA infection), (2) the patient was ≥18 years old, and (3) the patient had been discharged from one of the 15 participating acute-care hospitals in the 12 weeks before the invasive MRSA culture. Case patients were not eligible if invasive MRSA had developed during the prior hospitalization or if the prior hospitalization was ≤3 calendar days and therefore not long enough for the patients to have had more substantial hospital exposure.
For each case patient, 2 controls, matched for hospital, month of hospital discharge, and age in 10-year intervals, were randomly selected from hospital discharge databases. Patients were not eligible to be selected as controls if (1) their hospitalization was ≤3 calendar days, (2) they developed an invasive MRSA infection during hospitalization or anytime within 12 weeks after hospital discharge, or (3) they died between the discharge date and the date of the positive MRSA culture of the matched case patient.
During the enrollment process, other excluded case patients or controls included patients hospitalized in non acute-care locations only, those self-reporting hospitalizations in nonparticipating hospitals, those whose medical records were not available for review after 3 attempts, and those who were not available for interview after 8 contact attempts, refused enrollment, or were prisoners.
Data Collection
Inpatient Period
Data from the hospitalization for case patients (ie, hospitalization from where the patient was discharged from prior to invasive MRSA culture) and controls were abstracted from medical records using standardized forms. Data collected included admission and discharge diagnoses, invasive procedures, placement of devices, discharge with any invasive device, antimicrobial exposures, MRSA infection or colonization status, wounds, patient disposition at discharge, and underlying conditions expressed by the Charlson comorbidity index [8]. Records for other healthcare encounters, microbiology results, and infection control were also reviewed.
Post-discharge Period
The post-discharge period for case patients was defined as the time from hospital discharge to positive invasive MRSA culture; for controls, it was defined as the time from hospital discharge to the date of the matched case patient’s positive invasive MRSA culture. Telephone interviews were conducted to ascertain exposures during the post-discharge period for patients who were discharged to home. For patients discharged to a nursing home, a standardized form was used to abstract data from nursing home medical records for the post-discharge period of interest. If the patient had exposure to both a nursing home and home during the post-discharge period, both telephone interview and nursing home medical record review were conducted to collect information from the entire post-discharge period. Both telephone interview and nursing home medical review forms captured information on device maintenance and duration after discharge, surgical procedures, antimicrobial exposures, healthcare facility visits, wound care, training of caregivers, and patient functional status.
Variable Definitions
Variables were collected from 3 time points: the inpatient period (eg, hospitalization), the day of hospital discharge, and the post-discharge period. Central venous catheters (CVCs) were defined as hemodialysis lines, non-tunneled short-term catheters, peripherally inserted central catheters, implanted central venous access devices, and tunneled CVCs. Non-CVC invasive devices included arterial lines, endotracheal or nasotracheal tubes, indwelling urinary catheters, gastrostomy tubes, nasogastric tubes, tracheostomies, and abdominal or surgical drains.
Wounds were classified into 3 non–mutually exclusive categories: chronic wounds related to poor circulation or pressure ulcers, surgical wounds related to any surgical procedure, and “other” wounds (eg, skin abscesses, boils, or traumatic wounds). Patients with a wound that was present at hospital admission or discharge were classified as having a wound during the hospitalization. Surgeries or invasive procedures were defined as intraoperative or bedside procedures that penetrated a sterile site. MRSA colonization was defined by documented history of non-invasive MRSA infection or colonization in the 12 months before hospital admission or recovery of MRSA from a nonsterile site during the hospitalization.
Statistical Analysis
All analyses were performed using SAS software (version 9.3; SAS Institute). Univariable conditional logistic regression was used to calculate matched odds ratios (mORs) and P values for all variables; variables were included as candidates in the multivariable model if the 2-sided P value was ≤.25.
Conditional logistic regression with backward selection was then performed. Continuous variables were included in the logistic regression models as continuous if they had a linear relationship with the outcome of interest; otherwise, they were included as categorical variables. Differences were considered statistically significant at P ≤ .05 (2 sided) in the final model. Because MRSA bloodstream infections are the most common type of invasive MRSA [1], a subanalysis restricting the study population only to case patients with MRSA bloodstream infections and their matched controls was performed to identify risk factors, using the statistical methods described above.
Human Subjects Review
The CDC and local institutional review boards approved the study. Because the study posed no greater than minimal risk to participants, a waiver of informed consent was granted to review medical records in both the hospitals and nursing homes. Verbal consent was obtained from all participants who were interviewed.
RESULTS
Demographics
Among the 15 participating hospitals, 7 (47%) were small (201– 500 beds), 7 (47%) were medium (501–1000 beds), and 1 (7%) was large (>1000 beds). Of the 12 hospitals that reported having active MRSA screening programs, 3 (25%) were performing universal screening on patient admission, and 9 (75%) were performing unit-specific or patient-specific (eg, for patients admitted from long-term care facilities) screening.
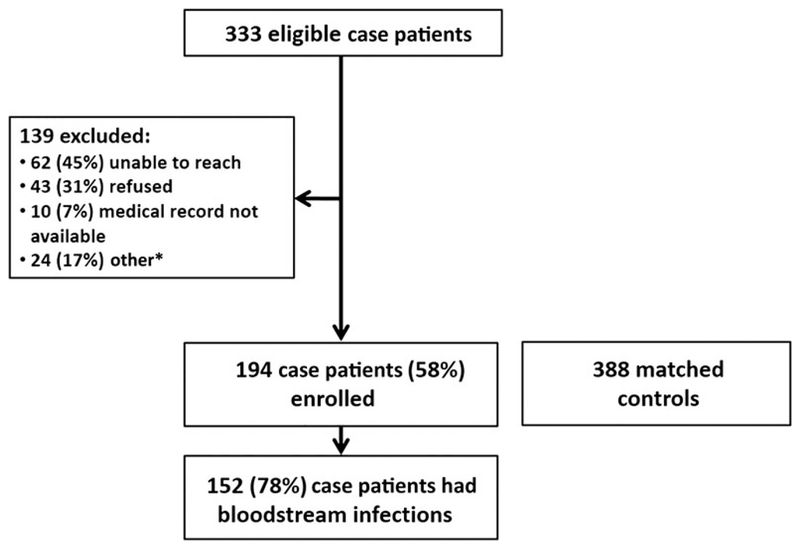
Of the 333 eligible case patients, 194 (58%) were enrolled in the study (Figure 1). Among the 194 case patients enrolled, 152 (78%) had MRSA isolated from blood, and 123 (63%) developed invasive MRSA infection within 30 days after hospital discharge. The median time from hospital discharge to invasive MRSA culture among case patients was 23 days (range, 1–83 days). Among the eligible case patients not enrolled, there were no significant differences in age, sex, or race compared with enrolled case patients.
Figure 1.
Case patient enrollment and final study population. *16 case patients excluded because sites did not identify 2 matched controls.
The 388 matched controls were similar in age to case patients but differed in many other characteristics (Table 1). Case patients were more likely than controls to be male (mOR, 2.55) and black (mOR, 1.88) and had more comorbid conditions (mOR, 3.08), as shown by the Charlson comorbidity index. In addition, MRSA colonization, according to our study definition, was strongly associated with case patient status (mOR, 11.04).
Table 1.
Univariable Comparison of Baseline Demographic and Clinical Characteristics in Case Patients Versus Controls
| Patients, No. (%)a | ||||
|---|---|---|---|---|
| Characteristic | Case Patients (n =194) | Controls (n = 388) | mOR | P Value |
| Age, median, y | 62.0 | 62.0 | … | .36 |
| Male sex | 122 (63) | 154 (40) | 2.55 | <.001 |
| Black race | 70 (36) | 104 (27) | 1.88 | .01 |
| Charlson comorbidity index >1b | 170 (88) | 279 (72) | 3.08 | <.001 |
| HIV infection | 7 (4) | 6 (2) | 2.33 | .13 |
| Hospitalization in prior year | 153 (79) | 184 (47) | 4.51 | <.001 |
| MRSA colonizationc | 78 (40) | 29 (8) | 11.04 | <.001 |
| MRSA recovered from nonsterile site during hospitalization | 43 (22) | 19 (5) | 6.80 | <.001 |
| Nasal swab sample | 30 (70) | 10 (52) | … | … |
| Sputum sample | 5 (12) | 2 (11) | … | … |
| Otherd | 8 (19) | 7 (37) | … | … |
| MRSA infection or colonization in prior 12 mo | 60 (31) | 17 (4) | 11.00 | <.001 |
| History of surgery in 30 d before hospital admission | 18 (9) | 24 (6) | 1.57 | .17 |
| BMI, median | 27.6 | 25.8 | … | .70 |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; mOR, matched odds ratio; MRSA, methicillin-resistant Staphylococcus aureus.
Data represent No. (%) of case patients or controls, unless otherwise specified.
Age was excluded from the Charlson index because it was a matching criterion.
MRSA colonization was defined as (1) MRSA recovered from a nonsterile site during the hospitalization or (2) MRSA infection or colonization in the prior 12 months.
Other nonsterile specimen sites include tracheal aspirate, bronchoalveolar lavage fluid, throat or nasopharynx, stool, urine, rectum, wound swab, catheter, and skin.
Factors Present During Hospitalization or at Discharge
Factors present during the hospitalization or at the time of discharge are listed in Tables 2 and 3. Exposure to a nursing home, either before hospitalization or after discharge, was associated with significant higher odds of being a case patient (mORs,4.28 and 4.55, respectively). Presence of a CVC at any period during the hospitalization (mOR, 2.68) or at discharge (mOR,4.16) was also associated with case patient status; the peripherally inserted central catheter line was the most common CVC type. Case patients were also more likely than controls to have received antibiotics during the hospitalization (mOR, 2.13), and most of the antibiotics were active against MRSA (Table 2). Although its not a rate exposure to a non-CVC invasive device was similar in case patients and controls during hospitalization, case patients were more likely than controls to be discharged with a non-CVC invasive device (mOR, 2.35). Finally, the presence of any type of wound during the hospitalization was associated with significantly higher odds of being a case patient (mOR,1.75); chronic wound showed the strongest association with case patient status (mOR, 4.93). Most of the patients (87% of case patients and 90% of controls) classified as having any type of wound during the hospitalization also had a wound present at discharge.
Table 2.
Univariable Comparison of Variables Present During Hospitalization in Case Patients Versus Controls
| Patients, No. (%)a | ||||
|---|---|---|---|---|
| Variable | Case Patients (n = 194) | Controls (n = 388) | Unadjusted mOR | P Value |
| Length of stay, median, d | 8.0 | 5.0 | … | <.001 |
| Admitted from LTACH or nursing home | 49 (25) | 27 (7) | 4.28 | <.001 |
| Admission diagnosis categoryb,c | ||||
| Infectious disease | 39 (20) | 38 (10) | 2.31 | <.001 |
| Surgical/trauma | 20 (10) | 58 (15) | 0.61 | .10 |
| Oncology | 11 (6) | 16 (4) | 1.39 | .41 |
| Medicald | 36 (19) | 102 (26) | 0.65 | .04 |
| Vascular | 9 (5) | 12 (3) | 1.53 | .35 |
| Neurology | 5(3) | 18 (5) | 0.56 | .24 |
| Obstetrics-gynecology | 0 (0) | 19 (5) | 0.0 | .90 |
| Othere | 74 (38) | 125 (32) | 1.33 | .14 |
| Admission to medical or surgical wardc | 134 (69) | 218 (56) | 1.82 | .002 |
| Admission to ICUc | 21 (11) | 53 (14) | 0.76 | .32 |
| MRSA noted on admission diagnosis | 2 (1) | 3 (1) | 1.33 | .75 |
| CVCf | 85 (44) | 89 (23) | 2.68 | <.001 |
| PICC line | 29 (15) | 36 (9) | … | … |
| Hemodialysis | 23 (12) | 8 (2) | … | … |
| Implanted and tunneled CVCs | 17 (9) | 18 (5) | … | … |
| Unknowng | 9 (5) | 7 (2) | … | … |
| Short-term catheter | 7 (4) | 20 (5) | … | … |
| Hemodialysish | 39 (20) | 15 (4) | 7.09 | <.001 |
| Woundsc | ||||
| Surgical | 79 (41) | 188 (49) | 0.72 | .07 |
| Chronic | 63 (33) | 36 (9) | 4.93 | <.001 |
| Otheri | 38 (20) | 47 (12) | 1.79 | .02 |
| Debridement | 25 (13) | 17 (4) | 3.04 | <.001 |
| Received any antimicrobialj | 168 (87) | 291 (75) | 2.13 | <.001 |
| MRSA active | 102 (53) | 114 (29) | … | … |
| Other | 48 (25) | 23 (6) | … | … |
| MRSA decolonization therapy | 11 (6) | 41 (11) | 0.49 | .05 |
| Urinary catheter | 94 (49) | 174 (45) | 1.16 | .40 |
| Physical therapy | 94 (49) | 175 (45) | 1.16 | .40 |
| Surgery/invasive procedures | 97 (50) | 205 (53) | 1.12 | .43 |
| Non-CVC invasive devicek | 70 (36) | 152 (39) | 0.87 | .45 |
| Endotracheal tube or tracheostomy | 35 (18) | 98 (25) | … | … |
| Gl tube | 21(11) | 40 (10) | … | … |
| Surgical drain | 15 (8) | 31 (8) | … | … |
| Other drain | 74 (38) | 125 (32) | … | … |
Abbreviations: CVC, central venous catheter; GI, gastrointestinal; ICU, intensive care unit; LTACH, long term acute care hospital; mOR, matched odds ratio; MRSA, methicillin-resistant Staphylococcus aureus; PICC, peripherally inserted central catheter.
Data represent No. (%) of case patients or controls, unless otherwise specified.
Patients could have multiple admission diagnoses.
Reference group is population without exposure.
Including GI, pulmonary, cardiovascular, and renal diagnoses.
Including patients with no diagnosis or multiple diagnoses.
The CVC could have been inserted before or during hospitalization.
Unknown CVC type includes central lines clearly indicated as CVCs in the medical chart but for which the indication was not clearly documented.
Including only patients who received long-term hemodialysis.
Other wounds include skin abscesses, boils, and traumatic wounds.
MRSA-active antimicrobials include vancomycin, linezolid, tigecycline, daptomycin, quinupristindalfopristin, rifampin, doxycycline, clindamycin, and trimethroprim-sulfamethoxazole; other antimicrobials, cephalosporins, carbapenems, penicillin, macrolides, aminoglycosides, and fluoroquinolones.
Patients could have ≥1 non-CVC in place. GI tubes include nasogastric, gastric, gastrojejunal, and rectal tubes, colostomies, jejunostomies, and ileostomies; surgical drains include Penrose, Jackson-Pratt, and biliary drains and cholecysolostomy tubes; and other drains include chest and nephrostomy tubes, epidural, peritoneal, and suprapubic catheters, and any other drains not already specified.
Table 3.
Univariable Comparison of Variables Present at Discharge in Case Patients Versus Controls
| Patients, No. (%) | ||||
|---|---|---|---|---|
| Variable | Case Patients (n = 194) | Controls (n = 388) | Unadjusted mOR | P Value |
| MRSA listed in discharge summary | 11 (6) | 6 (2) | 3.67 | .01 |
| Discharged to nursing home | 94 (49) | 88 (23) | 4.55 | <.001 |
| Urinary catheter | 18 (9) | 12 (3) | 3.56 | <.001 |
| Non-CVC invasive devicea | 29 (15) | 28 (7) | 2.35 | .003 |
| Endotracheal tube or tracheostomy | 7 (4) | 9 (2) | … | … |
| Gl tube | 6 (3) | 7 (2) | … | … |
| Surgical drain | 7 (4) | 9 (2) | … | … |
| Other drain | 10(5) | 5 (1) | … | … |
| CVC | 63 (33) | 42 (11) | 4.16 | <.001 |
| PICC line | 22 (11) | 18 (5) | … | … |
| Hemodialysis | 18 (9) | 7 (2) | … | … |
| lmplanted and tunneled venous catheter | 16 (8) | 14 (4) | … | … |
| Short-term catheter | 1 (1) | 1 (1) | … | … |
| Other or unknownb | 6 (3) | 2 (1) | … | … |
Abbreviations: CVC, central venous catheters; GI, gastrointestinal; mOR, matched odds ratio; MRSA, methicillin-resistant Staphylococcus aureus; PICC, peripherally inserted central catheter.
Patients could have ≥1 non-CVC in place. GI tubes include nasogastric, gastric, gastrojejunal, and rectal tubes, colostomies, jejunostomies, and ileostomies; surgical drains include Penrose, Jackson-Pratt, and biliary drains and cholecysolostomy tubes; and other drains include chest and nephrostomy tubes, epidural, peritoneal, and suprapubic catheters, and any other drains not already specified.
Including central lines clearly indicated as CVCs in the medical chart but for which the indication was not clearly documented.
Factors Present During Post-discharge Period
Case patients and controls discharged to home were interviewed a median (range) of 93 (21–289) and 179 (76–431) days after acute-care hospitalization, respectively. During the post-discharge period, case patients were more likely than controls to have a CVC inserted (mOR, 4.86) and to have received hemodialysis (mOR, 6.58) or any type of antimicrobial (mOR, 2.15) (Table 4). They were also more likely than controls to have a chronic wound during the post-discharge period (mOR, 8.14).
Table 4.
Univariable Comparison of Variables Present During Post-discharge Period in Case Patients Versus Controls
| Patients, No. (%) | ||||
|---|---|---|---|---|
| Variable | Case Patients (n = 194) | Controls (n = 388) | Unadjusted mOR | P Value |
| CVC insertion during postdischarge period | 17 (9) | 7 (2) | 4.86 | <.001 |
| Hemodialysisa | 31 (16) | 11 (3) | 6.58 | <.001 |
| Received any antimicrobialb | 112 (58) | 153 (39) | 2.15 | <.001 |
| MRSA active | 46 (24) | 45 (12) | … | … |
| Other | 48 (25) | 23 (6) | … | … |
| Woundsc | ||||
| Surgical | 65 (33) | 168 (43) | 0.66 | .02 |
| Chronic | 67 (35) | 24 (6) | 8.14 | <.001 |
| Otherd | 43 (22) | 28 (7) | 3.55 | <.001 |
| Debridement | 23 (12) | 9 (2) | 6.18 | <.001 |
| Surgery | 2 (1) | 3 (1) | 1.33 | .75 |
| Emergency department visit | 28 (14) | 39 (10) | 1.58 | .10 |
| Home health aide | 46 (24) | 92 (24) | 1.00 | 1.00 |
Abbreviations: CVC, central venous catheter; mOR, matched odds ratio; MRSA, methicillin-resistant Staphylococcus aureus.
Including patients receiving long-term hemodialysis.
MRSA-active antimicrobials include vancomycin, linezolid, tigecycline, daptomycin, quinupristin/dalfopristin, rifampin, doxycycline, clindamycin, and trimethroprim-sulfamethoxazole; other antimicrobials, cephalosporins, carbapenems, penicillin, macrolides, aminoglycosides, and fluoroquinolones.
Reference group is population without exposure.
Other wounds include skin abscesses, boils, and traumatic wounds.
Multivariable Analysis
In the multivariable analysis, after controlling for sex, Charlson comorbidity index and admission diagnosis, 5 factors remained significantly associated with invasive MRSA infections after discharge: MRSA colonization (mOR, 7.71; 95% confidence interval [CI], 3.60–16.51), CVC present at discharge (mOR, 2.16; 95% CI, 1.13–4.11), discharge to a nursing home (mOR, 2.65; 95% CI, 1.41–4.99), presence of non-CVC invasive device at discharge (mOR, 3.03; 95% CI, 1.24–7.39), and presence of a chronic wound in the post-discharge period (mOR, 4.41; 95%CI, 2.14–9.09) (Table 5). The risk factors with the highest odds ratio were not necessarily the most prevalent among case patients. For example, the odds of having a non-CVC invasive device at the time of discharge was 3.03 times higher among case patients than controls, but the prevalence of this exposure among case patients was only 15%. Therefore, intervening on this risk factor alone would be unlikely to produce a dramatic decline in MRSA infections after discharge.
Table 5.
Multivariable Analysis of Risk Factors for Postdischarge Invasive MRSA
| Variable | Adjusted mOR (95% Cl) | Prevalence of Risk Factor Among Case Patients, % |
|---|---|---|
| Admission diagnosisa | 1.84 (1.05–3.22) | 80 |
| Charlson comorbidity index >1 | 1.35 (1.17–1.55) | 88 |
| Male sex | 2.18 (1.31–3.63) | 63 |
| MRSA colonizationb | 7.71 (3.60–16.51) | 40 |
| CVC at discharge | 2.16 (1.13–4.11) | 33 |
| Discharge to nursing home | 2.65 (1.41–4.99) | 49 |
| Chronic wound during postdischarge period | 4.41 (2.14–9.09) | 35 |
| Discharge with non-CVC invasive device | 3.03 (1.24–7.39) | 15 |
Abbreviations: CI, confidence interval; CVC, central venous catheter; mOR, matched odds ratio; MRSA, methicillin-resistant Staphylococcus aureus.
Admission diagnoses that did not differ significantly were grouped together to simplify the model, which included the following diagnostic categories: infectious, oncology, vascular, pulmonary, orthopedic, obstetrics-gynecology, multiple diagnoses, and other.
MRSA colonization was defined as (1) MRSA recovered from a nonsterile site during the hospitalization or (2) MRSA infection/colonization in the prior 12 months.
Bloodstream Infections
Similar independent risk factors for pos-tdischarge invasive MRSA infection were observed when the analysis was restricted to case patients with MRSA bloodstream infections and their matched controls. After controlling for admission unit and Charlson comorbidity index, significant risk factors included MRSA colonization (mOR, 22.65; 95% CI, 6.68–76.80), discharge to a nursing home (mOR, 5.38; 95% CI, 2.19–13.22), and the presence of a chronic wound during the post-discharge period (mOR, 16.57; 95% CI, 4.96–55.34). Interestingly, in the bloodstream infection model, hemodialysis (mOR, 7.57; 95% CI, 1.28–44.62) replaced CVC present at discharge as a significant risk factor.
DISCUSSION
The growing delivery of healthcare in nonhospital settings and the dynamic movement of patients across healthcare settings makes it essential to identify populations at risk for MRSA infections during the post-discharge period. Using a large case-control study to assess exposures present during the hospitalization and post-discharge period and controlling for non-modiafiable risk factors, we found 5 independent risk factors for invasive MRSA infection occurring within 12 weeks after discharge from an acute-care hospital. The prevalence of these risk factors varied among case patients, with the most common being discharge to a nursing home (48%) and the least common being discharge with a non-CVC invasive device (15%). The magnitude of the odds ratio as well as the prevalence of risk factors should be considered when prioritizing interventions for prevention efforts.
Prior or concurrent MRSA colonization was the strongest risk factor for development of post-discharge invasive MRSA infections and one of the most prevalent risk factors among case patients, which is consistent with previous studies [9, 10]. Patients can carry MRSA in their nares for >1 year, with 1 study estimating the half-life of MRSA colonization to be 40 months [11]. Almost half of the case patients were determined to be colonized by MRSA, most of them through documented history of MRSA noninvasive infection or colonization in the prior year (77%) rather than through active MRSA screening during the acute-care hospitalization (23%). MRSA screening was not routinely performed across participating hospitals and there were probably additional patients with MRSA colonization missed among both case patients and controls. Given that MRSA screening policies vary across facilities and few facilities perform universal screening, our findings likely reflect the experience at most US acute-care facilities.
Discharge to a nursing home was also a strong independent risk factor for post-discharge invasive MRSA infections and highly prevalent among case patients (48%). Nursing homes can serve as a reservoir for MRSA, and the prevalence of MRSA carriage in nursing homes can be up to 50% [12], compared with approximately 5.1%–15.7% in hospital wards and 8.3%–24% in intensive care units [13–16]. Furthermore, the MRSA transmission risk among nursing home residents has been estimated to be 16% [17]. The need to discharge patients from hospitals to nursing homes may be unavoidable for various reasons. It is difficult to determine whether simply being discharged to a nursing home results in exposure to MRSA or whether it increases susceptibility to MRSA infections based on sustained nursing care required because of older age, chronic illness, or other comorbid conditions. However, in our analysis, discharge to a nursing home remained a risk factor for invasive MRSA infection even after we controlled for age and comorbid conditions included in the Charlson comorbidity index. Reinforcement of infection control policies and procedures to reduce MRSA transmission and optimal strategies to decrease MRSA colonization in nursing home patients need to be evaluated and will probably result in substantial declines in post-discharge invasive MRSA infections [18, 19] as well as MRSA infection rates in hospital settings, given that approximately 20%–40% of hospitalized patients with MRSA infections are reported to have recently been exposed to nursing homes [7, 18, 20, 21].
Previous studies have also shown a correlation between the presence of a chronic wound and MRSA bacteremia [22, 23]. Patients with wounds are often persistently colonized and respond poorly to decolonization therapy, which increases their risk to progress to more invasive infections [24, 25]. Therefore, aggressive management of chronic wounds to remove devitalized tissue and decrease bacterial bioburden, in association with strict compliance with infection control procedures among healthcare workers caring for patients with chronic wounds in the post-discharge setting, may decrease the risk of progression to invasive MRSA infection.
Both CVC and non-CVC invasive devices are known risk factors for MRSA infections in hospitalized patients [26–28]. Our study demonstrated that these factors, if present at the time of discharge, also increased the risk of post-discharge MRSA infections. The presence of any invasive device at discharge, either CVC or non-CVC, may be an indicator of severity of illness, which may increase patient’s vulnerability to post-discharge MRSA infections. Alternatively, invasive devices can become colonized with MRSA due to manipulation during the outpatient period and can serve as a portal of entry for MRSA into the bloodstream. Substantial progress has been made in acute-care settings to decrease central line–associated MRSA bloodstream infections [29]. Therefore, emphasis on alternative therapies that do not require CVCs at discharge, improvement in outpatient CVC maintenance practices, and prompt removal of unnecessary CVCs combined with targeted MRSA decolonization therapy at discharge may have a great impact on MRSA infections during the post-discharge period.
Patients receiving hemodialysis have a higher risk of invasive MRSA infection than the general population owing to the use of CVCs for vascular access as well as frequent healthcare exposures [30]. Although there was an association between hemodialysis and post-discharge invasive MRSA infection in both the univariable analysis and the analysis restricted to case patients with bloodstream infections, this association did not remain significant in the multivariable analysis including all invasive infections when other risk factors, such as the presence of a CVC at discharge, were taken into consideration. This is probably due to collinearity between CVC present at discharge and hemodialysis, given that nearly 30% of the case patients with CVC present at discharge from the hospital had a CVC for hemodialysis.
Our study is subject to several limitations. First, 139 eligible case patients (42%) were unable to be enrolled, largely owing to refusals or inability to be contacted for a telephone interview. A small percentage (11%) were excluded because matched case patients could not be identified within 6 months of hospital discharge; this exclusion criterion was not applied systematically throughout the study period. However, enrolled and non-enrolled case patients were similar with regard to age, sex, and race (data not shown). Second, the median time between hospital discharge and interviews differed between case patients and controls. Therefore, some exposures during the outpatient period may have been misclassified owing to recall bias. Nevertheless, this study assessed exposures for both case patients and controls using medical record reviews and telephone interviews, which may provide a more accurate description of exposures in and out of acute-care settings than studies relying solely on medical record data.
Third, although 15 hospitals across 6 US states were included in the study, our results may not be generalizable, given that most of the hospitals included had an academic affiliation and therefore may care for a different patient population or perform more complex procedures than hospitals without an academic affiliation. In addition, patients’ MRSA colonization status in our study population may have been misclassified, given that MRSA screening policies differed among participating hospitals. Finally, it is important to recognize that we cannot extrapolate the results of this analysis to time periods beyond 12 weeks after discharge because risk factors may change over a longer post-discharge period.
Efforts to decrease invasive MRSA infections in the post-discharge period should prioritize CVC care in the post-discharge settings, better chronic wound management, and evaluation of MRSA-specific interventions at the time of discharge from acute-care hospitals. Although universal MRSA decolonization of patients in intensive care units has been shown to successfully reduce MRSA-positive clinical cultures by 37% during a hospitalization [31], it is still unclear whether widespread decolonization or targeting specific high-risk populations during the hospitalization could also prevent post-discharge invasive MRSA infections. In addition, the optimal decolonization regimen has not been established yet. Specific efforts to prevent post-discharge MRSA infections should be part of a comprehensive nursing home infection control strategy that addresses all healthcare-associated infection risks to patient safety.
Acknowledgments.
The authors acknowledge the following persons for their contributions to data collection or input on study design: Art Reingold, MD, California Emerging Infections Program; Matthew Cartter, MD, Connecticut Department of Public Health; Betsy Stein, RN, Chris Bower, MPH, Lewis Perry, RN, MPH, Jessica Reno, MPH, Nicole Romero, and Wendy Baughman, MSPH, Georgia Emerging Infections Program; Emily Hallberg, MPH, Jane Harper, RN, MS, CIC, and Lindsey Lesher, MPH, Minnesota Emerging Infections Program; Anita Gellert, RN, New York Emerging Infections Program; and Wendi Welch, Tennessee Emerging Infections Program. We also acknowledge the following Centers for Disease Control and Prevention (CDC) contributors: Alyssa Parr, MPH, and Lisa LaPlace, MPH, for data entry and cleaning, Sandra Bulens, MPH, for her contribution to study design and training, Ronda Sinkowitz-Cochran, MPH for her contributions with the telephone interview questionnaires and training, and Shirley Zhang, MS for study database development.
Financial support. This study was partially funded by Stiefel/GlaxoSmithKline, Astellas Pharma Global Development, and Theravance through the CDC Foundation.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in the manuscript are those of the authors and do not necessarily represent the official position of the US CDC. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of the US government.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 2013; 173:1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filice GA, Nyman JA, Lexau C, et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2010; 31:365–73. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/. Accessed 1 November 2014.
- 4.The Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug resistant organisms in healthcare settings, 2006. Available at: http://www.cdc.gov/hicpac/pdf/MDRO/MDROGuideline2006.pdf. Accessed 14 September 2015.
- 5.Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillinresistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35(suppl 2):S108–32. [DOI] [PubMed] [Google Scholar]
- 6.Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA 2010; 304:641–8. [DOI] [PubMed] [Google Scholar]
- 7.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298: 1763–71. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 9.Duffy J, Dumyati G, Bulens S, et al. Community-onset invasive methicillin-resistant Staphylococcus aureus infections following hospital discharge. Am J Infect Control 2013; 41:782–6. [DOI] [PubMed] [Google Scholar]
- 10.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis 2003; 36:281–5. [DOI] [PubMed] [Google Scholar]
- 11.Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP. Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 1994; 19:1123–8. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds C, Quan V, Kim D, et al. Methicillin-resistant Staphylococcus aureus (MRSA) carriage in 10 nursing homes in Orange County, California. Infect Control Hosp Epidemiol 2011; 32:91–3. [DOI] [PubMed] [Google Scholar]
- 13.Jain R, Kralovic SM, Evans ME. Veterans Affairs initiative to prevent methicillinresistant Staphylococcus aureus infections. NEJM 2011; 364:1419–30. [DOI] [PubMed] [Google Scholar]
- 14.Harbarth S, Fankhauser C, Schrenzel J. Universal screening for methicillinresistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA 2008; 299:1149–57. [DOI] [PubMed] [Google Scholar]
- 15.Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007; 195:330–8. [DOI] [PubMed] [Google Scholar]
- 16.Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008; 148:409–18. [DOI] [PubMed] [Google Scholar]
- 17.Murphy CR, Quan V, Kim D, et al. Nursing home characteristics associated with methicillin-resistant Staphylococcus aureus (MRSA) burden and transmission. BMC Infect Dis 2012; 12:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BY, Bartsch SM, Wong KF, et al. The importance of nursing homes in the spread of methicillin-resistant Staphylococcus aureus (MRSA) among hospitals. Med Care 2013; 51:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mody L, Bradley SF, Huang SS. Keeping the “home” in nursing home: implications for infection prevention. JAMA Intern Med 2013; 173:853–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BY, Singh A, Bartsch SM, et al. The potential regional impact of contact precaution use in nursing homes to control methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2013; 34:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SS, Hinrichsen VL, Datta R, et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PloS One 2011; 6:e24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Big C, Malani PN. Staphylococcus aureus bloodstream infections in older adults: clinical outcomes and risk factors for in-hospital mortality. J Am Geriatr Soc 2010; 58:300–5. [DOI] [PubMed] [Google Scholar]
- 23.Lavery LA, Fontaine JL, Bhavan K, Kim PJ, Williams JR, Hunt NA. Risk factors for methicillin-resistant Staphylococcus aureus in diabetic foot infections. Diabet Foot Ankle 2014; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vriens MR, Blok HE, Gigengack-Baars AC, et al. Methicillin-resistant Staphylococcus aureus carriage among patients after hospital discharge. Infect Control Hosp Epidemiol 2005; 26:629–33. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TJ, Standiford HC, Reboli AC, et al. Randomized double-blinded trial of rifampin with either novobiocin or trimethoprim-sulfamethoxazole against methicillin-resistant Staphylococcus aureus colonization: prevention of antimicrobial resistance and effect of host factors on outcome. Antimicrob Agents Chemother 1993; 37:1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coello R, Glynn JR, Gaspar C, Picazo JJ, Fereres J. Risk factors for developing clinical infection with methicillin-resistant Staphylococcus aureus (MRSA) amongst hospital patients initially only colonized with MRSA. J Hosp Infect 1997; 37: 39–46. [DOI] [PubMed] [Google Scholar]
- 27.Harinstein L, Schafer J, D’Amico F. Risk factors associated with the conversion of meticillin-resistant Staphylococcus aureus colonisation to healthcare-associated infection. J Hosp Infect 2011; 79:194–7. [DOI] [PubMed] [Google Scholar]
- 28.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–44. [DOI] [PubMed] [Google Scholar]
- 29.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. Methicillin-resistant Staphylococcus aureus central line–associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009; 301:727–36. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen DB, Lessa FC, Belflower R, et al. Invasive methicillin-resistant Staphylococcus aureus infections among patients on chronic dialysis in the United States, 2005–2011. Clin Infect Dis 2013; 57:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. NEJM 2013; 368:2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]