Summary
"Poor accessibility to drugs" is the most problematic issue for patients with rare diseases in China. In recent years, China has issued a number of policies, such as prioritizing speeding up the evaluation for rare disease drugs, publishing national rare disease lists and giving priority to treatments for severe diseases like rare diseases during annual adjustments of National Medical Insurance Medicine Catalogue to improve the accessibility of rare disease drugs. From the outcome perspective, the evaluation of rare disease drugs takes 3 months shorter than ordinary drugs, basic research projects have been started and the number of rare disease drugs included in National Medical Insurance Medicine Catalogue has increased to 50. However, the policies' effects on new drug research and development, rare disease diagnosis and treatment as well as drug pricing are limited. It is recommended to learn the tilt policy of research and development for rare disease drugs from foreign countries and the mechanism of medical insurance funding and patient co-payments. Thus it is important to improve the availability, accessibility and affordability of rare diseases drugs based on the Chinese context.
Keywords: Rare disease, accessibility, orphan drug, policy, medical insurance
1. Introduction
Difficult to diagnose, difficult to treat and difficult to get medications are embarrassing situations for patients with rare diseases not only in China but also for rare disease patients all over the world. Due to previous hospital procurement restrictions, physician prescription restrictions, and outpatient reimbursement restrictions, having no access to drugs is a problem crying for solution for patients with rare diseases in China (1). Generally, accessibility includes availability, which involves research, development and market access of drugs, adaptability, which involves the construction of a diagnosis and treatment system, and affordability, which involves the pricing and inclusion in medical insurance (2-4). Thus, the following discussion about the current situation of accessibility to rare disease drugs in China will be from three perspectives, availability, adaptability and affordability.
2. The current national policies on orphan drugs
China's attention to rare diseases started late. There are no special rare disease laws and policies at the national level, and there is no specific rare disease drug (including orphan drug) policy. However, in recent years, as society's attention to rare diseases has increased, the importance of orphan drugs has gradually appeared in various policy documents (Table 1).
Table 1. Current effective national policies about rare diseases.
| Item | Category | Effective time | Policy & Regulation | Content about Rare Diseases |
|---|---|---|---|---|
| Availability | Development and imitation of drugs | 2012 (5) | The Notice of the State Council on National Drug Safety During the 12th Five-Year Plan issued by the State Council. | To encourage the development of orphan drugs and suitable dosage forms for children. |
| 2017 (6) | The Opinions of the State Council on Reform of the System of Evaluation, Review and Approval of Drugs and Medical Devices issued by the General Office of the State Council. | To support the development of drugs and medical devices for rare diseases. | ||
| Registration and approval | 2007 (7) | The Measures for the Administration of Drug Registration issued by former China Food and Drug administration. | Special approval for new drugs with obvious clinical efficacy in the treatment of AIDS, malignant tumors, rare diseases etc. | |
| 2009 (8) | The Notice of the China Food and Drug Administration for the Special Approval and Management of New Drugs issued by former Chinese Food and Drug Administration. | Special approval process for the application of new drugs for the treatment of AIDS, malignant tumors, rare diseases and other diseases with obvious clinical advantages. | ||
| 2013 (9) | The Opinions of the China Food and Drug Administration on deepening the reform of evaluation and approval systems and encouraging innovation on drugs issued by former China Food and Drug Administrations. | Prioritize and speed up the evaluation of rare disease drugs. | ||
| 2015 (10) | The Opinions of the State Council on Reform of the System of Evaluation, Review and Approval of Drugs and Medical Devices issued by the State Council. | Accelerated evaluation and approval of innovative drugs for the prevention and treatment of diseases such as rare diseases, sub-neoplastic diseases, AIDS and major infectious diseases. | ||
| 2016 (11) | The Notice of the General Office of the State Council on issuing the 2015 Major Task List on Deepening the Medical and Health Care System reform issued by the General Office of the State Council. | Further smooth the special channel for evaluation and approval of rare disease drugs and clinical urgently needed drugs. | ||
| 2017 (12) | Several Opinions of the General Office of the State Council on Further Reforming and Improving the Policies on Drug Production, Circulation and Use issued by the General Office of the State Council. | Classified Evaluation and approval of drugs for rare diseases, children, the elderly, emergency (rescuing) and Chinese medicine (classical prescription). | ||
| 2017 (13) | The Policies of the China Food and Drug Administration regarding Encouraging Innovation and Accelerating the Evaluation and Approval Systems on Drugs and Medical Devices (Consultation Paper) issued by the former China Food and Drug Ministration. | Applicants for rare disease treatments and medical devices may apply for clinical trials for reduction and exemption; and rare-drug treatment drugs and medical devices that have been approved for marketing abroad, supplement relevant research within the prescribed time after listing. | ||
| 2017 (14) | Opinions of the China Food and Drug Administrationon encouraging innovative implementation of prioritized evaluation and approval on drugs issued by the former China Food and Drug Administration. | Drug registration for rare diseases can be included in the scope of prioritized evaluation and approval. | ||
| 2018 (15) | Notice of the National Medical Products Administration and the National Health Commission on Optimizing Review and Approval of Registration of Medical Products issued by the National Medical Product Administration and the National Health Commission. | Orphan drugs can submit clinical trial data obtained overseas and directly apply for drug listing registration, which meeting the requirements of the Drug Registration Management Measures and related documents may directly approve the import. | ||
| 2018 (16) | Interim Measures of the National Medical Products Administration for Protection of Pharmaceutical Test Data (Consultation Paper) issued by the former China Food and Drug Administration. | Orphan drugs are listed as the target of data protection and a 6-year data protection period is granted to them since the indication firstly approved in China. | ||
| Adaptability | Diseases list | 2017 (17) | Policies of the China Food and Drug Administration regarding Encouraging Innovation and Accelerating the Evaluation and Approval Systems on Drugs and Medical Devices (Consultation Paper) issued by the former China Food and Drug Ministration. | The Health and Family Planning Department would publish a list of rare diseases and establish a registry system for rare patients. |
| 2018 (18) | The National Rare Disease List by five Authority bodies. | 121 rare diseases. | ||
| Diagnosis and Treatment | 2018 (19) | The Notice of the National Medical Products Administration of Requesting the List of Urgently Needed New drugs in Clinical (the First Batch) issued by the National Medical Product Administration. | There are 20 drugs' indications related to 12 diseases in the National Rare Disease List. | |
| 2019 (20) | The Notice of the National Medical Products Administration of Requesting the List of Urgently Needed New drugs in Clinical (the Second Batch) issued by the National Medical Product Administration. | Involving 13 drugs treating rare diseases. | ||
| 2019 (21) | The Guidelines for the Diagnosis and Treatment of Rare Diseases (2019 Edition) issued by the National Health Commission. | The diagnosis and treatment of 121 rare diseases. | ||
| Affordability | Pricing | 2019 (22) | The Notice by the Ministry of Finance regarding the Value Added Tax Policies on Drugs for Rare Diseases issued by the Ministry of Finance. | For the first batch of 21 orphan drugs and 4 drug substances, value added tax will be reduced to 3% referring to the anti-cancer drug in import process, and the domestic value added tax may choose the simple method, levied by 3%. |
| Inclusion in Medical Insurance | 2012 (23) | The Notice of the Ministry of Health, the Ministry of Finance, and the Ministry of Civil Affairs on Effectively Conducting Work for the New rural cooperative Medical System issued by three authority bodies. | 12 diseases including chronic myeloid leukemia, hemophilia and cleft lip and palate are preferentially included in the pilot area of major illness prevention. | |
| 2019 (24) | The Work Plan for the adjustment of Medicines List for National Medical Insurance in 2019 (Consultation Paper) issued by the National Healthcare Security Administration. | The national medical Insurance list of drug is adjusted to give priority to the treatment of serious diseases such as national essential drugs, cancer and rare diseases. |
It can be seen from various policies that China has increased the accessibility of orphan drugs and implemented priority evaluation. Especially, the evaluation of rare disease drugs is required to be done in 3 months, which has greatly accelerated the speed of new drug listings. In the importing process, value added tax is levied at 3%. In terms of basic research, the publication of National Rare Disease List involved 121 diseases, which greatly increased the attention to rare diseases (18).
3. The current accessibility to rare disease drugs in China
3.1. Availability of rare disease drugs
3.1.1. The level of research, development and imitation is still low
In 2012, "the Notice of the State Council on National Drug Safety During the 12th Five-Year Plan" (5) encouraged research and development of rare disease drugs and suitable dosages for children. Furthermore, "the Opinions of the State Council on Reform of the System of Evaluation, Review and Approval of Drugs and Medical Devices" (6) encouraged research and development of pharmaceuticals and medical devices for the treatment of rare disease. In the 2017 and 2018 new drug project application guidelines, urgently needed drugs for rare disease treatment were included in the "imperatively needed clinical drugs that should be researched and developed" and 9 research and development projects were carried out with a central government's financial budget of more than RMB 50 million (25). On May 29, 2019, the Center for Drug Evaluation, National Medical Product Administration published "The Basic Consideration of Using Real World Evidence to Support the Research and Development of Drugs (Consultation Paper)" and proposed that the clinical trials of rare disease drugs can use the real world data formed by the natural disease cohort as an external control to help the research (26). However, due to the weak foundation of China's drug research and development, coupled with slow progress in basic research and no special incentives from the country (such as internationally-recognized research and development grants, tax reduction policies, etc.), the company lacks research and development incentives. At present, there are no new drugs for rare diseases coming out in China.
3.1.2. Registration and approval are speeding up and the number is increasing
In terms of speed, it was proposed that rare disease drugs should be reviewed and evaluated within three months (27). From the quantity perspective, more than 40% of around 400 orphan drugs listed in the US haven't been applied in China (28). Due to the lack of a unified definition of rare disease in the world and there is no clear definition of rare diseases in China, China Organization for Rare Disorders used the National Rare Disease List as a sample to organize the pharmaceuticals globally listed for the 121 rare diseases from the list (Figure 1) (1). From the result, out of the 121 rare diseases, 47 diseases also have no therapeutic drugs (mainly in the US/EU/Japan). However, there are 79 drugs outside the country but not listed in the country, involving 21 diseases. There are still 35 listed drugs that have no indications from the list.
Figure 1.
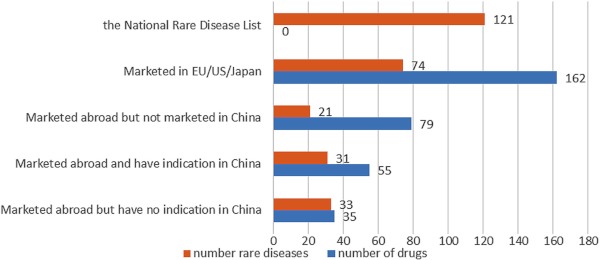
The market status of therapeutic drugs for rare diseases in the National Rare Disease List (1).
It is worth noting that in 2018, 13 rare disease drugs involving 10 rare diseases were successfully applied for listing through priority review and approval. There are 20 rare disease drugs involving 12 rare diseases included in the list of Clinically-needed Foreign New Drugs (first batch) (19) issued by Centre for Drug Evaluation, National Medical Products Administration. In 2019, 14 rare disease drugs were included in the list of clinically urgent new drugs (second batch) (20).
3.2. Adaptability of rare disease drugs
3.2.1. Basic research projects started
Although basic research on rare diseases in China is weak (29), with the continuous attention of society to rare diseases, the basic data is constantly improving. In December 2016, the China Research Hospital Association Rare Diseases Branch was established in Beijing. At the same time, the National Key Research and Development Program "Rare Disease Clinical Cohort Study" and "Rare Diseases Precision Diagnosis and Treatment Technology and Clinical Standard Research" project were officially launched (30). In June 2017, the National Key Research and Development Program "Chinese Severe Diseases and Rare Diseases Clinical and Life Omics Database" was launched (31). In the same year, the National Rare Disease Registration System (NRDRS) was officially launched, which would create an information resource platform and a biobank with gene, protein, metabolomics and molecular imaging diagnostic platforms. By the end of 2018, NRDRS had registered more than 100 rare diseases and more than 30,000 cases (32).
3.2.2. The current situation of diagnosis and treatment is not optimistic
Although in recent years, China has made many breakthroughs in the construction of a rare disease diagnosis and treatment system, in 2017, Shanghai edited the first rare disease monograph "Treatable Rare Disease", which provided diagnosis and treatment guidelines for 117 different rare diseases (33). In 2018, the China Alliance of Rare Diseases was established which became the first national and non-profit communication platform for rare diseases (34). In 2019, the Rare Diseases Diagnosis and Treatment Guidelines (2019 Edition) involving 121 rare diseases were released. In the same year, the National Health Commission announced that 324 hospitals with strong diagnosis and treatment ability as well as relatively more cases would be selected to establish a rare disease diagnosis and treatment collaboration network (35). However, patients with rare diseases still face the dilemma of having no pharmaceuticals for treatment. Among the more than 7,000 known rare diseases, less than 10% of the rare diseases have approved therapeutic drugs or interventions. From 2014 to 2018, the Chinese Organization for Rare Disorders surveyed 5,810 patients with rare diseases (36). The results showed that 42% of patients did not receive any treatment, and most of the patients who received treatment failed to take a sufficient amount of medicine in a timely way. In the National Rare Disease List, only 53 rare diseases have therapeutic drugs listed, and 43 kinds of drugs involving 33 rare diseases have been listed in China but have not registered corresponding rare disease indications, which indirectly leads to clinicians making over-indicated prescriptions. But this undoubtedly brings great risk of medication. Finally, the "last mile" of rare disease drugs is still full of challenges, such as bidding and purchasing, hospital purchase list, prescription restrictions, outpatient reimbursement, and restrictions on designated medical institutions as well as pharmacies.
3.3. Affordability of rare disease drugs
3.3.1. Pricing policy needs to be improved
Since June 2015, China has eliminated the way in which the government manages the price of medicines in a unified manner. The price of different types of drugs will be set by different methods. For rare diseases drugs, there are the following types: those included in the medical insurance list, the National Healthcare Security Administration formulates the medical insurance payment standard; for drugs with patents and exclusive production, the price is determined by multiple parties to negotiate and set the price; for other drugs, the enterprises will mainly price them independently. Because the current domestic rare disease drugs are mainly imported from abroad, most of them are patented drugs or exclusive products, which leads to a high price. For this reason, the State Council issued "the Notice of the Customs Tariff Commission of the State Council on the Provisional Import and Export Tariff Rate and Other Tariff Rate Adjustment Plan for 2019" (37), involving some raw materials of rare disease drugs to implement zero tariff. In 2019, the Ministry of Finance issued the "Notice by the Ministry of Finance, the General Administration of Customs, the State Administration of Taxation and the National Medical Products Administration of the VAT Policies on Drugs for Rare Diseases" (38), the value added tax of 21 rare diseases and 4 active pharmaceutical ingredients were reduced. Despite this, high-priced drugs still face difficulties entering medical insurance list, while low-cost drugs face a crisis of being discontinued or even having production stopped.
3.3.2. Number of rare disease drugs continuously increases
In 2017, China first introduced two orphan drugs into the medical insurance catalogue through national negotiations (39), and achieved a certain breakthrough in market access for orphan drugs. By the end of 2018, out of 121 rare diseases, 50 drugs for rare diseases had been included in medical insurance, of which 17 were classified as Class A medical insurance (Drugs in Class A medical insurance are fully reimbursed) and 33 were classified as Class B medical insurance (Drugs in Class B medical insurance are partly reimbursed. The percentage depends on the local policies and the type of drugs).
Some provinces and cities in China have guaranteed orphan drugs excluded from the national medical insurance list, but this exploration is still limited to a small number of provinces and cities and a small number of rare diseases/drugs (Table 2).
Table 2. The rare disease drugs which are reimbursed by local government.
| Province/City | The Rare Diseases Involved | Main Policy |
|---|---|---|
| Qingdao (39) |
Multiple sclerosis: Recombinant human interferon for injection (β-1b); Hyperphenylalanemia: Sapropterin Dihydrochloride Tablets; Idiopathic Pulmonary Artery Hypertension: Bosentan Tablets; Gaucher disease: Imiglucerase for Injection; |
▪) Supplementary Medical Insurance Funds reimbursed 80%. ▪) Large amount of security section, personal burden of more than 50,000 yuan/year, reimbursed 70%, annual maximum payment was 200,000 yuan. ▪) Insured people in low-and middle-income families can also enjoy the special medical subsidy provided by the civil administration. |
| Shanghai (40) |
Haemophilia: Recombinant Human Coagulation Factor Ⅸ for Injection. Children's Hospitalization Fund: Pompeii Disease, Fabre Disease, Gaucher Disease, Mucopolysaccharidosis; Special Rescue Funds for Lysosomal Storage: Diseases Associated with Lysosomal Storage. |
▪) Children's Hospitalization Funds reimbursed RMB10,000 (children only). ▪) Special Rescue Funds for Lysosomal Storage provide relief for the remaining co-payments of patients. |
| Zhejiang (41) |
Gaucher Disease: Imiglucerase for Injection; Hyperphenylalanemia: Sapropterin Dihydrochloride Tablets; Idiopathic Pulmonary Fibrosis: Nintedanib; Idiopathic Pulmonary Artery Hypertension: Ambrisentan tablets; Amyotrophic Lateral Sclerosis: Riluzole. |
▪) Medical expenses other than medicare reimbursement were resolved by financial arrangement funds through special assistance. ▪) Special funds were subsidized by provincial finance through civil assistance |
| Henan (42) | Haemophilia, Phenylketonuria etc. | ▪) Included in the list of severe diseases |
| Shenzhen (43) |
Idiopathic Pulmonary Arterial Hypertension: Bosentan Tablets; Crohn's Disease: Infliximab for Injection. |
▪) Included in the supplementary medical insurance list, reimburse 70% |
| Chengdu (44) |
Idiopathic Pulmonary Arterial Hypertension: Bosentan Tablets; Crohn's Disease: Infliximab for Injection |
▪) Included in the medical insurance drug list for severe diseases, reimburse 70% and the limitation is 150,000 yuan at most. |
4. Discussion and Suggestions
Despite the fact that rare diseases have received much attention and favorable policies have been introduced in China, drugs for rare diseases have been imported, but they are mainly concentrated in the drug registration and approval process, and other processes are still weak (Figure 2).
Figure 2.
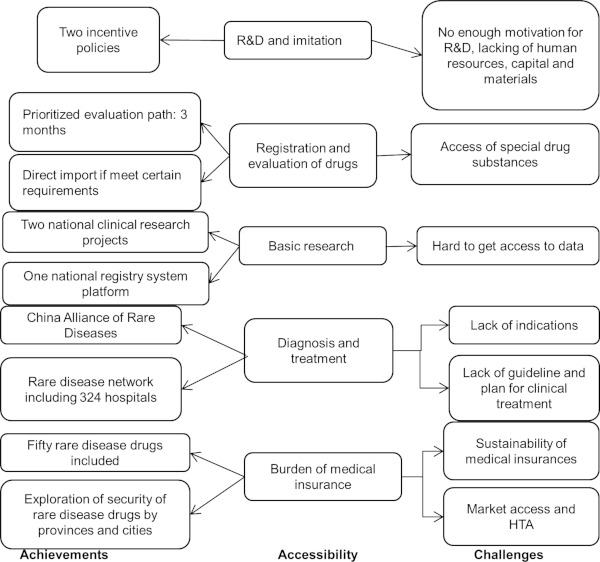
The current situation and challenges of rare disease drug accessibility in China.
4.1. Formulate a tilt policy for orphan drug research and development
From the perspective of research and development of orphan drugs, although China has introduced relevant incentive policies, it lacks substantial preferential measures, resulting in a lack of research and development incentives. At present, most of the rare diseases in the world lack effective treatment, and there are a large number of blank areas that need to be filled, which leaves room for Chinese biomedical innovation attention and favorable policies have been introduced in China, drugs for rare diseases have been imported, but they are mainly concentrated in the drug registration and approval process, and other processes are still weak (Figure 2).
4.2. Establish special drug classification and approval
In the drug registration and approval process, China has accelerated registration through priority evaluation and accelerated approval, which has greatly facilitated the introduction of orphan drugs abroad. It is recommended to give priority to the evaluation and approval of drugs with clear diagnosis and treatable rare diseases. Learn from the experience of introducing anticancer drugs, further render preferential tariffs and VAT. Establish special approval channels for some blood products related drugs to secure rare disease patients, which rely on blood products for intervention or treatment.
4.3. Implement dynamic adjustment to the National Rare Disease List and construct a centers of excellence
Basic research work and diagnosis and treatment capacity construct can directly affect the accessibility of rare diseases drugs. At present, a large-scale clinical cohort study and registration system has been established at the national level (49), but the number of rare diseases is numerous. Therefore, it is recommended to further strengthen the previous epidemiological research and related basic research, implement the dynamic update of the rare disease list. Strengthen the construction of diagnosis and treatment ability, and gradually establish the state and provincial center of excellence to further empower medical staffs to identify, diagnose, and treat rare diseases.
4.4. Try implementation of multi-party co-payment security mode
The cost of rare disease drugs is extremely high. With the main policy of medical insurance in China, how to reduce the burden of patients' drug costs is an important issue to be urgently solved. It is recommended to learn from local experience and establish a government-led, medical insurance covered and multi-party payment security model. First, set up a special fund within medical insurance for rare diseases, encourage social forces to participate through multi-funding and risk sharing. The second is to include rare disease drugs in medical insurance in batches, and to ensure that the rare drugs with the exact effect are preferentially included in the medical insurance. Finally, health technology assessment methods should be introduced to establish a special evaluation process for orphan drugs (50).
In conclusion, rare diseases are not just medical problems, but also social problems. How to promote innovation in the pharmaceutical industry? How to ensure the equity, equality and efficiency and how to solve the sustainability of funds? Despite the valuable experience of other countries and regions, and the exploration of domestic success in some provinces and cities, how to secure the accessibility of rare disease drugs based on the Chinese context still needs much effort.
Acknowledgements
This study was supported by a grant from the Shanghai Municipal Health Commission for a Special Research Project related to Health Policies entitled "Study on the disease burden of and social security for the vulnerable population in Shanghai (Project number: 19Y04017)".
References
- 1. Huang RF, Shao WB. The report on accessibility of rare diseases drugs in China, 2019. China Organization for Rare Disorders, IQVIA. 2019; pp.1-20. (in Chinese) [Google Scholar]
- 2. Ding JX, Ji N, Bai GL. Study of orphan drug market protection policy in China. Chinese Journal of Pharmaceuticals. 2012; 43:959-965. (in Chinese) [Google Scholar]
- 3. Gong SW, Xu Y, Zhang L. Study on evaluation indicator system of drug accessibility. Chinese Health Economics. 2011; 30:72-74. (in Chinese) [Google Scholar]
- 4. Ding J, Wang L. Chinese rare disease research report (2018). China Medical Science Press, Beijing, China, 2018; pp.1-22. (in Chinese) [Google Scholar]
- 5. The State Council. The Notice of the State Council on National Drug Safety During the 12th Five-Year Plan. http://www.gov.cn/zwgk/2012-02/13/content_2065197.htm (accessed April 5 , 2019). (in Chinese).
- 6. The General Office of the State Council. The Opinions of the State Council on Reform of the System of Evaluation, Review and Approval of Drugs and Medical Devices. http://www.gov.cn/xinwen/2017-10/08/content_5230105.htm (accessed April 6, 2019). (in Chinese).
- 7. The former China Food and Drug administration. The Measures for the Administration of Drug Registration. http://samr.cfda.gov.cn/WS01/CL1031/24529.html (accessed May 5, 2019). (in Chinese).
- 8. The former Chinese Food and Drug Administration. The Notice of the China Food and Drug Administration for the Special Approval and Management of New Drugs. http://samr.cfda.gov.cn/WS01/CL0058/35157.html (accessed April 6, 2019). (in Chinese).
- 9. The former China Food and Drug Administrations. The Opinions of the China Food and Drug Administration on deepening the reform of evaluation and approval systems and encouraging innovation on drugs. http://zw.hainan.gov.cn/data/news/2013/02/9412/ (accessed April 6, 2019). (in Chinese).
- 10. The State Council. The Opinions of the State Council on Reform of the System of Evaluation, Review and Approval of Drugs and Medical Devices. http://www.gov.cn/zhengce/content/2015-08/18/content_10101.htm (accessed April 8, 2019). (in Chinese).
- 11. The General Office of the State Council. The Notice of the General Office of the State Council on issuing the 2015 Major Task List on Deepening the Medical and Health Care System reform. http://www.scio.gov.cn/32344/32345/33969/34689/xgzc34695/Document/1480540/1480540_2.htm (accessed April 8, 2019). (in Chinese).
- 12. The General Office of the State Council. Several Opinions of the General Office of the State Council on Further Reforming and Improving the Policies on Drug Production, Circulation and Use. http://www.gov.cn/zhengce/content/2017-02/09/content_5166743.htm (accessed April 10, 2019). (in Chinese).
- 13. The former China Food and Drug Administration. The Policies of the China Food and Drug Administration regarding Encouraging Innovation and Accelerating the Evaluation and Approval Systems on Drugs and Medical Devices (Consultation Paper). https://www.cmde.org.cn/CL0101/6388.html (accessed April 8, 2019). (in Chinese).
- 14. The former China Food and Drug Administration. Opinions of the China Food and Drug Administration on encouraging innovative implementation of prioritized evaluation and approval on drugs. http://www.zjjxw.gov.cn/art/2018/1/31/art_1086725_15436535.html (accessed April 8, 2019). (in Chinese).
- 15. The National Medical Product Administration and the National Health Commission. Notice of the National Medical Products Administration and the National Health Commission on Optimizing Review and Approval of Registration of Medical Products. http://www.sdaqh.gov.cn/html/2018523/n938424866.html (accessed April 11, 2019). (in Chinese).
- 16. The former China Food and Drug Administration. Interim Measures of the National Medical Products Administration for Protection of Pharmaceutical Test Data (Consultation Paper). http://yjj.sc.gov.cn/CL3614/135466.html (accessed April 9, 2019). (in Chinese).
- 17. The former China Food and Drug Administration. Policies of the China Food and Drug Administration regarding Encouraging Innovation and Accelerating the Evaluation and Approval Systems on Drugs and Medical Devices (Consultation Paper). http://www.sdaqh.gov.cn/html/2017512/n025019717.html (accessed April 8, 2019). (in Chinese).
- 18. He JJ, Kang Q, Hu JH, Song PP, Jin CL. China has officially released its first national list of rare diseases. Intractable Rare Dis Res. 2018; 7:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The National Medical Product Administration. The Notice of the National Medical Products Administration of Requesting the List of Urgently Needed New drugs in Clinical (the First Batch). http://www.cde.org.cn/news.do?method=largeInfo&id=313990 (accessed April 11, 2019). (in Chinese).
- 20. The National Medical Product Administration. The Notice of the National Medical Products Administration of Requesting the List of Urgently Needed New drugs in Clinical (the Second Batch). http://www.cde.org.cn/news.do?method=largeInfo&id=314835 (accessed April 11, 2019). (in Chinese).
- 21. The National Health Commission. Guidelines for the Diagnosis and Treatment of Rare Diseases. http://www.gov.cn/fuwu/2019-02/28/content_5369203.htm (April 12, 2019). (in Chinese).
- 22. The Ministry of Finance. The Notice by the Ministry of Finance regarding the Value Added Tax Policies on Drugs for Rare Diseases. http://szs.mof.gov.cn/zhengwuxinxi/zhengcefabu/201902/t20190222_3176415.html (accessed April 13, 2019). (in Chinese).
- 23. The Ministry of Health, the Ministry of Finance, the Ministry of Civil affairs. The Notice of the Ministry of Health, the Ministry of Finance, and the Ministry of Civil Affairs on Effectively Conducting Work for the New Rural Cooperative Medical System. http://www.gov.cn/zwgk/2012-05/25/content_2145389.htm (accessed April 13, 2019). (in Chinese).
- 24. The National Healthcare Security Administration. The Work Plan for the adjustment of Medicines List for National Medical Insurance in 2019 (Consultation Paper). http://www.gov.cn/xinwen/2019-04/19/content_5384349.htm (accessed April 14, 2019). (in Chinese).
- 25. The National Health Commission. Reply to the recommendation No. http://www.nhc.gov.cn/wjw/jiany/201901/5025c4643964472a83f69318fec18a82.shtml (accessed April 14, 2019). (in Chinese).
- 26. The Center for Drug Evaluation, National Medical Product Administration. The Basic Consideration of Using Real World Evidence to Support the Research and Development of Drugs (Consultation paper). http://www.cde.org.cn/news.do?method=viewInfoCommon&id=314865 (accessed May 29, 2019). (in Chinese).
- 27. People's Daily. Patients use national medical insurance negotiating drugs, and the average review time of new drugs is cut in half. http://www.gov.cn/zhengce/2019-02/20/content_5367007.htm (accessed April 8, 2019). (in Chinese).
- 28. Li YM. Orphan drug payment security system needs a "guarantee". Medical Economics Reporter, 2019-2-21. (in Chinese)
- 29. Kang Q, Yang Y, He JJ. Progress, problems and suggestions on rare diseases security work in China. Soft Science of Health. 20-23. [Google Scholar]
- 30. Chinese Research Hospital Association. Chinese Research Hospital Association Rare Disease Branch was launched in Beijing. http://www.crha.cn/NewsView_4391.html (accessed April 11,2019). (in Chinese).
- 31. National Science and Technology Management Information System Public Service Platform. Notice on the Project Arrangement of the National Key Research and Development Program "Precision Medical Research" and "Reproductive Health and Major Birth Defect Prevention and Control Research" in 2017. http://service.most.gov.cn/2015tztg_all/20170602/2189.html (accessed April 13, 2019). (in Chinese).
- 32. China National Rare Disease Registration System. China National Rare Disease Registration System Project Memorabilia. https://www.nrdrs.org.cn/app/rare/project-schedule.html (accessed April 12, 2019). (in Chinese).
- 33. China News Net. The first rare disease monograph in China, which is based on clinical examples, has been published. http://www.sh.chinanews.com/kjws/2017-02-27/19230.shtml (accessed April 5, 2019). (in Chinese).
- 34. CCTV. China Alliance of rare disease was established. http://news.cctv.com/2018/10/25/ARTIR7VBxoCoL2lauI0qfp2J181025.shtml (accessed April 5,2019). (in Chinese).
- 35. CNR News. National Health Commission announced the establishment of a national network of rare disease diagnosis and treatment to improve the level of diagnosis and treatment of rare diseases. https://baijiahao.baidu.com/s?id=1625699291454805235&wfr=spider&for=pc (accessed April 5,2019). (in Chinese).
- 36. Business Times. 20 million rare disease patients are waiting for drugs, orphan drug commercial insurance is almost blank in China. https://www.businesstimes.cn/articles-158619-20190228-7292245218.htm?page=6 (accessed April 5, 2019). (in Chinese).
- 37. The State Council. The Notice of the Customs Tariff Commission of the State Council on the Provisional Import and Export Tariff Rate and Other Tariff Rate Adjustment Plan for 2019. http://www.gov.cn/xinwen/2018-12/24/content_5351532.htm (accessed March 16, 2019). (in Chinese).
- 38. The Ministry of Finance, the General Administration of Customs, the State Administration of Taxation and the National Medical Products Administration. Notice by the Ministry of Finance, the General Administration of Customs, the State Administration of Taxation and the National Medical Products Administration of the VAT Policies on Drugs for Rare Diseases. http://szs.mof.gov.cn/zhengwuxinxi/zhengcefabu/201902/t20190222_3176415.html (accessed March 17, 2019). (in Chinese).
- 39. Health Times. The second batch of drugs negotiated by the nation, the price of 36 drugs will decrease. http://www.jksb.com.cn/index.php?m=wap&a=show&catid=49&id=120679 (accessed April 3, 2019). (in Chinese).
- 40. Qingdao People's Government. Implementation Opinions on Establishing Supplementary Medical Insurance System. http://hrss.qingdao.gov.cn/n28356070/n32563353/n32563396/63242.html (accessed April 6, 2019). (in Chinese).
- 41. Shanghai Foundation for Rare Disease. Notice on the inclusion of rare disease-specific drugs in the payment range of children's hospitalization mutual funds. http://gobest1.w226.mc-test.com/Reception/GuidanceInfo.aspx?id=110&mid=18 (accessed April 5, 2019). (in Chinese).
- 42. Zhejiang Provincial Department of Human Resources and Social Security. Notice on Strengthening Medical Care for Rare Diseases. http://www.zjmz.gov.cn/il.htm?a=si&id=8aaf80155717920d0157417ef0e202a9 (accessed April 6, 2019). (in Chinese).
- 43. Henan Provincial Department of Human Resources and Social Security Henan Province Basic Medical Insurance and Maternity Insurance Medical Treatment Projects and Medical Service Facilities Directory (Trial). Dec 12, 2016. http://www.ha.hrss.gov.cn/sitegroup/root/html/4aef140825e3728f01261be1c51101b5/f193b6bf7b8f400c93a591c37f7685c5.html (accessed April 2, 2019). (in Chinese).
- 44. Shenzhen Municipal Bureau of Human Resources and Social Security. Notice on Printing and Distributing the List of Drugs for Supplementary Medical Insurance in Shenzhen City. http://www.sz.gov.cn/szrbj/tzgg/201511/t20151102_3337662.htm?17a7ca90 (accessed April 3, 2019). (in Chinese).
- 45. Chengdu Municipal People's Government. Notice on Improving the Medical Insurance System for Serious and Severe Diseases. http://gk.chengdu.gov.cn/govInfoPub/detail.action?id=80749&tn=6 (accessed April 4, 2019). (in Chinese).
- 46. MHLW. Overview of Orphan Drug /Medical Device Designation System. http://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/orphan_drug.html (accessed April 9, 2019).
- 47. FDA. Clinical Studies of Safety and Effectiveness of Orphan Products April 8, 2012. http://edocket.Access.gpo.gov/2008/pdf/E8-30061.pdf (accessed March 22, 2019).
- 48. Ma Z, Gao JM, Zhao YH, Dai WW, Zheng XY. Policy of rare diseases: International experience and its implication for China. Chinese Journal of Health Policy. 61-67. (in Chinese) [Google Scholar]
- 49. Song PP, He JJ, Li F, Jin CL. Innovative measures to combat rare diseases in China: The national rare diseases registry system, larger-scale clinical cohort studies, and studies in combination with precision medicine research. Intractable & Rare Diseases Research. 2017; 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yuan N, Tian TT, Zhang HJ, Wang Y. Major routes for orphan drugs to be incorporated into the reimbursement system of UK and the enlightenment to China. Journal of China Pharmaceutical University. 113-119. (in Chinese) [Google Scholar]


