Abstract
Tetrathiatriarylmethyl (TAM) radicals represent soluble paramagnetic probes for biomedical electron paramagnetic resonance (EPR)-based spectroscopy and imaging. There is an increasing demand in the development of multifunctional, biocompatible and targeted trityl probes hampered by the difficulties in derivatization of the TAM structure. We proposed a new straightforward synthetic strategy using click chemistry for the covalent conjugation of the TAM radical with a water-soluble biocompatible carrier exemplified here by dextran. A set of dextran-grafted probes varied in the degrees of Finland trityl radical loading and dextran modification by polyethelene glycol has been synthesized. The EPR spectrum of the optimized macromolecular probe exhibits a single narrow line with high sensitivity to oxygen and has advantages over the unbound Finland trityl of being insensitive to interactions with albumin. In vivo EPR imaging of tissue oxygenation performed in breast tumor-bearing mouse using dextran-grafted probe demonstrates its utility for preclinical oximetric applications.
Synthesis of the first organic free radical, triphenylmethyl, was reported by Gomberg in 19001. By the late 1990s, these compounds with sterically protected trivalent carbon regained attention as the core structural fragment for the synthesis of stable organic radicals. Nycomed Innovation designed the sterically crowded trityl radicals, TAMs (tetrathiatriarylmethyl), in order to avoid hydrogen hyperfine coupling and enhance radical stability and water solubility2–3. Currently TAMs represent one of the major classes of soluble paramagnetic probes characterized by extraordinary stability toward tissue redox processes, long relaxation time and narrow line width making them particularly attractive for electron paramagnetic resonance (EPR)-based spectroscopy and imaging applications3–7. Figure 1 shows the most popular structures of TAM oximetric probes, Finland trityl (FTr)8–9 and OX0634, their deuterated derivatives5, 10–12, and the recently synthesized multifunctional monophosphonated probe, HOPE13–14 (sensor for pH, Oxygen and Phosphate in Extracellular microenvironment). Wide application of the highly hydrophilic TAM probes, Ox0634 and its deuterated analog, Ox07112, is hampered by the lack of affordable large-scale syntheses and difficulties in functional derivatization of the core structure for extended multifunctional applications.
Figure 1.
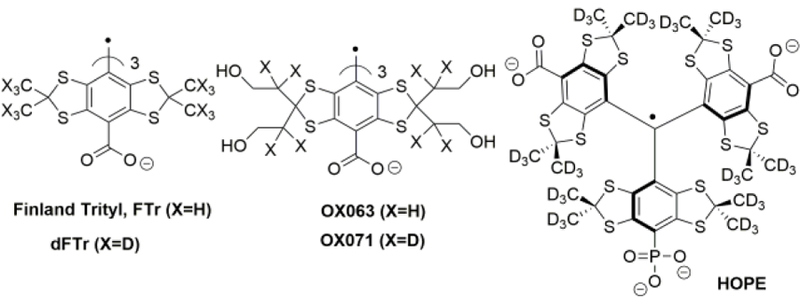
Representative structures of TAM radicals.
The recent progress in the development of multifunctional6, 15–17 (e.g., HOPE, Figure 1) and targeted trityl probes18–21 is mostly associated with the use of the Finland trityl core structure allowing for a large range of synthetic modifications. However, the relatively lipophilic nature of the aryl core of the Finland trityl is responsible for its hydrophobic interaction with bio-macromolecules such as albumin22, resulting in a signal loss and preventing systemic delivery of the corresponding spin probes.
Here we report a proof-of-concept of a new strategy for the development of multifunctional, biocompatible and targeted TAM structures based on covalent conjugation of the deuterated Finland radical core (dFTr) with a water-soluble biocompatible carrier. Scheme 1 illustrates this strategy utilized in the current work for the dextran polymer grafted with dFTr and polyethelene glycol (PEG), the latter allowing for the enhancing aqueous solubility of the probe. Previously, dextran has been widely explored as biocompatible carrier for probe and drug conjugation23.
Scheme 1.
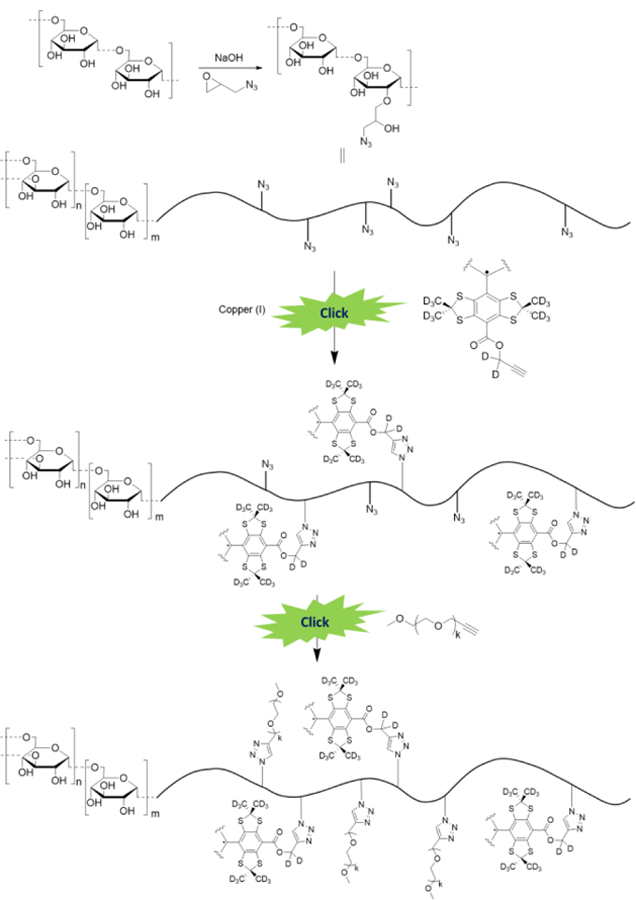
Schematic representation of the synthesis of dextran-conjugated TAM radicals using a click chemistry approach. The dextran grafting by the dFTr radical was achieved by copper-catalyzed azide-alkyne cycloaddition (CuAAC) of a TAM mono-propargyl ester followed by addition of excess of alkyne-PEG. Varying the trityl radical/dextran ratio allows for the tuning of the radical loading onto the polymer. Incorporation of PEG chains enhances the solubility of the grafted dextran and prevents spin-spin interactions between trityl radicals.
The dextran biopolymer with an average molecular weight of 20 kDa was functionalized through the etherification of the alcohol groups of the polysaccharide with 1-azido-2,3-epoxypropane (see Scheme 1)24. According to quantitative 13C NMR spectral analysis illustrated in Figure 2, about 8% of 125 sugar units of 20 kDa dextran chain were modified with 1-azido-2-hydroxypropyl chains, therefore resulting in total in 10 azide groups per dextran molecule.
Figure 2.
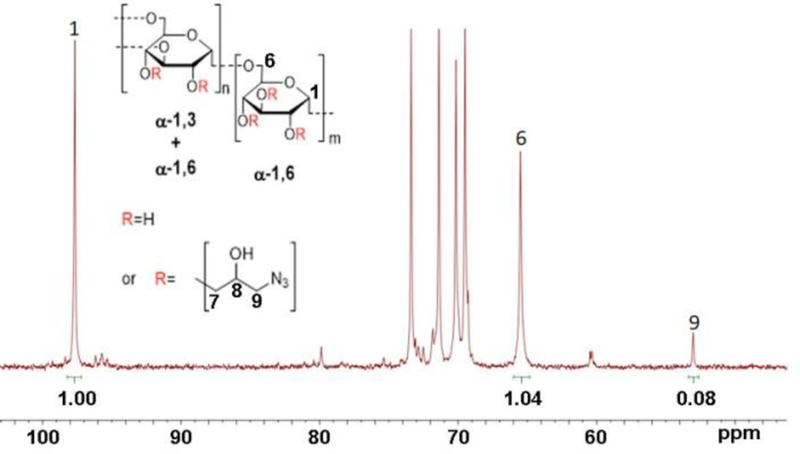
13C NMR spectrum of azidified dextran. Ratio of integrals of the peaks 9 and 1 gives the fraction of modification of sugar units by azide groups. Acquisition parameters: 100 MHz, D2O, time delay=15 s, 8000 scans. Insert: schematic structure of azidified dextran showing α−1,6 glycosidic linkages between glucose monomers with branches from α−1,3 linkages. The numbering of the carbon in the dextran structure corresponds to the corresponding peak numbering in the NMR spectrum.
Dextran grafting by the dFTr radical was achieved by a copper-catalyzed azide-alkyne cycloaddition (CuAAC) of a TAM mono propargyl ester 1 followed by addition of excess of commercially available alkyne-PEG (1 kDa, see Scheme 1 and SI). The reaction crude was easily purified by dialysis, and the completion of the reaction was confirmed by the disappearance of the azide peak on the IR spectrum (2110 cm−1). The TAM mono propargyl ester 1 was synthesized by esterification of dFTr using a deuterated propargyl tosylate 2 as depicted in Scheme 2 (see SI for the details).
Scheme 2.
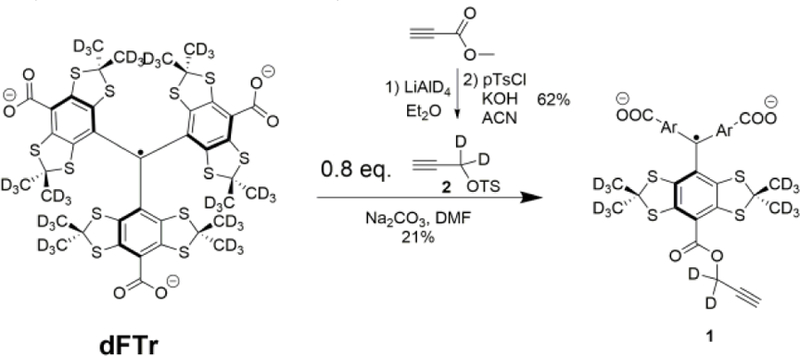
Synthesis of TAM mono propargyl ester (See SI for details).
Taking into account that all ten azide groups of azidified dextran were modified with 1 kDa dFTr or by 1 kDa PEG, the resulting grafted dextran has a molecular weight of approximately 30 kDa and its composition is described by the general formula Dextran-TAMxPEG10-x. To study the structure-function relationship of new macromolecular spin probes, we synthesized a set of the Dextran-TAMxPEG10-x that range from low to high trityl radical loading (see Table 1 and SI for the details).
Table 1.
Experimental conditions and final trityl radical loading, x, for a series of grafted dextran probes, Dextran-TAMxPEG10-x*.
| Loading | TAM | Dextran-N3 | PEG | x |
|---|---|---|---|---|
| Low | 3 mg | 20 mg | 60 mg | 3.6 |
| Medium | 6 mg | 20 mg | 60 mg | 6.5 |
| High | 12 mg | 20 mg | 60 mg | 8.1 |
An average number of TAM radicals bound to one dextran molecule, x, was measured using UV absorbance of Dextran-TAMxPEG10-x at 490 nm (characteristic for trityl radical, 16000 cm−1M−1) and supposing molecular weight of the grafted dextran equal to 30 kDa based on the initial molecular weight of 20 kDa and additional 10 azide sites modified by 1 kDa substitutes, dFTr or PEG. Figure 3 shows reverse phase HPLC chromatograms and UV spectra of the integrated peak of TAM mono-propargyl ester 1 and Dextran-TAM3.6PEG6.4 sample (low TAM loading).
Figure 3.
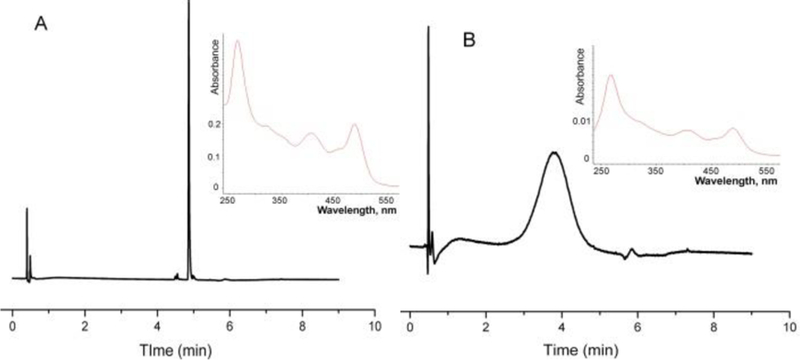
HPLC chromatograms of (A) TAM mono-propargyl ester radical and (B) dextran grafted with TAM radicals and PEG chains. Inserts: the UV spectrum of the main peak for each chromatogram (See SI for details).
Figure 4 shows the L-band EPR spectra of two Dextran-TAMxPEG10-x probes with low and intermediate TAM loading. An increase in spin density from x=3.6 (Figure 4A) to x=6.5 (Figure. 4B) did not significantly affect linewidth but resulted in an increase of signal intensity of about 1.6 times. Further increase in spin density in the Dextran-TAM8.1PEG1.9 probe resulted in the strong broadening effect of its L-band EPR spectrum apparently due to intramolecular spin-spin interaction, therefore we excluded this probe from the following studies. EPR spectra of both Dextran-TAM3.6PEG6.4 and Dextran-TAM6.5PEG3.5 demonstrated similar linear dependences on oxygen concentration (Figure 4C and 4D) making them useful oxygen-sensitive spin probes. Dextran-TAM3.6PEG6.4 demonstrated an important advantage of lacking an interaction with albumin (cf. red and black EPR spectra in Figures 4A) compared with significant albumin-induced line broadening for TAM6.5PEG3.5 (Figure 4B) apparently due to a larger number of hydrophilic PEG chains. Therefore, Dextran-TAM3.6PEG6.4 probe has been selected for testing its ability for tissue oxygen mapping, in vivo.
Figure 4.
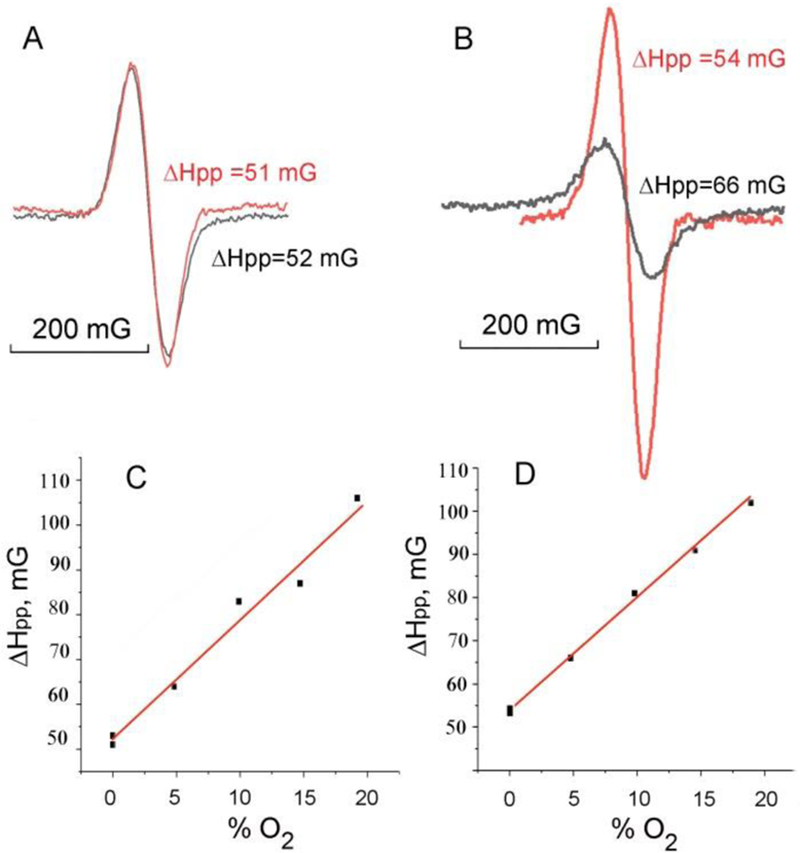
L-band (1.2 GHz) EPR spectra of Dextran-TAM3.6PEG6.4 (A) and Dextran-TAM6.5PEG3.5 (B) spin probes, and the corresponding dependences of their linewidth on oxygen concentration, (C) and (D). The spectra were measured for 0.5 ml samples of 65 µM solutions of the probes in deoxygenated 100 mM phosphate buffer in the absence (red lines) and presence (black lines) of 500 µM bovine serum albumin. The values of the peak-to-peak linewidth, ΔHpp, are shown near the spectra. The linear fits of the linewidth dependences on oxygen yield the spectral sensitivities to oxygen being equal to 2.7 mG/(% O2) (C) and 2.6 mG/(% O2) (D). The spectrometer settings were as follows: sweep time, 30 sec, sweep width, 480 mG; modulation amplitude, 30 mG; modulation frequency, 100 kHz; power attenuation, 15 dB.
Figure 5A shows the oxygen distribution in a breast cancer tumor measured in PyMT tumor-bearing mouse using rapid scan 800 MHz EPR imager after intratissue injection of the Dextran-TAM3.6PEG6.4 probe. The histogram of pO2 distribution (Figure 5B) clearly shows the presence of normoxic and hypoxic areas characteristic for highly heterogeneous tumor microenvironment5, 25–26. Interestingly, the intensity of the EPR signal was not significantly decreased over more than 1 hour, suggesting comparatively slow probe clearance from the tumor tissue of the anesthetized animal.
Figure 5.
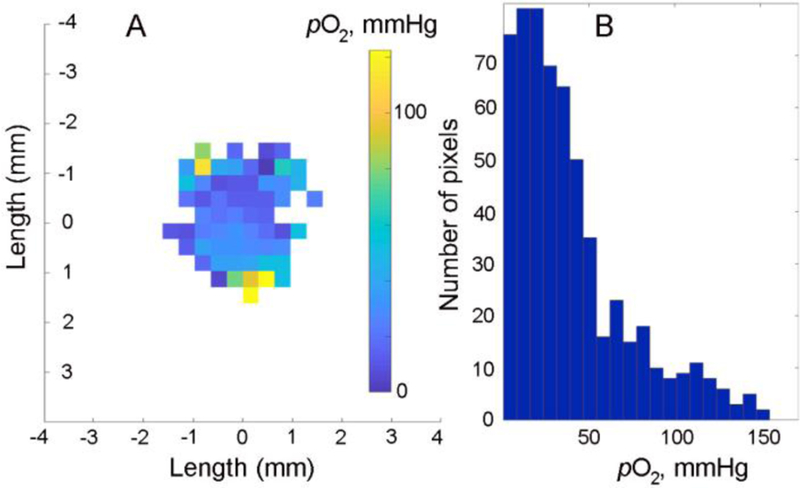
In vivo 4D (1-spectral-3-spatial) rapid scan 800 MHz EPR image of oxygen distribution in a breast tumor of a PyMT tumor-bearing mouse. An image acquisition was started 25 minutes after intratissue injection of 20 µl solution of 0.75 mM probe in 10 mM phosphate buffered saline; tumor volume, 214 mm3. Data acquisition parameters were as follows: acquisition time, 16.5 minutes; number of projections, 2546; rapid scan frequency, 9.4 kHz; and maximum gradient, 3G/cm. An integral intensity threshold of 30% was implemented to remove low signal-to-noise data. A. A two-dimensional slice (xz-plane) of the image is shown. B. A histogram of pO2 distribution within the entire image.
In summary, we synthesized a series of new dextran-conjugated trityl probes varied in the degree of Finland trityl radical loading and dextran modification by polyethylene glycol using a straightforward click chemistry approach. An optimized dextran-conjugated trityl probe demonstrated advantage over free Finland trityl in biocompatibility being more hydrophilic and lacking interaction with albumin. The probe exhibits an oxygen-sensitive narrow EPR spectral line allowing for in vivo oxygen mapping. The proposed strategy based on easy covalent conjugation of the deuterated Finland radical core with water-soluble biocompatible carriers such as dextran or chitosan, may result in the development of multifunctional6, biocompatible and targeted TAM structures with potentially favorable/tunable pharmacokinetics. It can be easily extended toward incorporation into the biopolymer structure of multifunctional trityls6, targeting moieties19 and/or therapeutic agents for the synthesis of theranostic probes.
Supplementary Material
Acknowledgements.
This work was partially supported by the NIH grants (USA) EB023990, CA194013, CA192064, EB022775, U54GM104942. The WVCTSI is acknowledged for start-up to V.V.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References and Notes
- 1.Gomberg M, An instance of trivalent carbon: triphenylmethyl. J. Am. Chem. Soc 1900, 22, 757–771. [Google Scholar]
- 2.Anderson S; Golman K; Rise F; Wikström H; Wistrand L-G, Free radicals. US Patent 1996, 5,530,140. [Google Scholar]
- 3.Ardenkjaer-Larsen JH; Laursen I; Leunbach I; Ehnholm G; Wistrand LG; Petersson JS; Golman K, EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J Magn Reson 1998, 133 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- 4.Krishna MC; English S; Yamada K; Yoo J; Murugesan R; Devasahayam N; Cook JA; Golman K; Ardenkjaer-Larsen JH; Subramanian S; Mitchell JB, Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A 2002, 99 (4), 2216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epel B; Krzykawska-Serda M; Tormyshev V; Maggio MC; Barth ED; Pelizzari CA; Halpern HJ, Spin Lattice Relaxation EPR pO(2) Images May Direct the Location of Radiation Tumor Boosts to Enhance Tumor Cure. Cell Biochemistry and Biophysics 2017, 75 (3–4), 295–298. [DOI] [PubMed] [Google Scholar]
- 6.Dhimitruka I; Bobko AA; Eubank TD; Komarov DA; Khramtsov VV, Phosphonated Trityl Probe for Concurrent In Vivo Tissue Oxygen and pH Monitoring Using EPR-based Techniques. JACS 2013, 135, 5904–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobko AA; Eubank TD; Driesschaert B; Dhimitruka I; Evans J; Mohammad R; Tchekneva EE; Dikov MM; Khramtsov VV, Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression. Sci Rep 2017, 7, 41233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy TJ; Iwama T; Halpern HJ; Rawal VH, General synthesis of persistent trityl radicals for EPR imaging of biological systems. Journal of Organic Chemistry 2002, 67 (14), 4635–4639. [DOI] [PubMed] [Google Scholar]
- 9.Dhimitruka I; Velayutham M; Bobko AA; Khramtsov VV; Villamena FA; Hadad CM; Zweier JL, Large-scale synthesis of a persistent trityl radical for use in biomedical EPR applications and imaging. Bioorg Med Chem Lett 2007, 17 (24), 6801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ardenkjaer-Larsen JH; Laursen I; Leunbach I; Ehnholm G; Wistrand LG; Petersson JS; Golman K, EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. Journal of Magnetic Resonance 1998, 133 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- 11.Dhimitruka I; Grigorieva O; Zweier JL; Khramtsov VV, Synthesis, structure, and EPR characterization of deuterated derivatives of Finland trityl radical. Bioorganic & Medicinal Chemistry Letters 2010, 20 (13), 3946–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epel B; Halpern HJ, In Vivo pO2 Imaging of Tumors: Oxymetry with Very Low-Frequency Electron Paramagnetic Resonance. Methods Enzymol 2015, 564, 501–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khramtsov VV; Bobko AA; Tseytlin M; Driesschaert B, Exchange Phenomena in the Electron Paramagnetic Resonance Spectra of the Nitroxyl and Trityl Radicals: Multifunctional Spectroscopy and Imaging of Local Chemical Microenvironment. Analytical Chemistry 2017, 89 (9), 4758–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bobko AA; Dhimitruka I; Zweier JL; Khramtsov VV, Fourier Transform EPR of Trityl Radicals for Multifunctional Assessment of Chemical Microenvironment. Angew. Chem. Int. Edit 2014, 53, 2735–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driesschaert B; Marchand V; Leveque P; Gallez B; Marchand-Brynaert J, A phosphonated triarylmethyl radical as a probe for measurement of pH by EPR. Chem Commun 2012, 48 (34), 4049–4051. [DOI] [PubMed] [Google Scholar]
- 16.Liu YP; Song YG; Rockenbauer A; Sun J; Hemann C; Villamena FA; Zweier JL, Synthesis of Trityl Radical-Conjugated Disulfide Biradicals for Measurement of Thiol Concentration. Journal of Organic Chemistry 2011, 76 (10), 3853–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YP; Villamena FA; Zweier JL, Highly stable dendritic trityl radicals as oxygen and pH probe. Chem Commun 2008, (36), 4336–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driesschaert B; Levêque P; Gallez B; Marchand-Brynaert J, Tetrathiatriarylmethyl Radicals Conjugated to an RGD-Peptidomimetic. European Journal of Organic Chemistry 2014, 2014 (36), 8077–8084. [Google Scholar]
- 19.Driesschaert B; Levêque P; Gallez B; Marchand-Brynaert J, RGD-conjugated triarylmethyl radical as probe for electron paramagnetic imaging. Tetrahedron Letters 2013, 54 (44), 5924–5926. [Google Scholar]
- 20.Driesschaert B; Bobko AA; Eubank TD; Samouilov A; Khramtsov VV; Zweier JL, Poly-arginine conjugated triarylmethyl radical as intracellular spin label. Bioorganic & Medicinal Chemistry Letters 2016, 26 (7), 1742–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YP; Villamena FA; Sun J; Wang TY; Zweier JL, Esterified trityl radicals as intracellular oxygen probes. Free Radical Bio Med 2009, 46 (7), 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song YG; Liu YP; Liu WB; Villamena FA; Zweier JL, Characterization of the binding of the Finland trityl radical with bovine serum albumin. Rsc Adv 2014, 4 (88), 47649–47656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varshosaz J, Dextran conjugates in drug delivery. Expert Opin Drug Del 2012, 9 (5), 509–523. [DOI] [PubMed] [Google Scholar]
- 24.Pahimanolis N; Vesterinen AH; Rich J; Seppala J, Modification of dextran using click-chemistry approach in aqueous media. Carbohyd Polym 2010, 82 (1), 78–82. [Google Scholar]
- 25.Khramtsov VV, In Vivo Molecular Electron Paramagnetic Resonance-Based Spectroscopy and Imaging of Tumor Microenvironment and Redox Using Functional Paramagnetic Probes. Antioxid Redox Signal 2018, 28 (15), 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishimoto S; Matsumoto KI; Saito K; Enomoto A; Matsumoto S; Mitchell JB; Devasahayam N; Krishna MC, Pulsed Electron Paramagnetic Resonance Imaging: Applications in the Studies of Tumor Physiology. Antioxid Redox Sign 2018, 28 (15), 1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


