Abstract
Background:
Drug overdose deaths have been rising since the early 1990s and is the leading cause of injury death in the United States. Overdose from prescription opioids constitutes a large proportion of this burden. State policy and systems-level interventions have the potential to impact prescription drug misuse and overdose.
Methods:
We searched the literature to identify evaluations of state policy or systems-level interventions using non-comparative, cross-sectional, before-after, time series, cohort, or comparison group designs or randomized/non-randomized trials. Eligible studies examined intervention effects on provider behavior, patient behavior, and health outcomes.
Results:
Overall study quality is low, with a limited number of time-series or experimental designs. Knowledge and prescribing practices were measured more often than health outcomes (e.g., overdoses). Limitations include lack of baseline data and comparison groups, inadequate statistical testing, small sample sizes, self-reported outcomes, and short-term follow-up. Strategies that reduce inappropriate prescribing and use of multiple providers and focus on overdose response, such as prescription drug monitoring programs, insurer strategies, pain clinic legislation, clinical guidelines, and naloxone distribution programs, are promising. Evidence of improved health outcomes, particularly from safe storage and disposal strategies and patient education, is weak.
Conclusions:
While important efforts are underway to affect prescriber and patient behavior, data on state policy and systems-level interventions are limited and inconsistent. Improving the evidence base is a critical need so states, regulatory agencies, and organizations can make informed choices about policies and practices that will improve prescribing and use, while protecting patient health.
Keywords: Prescribing, Opioids, Overdose, Policy, Evaluation, Pain
1. Introduction
In 2011, drug overdose was the leading cause of injury death, reaching epidemic levels in the United States. Among deaths where the drugs involved were specified, three quarters (over 16,000) of prescription drug overdoses involved opioid analgesics (CDC, 2014). While effective in treating cancer pain (Wiffen et al., 2013) and acute pain, such as in the perioperative setting (American Society of Anesthesiologists Task Force on Acute Pain Management, 2012), the evidence that opioids are effective at treating chronic, non-cancer pain safely over time is limited in quantity and quality (Haroutiunian et al., 2012; Noble et al., 2010). There are risks to opioid use including dependence, withdrawal, and overdose (Inturrisi, 2002). Because of their euphoric properties, they are also a candidate for diversion for nonmedical use. Yet, opioids are commonly prescribed: In 2010, an estimated 20% of patients presenting to physician offices in the United States with pain symptoms or diagnoses were prescribed opioids (Daubresse et al., 2013).
More than 125,000 people have died from overdoses involving prescription opioids during 1999–2010, and the number of such deaths per year quadrupled during this time period (CDC, 2011). Interestingly, opioid sales have increased in lock step during this period (CDC, 2011). While prescribing of opioids has increased and prescribing of non-opioid pain medications (e.g., non-steroidal anti-inflammatory drugs; NSAID) has decreased, changes in patient-reported pain severity seem to be insufficient in explaining shifts in prescribing (CDC, 2011; Chang et al., 2014).
Although it is a complicated picture, many overdose deaths can be linked to prescriptions from medical providers. For example, in a study of drug overdose fatalities in North Carolina, nearly half filled a prescription for at least one of the drugs that contributed to their death within 60 days of dying (Hirsch et al., 2014). In a study of opioid analgesic overdoses in an employer-sponsored insurance claims database, one-quarter of nonfatal overdoses were daily users with a prescription, 43.5% were other (intermittent) users with a prescription, and 31% used the opioid without a prescription (Paulozzi et al., 2014).
Several factors increase risk for drug overdose at the individual, community, and systems level. Individuals at higher risk include men; 35–54 year olds; whites and American Indians/Alaskan Natives; individuals at lower incomes; patients with mental health conditions; and patients receiving a high daily dose, prescriptions from multiple prescribers/pharmacies, and opioids combined with benzodiazepines. At the community level, those living in rural areas and communities with higher levels of use of prescription drugs prone to abuse are at higher risk (Paulozzi, 2012). Factors at the systems level include payer (with Medicaid incurring a higher rate of opioid prescriptions and adverse events such as ED visits and neonatal abstinence syndrome compared to other payers; Creanga et al., 2012; Raofi and Schappert, 2006) and prescriber volume (with those at high prescribing rates accounting for a greater proportion of patient deaths; Dhalla et al., 2011).
States operate the major levers that control access to drugs through prescription origination points (such as physician practices, emergency departments, hospitals, and pharmacies), payment and reimbursement (such as through insurers and pharmacy benefit managers), and public education (such as through campaigns and community initiatives). Innovative state policy and systems-level preventive interventions have been proposed to address the problem of opioid analgesic overdose at a population level. Table 1 summarizes these interventions and explains the state role. We sought to understand the evidence available on the effectiveness of such interventions on intermediate outcomes, such as provider and patient behavior, as well as health outcomes, such as fatal and nonfatal overdose. Previous reviews have investigated specific interventions (e.g., PDMPs), but none have integrated the strategies within one comprehensive, broad-scoped review across multiple strategies—a unique focus of the current paper.
Table 1.
State policy and systems-level prevention toolbox.
| Intervention | State role in implementation | Description |
|---|---|---|
| Prescription drug monitoring programs | Operated by state health departments; state law enforcement agencies; state boards of pharmacy |
Programs that require state pharmacies to submit all information on prescriptions filled for controlled substances electronically to a central office, such as the health department or board of pharmacy;information is provided to prescribers about patients using multiple prescribers or pharmacies, and in some cases to law enforcement about aberrant prescribing |
| Insurer and pharmacy benefit manager strategies | Implemented by state Medicaid programs and pharmacy benefit managers that assist state Medicaid |
Patient review and restriction programs that require patients suspected of misusing controlled substances to use a single prescriber/pharmacy; drug utilization review programs that review claims data to identify problematic use and notify prescribers; prior authorization and medication quantity limits |
| State legislation | Developed by the state legislature with education and information supplied by state health departments and law enforcement, among others | Pain clinic regulation that limits clinic ownership, prescribing, and dispensing combined with mandated registration and inspection; good samaritan laws that provide immunity from prosecution for possessing a controlled substance while seeking help for himself or another person experiencing an overdose; doctor shopping laws that prohibit patients from withholding information from providers about receipt of controlled substances from other providers |
| Clinical guidelines | Developed by state health departments in collaboration with other stakeholders for providers and health systems within the state | Guidance documents that provide recommendations to providers about clinical practice; focus on opioid prescribing; recommendations vary but typically include dose limits, medications and formulations, initiation and titration of dose, drug switching, drug interactions, screening tools, written treatment agreements, and urine drug testing |
| Naloxone distribution | Supported by state health departments and distributed by funded community organizations | Programs that provide naloxone and other opioid overdose prevention services to individuals who use drugs, their families and friends, and service providers; include education about overdose risk factors, signs of overdose, appropriate response, and administration of naloxone |
| Safe storage and disposal | Supported by state health departments and law enforcement agencies in collaboration with local stakeholders | Programs that inform the general public about safe storage and disposal of prescription drugs; collection of drugs by officials at permanent return programs or one-day events |
| Education: patients and providers | Supported by state health departments, in collaboration with community organizations, health systems, and schools | Programs that educate patients and providers about prescription opioid use and misuse; patient education ranges from informational materials to intensive family and school-based prevention; provider education focuses on opioid prescribing and includes tools, workshops, lectures, case discussions, consultant support, and continuing education credit |
2. Material and methods
2.1. Data sources and searches
With the assistance of a librarian, MEDLINE was searched for research articles evaluating on state policy and systems-level interventions published from 1946 to 2014 with search terms including, but not limited to, “drug overdose”, “analgesics/opioid”, “health education”, “patient education”, “organizational policy”, “prescription”, “monitoring”, “guideline”, “legislation”, “insurer”, “formulary”, and “drug utilization review”, resulting in over 500 citations. Additional articles were identified through searches of the references of retrieved articles, as well as relevant federal and organizational websites.
2.2. Selection criteria
Article abstracts were reviewed for relevance. Articles were selected for the review if they were written in English and evaluated a state or system policy or practice using a non-comparative, cross-sectional, before-after, time series, cohort, or comparison group study or a randomized/non-randomized trial. Studies were excluded if they were purely descriptive (e.g., characterized practices in a health system) without aiming to evaluate the influence of a state or system-level policy or practice. Eligible studies included the following intermediate and/or distal outcomes: provider behavior (e.g., controlled substance prescribing patterns, dose, guideline-concordant care), patient behavior (e.g., use of multiple providers or pharmacies, number of prescriptions), and health outcomes (e.g., adverse effects, misuse, abuse, non-fatal overdose, death). We prioritized interventions that offer prevention effects at a population level over substance abuse treatment interventions. Although there are effective strategies that focus on underlying substance use disorders and assist in recovery (e.g., expanding access to medication-assisted therapies; Volkow et al., 2014), substance use treatment is part of a larger strategy to address drug overdose and has been reviewed at length in the published literature; as a result, it was determined to be beyond the scope of the current review. We primarily relied on studies that were conducted in the United States (with an exception for Canada) given the variation in state infrastructure and health systems across countries.
2.3. Data extraction and synthesis
Categories of state policy and systems-level interventions were identified through the literature search: prescription drug monitoring programs (PDMPs), insurer and pharmacy benefit manager strategies, state legislation, clinical guidelines, naloxone distribution programs, safe storage and disposal strategies, and patient/provider education (see Table 1). These interventions are broad and represent primary, secondary, and tertiary prevention approaches. For example, patient education interventions can be seen to represent primary prevention, aiming to teach about the dangers of opioid misuse. Clinical guidelines can represent secondary prevention when they aim to change provider behavior to mitigate potential harm for patients at risk for opioid misuse. Naloxone distribution programs represent tertiary prevention, aiming to reduce risk of death among those misusing prescription opioids.
Intervention evidence tables were constructed with effects categorized by provider behavior, patient behavior, and health outcome. For each outcome, the study designs, number of studies, and key outcomes were compiled. Only outcomes relevant to the purpose of this review were included. For some studies, particularly studies employing descriptive epidemiology or before/after designs, statistical testing was not conducted. To provide a thorough review, outcomes were included in evidence tables when statistical testing was employed and when change was noted but no tests of significance were performed. Statistical testing is noted in the tables. Given the variation in interventions, study designs, and outcomes assessed, it was not practicable to synthesize the results through systematic analytic methods (e.g., meta-analysis) for any of the interventions evaluated. Hence, narrative reviews were constructed for each intervention, noting intervention components and key outcomes and summarizing process outcomes when feasible (e.g., implementation).
Quality of evidence judgments were made for each outcome type (provider behavior, patient behavior, health outcomes) for each intervention, inspired by the GRADE approach (see Balshem et al., 2011 for more details). This validated approach weighs the quality of the evidence across studies from systematic reviews, typically in the context of making recommendations for practice. Observational studies (e.g., before-after; time series) are initially assigned a rating of low evidence quality, while randomized controlled trials are initially assigned a rating of high evidence quality. Ratings are modified downward based on study limitations, imprecision, inconsistency of results, indirectness of evidence, and publication bias; ratings are modified upward based on large magnitude of effect, dose response, and when confounders likely minimize the effect. Final ratings possible for each outcome are high, moderate, low, or very low, considering the set of the studies that address the outcome. For example, a set of observational studies with a high risk of bias (e.g., no adjustment for potential confounders) and inconsistent findings would result in an evidence rating of very low. A set of studies including one or two RCTs with study limitations that indirectly assess the outcome of interest mixed with a large number of observational studies with inconsistent results would result in an evidence rating of low. When quality of evidence is high, there is confidence that the true effect lies close to the estimate of the effect. When the quality of evidence is low, the confidence in the effect is limited and further research is likely to have an impact on our confidence in the estimate. Given that the overwhelming majority of studies were observational and a limited number were RCTs, summary outcome tables were visually inspected by the authors to assign evidence ratings.
3. Results
Fig. 1 illustrates the number of studies reviewed by type of intervention, and the type of outcomes measured in the studies. There was substantial variation in the number of studies by intervention, with a greater number of studies found for PDMPs, naloxone education and distribution programs, and clinical guidelines than for insurer strategies, state legislation, safe storage and disposal, and provider/patient education. There also were large differences in the types of outcomes studied, with health outcomes being examined more often for naloxone distribution programs than for the other interventions.
Fig. 1.
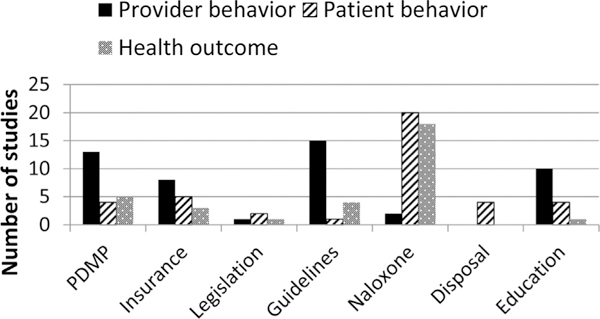
Number of studies by strategy type and outcomes measured.
3.1. Prescription drug monitoring programs
Background:
As of August, 2014, 49 states, the District of Columbia, and one U.S. territory (Guam) had statutes authorizing the creation of a PDMP, and 48 states and Guam had an operational PDMP. Missouri did not have a PDMP, and the PDMPs in New Hampshire and DC were not yet operational. The first PDMP began in California in the 1940s, but widespread adoption did not occur until the first decade of the 21st century. First-generation states (California, New York, and Texas) paired their PDMPs with requirements for use of special serialized triplicate prescription forms, a practice now largely abandoned. PDMPs now require state pharmacies to submit all the information on prescriptions filled for controlled substances electronically to a central office such as the health department or the board of pharmacy (Brandeis University Prescription Drug Monitoring Program Training and Technical Assistance Center, 2014a,b). All PDMPs other than Pennsylvania’s monitor controlled substance schedules II-IV, and most monitor schedules II-V. Providers can proactively search PDMP data to determine if their patients are using multiple prescribers and/or pharmacies for these drugs. Some PDMPs report data on aberrant prescribing proactively to law enforcement or health care licensure boards. Some states require prescribers and dispensers to register with the PDMP, and a small but growing number now require prescribers to check the PDMP before prescribing. Efforts are underway to incorporate PDMP data into electronic health records.
Findings:
Evaluations have focused on the prescribing of opioid analgesics, benzodiazepines, or both. Outcomes have included population-based prescribing rates for these drug classes, problematic prescribing (e.g., pill mills), or problematic use by patients (e.g., use of multiple prescribers or pharmacies). Less commonly, studies have evaluated health outcomes related to abuse of controlled prescription drugs such as fatal or nonfatal overdoses. Three studies also used state rates of substance abuse treatment admissions as an additional outcome (Reifler et al., 2012; Reisman et al., 2009; Simeone and Holland, 2006; see Table 2).
Table 2.
Prescription drug monitoring programs.
| Type outcomes | Study design | Number of studies |
Findings |
|---|---|---|---|
|
Provider behavior *Low |
Descriptive/before-after | 3 | Lower use of CSII drugs (Curtis et al., 2006; Simoni-Wastila and Qian, 2012; Wastila and Bishop, 1996)SS |
| Greateruse of CSIII drugs (Simoni-Wastila and Qian, 2012; Wastila and Bishop, 1996)SS | |||
| Time series | 10 |
Decrease in CSII opioid prescribing (Reisman et al., 2009; Simeone and Holland, 2006)SS (Sigleret al., 1984)NT Increase in CSIII prescribing (Sigler et al., 1984)NT |
|
| Decrease in benzodiazepine prescribing (Pearson et al., 2006; Weintraub et al., 1991; | |||
| Wolfe and Lurie, 1992)NT, (Ross-Degnan et al., 2004)SS | |||
| Decrease in “inappropriate” opioid use (Dormuth et al., 2012)SS | |||
| Decrease in “problematic” benzodiazepine use (Ross-Degnan et al., 2004)SS | |||
| No change in CSII-IV opioid prescribing (Brady et al., 2014; Paulozzi et al., 2011)NS | |||
| RCT | 0 | ||
| Patient behavior | Descriptive/before-after | 0 | |
| *Low | Time series | 4 | Decrease in benzodiazepine use by drug diverters (Wolfe and Lurie, 1992)NT |
| Decrease in use of multiple pharmacies (Ross-Degnan et al., 2004)SS | |||
| Decrease in use of multiple prescribers (Pearson et al., 2006)NT, (Dormuth et al., 2012)SS | |||
| RCT | 0 | ||
| Health outcomes | Descriptive/before-after | 0 | |
| *Low | Time series | 5 | Decrease in ED visits for benzodiazepines (Wolfe and Lurie, 1992)NT |
| Decrease in substance abuse treatment admissions (Simeone and Holland, 2006)SS, | |||
| (Reifler et al., 2012; Reisman et al., 2009)NS | |||
| No change in drug overdose mortality (Paulozzi et al., 2011)NS | |||
| Decrease in oxycodone poison center report rates (Reifler et al., 2012)SS | |||
| RCT | 0 | ||
Note:
= Tested and statistically significant.
= Tested and not statistically significant.
= No statistical testing conducted.
= Evidence level.
Evaluation studies during the 1980s largely focused on the New York PDMP and its addition of benzodiazepines to the program in 1989. Those studies found dramatic declines (20–80%) in use and problematic use of benzodiazepines with this addition (Pearson et al., 2006; Ross-Degnan et al., 2004; Weintraub et al., 1991; Wolfe and Lurie, 1992). One study (Wastila and Bishop, 1996) examined the CA, TX, and NY PDMPs that used triplicate forms and found lower Schedule II prescribing, higher Schedule III prescribing, and overall lower use of any prescribed analgesics in those states, although part of this finding may be attributable to the fact that the PDMPs only tracked schedule II drugs at that point in time. Studies published after 2000, which focused on opioid analgesics, confirmed lower Schedule II rates in PDMP states in general (Curtis et al., 2006; Reisman et al., 2009; Simeone and Holland, 2006). Lower schedule II prescribing rates have been shown to be offset by higher Schedule III prescribing in other studies (Paulozzi et al., 2011; Simoni-Wastila and Qian, 2012). Again, results might have differed if PDMPs in all states had tracked Schedule II and III during the study periods. The most recent study found no significant overall difference in opioid prescribing (Brady et al., 2014). One study found no reduction of overdose mortality in PDMP states (Paulozzi et al., 2011) while another found a slower rate of increase in oxycodone overdoses in PDMP states (Reifler et al., 2012).
Overall, the earliest evaluation studies of PDMPs were unable to disentangle the use of special forms from the use of PDMPs, while later studies, using data through 2008 in one case, have not clearly established significant effects on total opioid prescribing or health outcomes with PDMPs. The largest limitation is the lack of detailed data on prescribing volume and patterns prior to PMDP implementation, which forced the use of cross-sectional, observational study designs. The effect sizes in the most recent studies have been small, making it conceivable that the differences are due to unaddressed confounding variables. There is yet little data to settle the question of whether specific actions of PDMPS (e.g., proactive reporting) add to their effectiveness. However, recent adoption of mandates for prescriber use of PDMP data could demonstrate substantial positive effects of PDMPs, including increased registration and use, and subsequent decreases in prescribing of controlled substances (Brandeis University Prescription Drug Monitoring Program Training and Technical Assistance Center, 2014a,b).
3.2. Insurer and pharmacy benefit manager strategies
Background:
Insurers (e.g., Medicaid, private insurance offered through employers) and pharmacy benefit managers (PBMs; groups that process prescriptions for insurers) have access to detailed medical and pharmacy claims data and therefore are a good source for identifying inappropriate prescribing by providers and prescription drug abuse by patients (Katz et al., 2013; Sacciccio, 2011). Patient Review and Restriction (PRR) programs (also called “Lock-In” Programs), Drug Utilization Review (DUR) programs, Prior Authorization (PA), and medication Quantity Limits (QL) may be potential levers to change provider and patient behavior (CDC, 2013; Academy of Managed Care Pharmacy et al., 2010). PRRs require patients suspected of misusing controlled substances to use a single prescriber and/or pharmacy to obtain controlled substance prescriptions. DUR programs include review of claims data retrospectively to identify problematic use and notify providers about such use. Prior authorization requires review of medical justifications before drugs are covered by the insurer. Medication quantity limits are used to limit the amount of drug that can be dispensed within a given time frame.
Findings:
The limited studies on the effectiveness of insurer and PBM strategies have examined cost savings and changes in utilizations; few have evaluated impact on health outcomes (See Table 3). A total of eight PRR evaluations were identified with the earliest studies beginning in the 1970s and the most recent in 2012. Four reports contain only information on cost savings (Chinn, 1985; Colburn et al., 2008; Medicaid, 2005; Singleton, 1977). An evaluation of Louisiana’s PRR found reductions in polypharmacy (use of multiple medications), use of Schedule II narcotics, and pharmaceutical costs after enrollment in the PRR (Blake, 1999). Ohio’s Medicaid PRR reported monthly dosage reductions of 40.8% for narcotic analgesics and 36.3% for sedatives after patients enrolled in the PRR (Tanenbaum and Dyer, 1990). A 2009 evaluation found decreased use of narcotic medications, multiple pharmacies and physicians, and emergency department visits among patients in Oklahoma’s Medicaid PRR (Mitchell, 2009). Among patients in Washington’s PRR in 2006, the average number of narcotic prescriptions decreased from 3.07 to 1.63 and total morphine milligram equivalent (MME) doses decreased from 312 MME/day to 185 MME/day following enrollment. A follow-up analysis found, after one year, significant reductions in hospital costs, ED visits for injuries from any cause, physician visits and costs, and narcotic prescriptions among PRR patients. No differences in mortality were seen between the PRR and comparison groups (CDC, 2013).
Table 3.
Insurer and pharmacy benefit manager strategies.
| Type outcomes | Study design | Number of studies | Findings |
|---|---|---|---|
|
Provider behavior *Low |
Descriptive/before-after | 4 |
Decrease in mean number of CII-CV prescription drug claims PMPM (Hoffman et al., 2003)SS Decrease in mean number of prescribers per patient (Hoffman et al., 2003)SS No change in mean numberoftotal prescriptions PMPM (Hoffman et al., 2003)NS Decrease in long-acting opioid prescriptions and opioid duplication (Oregon State University, 2012)NT Decrease in percent of patients taking >100 morphine milligram equivalents of methadone (Oregon State University, 2012)NT Decrease in sedative/hypnotic prescriptions and quantities per prescription (Oregon State University, 2004b)NT Decrease in carisoprodol prescriptions, quantities per prescription, and average daily dose (Oregon State University, 2004a)NT |
| Time series | 3 |
Decrease in rates of poly-pharmacy events for opioids and benzodiazepines (Zarowitz et al., 2005)SS Decrease in mean controlled substance score driven by reduced number of controlled substance prescriptions and reduced numbers of prescribers and pharmacies utilized (Daubresse et al., 2014)SS Decrease in controlled-release oxycodone use in states with strict prior authorization criteria (Morden et al., 2008)SS No change in controlled-release oxycodone use in states with lenient prior authorization criteria (Morden et al., 2008)SS |
|
| RCT | 1 | Greater reduction in number of prescribers, dispensing pharmacies, and filled opioid prescriptions compared to control group (Gonzalez and Kolbasovsky, 2012)SS | |
|
Patient behavior *Low |
Descriptive/before-after | 2 |
Decrease in provider visits, number of opioid prescriptions, and mean daily morphine milligram equivalent dose (CDC, 2013)SS Decrease in opioid and sedative use (Tanenbaum and Dyer, 1990)SS |
| Time series | 3 |
Decrease in rates of poly-pharmacy events for opioids and benzodiazepines (Zarowitz et al., 2005)SS Decrease in numberof prescriberand pharmacies (Blake, 1999; Mitchell, 2009)SS Decrease in poly-pharmacy and CII opioid prescriptions (Blake, 1999)SS Decrease in number of opioid prescriptions (Mitchell, 2009)SS |
|
| RCT | 0 | ||
| Health outcomes *Low | Descriptive/before-after | 2 |
No change in rate ofED services, hospitalizations, or office visits among carisoprodol patients (Oregon State University, 2004a)NT Decrease in ED visits for injuries from any cause (CDC, 2013)SS No change in mortality (CDC, 2013)NS |
| Time series RCT |
1 0 |
Decrease in ED visits (Mitchell, 2009)SS | |
Note:
= Tested and statistically significant
= Tested and not statistically significant
= No statistical testing conducted
= Evidence level.
Four studies published between 2003 and 2013 evaluated DUR programs. A randomized trial evaluating the impact of proactive alerts sent to providers on patients receiving opioid prescriptions from ≥3 prescribers at ≥3 pharmacies in a 3-month period found that patients in the intervention group had a 24% reduction in number of prescribers, 16% reduction in number of dispensing pharmacies, and 15% reduction in filled opioid prescriptions over the one-year evaluation period compared to the control group (Gonzalez and Kolbasovsky, 2012). Daubresse et al. (2014) reported a significant decline in mean controlled substance score-a measure of controlled substance abuse risk-among patients whose providers were sent a letter describing the patients’ controlled substance history compared to patients whose providers were not sent letters. Hoffman et al. and Zarowitz et al. also reported reduced drug utilization after DUR program intervention (Hoffman et al., 2003; Zarowitz et al., 2005). None of these studies examined changes in health outcomes.
Four evaluations of PA and/or QL programs were identified, published between 2004 and 2012. A 2008 study examined the impact of PA on controlled-release oxycodone use by Medicaid enrollees in 49 states and the District of Columbia. Twenty-one states implemented a PA for controlled-release oxycodone during the study period. States with more strict PA criteria experienced a significant 34% decrease in controlled-release oxycodone use, while states with more lenient PAs experienced a nonsignificant increase of 6% (Morden et al., 2008). Oregon Medicaid’s long-acting opioid PA and methadone dose limit programs reported a 32% reduction in use of long-acting opioids after the first year of the program, and the percent of patients taking ≥100 MME per day of methadone decreased from 29% to 9% (Oregon State University, 2012). Oregon Medicaid also implemented QL/PA programs for non-opioid drugs of abuse—carisoprodol and sedative/hypnotics. The carisoprodol QL/PA resulted in a decrease in the rate of prescriptions per 1000 members from 7.07 to 2.03; average daily dose from 1110 mg to 956 mg; and average number of tablets per prescription from 63 to 40 after program implementation. No significant increase or decrease in the rate of ED visits, hospitalizations, or office visits was observed among carisoprodol users after program implementation (Oregon State University, 2004a). The sedative/hypnotic QL/PA program was less robust. Minimal impact on utilization likely resulted from generous “grandfathering” for patients previously prescribed these medications (Oregon State University, 2004b).
Overall, the quality of evidence is low for the impact of insurer and PBM strategies on prescription drug abuse and overdose because of the lack of comparison groups in most studies, short-term follow-up, inadequate statistical testing in several studies, unassessed health outcomes, and other events occurring simultaneously that could be responsible for effects. Despite this limited evidence base, insurer and pharmacy benefit manager strategies do show promise for changing certain prescribing and use behaviors linked to prescription drug abuse and overdose.
3.3. State legislation
Background:
Policies such as pain clinic regulation (“pill mill” laws), legislation that limits the use of multiple providers (“doctor shopping” laws), and laws that provide immunity from prosecution (“Good Samaritan” laws) are being considered by states to reduce diversion, abuse, and overdose. Eleven states have a pill mill law (as of January, 2014), 16 states have a specific doctor shopping law (as of August, 2010), and 18 states have a Good Samaritan law, including 9 that have laws that specifically create immunity from prosecution for people who call for help in the event of an overdose (as of September, 2012).
Findings:
Published studies reporting on evaluations of state policy strategies are extremely limited (see Table 4). Informal cts on number of pain clinics and opioid analgesic supply (DeRosier, 2008; Forrester, 2011). In 2010, Florida enacted legislation that limited pain clinic ownership, mandated registration and inspection of pain clinics, placed limits on prescribing with cash transactions, and restricted on-site dispensing of controlled substances; additional components were added to enhance implementation in 2011. A trend analysis revealed a significant decline in diversion for oxycodone, morphine, and methadone, as measured by prescription drug diversion investigations conducted by police departments, sheriff offices, state agencies, and drug task forces (Surratt et al., 2014). Another study showed that opioid analgesic overdose death rates decreased 27% from 2010 to 2012 after enactment of the law (Johnson et al., 2014). Although these findings are promising, several activities were occurring at the same time that could have contributed to changes in diversion and overdose (e.g., PDMP implementation, regional strike forces), making it difficult to identify effects uniquely attributable to the legislation.
Table 4.
State legislation (Pill Mill, Doctor Shopping, Good Samaritan).
| Type outcomes | Study design | Number of studies |
Findings |
|---|---|---|---|
|
Provider behavior *Very low |
Descriptive/before-after | 1 |
Providers willing to report doctor shopping, particularly if immunity was granted (Shaffer and Moss, 2010)NT |
| Time series | 0 | ||
| RCT | 0 | ||
| Patient behavior | Descriptive/before-after | 1 | Users willingto call 911 (Banta-Green et al., 2011)NT |
| *Low | Time series | 1 | Decline in diversion for oxycodone, morphine, and methadone (trends for hydrocodone were not statistically significant) after pill mill legislation enactment (Surratt et al., 2014)SS |
| RCT | 0 | ||
| Health outcomes | Descriptive/before-after | 1 | Decline in overdose death rates (Johnson et al., 2014)NT |
| *Very low | Time series | 0 | |
| RCT | 0 | ||
Note:
= Tested and statistically significant.
= Tested and not statistically significant.
= No statistical testing conducted.
= Evidence level.
There is very little evidence on immunity from prosecution or laws related to use of multiple providers (also known as “doctor shopping” laws). An initial evaluation of Washington’s Good Samaritan law found that drug users in Seattle were more comfortable calling 911 after implementation of the law, but law enforcement had low awareness of the law, and opinions on the law were mixed (Banta-Green et al., 2013, 2011). A study in West Virginia of general practice and emergency medicine physicians related to multiple provider laws found that 37% of the respondents had ever reported a patient to law enforcement, and 22% stated they currently report use of multiple providers. The physicians also reported that they would be more likely to report such behavior if they were granted immunity from reporting (Shaffer and Moss, 2010).
Overall the quality of evidence for the impact of state legislation on provider behavior, patient behavior, and health outcomes is low. Evaluation data are only available from three states, multiple efforts were in place at the time legislation was enacted, and causal conclusions about the impact of specific strategies are limited.
3.4. Clinical Guidelines
Background:
National medical organizations issue clinical practice guidelines to improve use ofevidence-based strategies and quality of care (e.g., the American Pain Society and the American Academy of Pain Medicine joint guidelines on the use of chronic opioid therapy in chronic noncancer pain; Chou et al., 2009). Large health systems (e.g., Veteran’s Administration/Department of Defense), health maintenance organizations, hospitals, and now states have followed suit in recommending ways to mitigate the risk of opioid therapy. Recommendations vary, but typically include dose limits, medications and formulations, initiation and titration of dose, drug switching, drug-interactions, screening tools to assess risk for misuse, written treatment agreements, and urine drug testing (Nuckols et al., 2014). Implementation strategies differ across states and systems, ranging from limited information dissemination efforts to intensive academic detailing, quality improvement, and enforcement through state regulation.
Findings:
Limited evaluations have assessed both process and outcome measures, employing a range of study designs: non-comparative descriptive epidemiological, before-after, and time-series designs, as well as randomized trials (see Table 5). Descriptive epidemiological studies of adherence to state, university clinic system, and VA guidelines illustrate moderate knowledge of recommendations and low level of provider adoption, particularly the use of assessment tools, written treatment agreements, and drug testing; However, some studies report that smaller percentages of patients are managed with high dose opioids; higher percentages of providers report avoiding long-acting opioids for acute pain or in combination with benzodiazepines; and physicians are more likely to use tools like drug screens in patients with substance use disorder, all beneficial findings (Cochella and Bateman, 2011; Krebs et al., 2011; Morasco et al., 2011; Morse et al., 2012; Porucznik et al., 2013; Sekhon et al., 2013; Victor et al., 2009). Findings from before-after studies of state, emergency department, and hospital guidelines are promising, and show declines in number and rate of opioid prescribing, lower average daily doses, and decreases in ED visits and deaths (Cochella and Bateman, 2011; Fox et al., 2013; Franklin et al., 2013,b; Gordon et al., 2000; Humphries et al., 1997). Yet, given the methodological limitations of these studies, conclusions are uncertain. The most rigorous evaluations of the Washington State Opioid Dosing Guideline using time-series designs with a workers compensation population illustrated significant declines in the proportion of incident users who became chronic users and who received a dosage of >120 mg MED/day; however no significant changes were detected in opioid poisonings or adverse effects. Two randomized trials have investigated the use of training and education approaches in enhancing guideline adoption, revealing mixed effects: Although enhanced education approaches may lead to improvements in provider reports of recommendation knowledge and use, this does not necessarily translate to changes in guideline-concordant care (Corson et al., 2011; McCracken et al., 2012).
Table 5.
Clinical guidelines.
| Type outcomes | Study design | Number of studies |
Findings |
|---|---|---|---|
|
Provider behavior *Low |
Descriptive/before-after | 12 |
Increase in reading and/or applying guideline (Franklin et al., 2013a)SS
Prescribes higher doses less often (Franklin et al., 2013a)NT Low percentage of providers with pharmacist collaborative drug therapy agreement (Franklin et al., 2013a)NT Decrease in/less opioid prescribing (Fox et al., 2013; Franklin et al., 2013a; Franklin et al., 2013b; Gordon et al., 2000)NT Increase in/higherprevalence of correct dose and frequency (Humphries et al., 1997)SS No differences in dose or long-acting opioid use for at-risk patients (Morasco et al., 2011)NS More likely to prescribe ER formulation for chronic episodes, but still underutilized (Victor et al., 2009)SS Limited actual use of recommended practices (e.g., treatment agreement, assessment of pain, urinetesting) (Cochellaand Bateman, 2011; Krebs et al., 2011; Morse et al., 2012; Porucznik et al., 2013; Sekhon et al., 2013)NT |
| Time series | 1 | Less likely to prescribe high dose among new users (Garg et al., 2013)SS | |
| RCT | 2 |
No changes in prescribing frequency (McCracken et al., 2012)NS
Increase in self-report use of guidelines (McCracken et al., 2012)SS No differences between clinicians in the Assistance with Pain Treatment intervention and clinicians in the treatment as usual group (overall limited use of recommended practices) (Corson et al., 2011)NS |
|
| Patient behavior | Descriptive/before-after | 0 | |
| *Low | Time series | 1 | Lower number of incident users who become chronic and statistically significant reduced likelihood of receiving high dose opioids with incident users (Garg et al., 2013)SS |
| RCT | 0 | ||
|
Health outcomes *Very low |
Descriptive/before-after | 3 |
Decrease in ED visits (Fox et al., 2013)NT Decrease in deaths (Cochella and Bateman, 2011; Franklin et al., 2013b)NT |
| Time series | 1 | No changes in poisonings or opioid adverse effects (Fulton-Kehoe et al., 2013)NS | |
| RCT | 0 | ||
Note:
= Tested and statistically significant.
= Tested and not statistically significant.
= No statistical testing conducted.
= Evidence level.
Overall, the quality of evidence for the impact of clinical guidelines at the state and system level on provider behavior and patient outcomes is low. Study limitations include lack of baseline data and comparison groups, inadequate statistical testing, small sample sizes, self-reported outcomes, short-term follow-up, and other events occurring simultaneously that could be responsible for effects. It is possible that more advanced methods of translating and disseminating guidelines could lead to increases in adoption and implementation; however, more translational research is needed to identify best practices.
3.5. Naloxone distribution programs
Background:
Naloxone has been used for many years by healthcare and emergency medical service providers to reverse the potentially fatal respiratory depression associated with opioid overdoses. Community-based overdose education and naloxone distribution (OEND) programs that provide naloxone and train at-risk individuals and their friends, family-members, or caregivers on overdose prevention and response have been implemented in the US in recent years. At least 188 community-based programs were in existence in the US in 2010 (Wheeler et al., 2012). In addition, some healthcare providers co-prescribe naloxone to patients taking high doses of opioids or to patients who are otherwise at risk for opioid overdose.
Findings:
Evaluations of OEND programs in the US appearing in the 2000s have focused on program implementation; ability to train non-medical personal to recognize and respond to an overdose, including the proper administration of naloxone; and number of individuals trained, number of vials of naloxone distributed, and number of overdose reversals reported by trained individuals (See Table 6). The majority of individuals trained have been people who injected drugs, primarily heroin or other illicit opioids, and their friends or family members. Two reports provide information on naloxone as part of a broader prescription opioid overdose prevention strategy. A single study (Walley et al., 2013b) specifically evaluated changes in overdose mortality over time after OEND program implementation.
Table 6.
Naloxone distribution programs.
Note:
= Tested and statistically significant.
= Tested and not statistically significant.
= No statistical testing conducted.
= Naloxone distribution is one component of the Project Lazarus program. An evaluation to determine the effects of the naloxone component alone has not been conducted.
= Evidence level.
Evaluation settings have primarily been in large urban center syringe exchange or harm reduction programs, methadone programs, or other addiction treatment or detoxification programs. A total of 12 studies provided information on OEND program evaluations in New York City (Galea et al., 2006; Heller and Stancliff, 2007; Piper et al., 2007, 2008), Massachusetts (Doe-Simkins et al., 2009; Walley et al., 2013a), Los Angeles (Wagner et al., 2010), San Francisco (Enteen et al., 2010), Chicago (Maxwell et al., 2006), Rhode Island (Yokell et al., 2011), Pittsburgh (Bennett et al., 2011), and Baltimore (Tobin et al., 2009). The outcomes typically focused on the number of trained individuals and overdose reversals reported, making it difficult to describe the population-level impact of these individual programs. However, a 2010 survey reported that 48 OEND programs in the US had trained and provided naloxone to over 50,000 individuals between 1996 and 2010. Among these programs, over 10,000 opioid overdose reversals were reported during the same time period, likely an underestimate since reporting is voluntary. The programs also reported that nearly 40,000 vials of naloxone had been provided to participants over the past year (Wheeler et al., 2012).
Six additional studies evaluating changes in overdose recognition and response knowledge and/or behaviors as a result of training were identified (Doe-Simkins et al., 2014; Green et al., 2008; Jones et al., 2014; Lankenau et al., 2013; Seal et al., 2005; Sherman et al., 2009). Taken together, with the 12-program evaluation studies, these data demonstrate that people at high-risk for opioid-related overdose (primarily heroin) and their friends or family members can successfully be trained to recognize and respond to an overdose and appropriately administer naloxone to reverse an opioid-related overdose. Importantly, the studies did not find an increase in drug use or high-risk behavior as a result of being provided naloxone.
Two studies describe the Project Lazarus program in North Carolina. The program, created in 2008, includes the co-prescription of naloxone to people at risk for opioid overdose as one component of a broader prescription opioid overdose strategy that included community coalition building and outreach, clinical practice changes, school-based education, surveillance, and evaluation (Albert et al., 2011; Brason et al., 2013). An initial evaluation of Project Lazarus in Wilkes County, North Carolina found significant declines in the unintentional drug overdose death rate from a peak of 46.6 deaths per 100,000 population in 2009 to 29.0 deaths per 100,000 in 2010 and 14.4 deaths per 100,000 in 2011. An evaluation of Project Lazarus that disentangles the impacts of its various components has not been published. Therefore, it is difficult to determine the exact role naloxone played in the reduction of Wilkes County’s unintentional drug overdose deaths.
The most robust evaluation examining changes in health outcomes as a result of OEND program implementation is by Walley et al. (2013b). The authors employed an interrupted time-series analysis to evaluate the impact of Massachusetts’ OEND program on opioid-related overdose deaths and non-fatal opioid overdose related acute care hospital utilization rates from 2002 to 2009. Communities that implemented OEND programs during the study time period trained 2912 individuals, and 327 overdose reversals were reported. In adjusted models, these communities had statistically significantly reduced opioid-related overdose death rates compared to communities that did not implement OEND programs. Acute care hospital utilization did not differ between OEND program communities and those that did not implement one.
Naloxone is a promising strategy with some evidence of effectiveness in reducing opioid overdose mortality rates. However, the data almost exclusively pertain to reversals of overdoses from heroin and not among people using prescription opioids. Overall, the quality of evidence for the impact of naloxone on opioid overdose is low. Study limitations include lack of randomization; lack of generalizability because the data are almost exclusively based on people who inject drugs, primarily heroin; self-reported outcomes; short-term follow-up; significant loss to follow-up; and lack of control for other events occurring simultaneously that could be responsible for effects.
3.6. Safe storage and disposal
Background:
Safe storage and disposal of prescription drugs has been promoted traditionally as a strategy for reducing unintentional poisonings among young children. States, communities, and organizations have recognized more recently that the strategy might reduce access to and misuse of controlled substances by adults without a prescription. States have sponsored public media campaigns that incorporate messaging about safe storage and disposal; communities have sponsored “drug take-back” events to allow for promote safe, convenient, and responsible disposal; and organizations have developed web-based interventions to educate patients.
Findings:
Although such programs are popular, evaluations are extremely limited and employ non-comparative descriptive epidemiological designs or before-after designs with small sample sizes, and information about health outcomes is lacking (see Table 7). For example, the “Use Only as Directed” campaign in Utah targeted adults with TV and radio spots, posters, patient information cards, bookmarks, and a website. This campaign promoted storage of medications in a safe place and disposal of unused or expired medications. In a before-after evaluation of the campaign, 18% of respondents reported disposal of medications because of the media message, and 5% reported disposal of prescription medication at a drop box or collection event (compared to less than 1% prior to the campaign). Respondents were also less likely to take a prescription medication that was not prescribed to them by a physician after the campaign; however it is unclear whether the campaign components related to safe storage and disposal were responsible for this effect (Johnson et al., 2011). In a non-comparative descriptive epidemiological study of a drug take-back event in Tennessee and Virginia, 9% of donated prescription medications were controlled substances. Of these, 32% were hydrocodone combinations, 11% were oxycodone and oxycodone combinations, and 5% were methadone formulations (Gray and Hagemeier, 2012). A similar descriptive study in Hawaii found that 10% of drugs returned during take-back events at a health care expo and at Kaiser Permanente (KP) clinics were controlled substances; overall 6% were narcotic analgesics with the most common substances including hydrocodone/acetaminophen, oxycodone, oxycodone/acetaminophen, and codeine/acetaminophen (Ma et al., 2004). Finally, a before-after study of an outpatient, clinic-based web Script Safety Intervention that shared information with patients about proper handling and disposal of opioid medications illustrated significant increases in knowledge and behavior change. At one-month follow-up, patients showed increased knowledge regarding safe storage and disposal, reported that they were less likely to lend or borrow pills from others, consume more opioids than prescribed, or save unused medications; However, there was no change in saving or using medications for reasons other than those for which they were prescribed (McCauley et al., 2013).
Table 7.
Safe disposal and drug take-back.
| Type outcomes | Study design | Numberof studies |
Findings |
|---|---|---|---|
| Provider behavior | Descriptive/before-after | 0 | |
| * None | Time series | 0 | |
| RCT | 0 | ||
|
Patient behavior *Very low |
Descriptive/before-after | 4 | Statistically significant increase in knowledge regarding safe use of prescription opioids (McCauley et al., 2013)SS |
| Decrease in likelihood to lend or borrow pills from others, consume more than prescribed, or save unused medication (Johnson et al., 2011; McCauley et al., 2013)SS+ | |||
|
No change in saving or using medication for other reasons than prescribed (McCauley et al., 2013)NS |
|||
| Disposal of medications, particularly in drop box/collection (Gray and Hagemeier, 2012; Johnson et al., 2011;Maet al., 2004)NT+ | |||
| Time series | 0 | ||
| RCT | 0 | ||
| Health outcomes | Descriptive/before-after | 0 | |
| *None | Time series | 0 | |
| RCT | 0 | ||
Note:
= Tested and statistically significant.
= Tested and not statistically significant.
= No statistical testing conducted.
= Safe disposal one component of Utah’s Prescription Safety Program. An evaluation to determine the effects of the safe disposal component alone has not been conducted.
= Evidence level.
Overall, the quality of evidence for the impact of safe storage and disposal efforts on prescription drug overdose is extremely low. Only a handful of studies have been reported, and study limitations include lack of baseline data and comparison groups, small sample sizes, self-reported outcomes, short-term follow-up, unassessed health outcomes, and other events occurring simultaneously that could be responsible for effects.
3.7. Patient education and provider education
Background:
Education approaches attempt to change knowledge and attitudes in an effort to motivate behavior change. Patient education has included both primary prevention approaches (educating youth and young adults about the dangers of substance use prior to misuse or abuse) and secondary/tertiary prevention approaches (educating at-risk populations with substance use disorder or engaging in methadone treatment). Strategies range from limited awareness-raising efforts (e.g., leaflets, posters) to intensive family and school-based programs. Provider education has focused on opioid prescribing because the medical school curriculum is often limited and produces providers lacking comprehensive training in pain management (Heavner, 2009). Education approaches encompass a wide spectrum of content delivery modalities, including use of educational tools, workshops, lectures, interactive case discussions, and consultant support. An incentive such as continuing medical education is usually offered for participation and is awarded after completing coursework, attending presentations, and trainings.
Findings:
Published evaluations of education prevention efforts aimed at patients and providers are small in number (see Table 8). A targeted evaluation of opioid intravenous drug users and their knowledge gained from viewing posters and leaflets throughout an addiction treatment center illustrated improvements in knowledge for recognizing overdoses and how to deal with them (Branagan and Grogan, 2006). In a small randomized trial, mothers and daughters completed an online family-based interactive intervention and were assessed for past 30 day drug use, family communication, and skill building to avoid drug use at follow-up. Significant decreases in prescription drug nonmedical use were reported 2 years after the intervention, though the low potential for misuse and overdose in this population should be noted (Fang and Schinke, 2013). Both studies are limited by small sample sizes and difficulty in generalizing results beyond the target population. Spoth et al. (2013) reported on evaluation findings from three large randomized studies of universal, family and school-based drug prevention interventions to decrease risk factors for prescription drug misuse in adolescents. When adolescents participating in the prevention programs were followed into young adult hood, significant reductions were seen in prescription opioid misuse overall and among higher risk subsets, compared to adolescents not in the programs. Although these results are extremely promising, the sample sizes were small, there was an overall low rate of prescription opioid misuse, and it is yet unclear how such findings might generalize to populations broader than those studied.
Table 8.
Patient/public & provider education.
| Type outcomes | Study design | Number of studies |
Findings |
|---|---|---|---|
|
Provider behavior *Low |
Descriptive/before-after | 7 |
Improved providerconfidence or knowledge (Elhwairis and Reznich, 2010)NT
Limited adoption of select safe opioid prescribing practices (Crozier et al., 2010)SS,(Srivastava et al., 2012; Ury et al., 2002; Young et al., 2012)NT Decrease in/lower risky opioid prescribing practices (Gugelmann et al., 2013; Hoffman et al., 2003)SS |
| Time series | 2 |
Little to no change in opioid prescribing practices (Kahan et al., 2013)NS Improved provider knowledge (Lofwall et al., 2011 )SS Change in safe opioid prescribing behavior (Lofwall et al., 2011)SS |
|
| RCT | 1 | Limited adoption of select safe opioid prescribing practices (Corson et al., 2011 )NS | |
|
Patient behavior * Moderate |
Descriptive/before-after | 2 |
Increased awareness of factors contributing to an opioid overdose (Johnson et al., 2011)*SS,(Branagan and Grogan, 2006)NT
Increased knowledge to manage an overdose (Branagan and Grogan, 2006)NT Decrease in providing medication to family/friends (Johnson et al., 2011)+NS Decrease in taking medication not prescribed (Johnson et al., 2011)+SS |
| Time series | 0 | ||
| RCT | 2 |
Lowered substance use intentions (Fang and Schinke, 2013)SS
Decrease in/lowerprescription drug nonmedical use (Fang and Schinke, 2013; Spoth et al., 2013)SS |
|
| Health outcomes | Descriptive/before-after | 0 | |
| *High | Time series | 0 | |
| RCT | 1 | No improvement in pain (Corson et al., 2011)SS | |
Note:
= Tested and statistically significant.
= Tested and not statistically significant.
= No statistical testing conducted.
= Patient education is one component of Utah’s Prescription Safety Program. An evaluation to determine the effects of the patient education component alone has not been conducted.
= Evidence level
For provider education, evaluations of Continuing Medical Education (CME) credit programs suggest a gain in knowledge but limited adoption of select safe opioid practices like assessing patient risk factors, treatment contracts, and referral to treatment when indicated (Crozier et al., 2010; Lofwall et al., 2011). Randomized case-based training among a small sample of Veterans Affairs providers facilitated the adoption of safe opioid prescribing practices, specifically among primary care clinicians, but did not improve patient response to pain treatment (Corson et al., 2011). A small pilot project in a Michigan community hospital targeted internal medicine residents with a pain management course over several weeks complete with examinations (Elhwairis and Reznich, 2010). Case discussions and role-playing activities proved useful in raising confidence in managing chronic pain patients and pain management knowledge. Additionally, cased based teachings to medical residents and ED providers were successful in altering the quantity of opioids prescribed (Ury et al., 2002). HMO drug claim reviews lead to quarterly mailings of flagged patient prescription profiles and suggestions for treatment (Hoffman et al., 2003). Reductions in the number of high-abuse prescription drug claims were seen 6 months following intervention mailings. Changes in physician practices were also suggested following mailings of an opioid guide book (Young et al., 2012). A Canadian opioid prescribing course offering multiple educational approaches did not succeed in changing behavior and had no effect on opioid prescribing up to two years following the intervention (Kahan et al., 2013).
Overall, the quality of evidence for the effect of patient and provider education is moderate to low. Few studies evaluated patient education programs, the studies employed small sample sizes or special populations, and health outcomes (e.g., overdose) were not measured. Evaluations of provider education incorporate small samples and evaluate few provider specialties. Mixed findings have been found, with some changes in adoption of safer prescribing, but less impact on patient outcomes.
4. Discussion
States have a variety of tools they can use with the potential for curbing the prescription drug overdose epidemic, particularly overdose due to opioid analgesics. Over the past several years, as the overdose epidemic has received increased attention, states have made astounding gains in prevention innovation. State and systems-level strategies have much promise for changing opioid prescribing, influencing patient misuse, and reducing nonfatal and fatal overdose from opioid analgesics. Optimistically, evaluations signal that prevention strategies can change provider and patient knowledge, attitudes, and behaviors.
For example, PDMP evaluations have detected some positive changes in prescribing patterns, decreased use of multiple providers and pharmacies, and decreased substance abuse treatment admissions and poison center report rates (although findings are mixed). Insurer strategies including PRR, DUR, PA, and QL have been associated with reduced prescribing, daily dose, and number of pharmacies and physicians utilized. Pain clinic regulation may reduce prescribing and diversion, as well as death rates. When clinical guidelines are implemented, physicians illustrate improved knowledge of prescribing recommendations. Naloxone distribution programs result in overdose reversals. Drug take-back events and campaigns can lead to the donation of controlled substances, and campaigns and clinic-based interventions can result in increased patient knowledge about safe storage and disposal, as well as likelihood of taking medications that are not prescribed and lending/borrowing pills from others. Education of patients can increase knowledge and awareness, and prevention programs that include communication and skill building may reduce non-medical use. Finally, continuing medical education can result in increased provider knowledge.
It is important to recognize, unfortunately, that there is much we do not yet know about the impact of these strategies. Findings are mixed, changes in health outcomes are detected less consistently, and there are open questions about how the strategies can be best implemented. For example, findings for the effects of PDMPs on prescribing and overdose mortality differ across studies, and there is no evidence for reductions in mortality for insurance strategies, drug take-back events and campaigns, or patient or provider education. Only a single study has looked at changes in mortality over time after implementation of naloxone distribution programs, and most of the reversals were among patients using heroin, limiting our understanding of applicability to prescription opioid abuse. Multiple efforts operating within states that occur in concert with legislation changes have limited the ability to draw causal conclusions about individual state policy effectiveness. In addition, although clinical guidelines can set a standard for practice, recommendation compliance could be improved, and it is not yet known the degree to which high quality implementation could lead to decreases in overdose.
Thus, overall the quality of evidence for the effectiveness of the reviewed strategies is low. Our confidence in the effects is limited, the true effects may be different, and further research is likely to have an important impact in our confidence in the estimate of the effects. Few rigorous evaluations have been published in the empirical literature. Although there are a handful of time-series analyses, published evaluations include primarily descriptive epidemiology, pretest-posttest observational studies, and do not appropriately account for confounding variables and events occurring simultaneously with the interventions that could influence the outcomes of interest. Randomized controlled studies have provided indirect evidence about overdose (e.g., compare one intervention to another, rather than a true control, and measure proximal outcomes). Study limitations include lack of baseline data and comparison groups, inadequate statistical testing, small sample sizes, self-reported outcomes, and short-term follow-up. Common outcomes studied include knowledge, attitudes and prescribing practices of providers, and problematic use by patients; rarely, studies have evaluated health outcomes related to misuse and abuse, and fatal or nonfatal overdoses (see Fig. 1). A further challenge is the great heterogeneity in the structure, content, and focus of the policies and practices, even within the categories reviewed; hence, it is difficult to understand how state policy and systems level interventions are most effectively and efficiently structured.
The limitations of evaluations are not surprising-state policy and systems level interventions are difficult to evaluate. Randomization is rarely feasible, appropriate comparison groups are hard to identify, pre-intervention data can be challenging to obtain, and changes in the environment that are concurrent with intervention implementation are hard to measure. There are limitations in the availability and timeliness of data to allow for rigorous, real-time evaluation; it is possible that enhanced adoption of electronic health records could lead to more feasible evaluation protocols. Although states and systems have been leaders in innovation, professionals struggle to publish evaluation findings in the scientific literature due to capacity limitations (e.g., limited evaluation skills, competing priorities, funding, and time; and data quality, time lag, and availability).
Acknowledging the challenges, improvements in research and evaluation could strengthen the evidence base and provide states and organizations information they need to improve public health. Improvements in research would include the use of rigorous designs, including natural experiments, quasi-experimental designs with comparison groups, and time-series analyses. For example, an educational intervention for clinicians, such as one based on clinical guidelines, could be studied within a large randomized trial: one group of providers within a health system could be randomized to continuing education, academic detailing, and quality improvement activities, and compared with another group of providers that continue with traditional practice; patient outcomes could be measured through the electronic health record in the time periods before, during, and after intervention implementation. It is important to measure not only proximal outcomes (e.g., implementation, prescribing changes) but also distal health outcomes including nonfatal and fatal overdose, as well as unintended consequences (e.g., reduced access to pain treatment). Economic evaluation can estimate the costs and benefits of interventions. Very little information is available to inform states about the cost of implementing the reviewed interventions, as well as on return on investment. The limited information available on implementation costs (e.g., PDMP implementation; Maryland Advisory Council on Prescription Drug Monitoring, 2009) illustrates wide variation based on program requirements and structure. As we learn more about the costs, impacts, and return on investment of different approaches, it will become more important to understand variations in findings, and the drivers behind these variations.
In the meantime, action must be taken to reverse the continued increases in morbidity and mortality, placing priority on promising strategies that show the potential for reducing inappropriate prescribing and patient visits to multiple providers, and improving overdose outcomes including prescription drug monitoring programs, insurer strategies, state legislation providing oversight of pain clinics, clinical guidelines, and naloxone distribution programs. States and systems are encouraged to act on strong evidence, consider promising strategies, and evaluate innovations to build knowledge where it is needed and make better decisions.
Acknowledgments
We would like to thank Bill Thomas from the CDC Public Health Library and Information Center for assistance with the literature search and Noah Aleshire and Akshara Menon for their assistance in identifying state legislation. The conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Food and Drug Administration.
Role of funding source
No funding was provided for this work beyond salary support.
Footnotes
Conflict of interest
No conflict declared.
References
- Academy of Managed Care PharmacyShoemaker, S., Pozniak A, Subramanian R, Mauch D, 2010. Effect of six managed care pharmacy tools: A review of the literature. J. Manag. Care Pharm. 16 (6 Suppl), S3–S20. [PubMed] [Google Scholar]
- Albert S, Brason F, Sanford C, Dasgupta N, Graham J, Lovette B, 2011. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain Med. 12, S77–S85. [DOI] [PubMed] [Google Scholar]
- American Society of Anesthesiologists Task Force on Acute Pain Management, 2012. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American society of anesthesiologists task force on acute pain management. Anesthesiology 116, 248–273. [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schunemann H, Oxman A, Kunz R, Brozek J, Vist G, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt G, 2011. GRADE guidelines: 3. Rating the quality of evidence.J. Clin. Epidemiol. 64, 401–406. [DOI] [PubMed] [Google Scholar]
- Banta-Green C, Beletsky L, Schoeppe J, Coffin P, Kuszler P, 2013. Police officers’ and paramedics’ experiences with overdose and their knowledge and opinions of Washington state’s drug overdose-naloxone-good samaritan law. J. Urban Health 90, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta-Green C, Kuszler P, Coffin P, Schoeppe J, 2011. Washington’s 911 Good Samaritan Drug Overdose Law—Initial Evaluation Results Alcohol And Drug Abuse Institute University OfWashington, Seattle, WA. [Google Scholar]
- Bennett A, Bell A, Tomedi L, Hulsey E, Kral A, 2011. Characteristics of an over-dose prevention, response, and naloxone distribution program in Pittsburgh and Allegheny County, Pennsylvania. J. Urban Health 88, 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake S, 1999. The effect of the Louisiana medicaid lock-in on prescription drug utilization and expenditure In: Drug Benefit Trends. CDC, Atlanta. [Google Scholar]
- Brady J, Wunsch H, DiMaggio C, Lang B, Giglio J, Li G, 2014. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep. 129, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branagan O, Grogan L, 2006. Providing health education on accidental drug over-dose. Nurs. Times 102, 32–33. [PubMed] [Google Scholar]
- Brandeis University Prescription Drug Monitoring Program Training and Technical Assistance Center, 2014a. Mandating PDMP Participation By Medical Providers: Current Status And Experience In Selected States. Brandeis University, Waltham, MA. [Google Scholar]
- Brandeis University Prescription Drug Monitoring Program Training and Technical Assistance Center, 2014b. Prescription Drug Monitoring Frequently Asked Questions. 〈http://www.pdmpassist.org/content/prescription-drug-monitoring-frequently-asked-questions-faq〉 (accessed 14.05.14).
- Brason F, Roe C, Dasgupta N, 2013. Project Lazarus: an innovative community response to prescription drug overdose. N.C. Med.J. 74, 259–261. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2011Vital signs: overdoses of prescription opioid pain relievers-United States, 1999–2008. MMWR 60, 1487–1492. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2013. Patient Review and Restriction Programs: Lessons Learned From State Medicaid Programs. CDC, Atlanta, GA. [Google Scholar]
- Centers for Disease Control and Prevention, 2014. CDC WONDER (Wide-Ranging Online Data for Epidemiologic Research). 〈http://wonder.cdc.gov/〉 (accessed 08.07.14).
- Chang H, Daubresse M, Kruszewski S, Alexander G, 2014. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am.J. Emerg. Med. 32, 421–431. [DOI] [PubMed] [Google Scholar]
- Chinn F, 1985. Medicaid recipient lock-in program: Hawaii’s experience in sixyears. Hawaii Med.J. 44, 9–18. [PubMed] [Google Scholar]
- Chou R, Fanciullo G, Fine P, Adler J, Ballantyne J, Davies P, Donovan M, Fish-bain D, Foley K, Fudin J, Gilson A, Kelter A, Mauskop j., O’Connor P, Passik S, Pasternak G, Portenoy R, Rich B, Roberts R, Miaskoski KTC, 2009. Opioid treatment guidelines: clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain.J. Pain 10,113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochella S, Bateman K, 2011. Provider detailing: an intervention to decrease prescription opioid deaths in Utah. Pain Med. 12, S73–S76. [DOI] [PubMed] [Google Scholar]
- Colburn D, Coady J, Ellis A, Griffin H, Tripp M, 2008. Medicaid integrity report: Iowa Comprehensive Program Integrity Review Final Report. Available from: 〈https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/FraudAbuseforProfs/downloads/iacompfy08pireviewfinalreport.pdf〉.
- Corson K, Doak M, Denneson L, Crutchfield M, Soleck G, Dickinson K, Gerrity M, Dobscha S, 2011. Primary care clinician adherence to guidelines for the management of chronic musculoskeletal pain: results from the study of the effectiveness of a collaborative approach to pain. Pain Med. 12, 1490–1501. [DOI] [PubMed] [Google Scholar]
- Creanga A, Sabel J, Ko J, Wasserman C, Shapiro-Mendoza C, Taylor P, Barfield W, Cawthon L, Paulozzi L, 2012. Maternal drug use and its effect on neonates: a population-based study in Washington state. Obstet. Gynecol. 119, 924–933. [DOI] [PubMed] [Google Scholar]
- Crozier M, McMillan S, Hudson S, Jones S, 2010. The eastern North Carolina opioid prescribers project: a model continuing medical education workshop. J. Opioid Manag. 6, 359–364. [DOI] [PubMed] [Google Scholar]
- Curtis L, Stoddard J, Radeva J, Hutchison S, Dans P, Wright A, Woosley R, Schulman K, 2006. Geographic variation in the prescription of schedule II opioid analgesics among outpatients in the United States. Health Serv. Res. 41, 837–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubresse M, Chang H, Yu Y, Viswanthan S, Shah N, Stafford R, Kruszewski S, Alexander G, 2013. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med. Care 51, 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubresse M, Gleason P, Peng Y, Shah N, Ritter S, Alexander G, 2014. Impact of a drug utilization review program on high-risk use of prescription controlled substances. Pharmacoepidemiol. Drug Saf. 23, 419–427. [DOI] [PubMed] [Google Scholar]
- DeRosier J, 2008. Pain Clinic Legislation in Louisiana Promising Legal Responses to the Epidemic of Prescription Drug Overdoses in The United States, Atlanta, GA: CDC, Atlanta. [Google Scholar]
- Dhalla I, Mamdani M, Gomes T, Juurlink D, 2011. Clustering of opioid prescribing and opioid-related mortalityamong family physicians in Ontario. Can. Fam. Physician 57, e92–e96. [PMC free article] [PubMed] [Google Scholar]
- Doe-Simkins M, Quinn E, Xuan Z, Sorensen-Alawad A, Hackman H, Ozonoff A, Walley A, 2014. Overdose rescues by trained and untrained participants and change in opioid use among substance-using participants in overdose education and naloxone distribution programs: a retrospective cohort study. BMC Public Health 14, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe-Simkins M, Walley A, Epstein A, Moyer P, 2009. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am. J. Public Health 99, 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormuth C, Miller T, Huang A, Mamdani M, Juurlink D, 2012. Effectofacentral-ized prescription network on inappropriat prescriptions for opioid analgesics and benzodiazepines. CMAJ 6, e852–e856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhwairis H, Reznich C, 2010. An educational strategy for treating chronic, non-cancer pain with opioids: a pilot test. J. Pain 11, 1368–1375. [DOI] [PubMed] [Google Scholar]
- Enteen L, Bauer J, McLean R, Wheeler E, Huriaux E, Kral A, Bamberfer J, 2010. Overdose prevention and naloxone prescription for opioid users in San Francisco. J. Urban Health 87, 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Schinke S, 2013. Two-year outcomes of a randomized, family-based substance use prevention trial for Asian American adolescent girls. Psychol. Addict. Behav. 27, 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florida Medicaid, 2005. Medicaid prescribed drug spending control program initiatives: quarterly report January 1-March 31, 2005. Tallahassee, FL. [Google Scholar]
- Forrester M, 2011. Ingestions of hydrocodone, carisoprodol, and alprazolam in combination reported to Texas poison centers. J. Addict. Dis. 30, 110–115. [DOI] [PubMed] [Google Scholar]
- Fox T, Li J, Stevens S, Tippie T, 2013. A performance improvement prescribing guideline reduces opioid prescriptions for emergency department dental pain patients. Ann. Emerg. Med. 62, 237–240. [DOI] [PubMed] [Google Scholar]
- Franklin G, Fulton-Kehoe D, Turner J, Sullivan M, Wickizer T, 2013a. Changes in opioid prescribing for chronic pain in Washington state. J. Am. Board Fam. Med. 26,394–400. [DOI] [PubMed] [Google Scholar]
- Franklin G, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D, 2013b. Bending the prescription opioid dosing and mortality curves: impact of the Washington state opioid dosing guideline. Am. J. Ind. Med. 55,325–331. [DOI] [PubMed] [Google Scholar]
- Fulton-Kehoe D, Garg R, Turner J, Bauer A, Sullivan M, Wickizer T, Franklin G, 2013. Opioid poisonings and opioid adverse effects in workers in Washington State. Am. J. Ind. Med. 56,1452–1462. [DOI] [PubMed] [Google Scholar]
- Galea S, Worthington N, Piper T, Vandi V, Curtis M, Rosenthal D, 2006. Provision of naloxone to injection drug users as an overdose prevention strategy: early evidence from a pilot study in New York City. Addict. Behav. 31,907–912. [DOI] [PubMed] [Google Scholar]
- Garg R, Fulton-Kehoe D, Turner J, Bauer A, Wickizer T, Sullivan M, Franklin G, 2013. Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic non-cancer pain: 2004 to 2010.J. Pain 14,1620–1628. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Kolbasovsky A, 2012. Impact of a managed controlled-opioid prescription monitoring program on care coordination. Am. J. Manag. Care 18,512–516. [PubMed] [Google Scholar]
- Gordon D,Jones H, Goshman L, Foley D, Bland S, 2000. A quality improvement approachto reducing Meperidine. Jt. Comm. J. Qual. Improv. 26, 686–699. [DOI] [PubMed] [Google Scholar]
- Gray J, Hagemeier N, 2012. Prescription drug abuse and DEA-sanctioned drug takeback events: characteristics and outcomes in rural Appalachia. Arch. Intern. Med. 172, 1186–1187. [DOI] [PubMed] [Google Scholar]
- Green T, Heimer R, Grau L, 2008. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction 103,979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugelmann H, Shofer F, Meisel Z, Perrone J, 2013. Multidisciplinary intervention decreases the use of opioid medication discharge packs from 2 urban EDs. Am. J. Emerg. Med. 31, 1343–1348. [DOI] [PubMed] [Google Scholar]
- Haroutiunian S, McNicol E, Lipman A, 2012. Methadone for chronic non-cancer pain in adults. Cochrane Libr, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavner J, 2009. Teaching pain management to medical students. Pain Pract. 9, 85. [DOI] [PubMed] [Google Scholar]
- Heller D, Stancliff S, 2007. Providing naloxone to substance users for secondary administration to reduce overdose mortality in New York City. Public Health Rep. 122, 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A, Proescholdbell S, Bronson W, Dasgupta N, 2014. Prescription histories and dose strengths associated with overdose deaths. Pain Med, 10.1111/pme.12391 (epub). [DOI] [PubMed] [Google Scholar]
- Hoffman L, Enders J, Pippins J, Segal R, 2003. Reducing claims for prescription drugs with a high potential for abuse. Am. J. Health Syst. Pharm. 60, 371–374. [DOI] [PubMed] [Google Scholar]
- Humphries C, Counsell D, Pediani R, Close S, 1997. Audit of opioid prescribing: the effect of hospital guidelines. Anaesthesia 52, 745–749. [DOI] [PubMed] [Google Scholar]
- Inturrisi C, 2002. Clinical pharmacologyofopioids for pain. Clin. J. Pain 18, S3–S13. [DOI] [PubMed] [Google Scholar]
- Johnson E, Porucznik C, Anderson J, Rolfs R, 2011. State-level strategies for reducing prescription drug overdose deaths: Utah’s prescription safety program. Pain Med. 12, S66–S72. [DOI] [PubMed] [Google Scholar]
- Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B, 2014. Decline in drug overdose deaths after state policy changes-Florida, 2010–2012. MMWR 63, 569–574. [PMC free article] [PubMed] [Google Scholar]
- Jones J, Roux P, Stancliff S, Matthews W, Comer S, 2014. Brief overdose education can significantly increase accurate recognition of opioid overdose among heroin users. Int.J. Drug Policy 25,166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan M, Gomes T, Juurlink D, Manno M, Wilson L, Mailis-Gagnon A, Sri-vastava A, Reardon R, Dhalla I, Mamdani M, 2013. Effect of a course-based intervention and effect of medical regulation on physicians opioid prescribing. Can. Fam. Physician 59, e231–e239. [PMC free article] [PubMed] [Google Scholar]
- Katz N, Birnhaum H, Brennan M, Freedman J, Gilmore G, Dennis J, Kenna G, Madras B, McElhaney L, Weiss R, White A, 2013. Prescription opioid abuse: challenges and opportunities for payers. J. Manag. Care 19, 295–302. [PMC free article] [PubMed] [Google Scholar]
- Krebs E, Ramsey D, Miloshoff J, Bair M, 2011. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 12, 740–746. [DOI] [PubMed] [Google Scholar]
- Lankenau S, Wagner K, Silva K, Kecojevic A, Iverson E, McNelly M, Kral A, 2013. Injection drug users trained by overdose prevention programs: responses to witnessed overdoses. J. Community Health 38,133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall M, Wunsch M, Nuzzo P, Walsh S, 2011. Efficacy of continuing medical education to reduce the risk of buprenorphine diversion. J. Subst. Abuse Treat. 41, 321–329. [DOI] [PubMed] [Google Scholar]
- Ma C, Batz F, Juarez D, Ladao L, 2004. Drug take back in Hawaii: partnership between the University of Hawaii Hilo College of Pharmacy and the Narcotics Enforcement Division. Hawaii J. Med. Public Health 73, 26–31 (accessed 11.09.14) http://msa.maryland.gov/megafile/msa/speccol/sc5300/sc5339/000113/012000/012478/unrestricted/20100355e.pdf [PMC free article] [PubMed] [Google Scholar]
- Maxwell S, Bigg D, Stancyzkiewicz K, Carlgerg-Racich S, 2006. Prescribing naloxone to actively injecting heroin users. J. Addict. Dis. 25, 89–96. [DOI] [PubMed] [Google Scholar]
- McCauley J, Back S, Brady K, 2013. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addict. Behav. 38, 2230–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken L, Boichat C, Eccleston D, 2012. Training for general practitioners in opioid prescribing for chronic pain based on practice guidelines: a randomized pilot and feasibility trial. J. Pain 13, 32–40. [DOI] [PubMed] [Google Scholar]
- Mitchell L, 2009. Pharmacylock-in program promotes appropriate use of resources. J. Okla. State Med. Assoc. 102, 276. [PubMed] [Google Scholar]
- Morasco B, Duckart J, Dobscha S, 2011Adherencetoclinicalguidelines for opioid therapy for chronic pain in patients with substance use disorder. J. Gen. Intern. Med. 26, 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden N, Zerzan J, Rue T, Heagert P, Roughead E, Soumerai S, Ross-Degnan D, Sullivan S, 2008. Medicaid prior authorization and controlled-release oxycodone. Med. Care 46, 573–580. [DOI] [PubMed] [Google Scholar]
- Morse J, Stockbridge H, Egan K, Mai J, Wickizer T, Franklin G, 2012. Primary care survey of the value and effectiveness of the Washington state opioid dosing guideline. J. Opioid Manag. 7, 427–433. [DOI] [PubMed] [Google Scholar]
- Noble M, Treadwell J, Tregear S, Coates V, Wiffen P, Akafomo C, Schoelles K, 2010. Long-term opioid management for chronic noncancer pain. Cochrane Libr. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuckols T, Anderson L, Popescu I, Diamant A, Doyle B, Capua P, Chou R, 2014. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann. Intern. Med. 160,38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oregon State University, 2004a. Carisoprodol Quantity Limit Policy Impact Analysis. Oregon State University, Salem, OR. [Google Scholar]
- Oregon State University, 2004b. Sedative/Hypnotic Quantity Limit Policy Impact Analysis. Oregon State University, Salem, OR. [Google Scholar]
- Oregon State University, 2012. Drug Use Evaluation: Long-Acting Opioids (LAO). Oregon State University, Salem, OR. [Google Scholar]
- Paulozzi L, 2012. Prescription drug overdoses: a review. J. Saf. Res. 43, 283–289. [DOI] [PubMed] [Google Scholar]
- Paulozzi L, Kilbourne E, Daesai H, 2011. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 12, 747–754. [DOI] [PubMed] [Google Scholar]
- Paulozzi L, Zhang K, Jones C, Mack K, 2014. Risk of adverse health outcomes with increasing duration and regularity of opioid therapy. J. Am. Board Fam. Med. 27, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson S, Soumerai S, Mah C, Zhang F, Simoni-Wastila L, Salzman C, Cosler L, Fanning T, Gallagher P, Ross-Degnan D, 2006. Racial disparities in access after regulatory surveillance of benzodiazepines. Arch. Intern. Med. 166,572–579. [DOI] [PubMed] [Google Scholar]
- Piper T, Rudenstine S, Stancliff S, Sherman S, Nandi V, Clear A, Galea S, 2007. Overdose prevention for injection drug users: lessons learned from naloxone training and distribution programs in New York City. Harm Reduct. J 4,3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper T, Stancliff S, Rudenstine S, Sherman S, Nandi V, Clear A, Galea S, 2008. Evaluation of a naloxone distribution and administration program in New York City. Subst. Use Misuse 43, 858–870. [DOI] [PubMed] [Google Scholar]
- Porucznik C, Johnson E, Rolfs R, Sauer B, 2013. Opioid prescribing knowledge and practices: provider survey following promulgation of guidelines—Utah, 2011. J. Opioid Manag. 9, 217–224. [DOI] [PubMed] [Google Scholar]
- Raofi S, Schappert S, 2006. Medication Therapy in Ambulatory Medical Care: United States, 2003–2004 National Center for Health Statistics, Hyattsville, MD: (Report No. 13) (163). [PubMed] [Google Scholar]
- Reifler L, Droz D, Bailey J, Schnoll S, Fant R, Dart R, Bartelson B, 2012. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 13,355–356. [DOI] [PubMed] [Google Scholar]
- Reisman R, Shenoy P, Atherly A, Flowers C, 2009. Prescription opiate usage and abuse relationships: an evaluation of state prescription drug monitoring program efficacy. Subst. Abuse Res. Treat. 3, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Degnan D, Simoni-Wastila L, Brown J, Gao X, Mah C, Cosler L, Fanning T, Gallagher P, Salzman C, Shader R, Inui T, Soumerai S, 2004. A controlled study of the effects of state surveillance on indicators of problematic and non-problematic benzodiazepine use in a Medicaid population. Int. J. Psychiatry Med. 34, 103–123. [DOI] [PubMed] [Google Scholar]
- Sacciccio L, 2011. Costs of prescription drug abuse in the medicare part D program. Testimony before the US Senate Committee on Homeland Security and Governmental Affairs Subcommittee on Federal Financial Management. Government Information, Federal Services, and International Security. [Google Scholar]
- Seal K, Thawley R, Gee L, Bamberger J, Kral A, Ciccarone D, Downing M, Edlin B, 2005. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: a pilot intervention study. J. Urban Health 82, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon R, Aminjavahery N, Davis C, Roswarski M, Robinette C, 2013. Compliance with opioid treatment guidelines for chronic non-cancer pain (CNCP) in primary care at a Veterans Affairs Medical Center (VAMC). Pain Med. 14, 1548–1556. [DOI] [PubMed] [Google Scholar]
- Shaffer G, Moss A, 2010. Physicians’ perceptions of doctor shopping in West Virginia. W.Va. Med. J. 106, 10–14. [PubMed] [Google Scholar]
- Sherman S, Gann D, Tobin K, Latkin C, Welse C, Bielenson P, 2009. The life they save may be mine: diffusion of overdose prevention information from a city sponsored programme. Int. J. Drug Policy 20, 137–142. [DOI] [PubMed] [Google Scholar]
- Sigler K, Guernsey B, Ingrim N, Buesing A, Hokanson J, Galvan E, Doutre W, 1984. Effect of a triplicate prescription law on prescribing of Schedule II drugs. Am. J. Health Syst. Pharm. 41,108–111. [PubMed] [Google Scholar]
- Simeone R, Holland L, 2006. An Evaluation Of Prescription Drug Monitoring Programs. US Department of Justice, Office of Justice Programs, Washington, DC. [Google Scholar]
- Simoni-Wastila L, Qian J, 2012. Influence of prescription monitoring programs on analgesic utilization by an insured retiree population. Pharmacoepidemiol. Drug Saf. 21, 1261–1268. [DOI] [PubMed] [Google Scholar]
- Singleton T, 1977. Missouri’s lock-in: control of recipient misutilization. J. Medicaid Manag. 1,10–17. [PubMed] [Google Scholar]
- Spoth R, Trudeau L, Shin C, Ralston E, Redmond C, Greenberg M, Feinberg M, 2013. Longitudinal effects of universal preventive intervention on prescription drug misuse: three randomized controlled trials with late adolescents and young adults. Am. J. Public Health 103, 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Kahan M, Jiva A, 2012. Prescription opioid use and misuse: piloting an educational strategy for rural primary care physicians. Can. Fam. Physician 58, e210–e216. [PMC free article] [PubMed] [Google Scholar]
- Surratt H, O’Grady C, Kurtz S, Sitivers Y, Cicero T, Dart R, Chen M, 2014. Reductions in prescription opioid diversion following recent legislative interventions in Florida. Pharmacoepidemiol. Drug Saf. 23,314–320. [DOI] [PubMed] [Google Scholar]
- Tanenbaum S, Dyer J, 1990. The dynamics of prescription drug abuse and its correctives in one state Medicaid program In: Wilford BB (Ed.), Balancing the Response to Prescription Drug Abuse. American Medical Association, Chicago, pp. 229–238. [Google Scholar]
- Tobin K, Sherman S, Bielenson P, Welsh C, Latkin C, 2009. Evaluation of the staying alive programme: training injection drug users to properly administer naloxone and save lives. Int. J. Drug Policy 20, 131–136. [DOI] [PubMed] [Google Scholar]
- Ury W, Rahn M, Tolentino V, Pignotti M, Yoon J, McKegney P, Sulmasy D, 2002. Can a pain management and palliative care curriculum improve the opioid prescribing practices of medical residents? J. Gen. Intern. Med. 17, 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T, Alvarez N, Gould E, 2009. Opioid prescribing practices in chronic pain management: guidelines do not sufficiently influence clinical practice. J. Pain 10, 1051–1057. [DOI] [PubMed] [Google Scholar]
- Volkow N, Frieden T, Hyde J,Cha S, 2014. Medication-assisted therapies: tackling the opioid-overdose epidemic. N. Engl. J. Med. 370, 2063–2066. [DOI] [PubMed] [Google Scholar]
- Wagner K, Valente T, Casanova M, Partovi S, Mendenhall B, Hundley J, Gonzalez M, Unger J, 2010. Evaluation of an overdose prevention and response training programme for injection drug users in the Skid Row area of Los Angeles, CA. Int. J. Drug Policy 21, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley A, Doe-Simkins M, Quinn E, Pierce C, Xuan Z, Ozonoff A, 2013a. Opioid overdose prevention with intranasal naloxone among people who take methadone.J. Subst. Abuse Treat. 44, 241–247. [DOI] [PubMed] [Google Scholar]
- Walley AZX, HH H, Quinn E, Doe-Simkins M, Sorensen-Alawad A, Ruiz S, Ozonoff A, 2013b. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interupted time series analysis. Br. Med. J. 346, f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastila L, Bishop C, 1996. The influence of multiple copy prescription programs on analgesic utilization. J. Pharm. Care Pain Symptom Control 4, 3–19. [Google Scholar]
- Weintraub M, Singh S, Byrne L, Maharaj K, Guttmacher L, 1991. Consequences of the 1989 New York State triplicate benzodiazepine prescription regulations. J. Am. Med. Assoc. 266, 2392–2397. [PubMed] [Google Scholar]
- Wheeler E, Davidson P, Jones T, Irwin K, 2012. Community based opioid overdose prevention programs providing naloxone—United States, 2010. MMWR61, 101–105. [PMC free article] [PubMed] [Google Scholar]
- Wiffen P, Wee B, Moore R, 2013. Oral morphine for cancer pain (review). Cochrane Libr., 7. [DOI] [PubMed] [Google Scholar]
- Wolfe S, Lurie P, 1992. Regulation of benzodiazepine prescription. J. Am. Med. Assoc. 268, 472. [PubMed] [Google Scholar]
- Yokell M, Green T, Bowman S, McKenzie M, Rich J, 2011. Opioid overdose prevention and naloxone distribution in Rhode Island. Med. Health R.I. 94, 240–242. [PMC free article] [PubMed] [Google Scholar]
- Young A, Alfred K, Davignon P, Hughes L, Robin L, Chaudhry H, 2012. Physician survey examining the impact ofan educational tool for responsible opioid prescribing. J. Opioid Manag. 8, 81–87. [DOI] [PubMed] [Google Scholar]
- Zarowitz B, Stebelsky L, Muma B, Romain T, Peterson E, 2005. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy 25, 1636–1645. [DOI] [PubMed] [Google Scholar]


