Abstract
Attapulgite nanoparticles have good chemical properties and can be modified easily for broad applications. In this work, for the first time, attapulgite nanoparticles were employed to modify the inner wall of separation capillaries for capillary zone electrophoresis-tandem mass spectrometry (CZE-MS/MS)-based top-down proteomics. The attapulgite nanoparticles and the inner wall of a fused silica capillary were first functionalized with γ-methacryloxypropyl trimethoxysilane. Then the modified nanoparticles and acrylamide were copolymerized in the fused silica capillary with the assistance of azobisisobutyronitrile and heat. The incorporation of high-surface-area nanoparticles in the linear polyacrylamide (LPA) coating resulted in significantly lower electroosmotic mobility compared with the typical LPA coating (3.48 × 10−5 vs. 9.03 × 10−5 cm2 V−1 S−1), most likely because more LPA molecules were immobilized on the inner wall of the separation capillary. The attapulgite nanoparticles functionalized separation capillaries have shown great stability and reproducibility across 43 discontinuous CZE-MS runs of a standard protein mixture. We applied the CZE-MS/MS system for top-down proteomics of Escherichia coli cells. In a proof-of-principle experiment, the CZE-MS/MS system achieved a 90-min separation window and a 1-μL sample loading volume, leading to nearly 300 proteoform and 135 protein identifications in a single run. Many post-translational modifications (PTMs) were identified, including methylation, acetylation, phosphorylation, biotinylation, succinylation, and disulfide bond.
Keywords: Attapulgite nanoparticles, Capillary zone electrophoresis, Tandem mass spectrometry, Top-down proteomics, Escherichia coli
Graphical Abstract
1. Introduction
Attapulgite nanoparticles, a fibrous morphology with a formula of [(OH2)4 (Mg, Al, Fe)5 (OH)·2Si8O20]·4H2O, has advantages of low cost, high surface area, adjustable surface chemistry, good mechanical strength, thermal resistance, and chemical inertness [1–4]. After acid treatment, many reactive Si-OH groups and exchangeable cation sites can be obtained, and they can react with agents (e.g., γ-methacryloxypropyl trimethoxysilane (γ-MAPS) [5], 3-aminopropyltriethoxysilane [6], 3-glycidyloxypropyltrimethoxysilane [7], and thionyl chloride [8], etc.), and electro-statically adsorb cation ions [9–11]. The attapulgite nanoparticles have various functional groups and can be decorated with various organic compounds, including poly(acrylic acid) [5], 4-butylaniline [7], N-methylimidazole [8], polyacrylamide [12], and polyethyleneimine [13]. Attapulgite nanoparticles have been used as adsorbents for removal of pollutants [3, 5, 6, 8, 10] and sample preparation [7, 11, 13, 14] due to good chemical properties. Attapulgite nanoparticles have also been embedded in monolithic materials for liquid chromatography (LC) to improve the separation efficiency of the column [15]. However, based on our knowledge, there is no report about using attapulgite nanoparticles in capillary zone electrophoresis (CZE)-mass spectrometry (MS).
Recently, CZE-MS/MS has been proved to be a useful tool for top-down proteomics due to its advantages of highly efficient separation and highly sensitive detection of intact proteins [16–23]. Modifications of the inner wall of separation capillaries are typically required for CZE-MS analysis of proteins to reduce the protein adsorption and better the protein separation. Linear polyacrylamide (LPA) is widely used for the modification of capillary inner wall for CZE-MS due to its hydrophilic and neutral features [16–20, 24, 25]. LPA coatings can reduce the protein adsorption and eliminate the electroosmotic flow in the capillary, leading to better performance of CZE for separation of intact proteins.
In this work, we incorporated the attapulgite nanoparticles in the LPA coating, and the high-surface-area nanoparticles allowed more LPA molecules to functionalize the inner wall of separation capillary, leading to a significantly lower electroosmotic mobility than typical LPA coating. We evaluated the stability and reproducibility of the attapulgite nanoparticles functionalized separation capillaries for CZE-MS analysis of a standard protein mixture. We also tested the CZE-MS/MS system for top-down proteomics of E.coli cells. This work represents the first report of CZE-MS/MS-based top-down proteomics using separation capillaries with attapulgite nanoparticles doped LPA coating. We need to note that in this work we employed separation capillaries with 75-μm i.d. for CZE-MS to get better sample loading capacity than the widely used 50 or 30-μm-i.d. capillaries.
2. Materials and methods
2.1. Reagents and materials
Standard proteins, ammonium bicarbonate (NH4HCO3), azobisisobutyronitrile, and γ-MAPS were obtained from Sigma-Aldrich (St. Louis, MO). Acrylamide was bought from Acros Organics (NJ, USA). LC/MS grade water, hydrofluoric acid, methanol, acetic acid, and formic acid were purchased from Fisher Scientific (Pittsburgh, PA). Toluene was obtained from Jade Scientific, Inc. (MI, USA). Bare fused silica capillaries (75 μm i.d., 360 μm o.d.) were obtained from Polymicro Technologies (Phoenix, AZ). The attapulgite nanoparticles were bought from Jiangsu Xuyi Anhalt Nonmetallic Mining Ltd. (Jiangsu, China).
2.2. Preparation of the attapulgite nanoparticles doped LPA-coated capillaries
The outer surface of attapulgite nanoparticles and the inner wall of the capillaries (75 μm i.d., 360 μm o.d.) were first modified by γ-MAPS according to the protocol described in the references [15] and [24]. The capillaries were coated with attapulgite nanoparticles doped polyacrylamide by a one-step polymerization. 5.0 mg of γ-MAPS-modified attapulgite nanoparticles were homogeneously dispersed in 20.0 mg of acrylamide, 1000.0 mg of formamide, and 1.0 mg of azobisisobutyronitrile mixture with sonication for one hour. The mixture was purged with nitrogen for 5 min. Then, the degassed mixture was introduced into an 80-cm-long or 110-cm-long γ-MAPS-modified capillary with a syringe. Both ends of capillary were sealed by silicon rubber, and then the capillary was incubated in a 75 °C water bath for 2 0 h. The obtained capillaries were flushed with methanol to remove residuals. After that, one end of the attapulgite nanoparticles doped LPA-coated capillary was etched with hydrofluoric acid according to the previous work to reduce the outer diameter of the separation capillary [26].
LPA-coated capillaries (75 μm i.d., 360 μm o.d.) without attapulgite nanoparticles were also prepared according to references [24] and [27]. The capillaries were compared with the attapulgite nanoparticles doped LPA-coated capillaries.
2.3. Sample preparation
A mixture of standard proteins containing cytochrome c (cyto.c, 12 kDa), bovine serum albumin (BSA, 66.5 kDa), myoglobin (16.9 kDa) and carbonic anhydrase (CA, 29 kDa) was prepared in 50 mM NH4HCO3 (pH 8.0) for CZE-MS analyses. The preparation of E. coli (strain K-12 substrain MG1655) samples was mainly based on the protocol described in the reference [16] with some modifications. The major modification was that the E. coli proteins were not reduced and alkylated in this work. After the E. coli proteins were desalted with a C4 trap column as described in the reference [16], the protein sample was lyophilized and redissolved in 50 mM NH4HCO3 (pH 8.0) to obtain about 1 mg/mL protein concentration for CZE-MS/MS analysis.
2.4. CZE-ESI-MS/MS
An automated CZE-ESI-MS/MS system consisting of an ECE-001 CE autosampler (CMP Scientific, Brooklyn, NY), a commercialized electro-kinetically pumped sheath flow interface (CMP Scientific, Brooklyn, NY) [28,29], and an LTQ-XL or a Q-Exactive HF mass spectrometer (Thermo Fisher Scientific) was used in the experiments.
The attapulgite nanoparticles doped LPA-coated capillaries (75 μm i.d., 360 μm o.d.) were used for CZE separation of standard proteins (70 cm in length) and the E. coli protein sample (95 cm in length). 5% (v/v) acetic acid and 0.2% (v/v) formic acid containing 10% (v/v) methanol were used for CZE as background electrolyte and sheath buffer, respectively. Sample injection was carried out via applying 5 psi of pressure for different injection periods for varied sample injection volumes. The sample loading volumes were calculated based on the Poiseuille’s law. 30 kV of high voltage was applied at the injection end of the capillary for CZE separation, and around 2 kV was applied for ESI. After CZE separation, the capillary was flushed with background electrolyte via applying 20-psi pressure for 10 min. ESI emitters, with around 20~30 μm orifice, was pulled from a borosilicate glass capillary (0.75 mm i.d., 1 mm o.d.) by a Sutter P-1000 flaming/brown micropipette puller.
Standard-protein-mixture experiments were performed on the LTQ-XL mass spectrometer. MS scans were acquired in the range of m/z 600−2000 with positive ion mode, and no protein fragmentation was used. The automatic gain control (AGC) target value was 30000 and the maximum ion injection time was 50 ms.
The E. coli experiment was performed on the Q-Exactive HF mass spectrometer using a data-dependent acquisition (DDA) method. For MS scans, three microscans, mass resolution of 240,000 at m/z 200, AGC target of 1E6, a maximum injection time of 50 ms were used. The MS scan range was 600–2000 m/z. For MS/MS scans, the mass resolution of 60,000 at m/z 200, three microscans, AGC target of 1E6, a maximum injection time of 200 ms were used. The five most intense ions with charge state ≥4 in one MS spectrum were selected in the quadrupole sequentially with an isolation window as 2 m/z, followed by high energy collision dissociation (HCD) in the HCD fragmentation cell with normalized collision energy (NCE) as 20%. The dynamic exclusion was 30 s, and the intensity threshold for triggering fragmentation was 1E5.
2.5. Data analysis
An Xcalibur software (Thermo Fisher Scientific) was used to get peak areas and base peak chromatograms of standard proteins. The RAW file of E. coli protein sample was analyzed with the ProSightPC 3.0 as a plug-in node within Proteome Discoverer 2.2 (Thermo Fisher Scientific). Data were searched against an E. coil database downloaded from the http://proteinaceous.net/database-warehouse-legacy/. The database search workflow used a three-tier search. In brief, tier one consisted of an absolute mass search with 2.0 Da precursor mass tolerance and 10 ppm fragment mass tolerance. Tier two consisted of a biomarker search with 10 ppm no-enzyme precursor mass tolerance and 10 ppm fragment mass tolerance. Tier three consisted of an absolute mass search with 1000 Da precursor mass tolerance and 10 ppm fragment mass tolerance. The target-decoy approach was used to evaluate the false discovery rates (FDRs) of proteoform identifications [30,31]. A 5% proteoform-level FDR was used to filter the proteoform identifications.
3. Results and discussion
3.1. Characterization of the attapulgite nanoparticles doped LPA coating
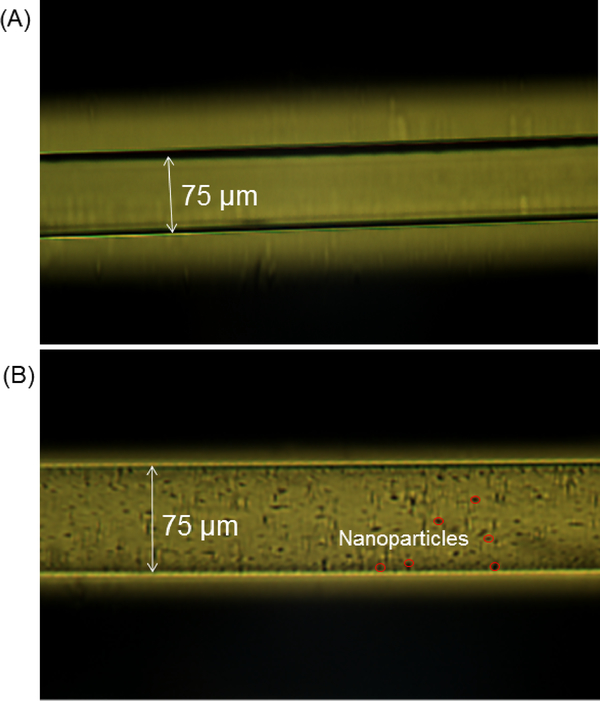
To better understand the morphological structure of the attapulgite nanoparticles doped LPA coating in the capillary, an optical microscope was utilized to characterize the capillary column. The LPA-coated capillary without attapulgite nanoparticles was transparent and had a smooth surface, Figure 1A. The optical microscope image of the attapulgite nanoparticles doped LPA-coated capillary after flushed with methanol is shown in Figure 1B. The attapulgite nanoparticles were evenly distributed on the inner wall of the capillary. The data indicated that the inner wall of the capillary was successfully modified with the attapulgite nanoparticles.
Figure 1.
Optical microscope images of the LPA-coated capillary (A) and the attapulgite nanoparticles doped LPA-coated capillary after flushed with methanol (B).
We speculated that much more LPA molecules could be immobilized on the inner wall of the separation capillary functionalized with attapulgite nanoparticles compared with capillaries without the nanoparticles because the attapulgite nanoparticles had a very high surface area (286 m2/g) [32]. Therefore, the attapulgite nanoparticles doped LPA-coated separation capillary should have lower electroosmotic mobility than the LPA-coated separation capillary. We determined the electroosmotic mobility in an LPA-coated capillary (75 μm i.d.) and an attapulgite nanoparticles doped LPA-coated capillary (75 μm i.d.) based on the method used in references [33] and [34]. The electroosmotic mobility in these two capillaries was 9.03 × 10−5 and 3.48 × 10−5 cm2 V−1 S−1, respectively. The data indicate that the introduction of attapulgite nanoparticles in the LPA coating can significantly reduce the electroosmotic mobility in the capillary.
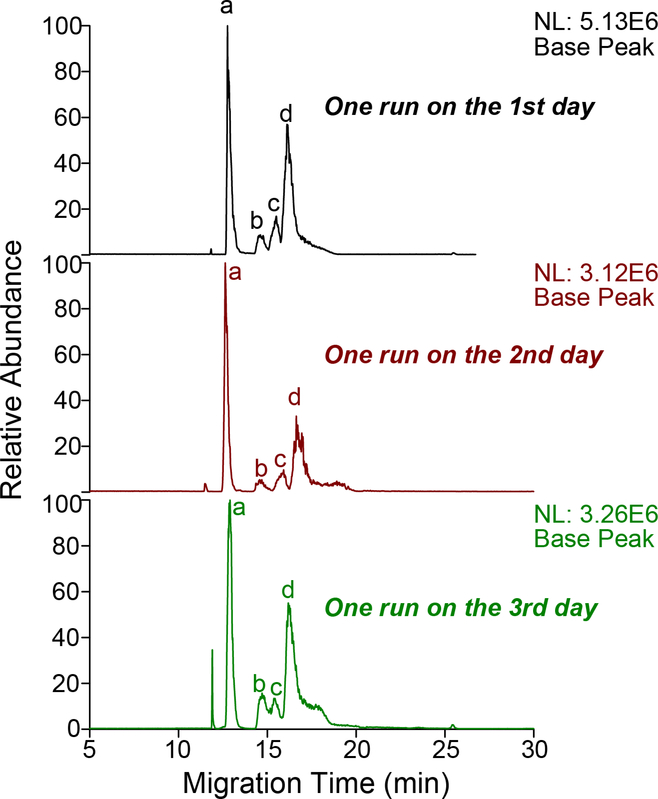
We investigated the attapulgite nanoparticles doped LPA-coated separation capillary for CZE-MS analysis of intact proteins. We performed CZE-MS analyses of a standard protein mixture for 43 discontinuous runs. In the analyses, the sample was analyzed continuously for 11 runs every day, and each run took 36 min, including 26 min for separation and 10 min for capillary flushing with the background electrolyte using a 20-psi pressure. After the final run of each day, the capillary was stored in the background electrolyte. The sample injection volume was 700 nL that corresponds to 20% of the total capillary volume for each CZE-MS run. The sample was dissolved in a buffer containing 50 mM NH4HCO3 (pH 8.0), and the background electrolyte of CZE was 5% acetic acid (pH 2.4). The large volume of injected sample in the capillary was first concentrated via a dynamic pH junction method before the CZE separation [16, 35–37]. Several selected electropherograms are shown in Figure 2. The four standard proteins were well separated with good reproducibility regarding the separation profile and migration time. The relative standard deviations (RSDs) of migration time of cyto.c, BSA, myoglobin, and CA across the 43 runs were 2.2%, 3.5%, 4.8% and 5.9%, respectively. The results here suggest that the attapulgite nanoparticles doped LPA-coating on the inner wall of the separation capillary is stable under the experimental condition. We noted that the protein intensity was not very consistent across those 43 runs, most likely because those CZE-MS runs were performed discontinuously in different days.
Figure 2.
Three selected base peak electropherograms of the standard protein mixture from 43 discontinuous CZE-MS runs using one attapulgite nanoparticles doped LPA-coated capillary. The sample injection volume was 700 nL per CZE-MS run. (a): cyto.c; (b): BSA; (c): myoglobin; (d):CA. The standard protein mixture contained cyto.c (0.003 mg/mL), BSA (0.03 mg/mL), myoglobin (0.003 mg/mL), and CA (0.016 mg/mL).
We further investigated the batch-to-batch reproducibility of the attapulgite nanoparticles doped LPA coating. Three batches of the attapulgite nanoparticles doped LPA-coated capillaries were prepared. The migration time and peak area of proteins were reproducible across the three CZE-MS runs representing the separation capillaries in three batches. The RSDs of migration time and peak area of proteins were less than 9% and less than 20%, respectively.
3.2. Calibration curve data: protein concentration vs. protein peak area
As mentioned in 3.1, a dynamic pH junction method was employed for online protein concentration. Here we investigated the performance of the dynamic pH junction method for protein stacking in our attapulgite nanoparticles doped LPA-coated separation capillary. We performed a calibration-curve experiment, studying the protein peak area change as a function of protein concentration for the four standard proteins using both 175 nL (5% of total capillary volume) and 700 nL (20% of total capillary volume) sample injection volumes, Figure 3A–3D. For this calibration curve experiment, one stock solution of the standard protein mixture in 50 mM NH4HCO3 (pH 8.0) was prepared, and it contained cyto.c (0.05 mg/mL), BSA (0.5 mg/mL), myoglobin (0.05 mg/mL), and CA (0.25 mg/mL). The stock solution was diluted by factors of 2, 4, 8, 16 and 32 with 50 mM NH4HCO3 (pH 8.0) for the experiment. The background electrolyte was 5% (v/v) acetic acid (pH 2.4).
Figure 3.
Calibration curve data of cyto. c, BSA, myoglobin and CA (A-D). Base peak electropherograms of the standard protein mixture with a sample injection volume 175 nL (E) and 700 nL (F). (a): cyto.c; (b): BSA; (c): myoglobin; (d):CA. The standard protein mixture for Figures (E) and (F) contained cyto.c (0.0125 mg/mL), BSA (0.125 mg/mL), myoglobin (0.0125 mg/mL), and CA (0.0625 mg/mL).
Good linear correlations between protein concentration and protein peak area were achieved with R2 ranging from 0.97 to 0.99. The data indicate that the CZE-MS system using the attapulgite nanoparticles doped LPA-coated capillary (75 μm i.d.) can efficiently stack protein samples with the various protein concentrations studied here. Figure 3E and 3F show two base peak electropherograms of the four-protein mixture after CZE-MS analyses with 175 nL and 700 nL sample injection volumes. The protein intensity from 700 nL sample injection volume is dramatically higher than that from 175 nL sample injection volume, suggesting that the CZE-MS method can concentrate proteins in the capillary efficiently with even a 700-nL sample injection volume that corresponds to 20% of the total capillary volume.
3.3. Top-down proteomics of E. coli cells using CZE-MS/MS
The CZE-MS/MS system with attapulgite nanoparticles doped LPA-coated separation capillary was applied for top-down proteomics of E. coli cells. A 1-mg/mL E. coli protein sample in 50 mM NH4HCO3 (pH 8.0) was used for the experiment. The background electrolyte was 5% acetic acid (pH 2.4). Roughly 1 μL of the E. coli sample was injected into the capillary for analysis.
As shown in Figure 4A, the CZE-MS/MS system achieved a 90-min separation window for the E. coli sample analysis. 286 proteoforms and 135 proteins were identified in a single CZE-MS/MS run with a 5% proteoform-level FDR. The identified proteins correspond to 134 E. coli genes. The list of identified proteoforms is shown in the Supporting Information I. As shown in Figure 4B, the mass of identified proteoforms ranged from 2000 Da to over 25000 Da. Many post-translational modifications (PTMs) were identified, such as methylation, acetylation, phosphorylation, biotinylation, succinylation, and disulfide bond, Figure 4C. As shown in Figure 4D, we detected multiple proteoforms of 50S ribosomal protein L7/L12: one proteoform without PTMs; one proteoform with methylation on a lysine; one proteoform with N-terminal acetylation; one proteoform with both the acetylation and methylation PTMs. Figure 4E shows the sequences and fragmentation patterns of the four different proteoforms of 50S ribosomal protein L7/L12. The lysine and protein N-termini with methylation and acetylation were highlighted in green and red, respectively. The –log (E-Values) of the identifications were better than 40, suggesting high confidence of these identifications. We can determine the relative abundance of those proteoforms in the sample by simply comparing their intensity in the mass spectrum, Figure 4D. The data highlight the value of top-down proteomics for characterization of proteoforms.
Figure 4.
Base peak electropherogram of the E. coli sample after CZE-MS/MS analysis (A). Mass distribution of the identified proteoforms (B). Summary of the number of proteoforms with various PTMs (C). The mass spectrum of four proteoforms of 50S ribosomal protein L7/L12 showing their mass differences (D). Sequences and observed fragmentation patterns of the four different proteoforms in Figure 4D (E). The lysine and protein N-termini with methylation and acetylation were highlighted in green and red, respectively.
We note that the separation profile of the E. coli sample in this work is different from our previous data in the reference [16] and the number of proteoform identifications here is also lower than that reported in the reference [16]. There are three possible reasons. First, in this work, the E. coli proteins were not reduced and alkylated. We identified disulfide bonds from several proteins. In our previous work, the proteins were reduced and alkylated, leading to complete unfolding of proteins [16]. The drastically different sample conditions influenced the separation profiles and proteoform identifications. Second, we employed a 75-μm-i.d. separation capillary for CZE-MS/MS in this work, and in reference [16], a 50 μm-i.d. separation capillary was used. A separation capillary with a larger i.d. can help to improve the sample loading capacity, but also can lead to more significant sample diffusion due to the much higher current. We also used a higher electric field in this work compared with our previous work (300 V/cm vs.180 V/cm). Third, we employed more microscans for data acquisition in this work, leading to a lower number of acquired MS/MS spectra.
4. Conclusions
We employed attapulgite nanoparticles for the modification of separation capillaries used for CZE-MS/MS. The attapulgite nanoparticles doped LPA-coated capillaries showed good stability and reproducibility for CZE-MS analysis of intact proteins. The CZE-MS/MS system produced nearly 300 proteoform identifications from an E. coli sample in a single run. The data demonstrate the great potential of the attapulgite nanoparticles doped LPA-coated capillary-based CZE-MS/MS for large-scale top-down proteomics.
Supplementary Material
Capillaries with attapulgite particles were used for CZE-MS/MS for the first time.
Single-shot CZE-MS/MS identified 300 proteoforms from the E. coli cells.
The CZE-MS system showed good stability and reproducibility.
Acknowledgments
We thank the support from the Zhejiang Province Public Welfare Technology Application Research Project (LGC19B050004) and the National Institute of General Medical Sciences, National Institutes of Health (NIH R01GM125991). Tingting Wang gratefully acknowledges the support of K.C.Wong Education, Hong Kong. We thank Prof. Heedeok Hong’s group at the Department of Chemistry, Michigan State University, for kindly providing the E. coli cells for our experiments.
Footnotes
Conflict of interest
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bradley WF, The structural scheme of attapulgite, Am. Miner 25 (1940) 405–410. [Google Scholar]
- [2].Frost RL, Ding Z, Controlled rate thermal analysis and differential scanning calorimetry of sepiolites and palygorskites, Thermochim. Acta 397 (2003) 119–128. [Google Scholar]
- [3].Ye H, Chen F, Sheng Y, Sheng G, Fu J, Adsorption of phosphate from aqueous solution onto modified palygorskites, Sep. Purif. Technol 50 (2006) 283–290. [Google Scholar]
- [4].Mu B, Wang A, One-pot fabrication of multifunctional superparamagnetic attapulgite/Fe3O4/polyaniline nanocomposites served as an adsorbent and catalyst support, J. Mater. Chem. A 3 (2015) 281–289. [Google Scholar]
- [5].Liu P, Jiang L, Zhu L, Wang A, Novel approach for attapulgite/poly(acrylic acid) (ATP/PAA) nanocomposite microgels as selective adsorbent for Pb(II) ion, React. Funct. Polym 74 ( 2014) 72–80. [Google Scholar]
- [6].Xue A, Zhou S, Zhao Y, Lu X, Han P, Effective NH2-grafting on attapulgite surfaces for adsorption of reactive dyes, J. Hazard. Mater 194 (2011) 7–14. [DOI] [PubMed] [Google Scholar]
- [7].Chu G, Cai W, Shao X, Preparation of 4-butylaniline-bonded attapulgite for pre-concentration of bisphenol A in trace quantity, Talanta 136 (2015) 29–34. [DOI] [PubMed] [Google Scholar]
- [8].Chang Y, Liu H, Zha F, Chen H, Ren X, Lei Z, Adsorption of Pb(II) by N-methylimidazole modified palygorskite, Chem. Eng. J 167 (2011) 183–189. [Google Scholar]
- [9].Shen L, Lin Y, Du Q, Zhong W, Yang Y, Preparation and rheology of polyamide-6/attapulgite nanocomposites and studies on their percolated structure, Polymer 46 (2005) 5758–5766. [Google Scholar]
- [10].Huang J, Liu Y, Jin Q, Wang X, Yang J, Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonication-surfactant-modified attapulgite clay, J. Hazard. Mater 143 (2007) 541–548. [DOI] [PubMed] [Google Scholar]
- [11].Yang X, Lin X, Mi Y, Gao H, Li J, Zhang S, Zhou W, Lu R, Ionic liquid-type surfactant modified attapulgite as a novel and efficient dispersive solid phase material for fast determination of pyrethroids in tea drinks, J. Chromatogr. B 1089 (2018) 70–77. [DOI] [PubMed] [Google Scholar]
- [12].Chen Y, Zhao Y, Zhou S, Chu X, Yang L, Xing W, Preparation and characterization of polyacrylamide/palygorskite, Appl. Clay Sci 46 (2009) 148–152. [Google Scholar]
- [13].Wang T, Chen Y, Ma J, Jin Z, Chai M, Xiao X, Zhang L, Zhang Y, A polyethyleneimine-modified attapulgite as a novel solid support in matrix solid-phase dispersion for the extraction of cadmium traces in seafood products, Talanta 180 (2018) 254–259. [DOI] [PubMed] [Google Scholar]
- [14].Wang T, Chen Y, Ma J, Qian Q, Jin Z, Zhang L, Zhang Y, Attapulgite nanoparticles-modified monolithic column for hydrophilic in-tube solid-phase microextraction of cyromazine and melamine, Anal. Chem 88 (2016) 1535–1541. [DOI] [PubMed] [Google Scholar]
- [15].Chai M, Chen Y, Xuan R, Ma J, Wang T, Qiu D, Zhang L, Zhang Y, Preparation of attapulgite nanoparticles-based hybrid monolithic column with covalent bond for hydrophilic interaction liquid chromatography, Talanta 189 (2018) 397–403. [DOI] [PubMed] [Google Scholar]
- [16].Lubeckyj RA, McCool EN, Shen X, Kou Q, Liu X, Sun L, Single-shot top-down proteomics with capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry for identification of nearly 600 Escherichia coli proteoforms, Anal. Chem 89 (2017) 12059–12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun L, Knierman MD, Zhu G, Dovichi NJ, Fast top-down intact protein characterization with capillary zone electrophoresis-electrospray ionization tandem mass spectrometry, Anal. Chem 85 (2013) 5989–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shen X, Kou Q, Guo R, Yang Z, Chen D, Liu X, Hong H, Sun L, Native proteomics in discovery mode using size-exclusion chromatography-capillary zone electrophoresis-tandem mass spectrometry, Anal. Chem 90 (2018)10095–10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McCool EN, Lubeckyj RA, Shen X, Chen D, Kou Q, Liu X, Sun L, Deep top-down proteomics using capillary zone electrophoresis-tandem mass spectrometry: identification of 5700 proteoforms from the Escherichia coli proteome, Anal. Chem 90 (2018) 5529–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhao Y, Sun L, Zhu G, Dovichi NJ, Coupling capillary zone electrophoresis to a Q Exactive HF mass spectrometer for top-down proteomics: 580 proteoform identifications from Yeast, J. Proteome Res 15 (2016) 3679–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Han X, Wang Y, Aslanian A, Bern M, Lavallée-Adam M, Yates JR, Sheathless capillary electrophoresis-tandem mass spectrometry for top-down characterization of Pyrococcus furiosus proteins on a proteome scale, Anal. Chem 86 (2014)11006–11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Han X, Wang Y, Aslanian A, Fonslow B, Graczyk B, Davis TN, Yates JR, In-line separation by capillary electrophoresis prior to analysis by top-down mass spectrometry enables sensitive characterization of protein complexes, J. Proteome Res 13 (2014) 6078–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Y, Compton PD, Tran JC, Ntai I, Kelleher NL, Optimizing capillary electrophoresis for top-down proteomics of 30–80 kDa proteins, Proteomics 14 (2014) 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhu G, Sun L, Dovichi NJ, Thermally-initiated free radical polymerization for reproducible production of stable linear polyacrylamide coated capillaries, and their application to proteomic analysis using capillary zone electrophoresis–mass spectrometry, Talanta 146 (2016) 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Busnel JM, Schoenmaker B, Ramautar R, Carrasco-Pancorbo A, Ratnayake C, Feitelson JS, Chapman JD, Deelder AM, Mayboroda OA, High capacity capillary electrophoresis-electrospray ionization mass spectrometry: coupling a porous sheathless interface with transient-isotachophoresis, Anal. Chem 82 (2010) 9476–9483. [DOI] [PubMed] [Google Scholar]
- [26].Sun L, Zhu G, Zhao Y, Yan X, Mou S, Dovichi NJ, Ultrasensitive and fast bottom-up analysis of femtogram amounts of complex proteome digests, Angew. Chem. Int. Ed 52 (2013) 13661–13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McCool EN, Lubeckyj R, Shen X, Kou Q, Liu X, Sun L, Large-scale top-down proteomics using capillary zone electrophoresis tandem mass spectrometry, J. Vis. Exp 2018. (140). doi: 10.3791/58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wojcik R, Dada OO, Sadilek M, Dovichi NJ, Simplified capillary electrophoresis nanospray sheath-flow interface for high efficiency and sensitive peptide analysis, Rapid. Commun. Mass Spectrom 24 (2010) 2554–2560. [DOI] [PubMed] [Google Scholar]
- [29].Sun L, Zhu G, Zhang Z, Mou S, Dovichi NJ, Third-generation electrokinetically pumped sheath-flow nanospray interface with improved stability and sensitivity for automated capillary zone electrophoresis-mass spectrometry analysis of complex proteome digests, J. Proteome Res 14 (2015) 2312–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Keller A, Nesvizhskii AI, Kolker E, Aebersold R, Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search, Anal. Chem 74 (2002) 5383–5392. [DOI] [PubMed] [Google Scholar]
- [31].Elias JE, Gygi SP, Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry, Nat. Methods 4 (2007) 207–214. [DOI] [PubMed] [Google Scholar]
- [32].Suárez Barrios M, Flores González LV, Vicente Rodríguez MA, Martín Pozas JM, Acid activation of a palygorskite with HCl: development of physico-chemical, textural and surface properties, Appl. Clay Sci 10 (1995) 247–258. [Google Scholar]
- [33].Williams BA, Vigh G, Fast, accurate mobility determination method for capillary electrophoresis, Anal. Chem 68 (1996) 1174–1180. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Z, Peuchen EH, Dovichi NJ, Surface-confined aqueous reversible addition-fragmentation chain transfer (SCARAFT) polymerization method for preparation of coated capillary leads to over 10 000 peptides identified from 25 ng HeLa digest by using capillary zone electrophoresis-tandem mass spectrometry, Anal. Chem 89 (2017) 6774–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Britz-McKibbin P, Chen DD, Selective focusing of catecholamines and weakly acidic compounds by capillary electrophoresis using a dynamic pH junction, Anal. Chem 72 (2000)1242–1252. [DOI] [PubMed] [Google Scholar]
- [36].Zhu G, Sun L, Yan X, Dovichi NJ, Bottom-up proteomics of Escherichia coli using dynamic pH junction preconcentration and capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry, Anal. Chem 86 (2014) 6331–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen D, Shen X, Sun L, Capillary zone electrophoresis-mass spectrometry with microliter-scale loading capacity, 140 min separation window and high peak capacity for bottom-up proteomics, Analyst 142 (2017) 2118–2127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.