Abstract
Background
Cross-sectional reporting of viral suppression rates within a population underestimates the community viral load (VL) burden. Longitudinal approaches, while addressing cumulative effects, may still underestimate viral burden if “churn” (movement in and out of care) is not incorporated. We examined the impact of churn on the cumulative community HIV viral burden.
Methods
All HIV+ patients followed in 2016–2017 at the Southern Alberta Clinic (Calgary, Canada) were categorized as follows: (1) in continuous care, (2) newly diagnosed, (3) diagnosed elsewhere transferring care, (4) returning to care, (5) lost-to-follow-up, (6) moved care elsewhere, or (7) died. Patient days were classified by VL as suppressed (≤200copies/ml), unsuppressed (>200 copies/ml), and transmittable (>1500 copies/ml).
Results
Of 1934 patients, 78.4% had suppressed VL; 21.4% had ≥1 unsuppressed VL, and 18.7% ≥1 transmittable VL. Of 1 276 507 total patient days in care, 92.1% were spent suppressed, 7.9% unsuppressed (101 459 days), and 6.4% (81 847 days) transmittable. 88.7% of category 1 patients had suppressed VL, 11.3% ≥1 unsuppressed VL, and 8.9% ever a transmittable VL. Of category 2 patients, 90% became suppressed on treatment (mean – 62 days). 38.5% of category 3 patients presented with a transmittable VL. Category 4 and 5 patients combined had high rates of unsuppressed (54.5%) and transmittable (51.2%) VL and, while representing only 6.2% of all patients, they accounted for 37.1% of unsuppressed and 41.5% of all transmittable days.
Conclusion
Focus on VL of patients continuously in care misses those with unsuppressed and transmittable VL in a community. Patients moving in and out of care pose an underappreciated risk for HIV transmissions.
Keywords: AIDS, antiretroviral therapy, Canada, churn, HIV; viral suppression
We examined the impact of “churn” on cumulative community HIV viral burden over a 2-year period. We found that patients engaging and disengaging to HIV care contribute disproportionately to the number of days patients spent virally unsuppressed, transmittable, or both.
Plasma viral load (VL) measurements currently are used to determine a baseline when starting antiretroviral therapy (ART), the success of ART in suppressing viral replication, and for measuring the viral risk for ongoing HIV transmission. They are used by HIV programs for determining local success in achieving “the last 90” of the UNAIDS 90-90-90 Cascade of Care (ie, VL suppression rates >90% for patients on ART) [1]. Suppressed VL (ie, <200copies/mL) is strongly associated with reduced risks of HIV morbidity, mortality, and viral transmission risk to others [2–5].
In a population, the most recent VL (ie, within the past year) commonly is used as an easy reproducible marker to document viral suppression. However, Marks et al [6]. reported that use of a single VL measure in surveillance overestimated stable VL suppression rates by 16% within a population. Longitudinal VL measures addressing the dynamic trajectories of VLs over longer time periods have been proposed as a preferable means to reflect such complexities [7–10]. Longitudinal VL also may capture an individual’s cumulative exposure to viral replication over time, reflecting inflammation and immune system activation as well as potential infectivity others [7, 8]. Time spent above a transmittable VL level (>1500 copies/mL) may be a more precise measurement of the length of time an individual may be experiencing immune damage as well as being potentially being infectious to others [7]. As such, it may be a superior metric for measuring the last 90.
Although longitudinal approaches have been developed to address cumulative effects of HIV viral burden [6–10], these measures may further underestimate viral burden if “churn” (the movements in andout of a population) [11–13] is not considered. We examined over a 2-year period of the impact of churn on both the traditional cumulative HIV viral burden metric and on the longitudinal approach in a clinical cohort population in care.
METHODS
The Southern Alberta Clinic (SAC) is the exclusive provider for HIV care in southern Alberta, Canada; the nearest HIV center is approximately 300 km (180 miles) away. SAC provides free access to all HIV services, including ART for eligible individuals (ie, Alberta residents) under universal health care. All adult (>15 years) patients followed by SAC between January 1, 2016, and December 31, 2017, with ≥1 clinic visit and >1 VL were included. Patients residing in the region but receiving HIV care elsewhere are not considered in care at SAC and were not included. Patients are considered followed or in care from the date of their first clinic visit until they either move outside the catchment area (the geographic area from which SAC’s patients are drawn), died, or until they had no contact for >12 consecutive months (ie, they became LTFU—lost to follow-up). Patients attending SAC voluntarily sign a written informed consent form authorizing the use of confidential administrative data for research purposes. The use of non-nominal demographic and clinical data has been approved for research purposes by the University of Calgary Conjoint Heath Research Ethics Board.
Sociodemographic variables, including gender (male,female, or transgender), age (as of January 1, 2016), self-identified ethnicity (Caucasian, Indigenous Canadian, African or Caribbean Black, Other); most likely HIV transmission risk (MSM, men having sex with men; MSW or WSM, men having sex with women; PWID, persons who inject drugs; Other), CD4 count at baseline visit, and time since HIV diagnosis were collected as of January 1, 2106,or at first SAC clinic visit. All VLs for patients under care were recorded between Janaury 1, 2016, and January 1, 2018.
Patients were placed into the following 7 categories: (1) patients continuously followed and in care with ≥1 regular clinic visit and ≥1 VL test in both 2016 and 2017; (2) patients newly diagnosed in southern Alberta and initiating care at SAC between Janaury 1, 2016, and December 31, 2017; (3) patients previously diagnosed and followed outside of southern Alberta who had moved and initiated care at SAC after January 1, 2016; (4) former SAC patients (prior to Janaury 1, 2016) returning after either being LTFU for >12 months or a temporary move outside the catchment area; (5) SAC patients becoming LTFU after January 1, 2016; (6) SAC patients moving HIV care outside of southern Alberta after Janaury 1, 2016; and (7) patients who died between Janaury 1, 2016, and December 31, 2017, ≤12 months after their final visit to SAC. “Continuously in care” was defined broadly in order to account for patients who might extend the time between visits beyond 6 months yet remain in active care.
The number of patients in each category, the number of days in care at SAC from either January 1, 2016,or their first clinic visit date subsequent to that date were determined. Patient days were classified following Marks et al [7] as either suppressed (<200 copies/ml), unsuppressed (≥200 copies/ml), or transmittable (≥1500 copies/ml). Briefly, the number of days between VL tests were first calculated for each patient and designated as T0, T1, T2, etc. T0 is defined as either Janaury 1, 2016, if the patient was actively followed on that date, or the date of first clinic visit if the patient entered SAC after this date. Tx (the end date of follow-up) is either December 31, 2017, if the patient remained actively followed at SAC, or the last clinic contact date before moving, becoming LTFU, or dying. If all values for consecutive VL fell within the same category (ie, <200; >200, >1500), the number of patient days spent in that category was the cumulative days between test dates. If 1 VL increased or decreased outside the category, the number of days between tests was halved, thereby assigning half of the days to each VL category. The total number of days spent in each VL category during the time spent under care at SAC was determined for each patient and the proportion of time spent within each category was calculated by dividing the total number of days spent in that category by the total number of days under care.
Basic descriptive statistics (ie, mean, standard deviation, medians, etc.) on all demographic and clinical characteristics were used. Where appropriate, we used Student t tests for normally distributed data and Mann Whitney tests for non-normally distributed variables. Statistical significance was defined as P < .05 and 95% confidence intervals. Statistical analyses were performed with SPSS v20.0 (IBM, Armonk, NY).
RESULTS
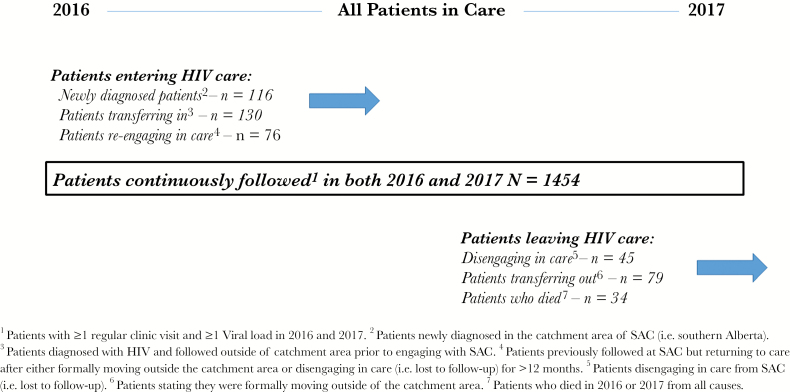
In 2016 and 2017, 1934 patients received HIV care at SAC for a total of 1 276 507 days of follow-up. Three quarters of these patients (n = 1454; 75.2%) received care for the entire study period. In total, 322 patients entered care at SAC; 116 (6.0%) were newly diagnosed, 130 (6.7%) had received HIV care elsewhere before initiating care, and 76 (3.9%) were LTFU but re-engaged in care. 158 (8.2%) patients left care either by disengaging (n = 45), formally transferring care outside the region (n = 79) or dying (n = 34). The movement in and out of care (ie, churn) produced a net increase of 164 patients from January 1, 2016, and December 31, 2017 (Figure 1).
Figure 1.
Patients Entering and Leaving HIV Care (ie, churn) for All Patients in Care at the Southern Alberta Clininc, Calgary, Canada, in 2016 and 2017.
SAC patients received 9593 VL tests (mean = 4.9 tests per person or 0.75 per 100 days followed or 2.75 per year, data not shown). The mean number of days between VL tests per patient was 131 days (+/- 23 days). Overall, 1518 (78.5%) patients had a continuously suppressed VL (ie, durable viral suppression) for the entire period; 21.4% of patients had at least 1 unsuppressed VL (>200 copies/mL), and 18.7% of all patients had at least 1 transmittable VL (>1500 copies/mL) (Table 1). Fifty three of the 414 patients (12.8%) had a an unsuppressed VL but were below the transmittable threshold. Patients with at least 1 detectable VL at any level were more likely to be female, younger, non-Caucasian, PWID, and have acquired HIV more recently (Table 2).
Table 1.
Number (%) of Patients Followed at the With Suppressed Viral Loads, At Least 1 Unsuppressed Viral Load (>200copies/mL), or At Least 1 Transmittable Viral Load (>1500copies/mL) Between 2016 and 2017a
| Category | N (%) | Continuously Suppressed | Ever Unsuppressed | Ever Transmittable |
|---|---|---|---|---|
| Total | 1934 (100) | 1518 (78.4) | 416 (21.4) | 361 (18.7) |
| Continuously followedb | 1454 | 1290 (88.7) | 164 (11.3) | 130 (8.9) |
| Patients entering SAC | ||||
| Newly diagnosedc | 116 | 11 (9.4) | 105 (90.5) | 100 (86.2) |
| Diagnosed elsewhered | 130 | 73 (56.2) | 57 (43.8) | 48 (36.9) |
| Return to caree | 76 | 24 (31.6) | 52 (68.4) | 50 (65.8) |
| Subtotal A | 322 | 108 (33.5) | 214 (66.5) | 198 (61.5) |
| Patients leaving SAC | ||||
| Disengaged from caref | 45 | 31 (68.9) | 14 (31.1) | 12 (26.7) |
| Movedg | 79 | 62 (78.4) | 17 (21.5) | 14 (17.8) |
| Diedh | 34 | 27 (79.4) | 7 (20.6) | 7 (20.6) |
| Subtotal B | 158 | 120 (75.9) | 38 (24.1) | 33 (20.9) |
Abbreviation: SAC, Southern Alberta Clinic.
aPatients with a transmittable viral load are included in the unsuppressed category.
bPatients with ≥1 regular clinic visit and ≥1 viral load in 2016 and 2017.
cPatients newly diagnosed in southern Alberta.
dPatients diagnosed with HIV and followed outside of southern Alberta prior to engaging with SAC.
ePatients previously followed at SAC but returning to care after either formally moving outside of the of southern Alberta or disengaging from care (ie, lost to follow-up) for >12 months.
fPatients disengaging from care from SAC (ie, lost to follow-up).
gPatients stating they were formally moving outside of southern Alberta.
hPatients who died in 2016 or 2017 from all causes.
Table 2.
Comparisons of Patients in Care at the Southern Alberta Clinic, Calgary, Canada, in 2016 and 2017 Who had Durable Viral Suppression (<200 copies/mL), Ever Were Unsuppressed (>200 copies/mL), or Ever had a Viral Load Above Transmittable Levels (>1500 copies/mL)
| Total in Care | Durable VL | Ever Unsuppressed | Ever Transmittable | P-value | |
|---|---|---|---|---|---|
| N (%) | 1934 | 1518 (78.4) | 416 (21.6) | 361 (18.7) | |
| Gender | |||||
| Male | 1433 (74.1) | 1146 (75.5) | 287 (68.9) | 255 (70.8) | |
| Female | 491 (25.4) | 364 (23.9) | 127 (30.5) | 104 (28.8) | <.001 |
| Transgendered | 10 | 8 | 2 | 2 | |
| Age (as of 1/1/2018) | |||||
| ≤30 years | 173 (8.9) | 109 (7.2) | 64 (15.4) | 57 (15.8) | |
| 31–40 | 468 (24.2) | 324 (21.3) | 144 (34.6) | 130 (36.0) | <.001 |
| 41–50 | 572 (29.6) | 453 (29.8) | 119 (28.6) | 100 (27.7) | |
| 51–60 | 514 (26.6) | 443 (29.2) | 71 (17.1) | 56 (15.5) | |
| >60 | 207 (10.7) | 189 (12.5) | 18 (4.3) | 18 (5.0) | |
| Median | 46 | 48 | 40 | 40 | <.001 |
| IQR | [38–54] | [39–55] | [34–49] | [33–48] | |
| HIV transmission risk a | |||||
| MSM | 885 (45.8) | 734 (48.4) | 151 (36.3) | 136 (37.7) | |
| WSM/MSW | 824 (42.6) | 621 (40.9) | 203 (48.8) | 169 (46.8) | <.001 |
| PWID | 177 (9.1) | 124 (8.2) | 53 (12.7) | 50 (13.8) | |
| Other | 45 (2.3) | 36 (2.4) | 9 (2.2) | 6 (1.7) | |
| Not reported | 3 | 3 | 0 | 0 | |
| Self-reported ethnicity b | |||||
| Caucasian | 1032 (53.3) | 867 (57.1) | 165 (39.7) | 143 (39.6) | |
| Indigenous | 152 (7.9) | 87 (5.7) | 65 (15.6) | 59 (16.3) | <.01 |
| ACB | 488 (25.2) | 377 (24.8) | 111 (26.7) | 89 (24.7) | |
| Other | 197 (10.2) | 152 (10.0) | 45 (10.8) | 43 (11.9) | |
| Not reported | 65 | 35 | 30 | 27 | |
| Most recent CD4 (prior to 1/1/2018) | |||||
| Median | 527 | 561 | 374 | 363 | <.001 |
| IQR | [360–724] | [408–746] | [210–556] | [190–568] | |
| Time in months since diagnosis (as of 1/1/2018) | |||||
| Median | 136 | 148 | 82 | 73 | <.001 |
| IQR | [72–215] | [87–226] | [19–159] | [18–156] |
Abbreviations: ACB, African/Caribbean Black;IQR, interquartile range;.MSM, men having sex with men; MSW, men having sex with women; PWID, persons who inject drugs; VL, viral load; WSM, women having sex with men.
a P-value compares patients with durable viral load to patients with ever transmittable viral load.
Patients in care spent 1 175 048 days (92.1% of all days followed) virally suppressed, 101 459 days (7.9%) unsuppressed, and 81 847 (6.4%) days with a transmittable VL (Table 3). However, the proportion of patients and the number of days spent suppressed, unsuppressed, or transmittable varied significantly between categories.
Table 3.
Number (%) of Estimated Days Spent With Suppressed Viral Loads (<200 copies/mL), Unsuppressed Viral Loads (≥200 copies/mL), or Transmittable Viral Loads (>1500 copies/mL) for Patients Followed at the Southern Alberta Clinic from January 1, 2016, to December 31, 2017.
| Category of Churn | Days (%) | Suppressed | Unsuppressed | Transmittable |
|---|---|---|---|---|
| Total | 1 276 507 (100) | 1 175 048 (92.1) | 101 459 (7.9) | 81 847 (6.4) |
| Continuously followeda | 1 061 473 | 1 013 880 (95.6) | 47 593 (4.5) | 35 122 (3.3) |
| Patients entering SAC | ||||
| Newly diagnosedb | 39 760 | 33 675 (84.7) | 6095 (15.3) | 4561 (11.4) |
| Diagnosed elsewherec | 47 975 | 42 110 (87.8) | 5865 (12.2) | 4483 (9.3) |
| Return to cared | 55 347 | 26 801 (48.4) | 28 546 (51.6) | 26 690 (48.2) |
| Subtotal Ah | 143 082 | 102 586 (75.8) | 40 506 (28.3) | 35 734 (25.0) |
| Patients leaving SAC | ||||
| Disengaged from Caree | 32 007 | 22 928 (71.6) | 9079 (28.4) | 7299 (22.8) |
| Movedf | 26 802 | 24 586 (91.7) | 2216 (8.2) | 1768 (6.5) |
| Diedg | 13 143 | 11 078 (84.3) | 2065 (15.7) | 1924 (14.6) |
| Subtotal Bi | 71 952 | 58 592 (81.4) | 13 360 (18.6) | 10 991 (15.3) |
a Patients with ≥1 regular clinic visit and ≥1 viral load tests in 2016 and 2017.
b Patients newly diagnosed in southern Alberta.
c Patients diagnosed with HIV and followed outside of southern Alberta prior to engaging with Southern Alberta Clinic.
d Patients previously followed at Southern Alberta Clinic but returning to care after either formally moving outside of southern Alberta or disengaging from care (ie, lost to follow-up) for >12 months.
e Patients disengaging from care from Southern Alberta Clinic (ie, lost to follow-up).
f Patients stating they were formally moving outside of southern Alberta.
g Patients who died in 2016 or 2017 from all causes.
hIncludes categories 2, 3, and 4.
iIncludes categories 5, 6, and 7.
Of the 1454 patients in continuous care (ie, category 1), 1290 (88.7%) maintained durable viral suppression (Table 1). The remaining 164 (11.3%) patients experienced at least 1 VL >200 copies/mL and 34 had VL >200 but <1500 copies/mL with subsequent recovery of suppression with any change in ART (ie, “blips” [14]). However, 130 patients (8.9% of patients in continuous care) had episodes above 1500 copies/mL. The majority (92%) of these patients were ART-experienced but not on ART; 83% had discontinued ART for a mean time of 183 days prior to their transmittable VL (data not shown). These patients were either erratically adherent or had delayed refilling their ART prescriptions. While in care, these patients had fewer VL tests (median 4 vs. 6) and longer time intervals between VL tests (172 vs. 121 days) compared to those with durable viral suppression. Category 1 patients accounted for 83.2% of total days followed; 95.6% (1 013 880 days) of days were virally suppressed, however, for 4.5% of days (ie, 47 593 days) these patients were unsuppressed, and for 3.3% of days (35 122 days) they had a VL that was transmittable.
Patients Entering HIV Care at SAC
Patients entering HIV care at SAC (categories 2, 3, and 4), while representing only 16.6% of the population under care, accounted for 51.7% of patients ever having an unsuppressed VL, and 54.7% of patients ever with a transmittable VL (Table 1, subtotal A). They spent 40 506 days (39.9% of all patient days unsuppressed) and 35 734 days (43.7%) transmittable (Table 3).
Of the 116 new locally diagnosed (category 2) patients initiating care, 105 had unsuppressed VL representing 25.3% of all patients who were ever unsuppressed, and 100 had transmittable VLs representing 27.8% of patients ever with a transmittable VL. In most cases, the high baseline VL had undetectable levels soon after ART was initiated. The median time from HIV diagnosis to a suppressed VL for these patients was 98 days [IQR 11–402]. Five patients did not achieve viral suppression; 4 patients initially suppressed who had a subsequent episode of transmittable VL were suppressed by the next VL. Newly diagnosed patients represented 3.1% of total days followed and 6.0% and 5.6% of days unsuppressed or transmittable (Table 3).
Category 3 patients (received initial HIV care elsewhere) now engaging to SAC accounted for 6.7% of all patients. Just under half (44.6%) presented with an unsuppressed VL and 38.5% had a level >1500/mL. Similar to newly diagnosed patients, ART-achieved viral suppression in 86% of patients with a median time of 68 [56–80] days; 8 patients did not achieve viral suppression; 3 patients who were initially virally suppressed had a subsequent episode of transmittable VL but were suppressed by next VL. Patients diagnosed elsewhere represented 3.8% of total days in care and 5.9% and 5.5% of days unsuppressed or transmittable (Table 3).
Category 4 patients (LTFU (87%) or transferring HIV care back to SAC (13%)) represented only 3.9% of patients (n = 76), but 12.6% of those were ever unsuppressed and 13.9% of patients were ever transmittable. They had been disengaged for a median of 546 days [191–744]. Patients re-engaging in care represented only 4.3% of all days followed but 28.1% (28 546 days) and 32.6% (26 690 days) of all days spent unsuppressed or transmittable while in care. Overall, 67% of these patients were transmittable on return. Of note, 85% of patients who had been LTFU had a transmittable VL compared to 12% of patients who returned care from elsewhere. Viral suppression was achieved by ART in 72% of category 4 patients by study end with a median time of 98 (66–120) days until suppression.
Patients Leaving or Disengaging in Care at SAC
One hundred and fifty eight patients (n = 158) disengaged from care by becoming LTFU, moved out the region, or died. They represent 8.2% of all patients in care and 9.2% of patients with a transmittable VL but 13.5% of total days spent with a VL >1500/mL (Table 3).
Patients disengaging from care (ie, LTFU) (category 5) represent only 2.3% (n = 45) of all patients and 3.3% of all patients ever with a transmittable VL; however, 28.2% of these patients had a transmittable VL at last clinic visit. Although representing 2.5% of all days followed in care, they constitute 8.9% of days ever unsuppressed or transmittable.
In contrast, patients who stated they were formally moving outside of the catchment area (category 6) comprised 4.1% (n = 79) of the total population in care and 3.9% of patients with a transmittable VL. Compared to patients disengaging (ie, 28.2% of category 5 patients), only 14% of patients who formally moved had an unsuppressed VL prior to moving. Overall, patients who moved accounted for only 2.2% (1768 days) of total days transmittable.
Thirty four patients died (category 7); 4 were AIDS–related deaths. All 4 of these patients had transmittable VLs. Only 3 patients who died of non-HIV-related conditions had transmittable VLs. Patients who died comprised 1.8% of the total population and 1924 days with a transmittable VL.
DISCUSSION
Our study has a series of implications and poses challenges. We have shown that churn adds complexity to measuring and reporting on HIV viral burden for those in care. The standard cross-sectional approaches to reporting VL rates within a population using a single, or latest VL measurement, while easy to use, may underestimate the VL burden. For example, using the last VL before the 31st of December for 2016 and 2017 within our population showed that 8.4% and 5.7% of patients had unsuppressed (ie, >200 copies/mL) VLs (data not shown). However, using the longitudinal approach present here at least twice as many (ie, 15%)patients had experienced unsuppressed or transmittable VLs during the past 2 years. The fluid nature of VL suppression over time among a specific subset of patients is of importance.
We found that overall 92.1% of days in-care patients were suppressed reinforcing findings that engagement and retention in care is effective [15–17]. However, for 7.9% of days patients continuously followed were unsuppressed, and 6.4% of days were transmittable. These proportions, while appearing modest, equate to 101 459 days over the 2 years unsuppressed, and 81 847 days spent above VL levels that increased the risk of onward transmission. Our proportions of patients experiencing durable viral suppression are higher (92% vs. 62%) and lower (8% vs. 43.2%) for patients with a transmittable VL than those reported by Crepaz et al [8] in a large study from the United States; although, we also found that women, younger individuals, non-Caucasians, and the type of HIV-risk acquisition (other than MSM) were more likely to experience transmittable VLs overall. Colasanti et al [18] found that the proportions of patients experiencing durable viral suppression decreased over a 3 year period. Our level of retention and suppression was higher albeit over a shorter duration; however, the findings by Colasanti and colleagues is important in stressing the need for longitudinal reporting on VLs.
Patients followed under continuous care spent the highest proportion of days (95.5%) with durable viral suppression. Of concern was our finding that nearly 9% (n = 130) of patients regularly followed in care had VLs >1500 copies/mL at some time, and they had spent over 35 000 days over the 2 years transmittable. Although these patients may have temporarily disengaged from care or failed to follow their ART regimen, they cannot be listed as “LTFU” by definition, and hence were still categorized as in care. We found that the longer time spent either between visits or between VL tests, the greater the likelihood of unsuppressed or transmittable VL levels.
Newly diagnosed HIV patients initially contribute disproportionately to viral burden before they are in care. Once in care, we found that >80% of our patients achieved viral suppression within 3 months of diagnosis. In contrast, Xia et al [19] found that the percentage of all persons with HIV achieving viral suppression within 3 months using surveillance data from New York City was 37% in 2016. Our proportions may be higher due to different health care systems and access to care as well as population and geographic differences; however, time to achieving viral suppression emphasizes the importance of widespread testing and accessing care so as to achieve viral suppression and reduce transmission [20].
We found that patients who transfer in or out of the population generally have high levels of viral suppression with over 80% achieving durable viral suppression; however, of concern. the remaining 20% who had unsuppressed or transmittable VLs either at last visit before leaving or at first visit moving in from elsewhere. We have previously reported interruptions in care due to a variety of practical and personal reasons often experienced by patients who move [21]. Transferring care may be contributing to transmittable VLs due to ART discontinuation during the transfer.
By far, the highest proportion of days patients spent with transmittable VLs was among patients who have in the past or will in the future disengage in care (ie, categories 4 and 5). Although consisting of only 6.2% of the all patients in care, they present 17.2% of all patients with transmittable VLs. Nearly 2 out of 3 (65%) returning patients had a transmittable VL at first visit after a gap of more than a year in care, and 1 in 4 (26%) patients who subsequently disengaged from care from SAC had a transmittable VL at last visit before disengaging. Combined, patients who disengaged from care and returned or those who disengaged and left spent 33 989 of 87 354 days (38.9%) in care with a transmittable VL. This does not account for days spent outside of care with transmittable VLs. Numerous challenges and barriers [12, 13, 22–24] exist that impact retention in care and thus impact the numbers of individuals with unsuppressed VLs who are disengaged from care, making retention an ongoing issue. However, because of the incomplete or interrupted aspect of these patients under care, they often are not included when cross-sectional surveillance is used, as shown by Jose et al [25].
Our study has several limitations. The study population, although covering a wide geographical area, represents only 1 site under universal health care within Canada. Access to, retention in, and disengagement from care (churn) may be different in other jurisdictions due to referral patterns, availability of alternative centers and care givers within the region, access to ART, and the socioeconomic and structural factors that impact churn. Although these factors may impact the numbers and proportions of individuals in each category, the effect of churn on the longitudinal VL should be considered in any local population study.
Longitudinal VL monitoring and surveillance provides a broader, more comprehensive perspective on the impact of unsuppressed and transmittable VLs within a population in care than cross-sectional reporting, particularly within the context of churn. Continual movements into and out of the population in care will effect positively or negatively the proportion of patients, and which patients, are suppressed or unsuppressed, especially as patients who change providers or HIV care centers often have unsuppressed VLs as we show in this study. Longitudinal approaches may better reflect actual changes impacting patients over time. Understanding churn within a population also affects which patients are captured or observed in a given time period much better than cross-sectional data that may exclude patients who are not active or in care on a particular chosen date. Although more complex, this approach provides a better understanding of real world populations dealing with HIV infection and allows for more targeted interventions. If we are to achieve better control in stopping HIV transmission though ART, then accurate metrics identifying areas of vulnerability will be required.
Notes
Financial support. None reported.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. 90-90-90. An ambitious treatment target to help end the AIDS epidemic. Available at: http://www.unaids.org/en/resources/909090. Accessed May 6, 2019. [Google Scholar]
- 2. Cohen MS, Chen YQ, McCauley M, et al. ; for the HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montaner JS, Lima VD, Harrigan PR, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV Treatment as Prevention” experience in a Canadian setting. PLOS ONE 2014; 9:e87872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simmons RD, Ciancio BC, Kall MM, et al. Ten-year mortality trends among persons diagnosed with HIV infection in England and Wales in the era of antiretroviral therapy: AIDS remains a silent killer. HIV Med 2013; 14:596–604. [DOI] [PubMed] [Google Scholar]
- 5. Attia S, Egger M, Müller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–404. [DOI] [PubMed] [Google Scholar]
- 6. Marks G, Patel U, Stirratt MJ, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications. J Acquir Immune Defic Syndr 2016; 73:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marks G, Gardner LI, Rose CE, et al. Time above 1500 copies: a viral load measure for assessing transmission risk of HIV-positive patients in care. AIDS 2015; 29:947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crepaz N, Tang T, Marks G, et al. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012-2013. Clin Infect Dis 2016; 63:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hughes AJ, Rector A, Jimenez V, et al. Cumulative plasma HIV burden disparities among adults in HIV care: implications for HIV transmission in the era of treatment as prevention. AIDS 2018; 32:1881–9. [DOI] [PubMed] [Google Scholar]
- 10. Jose S, Delpech V, Howarth A, et al. ; UK CHIC Study Steering Committee A continuum of HIV care describing mortality and loss to follow-up: a longitudinal cohort study. Lancet HIV 2018; 5:e301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gill MJ, Krentz HB. Unappreciated epidemiology: the churn effect in a regional HIV care programme. Int J STD AIDS 2009; 20:540–4. [DOI] [PubMed] [Google Scholar]
- 12. Rebeiro P, Althoff K, Buchacz K, et al. ; North American AIDS Cohort Collaboration on Research and Design. Retention among North American HIV-infected persons in clinical care, 2000–2008. JAIDS 2013; 62:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nosyk B, Laurenco L, Min JE, et al. Characterizing retention in HAART as a recurrent event process: insights into ‘cascade churn’. AIDS 2015; 29:1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. .Havlir DV, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination hiv therapy. JAMA 2001; 286:171–9. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah M, Risher K, Berry SA, et al. The epidemiologic and economic impact of improving HIV testing, linkage, and retention in care in the United States. Clin Infect Dis 2015; 62:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higa DH, Crepaz N, Mullins MM, et al. Identifying best practices for increasing linkage to, retention, and re-engagement in HIV medical care: findings from a systematic review, 1996–2014. AIDS Behav 2016; 20:951–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colasanti J, Kelly J, Pennisi E, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis 2016; 62:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xia Q, Coeytaux K, Braunstein SL, et al. Proposing a new indicator for the National HIV/AIDS Strategy: percentage of newly diagnosed persons achieving viral suppression within three months of diagnosis. J Infect Dis 2019; 219:851–5. [DOI] [PubMed] [Google Scholar]
- 20. Dombrowski JC, Baeten JM. It’s time to make the time to viral suppression after HIV diagnosis a metric of HIV care success. J Infect Dis 2019; 219:845–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krentz HB, Worthington H, Gill MJ. Adverse health effects for individuals who move between HIV care centers. J Acquir Immune Defic Syndr 2011; 57:51–4. [DOI] [PubMed] [Google Scholar]
- 22. Colasanti J, Stahl N, Farber EW, et al. An exploratory study to assess individual and structural level barriers associated with poor retention and re-engagement in care among persons living with HIV/AIDS. J Acquir Immune Defic Syndr 2017; 74(Suppl 2):S 113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giordano TP, Gifford AL, White AC Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis 2007; 44:1493–9. [DOI] [PubMed] [Google Scholar]
- 24. Hall HI, Gray KM, Tang T, et al. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr 2012; 60:77–82. [DOI] [PubMed] [Google Scholar]
- 25. Jose S, Delpech V, Howarth A, et al. ; UK CHIC Study Steering Committee A continuum of HIV care describing mortality and loss to follow-up: a longitudinal cohort study. Lancet HIV 2018; 5:e301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]