Abstract
This report describes a frail 92-year-old woman with dementia who presented with a year’s history of chronic watery non-bloody diarrhoea. She had abdominal bloating, weight loss, faecal urgency, nocturnal stools and developed faecal incontinence. Her serum C reactive peptide and faecal calprotectin were elevated. Flexible sigmoidoscopy was macroscopically normal, but demonstrated histological features of microscopic colitis (MC) in sigmoid colon and rectal biopsies. Polypharmacy was reviewed for possible medication-induced MC. Ranitidine, donepezil and simvastatin were discontinued. She was started on oral budesonide with improvement in the abdominal and bowel symptoms. Stool frequency and consistency normalised, and the faecal incontinence resolved with treatment. The outcomes were an improved quality of life, reduced functional dependency, reduced carer strain and avoidance of premature transition from her home into a long-term/institutional care setting. We briefly review terminology, basic epidemiology, notable associations, the importance of establishing a diagnosis and some treatment considerations for MC.
Keywords: long term care, inflammatory bowel disease, geriatric medicine, continence, drugs: gastrointestinal system
Background
This case is of clinical interest as it offers:
A reminder of an important clinical lesson (a BMJ Case Reports ‘Criterion of Interest’)
The case describes an initial presentation of microscopic colitis (MC) at a fairly late age at onset (in her early 90s) in a frail patient with dementia. This resulted in distressing symptoms for up to a year, with adverse effects on quality of life, carer stress and the risk of a premature need to move into institutional/long-term care.
The report highlights the important clinical lesson that diagnosis may be established even in frailer older patients. In the index case, we opted for a pragmatic investigation—in the form of flexible sigmoidoscopy (plus random rectal and sigmoidal biopsies to evaluate histologically for possible MC).
The case illustrates the lesson that following an established diagnosis, response to treatment for MC with targeted polypharmacy reviews and the institution of pharmacological treatment (such as oral budesonide) might prove clinically effective.
Achieving improvements to (or resolution of) significant symptoms (such as difficult to manage faecal incontinence (FI)), may potentially improve an older patient’s quality of life and also delay the premature transition into long-term institutional (nursing) care.
Case presentation
A 92-year-old woman presented to hospital with a 1-year history of chronic diarrhoea, faecal urgency, abdominal bloating and progressive weight loss of 5 kg (~9.5% of her presymptom body weight). The stools were watery and with a frequency of three to five times a day. She also experienced nocturnal loose stools. The stools contained neither blood nor mucus. There was no reported abdominal pain or fever.
Her notable medical history included mixed Alzheimer’s and vascular dementia, falls, osteoporosis, osteoarthritis, iron deficiency anaemia, diverticular disease, glaucoma, impaired glucose tolerance, hypertension and a previous transient ischaemic attack.
Regular medications included aspirin, calcichew-D3 forte, donepezil hydrochloride, duloxetine hydrochloride, ferrous fumarate, loperamide hydrochloride, ranitidine hydrochloride and simvastatin. She was not taking over-the-counter medications or complementary medicine.
Her occupational history, as well as more recent social and travel histories, was unremarkable. She was a non-smoker who drank minimal alcohol.
Her clinical and digital rectal examinations were unremarkable. There were no clinical signs of constipation, faecal loading, impaction or overflow diarrhoea.
Previous investigations in primary care and via a day hospital had noted a range of unremarkable basic blood tests. She had also undergone basic and more advanced imaging, but with no clear diagnosis to explain the ongoing symptoms. In particular, her quality of life and that of her primary caregiver (her husband) had been adversely affected by the recurrent FI. The FI was assessed to be functional; being largely linked to the combination of faecal urgency and chronic poor mobility from osteoarthritis. Her primary care physician had introduced regular oral loperamide hydrochloride to offer some symptom relief, but with minimal benefit to the diarrhoea.
On admission to acute hospital, she was triaged to medicine of the elderly. She was deemed too frail (physically and cognitively) to undergo a colonoscopy. However, we subsequently assessed her as suitable to undergo flexible sigmoidoscopy. This procedure was done specifically to secure a macroscopic review and towards colonic biopsies to exclude the possibility of MC.
Investigations
Haematology and biochemistry
Haemoglobin was 107 g/L (ref. 115–160), mean corpuscular volume 84 fL (ref. 78–98). She had a mild lymphopaenia 1.41×109/L (ref. 1.5–4). Otherwise, the total and other white cellular differentials were normal. Serum iron studies were compatible with a functional iron deficiency.
She had recurring hypokalaemia with a nadir of 2.1 mmol/L (ref. 3.5–5). Otherwise, her other electrolytes, urea, B12, folate, serum amylase, glucose, lactate, immunoglobulins and thyroid function tests were normal. Liver function tests were normal except for a low serum albumin of 29 g/L (ref. 36–47).
Serum C reactive peptide was 28 mg/L (ref. 0–5). Serum 7-alpha-hydroxycholestenone was normal. Faecal elastase was normal. Faecal calprotectin was markedly elevated at 795 μg/g (ref. 0–50).
Radiology
Chest X-ray was unremarkable. A recent contrast-enhanced CT scan of the chest, abdomen and pelvis showed mild bilateral lower lobe bronchiectasis. There was a degree of pancreatic atrophy with several benign-appearing cysts (all ≤15 mm) within the pancreatic head and neck. There was no size-significant lymphadenopathy. She had mild uncomplicated sigmoid diverticular disease.
Microbiology
Routine stool microscopy (for ova, cysts and parasites), and cultures (for pathological enteric bacteria) were unremarkable. Faecal screening for Clostridium difficile toxin was negative.
Targeted immunology
Serum IgA tissue transglutaminase was normal.
Flexible sigmoidoscopy and histopathology of colonic biopsies
The distal colon appeared macroscopically normal. Random rectal and sigmoid colon biopsies (six in total) demonstrated histological features consistent with a diagnosis of MC. There was a mild but diffuse increase in lymphocytes and plasma cells within the superficial half of the lamina propria. There was a mild increase in intraepithelial lymphocytes (IEL). In addition, some biopsy fragments also demonstrated thickened and irregular subepithelial collagen bands (see figures 1 and 2).
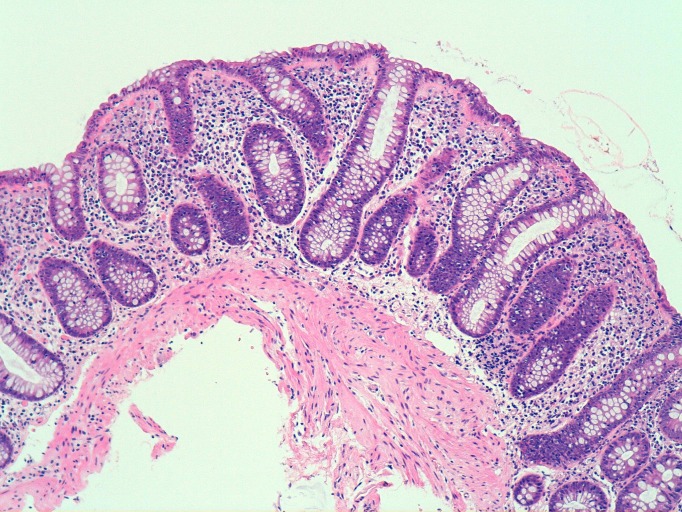
Figure 1.
Histology of index patient’s sigmoid colon—lymphocytic colitis. H&E section, x100 magnification. Image shows a general increase in lymphocytes and plasma cells within the lamina propria and an excess of intraepithelial lymphocytes. The subepithelial collagen band is slightly prominent but not significantly thickened. The features are consistent with the lymphocytic variant of microscopic colitis.
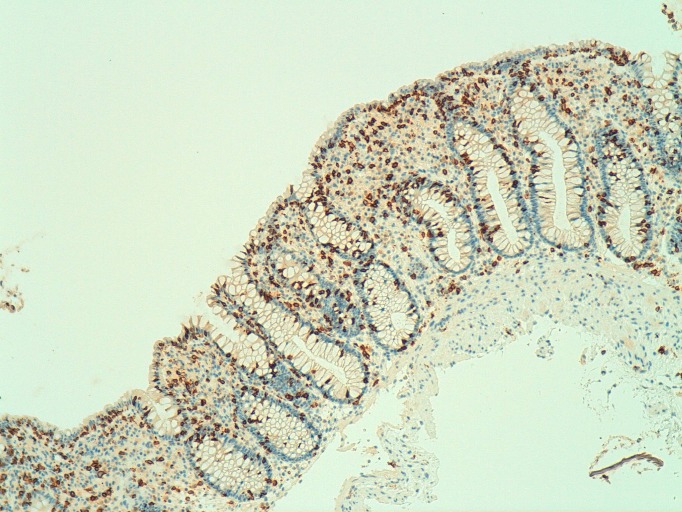
Figure 2.
Histology of index patient’s sigmoid colon—lymphocytic colitis. Immunohistochemistry (x100 magnification) for the cytotoxic T-cell marker CD8, highlighting the increase in lymphocytes within the surface epithelium and colonic crypts.
Differential diagnosis
The gastrointestinal differential diagnoses were progressively reviewed in an evolving manner, that is, guided by revisiting the clinical history and examination, phased investigation profile (as earlier outlined), and a staged review of her polypharmacy.
The ‘broader’ differentials considered included: lower gastrointestinal neoplasia, medication/polypharmacy associated diarrhoea (in particular: ranitidine, donepezil, iron supplements, statin and duloxetine therapy), infective diarrhoea, late-onset coeliac disease, biliary diarrhoea, pancreatic insufficiency and hyperthyroidism.
Serotonin syndrome (in view of duloxetine therapy), carcinoid syndrome and other hormonal gut disorders were considered, but deemed clinically unlikely.
In the context of the clinical symptoms, a raised C reactive protein and raised faecal calprotectin levels, we refocused our investigative strategy and diagnostic formulation.
The ‘narrowed down’ differentials were:
MC (deemed clinically ‘more likely’ in the context of duration of symptoms; nature of stools: non-mucoid and non-bloody diarrhoea; medication history (H2 receptor blocker use); raised inflammatory markers; etc).
Diverticular disease-related symptoms (deemed clinically ‘less likely’ in context of clinical examination and results of recent advanced imaging, that is, not suggestive of active diverticulitis).
Inflammatory bowel disease—presentation with either Crohn’s disease or ulcerative colitis. Despite the raised inflammatory markers, these were also deemed to be clinically ‘less likely’; in context of non-bloody and non-mucoid diarrhoea, etc).
In view of her physical and cognitive frailty, we aimed for pragmatic distal colonic visualisation and random colonic biopsies. In consultation with the patient and her next of kin, we opted for a flexible sigmoidoscopy (with premedication using inhaled nitrous oxide and oxygen: ENTONOX, EQUANOX) instead of a colonoscopy.
Treatment
General treatment modalities
Her polypharmacy was rationalised in a staged manner to monitor the clinical impact on the diarrhoea. We negotiated a phased discontinuation of ranitidine, donepezil and simvastatin.
Specific treatment modalities
Following histological confirmation of MC and a remote discussion with the local specialist gastroenterology team, she was commenced on a course of modified release oral budesonide capsules. Induction treatment was started at 9 mg daily for 3 months. The aim was to reduce to 6 mg daily for 3 months, and finally to reduce to 3 mg daily as maintenance therapy.
Outcome and follow-up
There was a mild improvement to her abdominal (bloating) and bowel symptoms (watery non-bloody diarrhoea, faecal urgency and incontinence) following the initial discontinuation of ranitidine.
Thereafter, all aforementioned symptoms improved significantly within 2 weeks of instituting oral budesonide (induction) therapy. Her stools became formed, and returned to a presymptom frequency of once every other day. She discontinued regular use of oral loperamide.
She was successfully discharged home with support, and her quality of life (both self-reported and as reported by her husband) improved considerably with budesonide treatment.
Discussion
Definition and terminology of MC
MC is an inflammatory disease affecting the colon, and which may be further histologically categorised into lymphocytic colitis and collagenous colitis.1 2 IEL infiltration is often noted in the colonic mucosa in both subtypes. In addition, collagenous colitis demonstrates thickened collagenous bands in the basement membrane of the colonic epithelia.1 2
Recognising the key role played by pathologists in the histological diagnosis of MC, previous authors have described evolutionary changes to both the diagnostic criteria and the diagnostic methods used over time, although placing particular emphasis on the viewpoint/perspective of pathologists.2
Brief epidemiology and notable associations of MC
MC is increasingly recognised as a cause of chronic watery diarrhoea in older people.3–5 The condition has been associated with increasing age, female gender (especially in relation to collagenous colitis), smoking, some autoimmune conditions (eg, coeliac disease, thyroid disease, rheumatoid arthritis, etc), malignancy and solid organ transplantation.3
An expanding list of medications are recognised to be associated with chronic diarrhoea, 3–5for example, non-steroidal anti-inflammatory drugs, selective serotonin receptor inhibitors (eg, sertraline, paroxetine, duloxetine), beta-blockers, statins, bisphosphonates, proton pump inhibitors (eg, lansoprazole), H2 receptor blockers (eg, ranitidine), ticlopidine hydrochloride—an antiplatelet agent, and flutamide—a non-steroidal antiandrogen. Some of these agents have also been linked to MC.3 6–11
The importance of establishing a diagnosis
MC is a significant cause of morbidity and also negatively impacts on quality of life, for example, when associated with FI.3 12–14 Particularly among older and frailer patients, functional dependency (including incontinence care) is an important contributor to institutionalisation in long-term care facilities.3 12–15
A colonoscopy has the distinct advantage of allowing for colonic biopsies from both the proximal and distal colon.15 16 However, in the index case, we restricted the colonic visualisation and biopsies to the distal colon (via a flexible sigmoidoscopy focusing on the sigmoid and rectum). The clinical rationale for the aforementioned decision was the patient’s physical and cognitive frailty.
We do not promote this approach (flexible sigmoidoscopy) for patients who are otherwise fit enough to undertake a colonoscopy, as there is clear evidence that the latter offers a higher yield for the histological diagnosis and subtyping of MC. This is particularly the case in relation to establishing the diagnosis of collagenous colitis: as biopsies taken from the proximal and transverse colon are deemed to offer a higher diagnostic yield to those taken from the distal/sigmoid colon and the rectum.15 16 Nevertheless, we note that the potential utility (although restricted) of distal colonic biopsies has been previously described in the diagnosis of MC.16 The pragmatic strategy of considering a flexible sigmoidoscopy in a frailer older patient with dementia proved effective in this index case. The decision translated into noteworthy and positive outcomes: as it impacted on the patient’s quality of life, reduced her functional dependency levels and also reduced the strain on her main caregiver. Furthermore, in the context of a challenging clinical scenario, it also obviated the need for premature transition from her home environment into a long-term/institutional care setting. We envisage that maintenance therapy with budesonide will be required in this patient for the MC, as symptom recurrence rates are considerable if therapy is stopped.
In this case, the duration (approximately 1 year) of the patient’s ongoing symptoms suggests that there were missed opportunities by a number of clinicians (in primary and secondary care), to facilitate an earlier discussion with a gastroenterologist. Gastroenterologists are well placed to offer support and guidance to generalists when considering the appropriate choice of investigations for patients with chronic diarrhoea.17 Gastroenterologists may also support clinical decision-making regarding whether or not the further investigation of an individual patient could potentially translate into any practical options for treatment. In selected cases, patients and clinicians may agree on targeted investigation as this might support anticipatory care planning and other palliative considerations.
We noted that this patient had undergone a contrast-enhanced CT chest, abdomen and pelvis a few months prior to our review. This had demonstrated a minor degree of sigmoid diverticula. The imaging had shown no signs of diverticulitis nor overt malignancy. An alternative non-invasive investigation such as a minimal bowel preparation CT colon would have been reasonable in this older and frail patient. This could have served as a reasonable screening test to investigate for other important differentials of chronic diarrhoea, for example, large bowel malignancy or significant diverticular disease. However, guided by the information we had from the preceding CT scan, we opted to proceed to a targeted invasive test, rather than to undertake a further minimal preparation CT colon. Radiologists may also offer support in the appropriate choice of imaging studies. This is especially relevant when clinicians are required to evaluate older and potentially frailer patients. A staged approach to investigation would concurrently factor in the relative likelihood of identifying the aforementioned commoner causes of chronic diarrhoea, for example, significant diverticular disease or malignancy. It is equally important to highlight that the strategy of adopting non-invasive before invasive tests supports the broader principles of realistic medicine.18
Treatment considerations for MC
Recommendations for the treatment of MC and its subtypes were historically guided by case reports, case series and some uncontrolled studies.19 However, some randomised controlled trials and systematic reviews have progressively added to the limited but overall supportive evidence base.13 19–25 Many of these studies cite demonstrable benefit with the use of specific medications such as budesonide in the symptomatic management of both subtypes of MC.19–25 In cases of medication-induced/associated MC, withdrawal of linked medications should also be considered.
The more general treatment options include symptom-relief options like antidiarrhoeal agents (eg, loperamide, bismuth subsalicylate or bismuth sulfate, methylcellulose, etc) and bile chelating agents in the context of coexistent biliary malabsorption (eg, cholestyramine, etc).19 Although the evidence base is currently also limited, in some cases where budesonide is ineffective, other anti-inflammatory agents (eg, mesalazine, beclometasone dipropionate, etc) and immune-modulating agents (eg, methotrexate, azathioprine, etc) have been trialled and may offer some patients symptom relief.19
Though subject to further research and testing (particularly around safety and efficacy), there is also limited evidence that some patients who do not respond adequately to initial pharmacotherapeutic options may respond to anti-tumour necrosis factor agents, for example, infliximab and adalimumab.26
Where deemed clinically indicated/appropriate, some patients may require surgical options (eg, colectomy or ileostomy) for managing intractable MC-related disease.
Learning points.
Microscopic colitis (MC) is an important but still under-recognised cause of chronic non-watery diarrhoea. Gastroenterologists may be more familiar with the condition, but there is a need for further awareness among primary care physicians/general practitioners and hospital physicians (general medicine and medicine of the elderly).
This is important given the described higher incidence and prevalence of MC in older patients, and the risk of resultant faecal incontinence and functional dependency. These features are common causes of institutional/long-term care in frailer older patients. This outcome might be temporised in some patients by the increased awareness of MC among non-gastroenterologists. Where appropriate, patients with chronic diarrhoea of undiagnosed aetiology may benefit from discussion with or referral to a gastroenterologist—to guide workup, diagnoses and relevant treatments.23
Some cases of MC are medication-induced/associated and where possible, these agents may need to be discontinued.3–11
Some other general treatment strategies include use of symptom-relief options like antidiarrhoeal agents (eg, loperamide, bismuth); bile chelating agents if there is coexistent biliary malabsorption (eg, cholestyramine, etc); and/or immune-modulating agents such as budesonide.
Oral budesonide has been demonstrated to be an effective and evidence-based treatment for both induction and maintenance therapy in MC.13 19–25
Acknowledgments
The authors would like to acknowledge the patient’s family who granted permission for this report to be written. The authors would also like to thank David Worrall (Consultant Histopathologist, RIE, Edinburgh) for his support in the preparation and interpretation of the patient’s histological sections included in this manuscript.
Footnotes
Contributors: OAO conceived the idea and design of the article. OAO took the lead role in preparation and review of the report. AC contributed to the review and editing of the manuscript. Both authors approved the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Lindström CG. ’Collagenous colitis' with watery diarrhoea--a new entity? Pathol Eur 1976;11:87–9. [PubMed] [Google Scholar]
- 2. Engel PJH, Fiehn AK, Munck LK, et al. The subtypes of microscopic colitis from a pathologist’s perspective: past, present and future. Ann Transl Med 2018;6:69 10.21037/atm.2017.03.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams JJ, Beck PL, Andrews CN, et al. Microscopic colitis -- a common cause of diarrhoea in older adults. Age Ageing 2010;39:162–8. 10.1093/ageing/afp243 [DOI] [PubMed] [Google Scholar]
- 4. Pilotto A, Franceschi M, Vitale D, et al. The prevalence of diarrhea and its association with drug use in elderly outpatients: a multicenter study. Am J Gastroenterol 2008;103:2816–23. 10.1111/j.1572-0241.2008.02107.x [DOI] [PubMed] [Google Scholar]
- 5. Trinh C, Prabhakar K. Diarrheal diseases in the elderly. Clin Geriatr Med 2007;23:833–56. 10.1016/j.cger.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 6. Hilmer SN, Heap TR, Eckstein RP, et al. Microscopic colitis associated with exposure to lansoprazole. Med J Aust 2006;184:185–6. [DOI] [PubMed] [Google Scholar]
- 7. Thomson R, et al. Lansoprazole-associated microscopic colitis: a case series. Am J Gastroenterol 2002;97:2908–13. 10.1016/S0002-9270(02)05483-7 [DOI] [PubMed] [Google Scholar]
- 8. Giardiello FM, Hansen FC, Lazenby AJ, et al. Collagenous colitis in setting of nonsteroidal antiinflammatory drugs and antibiotics. Dig Dis Sci 1990;35:257–60. 10.1007/BF01536772 [DOI] [PubMed] [Google Scholar]
- 9. Beaugerie L, Pardi DS. Review article: drug-induced microscopic colitis - proposal for a scoring system and review of the literature. Aliment Pharmacol Ther 2005;22:277–84. 10.1111/j.1365-2036.2005.02561.x [DOI] [PubMed] [Google Scholar]
- 10. Riddell RH, Tanaka M, Mazzoleni G. Non-steroidal anti-inflammatory drugs as a possible cause of collagenous colitis: a case-control study. Gut 1992;33:683–6. 10.1136/gut.33.5.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández-Bañares F, Esteve M, Espinós JC, et al. Drug Consumption and the Risk of Microscopic Colitis. Am J Gastroenterol 2007;102:324–30. 10.1111/j.1572-0241.2006.00902.x [DOI] [PubMed] [Google Scholar]
- 12. Bharucha AE, Zinsmeister AR, Locke GR, et al. Risk factors for fecal incontinence: a population-based study in women. Am J Gastroenterol 2006;101:1305–12. 10.1111/j.1572-0241.2006.00553.x [DOI] [PubMed] [Google Scholar]
- 13. Madisch A, Heymer P, Voss C, et al. Oral budesonide therapy improves quality of life in patients with collagenous colitis. Int J Colorectal Dis 2005;20:312–6. 10.1007/s00384-004-0660-y [DOI] [PubMed] [Google Scholar]
- 14. Harrison JK, Walesby KE, Hamilton L, et al. Predicting discharge to institutional long-term care following acute hospitalisation: a systematic review and meta-analysis. Age Ageing 2017;46:547–58. 10.1093/ageing/afx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thijs WJ, van BJ, Kleibeuker JH, et al. Microscopic colitis: prevalence and distribution throughout the colon in patients with chronic diarrhoea. Neth J Med 2005;63:137–40. [PubMed] [Google Scholar]
- 16. Tanaka M, Mazzoleni G, Riddell RH. Distribution of collagenous colitis: utility of flexible sigmoidoscopy. Gut 1992;33:65–70. 10.1136/gut.33.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arasaradnam RP, Brown S, Forbes A, et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut 2018;67:1380–99. 10.1136/gutjnl-2017-315909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calderwood C. Realistic medicine to improve the quality of care in Scotland. Bull World Health Organ 2017;95:395–6. 10.2471/BLT.17.030617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chande N, Al Yatama N, Bhanji T, et al. Interventions for treating lymphocytic colitis. Cochrane Database Syst Rev 2017;7:CD006096 10.1002/14651858.CD006096.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miehlke S, Madisch A, Bethke B, et al. Budesonide in lymphocytic colitis —a randomized, double-blind, placebo-controlled trial. Gastroenterology 2007:132A–131. [DOI] [PubMed] [Google Scholar]
- 21. Nyhlin N, Bohr J, Eriksson S, et al. Systematic review: microscopic colitis. Aliment Pharmacol Ther 2006;23:1525–34. 10.1111/j.1365-2036.2006.02913.x [DOI] [PubMed] [Google Scholar]
- 22. Bonderup OK, Hansen JB, Birket-Smith L, Vestergaard V, Teglbjaerg PS, Fallingborg J. Budesonide treatment of collagenous colitis: a randomised, double blind, placebo controlled trial with morphometric analysis. Gut 2003;52:248–51. 10.1136/gut.52.2.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Münch A, Langner C. Microscopic colitis: clinical and pathologic perspectives. Clin Gastroenterol Hepatol 2015;13:228–36. 10.1016/j.cgh.2013.12.026 [DOI] [PubMed] [Google Scholar]
- 24. Baert F, Schmit A, D’Haens G, et al. Budesonide in collagenous colitis: A double-blind placebo-controlled trial with histologic follow-up. Gastroenterology 2002;122:20–5. 10.1053/gast.2002.30295 [DOI] [PubMed] [Google Scholar]
- 25. Miehlke S, Heymer P, Bethke B, et al. Budesonide treatment for collagenous colitis: A randomized, double-blind, placebo-controlled, multicenter trial. Gastroenterology 2002;123:978–84. 10.1053/gast.2002.36042 [DOI] [PubMed] [Google Scholar]
- 26. Anderson RJ, Makins R. Successful use of adalimumab in patient with treatment-refractory microscopic colitis. BMJ Case Rep 2016;2016:bcr2016215639 10.1136/bcr-2016-215639 [DOI] [PMC free article] [PubMed] [Google Scholar]