Abstract
A male patient in his late 30s presented to our outpatient clinic at Mortimer Market Centre with worsening liver transaminases tests 2 months after a resolved acute hepatitis A infection. A diagnosis of parainfectious autoimmune-like hepatitis phenomena was made based on the history, laboratory and histological features.
Keywords: hepatitis other, hepatitis and other GI infections
Background
Autoimmune hepatitis (AIH) is an uncommon cause of persistent liver inflammation caused by the interaction of both genetic and environmental influences. Hepatitis A can be an external trigger for the initiation of AIH or may induce inflammation with autoimmune features that are not persistent or do not fulfil the classical diagnostic criteria of AIH. In the context of the current outbreak of hepatitis A in the UK and Europe, autoimmune phenomena should be considered when patients return with worsening liver enzyme levels after initial resolution.
Case presentation
A 38-year-old previously well man who has sex with men (MSM), presented to his general practitioner (GP) following 10 days of jaundice associated with fever, abdominal pain, dark urine and anorexia. He was subsequently diagnosed with acute hepatitis A. His medical history included eosinophilic gastritis. He was immune to hepatitis B, reported minimal alcohol intake, no recreational or intravenous drug use and worked in computing. Identified risk factors for hepatitis A infection included MSM; however, he had only had oral sexual intercourse with one regular male partner. At this stage, there was no travel history of note. The patient had had normal liver function tests 2 years previously.
On examination, he had a tender, palpable liver edge 2-3 cm below the costal margin and was clinically jaundiced. His alanine transaminase (ALT) peaked around 2500 IU/L, and his liver profile was monitored weekly until it had normalised. He was discharged to his GP care after 1 month. His partner had also developed acute hepatitis A and was admitted to hospital due to abnormalities in his synthetic liver function.
Two months later, our patient re-presented with dyspepsia, diarrhoea and upper abdominal pain of 2 weeks’ duration. He had recently been prescribed omeprazole by his GP, which he had stopped due to the onset of nausea and vomiting. There had been no other changes to the drug history and no further sexual risk. His travel history revealed a journey to Southern France, where he potentially had ingested raw shellfish and meat 2 weeks prior to onset of these symptoms. Clinically, he was afebrile and appeared well, and there was no evidence of icterus or tenderness on abdominal examination.
Investigations
Initial results included reactive anti-hepatitis A virus immunoglobulin (Ig) G, reactive antihepatitis A virus IgM and detectable hepatitis A virus RNA. During the acute hepatitis A infection, the patient’s ALT peaked at 2426 IU/L before continuing to decrease to 62 IU/L at the point of discharge to his GP. Throughout this time, his synthetic liver function remained normal (platelets, albumin and coagulation studies remained within the normal range). Repeat liver profile on re-presentation showed a new increasing trend in ALT, as shown in table 1.
Table 1.
Blood results during the initial presentation with acute hepatitis A infection, resolution of this infection, re-presentation and following immunosuppression. During the acute hepatitis A infection, the abnormal bilirubin and ALT continued to improve towards normal at the point of discharge. During re-presentation, a new increasing trend of elevated ALT was seen with normal bilirubin and a mild, isolated elevation in IgG. Treatment with immunosuppression correlated with normalisation of both ALT and IgG. ALT, alanine transaminase.
| During acute hepatitis A infection | Point of discharge | Re-presentation | Treatment with immunosuppression | |||||||
| August–September | October–November | December–May | ||||||||
| Bilirubin (µmol/L) (normal 0.0–20.0) |
162 | 54 | 39 | 23 | 17 | 7 | 8 | 11 | 8 | 4 |
| ALT (IU/L) (normal 10–50) |
2426 | 528 | 273 | 105 | 62 | 314 | 455 | 474 | 32 | 20 |
| Immunoglobulin A (g/L) (normal 0.7–4.0) |
3.98 | 3.2 | 3.2 | |||||||
| Immunoglobulin G (g/L) (normal 7.0–16.0) |
17.01 | 12.1 | 13.5 | |||||||
| Immunoglobulin M (g/L) (normal 0.4–2.3) |
1.31 | 1.7 | 1.5 | |||||||
ALT, alanine transaminase.
Further tests did not show evidence of hepatitis C infection with negative antibody and antigen results and immunity to hepatitis B with surface antibody >100 IU/L, and no evidence of syphilis infection as treponemal antibody was negative. He was hepatitis A IgG antibody positive but hepatitis A IgM antibody negative, and hepatitis E IgG antibody positive with a negative IgM antibody and negative RNA. Similarly Epstein-Barr virus and cytomegalovirus antibodies were positive for IgG but not IgM, all indicating a past infection. A standard autoantibody screen was negative, which included gastric parietal antibodies, smooth muscle antibodies and mitochondrial antibodies. Antinuclear antibodies and antineutrophil cytoplasm antibodies were also negative. IgG levels were mildly raised at 17.01 g/L (normal range 7.0–16.0) and IgA and IgM levels were both within the normal range.
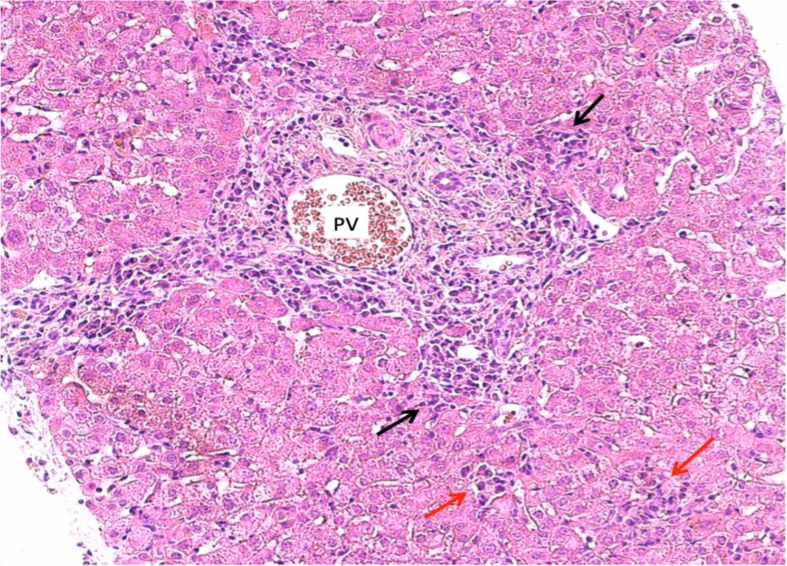
There were no abnormalities detected on liver ultrasound, which showed normal liver appearance and echotexture with normal hepatic and portal flow. A core liver biopsy showed plasma cell-rich inflammation of moderate degree within the portal tracts, at the interface plates and within the lobules, as displayed in figure 1. There was normal lobular architecture, and no lymphoid aggregate formation or fibrosis was seen. There was no evidence of copper-associated protein, liver cell siderosis or alpha-1-antitrypsin material. These pathological findings suggested moderate hepatitis with some features of AIH.
Figure 1.
Liver histology (H&E stain at magnification ×20) showing the portal tract expanded by a plasma cell rich inflammatory infiltrate. Plasma cells are also seen at the interface plates (interface hepatitis, black arrow) and within the lobules (spotty necrosis, red arrow).
Differential diagnosis
Viral causes of hepatitis were suspected including biphasic hepatitis A infection, hepatitis B and C in the context of MSM, as well as hepatitis E with the travel history and shellfish ingestion. However, there was no suggestion of recent infection in the serology results. As with all presentations of hepatitis, drug-induced hepatotoxicity was considered with the recent initiation of the protein pump inhibitor omeprazole. Given the absence of diagnostic serology, a liver biopsy was performed.
Treatment
Due to the findings of raised immunoglobulins and autoimmune features on biopsy, the patient was started on oral prednisolone at 40 mg daily. His ALT fell rapidly (within 5 days), and after satisfactory thiopurine methyltransferase levels, azathioprine 75 mg daily was added. The steroids were weaned by 5 mg weekly until cessation. His treatment course was uncomplicated apart from gastritis secondary to steroids and an unrelated episode of frank haematuria thought to be related to heavy exercise.
Outcome and follow-up
Within a month of starting immunosuppression, the patient’s ALT and IgG had normalised. Azathioprine treatment was weaned and was discontinued within 9 months. The patient remains in clinical and biochemical remission 3 months later, and the plan is to monitor his liver function tests annually with a repeat liver biopsy required only if there is a rise in transaminases or immunoglobulins.
Discussion
Hepatitis A infection is usually a self-limiting infection resolving after a few weeks.1 Less commonly biphasic or relapsing courses of infection can occur, which are described in 6%–10% of cases and are associated with persistence of hepatitis A IgM and RNA.1 However, chronic hepatitis is not a feature.1 The virus is transmitted by faecal–oral route through contaminated food and water or close physical contact including sexual contact. In July 2016, Public Health England identified a hepatitis A outbreak predominantly involving MSM.2 Three hepatitis A genotype Ia strains are in circulation that had not previously been detected in England.2 This outbreak continues, and there have been 4101 reported confirmed cases of the outbreak strains in 22 European countries from June 2016 to March 2018.3
Classical AIH was first described in young women, but it is seen in patients of all ages, sexes and ethnicities. A quarter of cases will present with acute hepatitis and fulminant hepatitis can also be part of the initial presentation.4 AIH is a chronic hepatitis typically associated with positive autoantibodies, hypergammaglobulinaemia and necroinflammatory features on liver histology.5 6 Although the exact aetiology of AIH is not fully understood, there is some evidence that genetic factors are involved, and an association with human lymphocyte antigen types DR3 and DR4 has been described.5 Viral triggers for AIH have also been reported including Epstein-Barr virus, herpes simplex virus, HIV and hepatitis A, B, C and D viruses, particularly in individuals with a genetic predisposition to autoimmunity.7 Additionally, hepatotropic viral infections are associated with similar autoimmune phenomena to AIH.7–9
AIH has two subtypes that differ in which autoantibodies are present; AIH type 1 characterised by positive antinuclear and/or antismooth muscle antibodies are positive, compared with AIH type 2 where antiliver kidney microsomal antibody type 1 and/or anti-liver cytosol type 1 antibody are positive.4 Other autoantibodies may be found in seronegative AIH such as atypical perinuclear antineutrophilic cytoplasmic antibodies. Antisoluble liver antigen/liver pancreas antigen are specific to AIH but only present in 20% of cases at the time of diagnosis,10 and these were not tested for in our patient. Autoantibodies alone are not required for the diagnosis of AIH but can indicate immune-mediated mechanisms,11 and typically antinuclear and/or antismooth muscle antibodies are positive in up to 80% of patients with AIH.12 Autoantibodies can be negative in up to 10% of patients with AIH and particularly in the acute setting may take several months to develop. Patients with seronegative AIH have classical biochemical and histological features of AIH in the absence of conventional autoantibodies.
The International Autoimmune Hepatitis Group previously recommended the use of the Revised Diagnostic Criteria (RDC) for AIH diagnosis.13 However, this was changed in 2008 to the Simplified Diagnostic Criteria (SDC) reporting high sensitivity and specificity.14 The diagnostic scoring criteria considers the presence of autoantibodies, histological features, raised IgG levels and a negative viral hepatitis screen.15 The SDC can make or exclude a diagnosis of probable or definite AIH in the majority of cases.15 An SDC of 6 or more makes the diagnosis of AIH likely. The SDC score for our patient was 5 based on raised IgG, typical features of AIH on histology and the absence of a viral hepatitis. However, there have been case reports of seronegative AIH, where the calculated SDC score has not fulfilled the diagnostic threshold for AIH but when employing the RDC scoring system, the score confirmed the diagnosis of AIH.12 An RDC pretreatment score of >15 and post-treatment score >17 gives a definite diagnosis of AIH.13 When employing the RDC for our patient, the pretreatment score was 16 and post-treatment score was 18, based on alkaline phosphatase/aspartate transaminase ratio, IgG level, no illicit drug use or hepatotoxic drug history, minimal alcohol intake, histological features, negative viral hepatitis markers and response to immunosuppression.
There are no features on histology that are pathognomonic of AIH.13 Typical histopathological features include the presence of lymphoplasmacytic infiltrates in the portal tracts, emperipolesis and rosette formation.15 Emperipolesis and rosettes are not specific for AIH and are seen in non-autoimmune cases.16 In addition, it has been argued that these characteristics are related to the degree of hepatitis as opposed to the underlying cause.17 Plasma cells, portal inflammation and piecemeal necrosis are also seen in hepatitis A infection and were demonstrated in our patient’s histology. Histological morphology on the follow-up of patients with hepatitis A infection has correlated well with aminotransferase levels.18 In this case, the patient’s ALT had almost normalised after the acute hepatitis A infection.
In our patient, we believe the aetiology of his hepatitis was an autoimmune phenomenon triggered by infection because it was temporally distinct from the infectious hepatitis and had several autoimmune features including plasma cell infiltrate on biopsy, raised IgG and steroid responsiveness. His disease course was different than that seen in chronic AIH in that we were able to rapidly wean immunosuppression without recurrence of transaminitis or raised immunoglobulins.
We cannot completely exclude a biphasic hepatitis A infection but typically this is associated with the persistence of IgM to hepatitis A, which was negative in this case on re-presentation.19 Histological follow-up of patients after an acute hepatitis A infection can also demonstrate a non-specific reactive hepatitis, which is seen up to 1 year after the initial infection.18
An observational study following healthy relatives of AIH showed three patients that developed AIH following subclinical infection of hepatitis A.20 Vento et al hypothesised that the underlying pathological mechanism for AIH following infection with hepatitis A virus was a possible defect in specific suppressor inducer T cells, which allowed an immune response to an antigen located on the surface of hepatocytes called asialoglycoprotein receptor.5 20 Hepatitis A virus is also known to be associated with other immune-mediated diseases such as Guillain-Barré syndrome and haemolytic anaemia.21
There are a small number of subsequent case reports describing the development of AIH posthepatitis A infection,22–28 and to our knowledge, there are 10 case reports described in the literature in the English language inclusive of our case. The patients in these case studies range from age 7 years to 75 years of age and the majority are female.6 AIH usually develops within 3 months of the initial viral infection, though the interval range is between 8 weeks and 1 year, and patients typically had features of type 1 AIH. In most of these cases, the use of steroids has resulted in prompt disease remission and about half of these patients also received azathioprine. In one case ursodeo-xycholic acid was used as monotherapy, which also resulted in disease remission.1
Learning points.
Autoimmune hepatitis (AIH) can be induced by hepatitis A infection, and this should be considered with re-emergent hepatitis following apparent resolution.
Liver biopsy has an important role in diagnosis, and features of classical AIH may not be present.
It is paramount that AIH is included in the differential diagnosis following hepatitis A infection in the current outbreak of hepatitis A infection experienced in the UK and Europe.
Footnotes
Contributors: HY-C-T has taken the lead role in authorship and writing of this article, including liaising with the patient and also collecting relevant clinical information from both centres of this patient’s care. HY-C-T also took a lead in organising a literature search for similar cases and associations. KK who also contributed to the planning and the writing of this case report including reviewing the literature. IG was one of the patient’s lead clinicians at the Mortimer Market Centre and identified the case for publication. IG also reviewed drafts of the case report for senior review and expert opinion. DM is the consultant of care at the Department of Hepatology at the Royal Free Hospital. DM has provided relevant clinical information and expert opinion regarding diagnosis and treatment of autoimmune hepatitis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Mikata R, Yokosuka O, Imazeki F, et al. Prolonged acute hepatitis A mimicking autoimmune hepatitis. World J Gastroenterol 2005;11:3791–3. 10.3748/wjg.v11.i24.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Public Health England. Health Protection Report. 2017;11(7). [Google Scholar]
- 3. European Centre of Disease prevention and Control. Epidemiological update: hepatitis A outbreak in the EU/EEA mostly affecting men who have sex with men. 2018. https://ecdc.europa.eu/en/news-events/epidemiological-update-hepatitis-outbreak-eueea-mostly-affecting-men-who-have-sex-men-1.
- 4. Manns MP, Lohse AW, Vergani D. Update Ahepatitis–. J Hepatol 20152015;62:S100–S111. [DOI] [PubMed] [Google Scholar]
- 5. Hilzenrat N, Zilberman D, Klein T, et al. Autoimmune hepatitis in a genetically susceptible patient: is it triggered by acute viral hepatitis A? Dig Dis Sci 1999;44:1950–2. 10.1023/A:1026645629103 [DOI] [PubMed] [Google Scholar]
- 6. Grünhage F, Spengler U, Fischer HP, et al. Autoimmune hepatitis--sequel of a relapsing hepatitis A in a 75-year-old woman. Digestion 2004;70:187–91. 10.1159/000082253 [DOI] [PubMed] [Google Scholar]
- 7. Manns MP. Hepatotropic viruses and autoimmunity 1997. J Viral Hepat 1997;4(Suppl 1):7–10. 10.1111/j.1365-2893.1997.tb00154.x [DOI] [PubMed] [Google Scholar]
- 8. Amin K, Rasool AH, Hattem A, et al. Autoantibody profiles in autoimmune hepatitis and chronic hepatitis C identifies similarities in patients with severe disease. World J Gastroenterol 2017;23:1345–52. 10.3748/wjg.v23.i8.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeman MV, Hirschfield GM. Autoantibodies and liver disease: uses and abuses. Can J Gastroenterol 2010;24:225–31. 10.1155/2010/431913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lohse AW, Hennes E. Diagnostic criteria for autoimmune hepatitis. Hepatology Research 2007;37:S509 10.1111/j.1872-034X.2007.00289.x [DOI] [PubMed] [Google Scholar]
- 11. Czaja AJ. Autoimmune hepatitis--approach to diagnosis. MedGenMed 2006;8:55. [PMC free article] [PubMed] [Google Scholar]
- 12. Sherigar JM, Yavgeniy A, Guss D, et al. Seronegative Autoimmune Hepatitis A Clinically Challenging Difficult Diagnosis. Case Rep Med 2017;2017:1–3. 10.1155/2017/3516234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929–38. 10.1016/S0168-8278(99)80297-9 [DOI] [PubMed] [Google Scholar]
- 14. Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169–76. 10.1002/hep.22322 [DOI] [PubMed] [Google Scholar]
- 15. Manns MP, Czaja AJ, Gorham JD, et al. American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193–213. [DOI] [PubMed] [Google Scholar]
- 16. Balitzer D, Shafizadeh N, Peters MG, et al. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol 2017;30:773–83. 10.1038/modpathol.2016.267 [DOI] [PubMed] [Google Scholar]
- 17. Gurung A, Assis DN, McCarty TR, et al. Histologic features of autoimmune hepatitis: a critical appraisal. Hum Pathol 2018;82:51–60. 10.1016/j.humpath.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 18. Kryger P, Christoffersen P. Liver histopathology of the hepatitis A virus infection: a comparison with hepatitis type B and non-a, non-b. J Clin Pathol 1983;36:650–4. 10.1136/jcp.36.6.650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine 1992;10(Suppl 1):S18–S20. 10.1016/0264-410X(92)90534-Q [DOI] [PubMed] [Google Scholar]
- 20. Vento S, Garofano T, Di Perri G, et al. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet 1991;337:1183–7. 10.1016/0140-6736(91)92858-Y [DOI] [PubMed] [Google Scholar]
- 21. Heurgué A, Bernard-Chabert B, Picot R, et al. Overlap syndrome triggered by acute viral hepatitis A. Eur J Gastroenterol Hepatol 2009;21:708–9. 10.1097/MEG.0b013e3282ffda23 [DOI] [PubMed] [Google Scholar]
- 22. Huppertz HI, Treichel U, Gassel AM, et al. Autoimmune hepatitis following hepatitis A virus infection. J Hepatol 1995;23:204–8. 10.1016/0168-8278(95)80336-X [DOI] [PubMed] [Google Scholar]
- 23. Kim YD, Kim KA, Rou WS, et al. [A case of autoimmune hepatitis following acute hepatitis A]. Korean J Gastroenterol 2011;57:315–8. 10.4166/kjg.2011.57.5.315 [DOI] [PubMed] [Google Scholar]
- 24. Muñoz Bertrán E, Rosa Salazar V, Hostalet Robles F, et al. [Autoimmune hepatitis caused by acute hepatitis due to hepatitis A virus]. Gastroenterol Hepatol 2002;25:501–4. [PubMed] [Google Scholar]
- 25. Rahaman SM, Chira P, Koff RS. Idiopathic autoimmune chronic hepatitis triggered by hepatitis A. Am J Gastroenterol 1994;89:106–8. [PubMed] [Google Scholar]
- 26. Singh G, Palaniappan S, Rotimi O, et al. Autoimmune hepatitis triggered by hepatitis A. Gut 2007;56:304 10.1136/gut.2006.111864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skoog SM, Rivard RE, Batts KP, et al. Autoimmune hepatitis preceded by acute hepatitis A infection. Am J Gastroenterol 2002;97:1568–9. 10.1111/j.1572-0241.2002.05751.x [DOI] [PubMed] [Google Scholar]
- 28. Tanaka H, Tujioka H, Ueda H, et al. Autoimmune hepatitis triggered by acute hepatitis A. World J Gastroenterol 2005;11:6069–71. 10.3748/wjg.v11.i38.6069 [DOI] [PMC free article] [PubMed] [Google Scholar]