Abstract
We describe a case of a 44-year-old woman with locally advanced aggressive angiomyxoma with a novel translocation high-mobility group AT-hook 2–yes-associated protein 1 (HMGA2-YAP1) fusion, implying a t(11;12)(q22.1;q14.3) translocation. She was started on gonadotropin-releasing hormone agonist injection and an aromatase inhibitor for persistent disease, which responded to treatment; she was subsequently treated with radiation before a more definitive operation was conducted. This case report indicates that HGMA2-YAP1–translocated aggressive angiomyxoma is responsive to oestrogen antagonism and hopefully will allow for the development of diagnostics useful for this rare but often morbid neoplasm. This case also highlights the importance of appropriate workup of a soft tissue mass.
Keywords: therapeutic indications, gynecological cancer
Background
Aggressive angiomyxoma (AA) was first reported by Steeper and Rosai in 1983.1 AAs are rare heterogeneous mesenchymal tumours with abundance of extracellular myxoid matrix,2 with ~250 cases reported in the literature.3 AA often occurs from female pelvic and perineal regions and demonstrates relatively slow growth.2 4 We describe a case of AA that was responsive to oestrogen antagonism. We identified a translocation in this locally aggressive neoplasm by molecular genomic testing, thus both describing the fusion partners for this translocation for the first time and linking the diagnosis to the translocation to therapy for the first time.
Case presentation
A 44-year-old woman without prior significant medical history or family history of cancer visited her local gynaecologist for a right vulvar nodule in 2016. The mass grew over time and was finally seen by a different gynaecological surgeon in January 2018. In both visits, she was thought to have a Bartholin gland cyst or lipoma. She underwent transvaginal ultrasound of the pelvis, demonstrating the mass but no other abnormalities. Subsequently, she underwent hysteroscopy, dilation and curettage. During surgery, the mass was found to be deeply rooted; she had ~300 mL of blood loss during the procedure. Postoperatively, she developed a large hematoma and hypotension and was brought back to the operating room for evacuation of 500 mL of blood clot, application of hemostatic agents and transfusion. The resected tumour measured 9.5 cm in greatest dimension. After discharge, she was referred to medical oncology for evaluation. She had no other significant past medical history, aside from irregular menstrual cycle period prior to her diagnosis. MRI after surgery showed residual tumour measuring 68×55×53 mm, extending for the right aspect of the vagina and vulva into the ischiorectal space.
Investigations
Pathology was consistent with AA (figure 1), demonstrating fairly bland spindle to stellate cells with plump to elongated nuclei with vesicular chromatin and occasional nucleoli in a delicate fibromyxoid stroma and a prominent vascular network showing perivascular hyalinisation. Microscopically, the tumour was very infiltrative, showing entrapment of adjacent muscle and adipose tissue without necrosis or a high proliferative index (up to one mitotic figure per 10 high-power fields was identified). Immunohistochemistry stained strongly positive for oestrogen/progesterone receptors (OR/PR), desmin and smooth muscle actin. A FoundationOne Heme test was ordered to further characterise her tumour, given its aggressive nature. Testing showed a novel high-mobility group AT-hook 2–yes-associated protein 1 (HMGA2-YAP1) fusion that creates an imputed t(11;12)(q22.1;q14.3) translocation, involving exons 1–3 of HMGA2 on chromosome 12 to exons 2–7 of YAP1 on chromosome 11 (figure 2). This translocation was identified in one AA previously,5 but not the partner YAP1.
Figure 1.
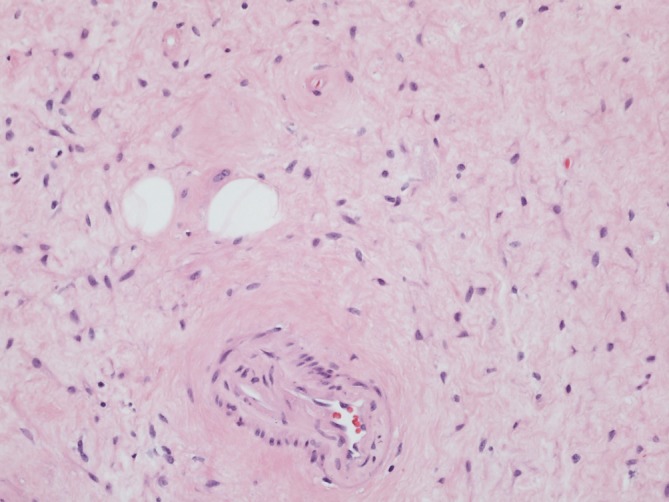
Imaging (×20) of the treated tumour, hematoxylin and eosin staining. Tumour shows delicate collagenised stroma. Lower centre of the image highlights a small calibre blood vessel with subtle perivascular hyalinisation. Two entrapped adipocytes are present.
Figure 2.
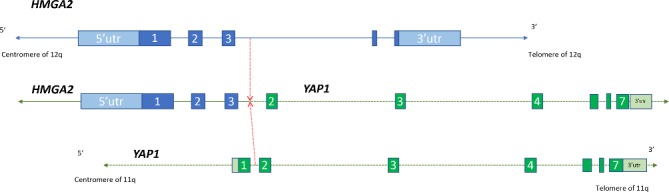
Translocation partners in the HGMA2-YAP1 t(11;12)(q22.1;q14.3) fusion. Selected exons of the two genes are indicated. The chromosomal breakpoint is indicated with a dotted red line. HMGA2-YAP1, high-mobility group AT-hook 2–yes-associated protein 1; utr, untranslated region.
Differential diagnosis
Myxoid tumours encompass a large spectrum of sarcomas and related neoplasms, including myxofibrosarcoma, myxoid liposarcoma, ossifying fibromyxoid tumour, extraskeletal myxoid chondrosarcoma and myxoid malignant peripheral nerve sheath tumour, among other tumours. For this anatomic site, beyond a Bartholin gland cyst, angiomyxoma, cellular angiofibroma and soft tissue sarcomas such as liposarcoma, gastrointestinal stromal tumour (GIST), leiomyosarcoma, malignant peripheral nerve sheath tumour, epithelioid sarcoma and extraskeletal myxoid chondrosarcoma all have at least some predilection for this anatomic site. Diagnosis is best made by pathologists with expertise in soft tissue pathology.
Treatment
AA is OR/PR positive in majority of cases and is responsive to oestrogen antagonism.6 7 Given the complex location of the tumour and size, she was placed on gonadotropin-releasing hormone (GnRH) leuprolide and aromatase inhibitor anastrozole to decrease tumour burden prior to a more definitive operation. On restaging imaging, after 4 months of therapy, her tumour measured 39 mm in maximum dimension, decreased from 68 mm pretreatment (figure 3). In addition, as seen in GIST, desmoid tumours or other tumours responding to therapy, dropout of the gadolinium contrast signal is seen, consistent with a responding tumour (Choi response).8 9
Figure 3.
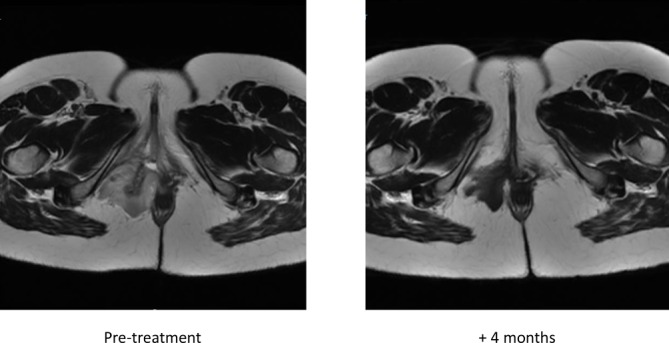
Pretreatment and 4-month post-treatment axial pelvis MRI images with contrast enhancement of the patient treated with oestrogen receptor antagonists. Note tumour shrinking and loss of contrast enhancement on therapy.
Outcome and follow-up
We managed the patient as though she had had an incisional biopsy, with the idea that a more definitive surgery was required. After initial treatment with GnRH antagonist and aromatase inhibitor, she experienced relatively debilitating hot flashes, diaphoresis, mood changes and weight. She was maintained on oestrogen antagonism while on radiation and was then sent for a more definitive operation. She remained without evidence of disease, but the margins on the re-resection were microscopically positive for tumour. Short of further resection, her primary therapeutic option will be ovarian suppression since she is premenopausal. She is being observed off treatment, having chosen not to receive further therapy at present.
Discussion
AA is an unusual tumour most frequently treated by gynaecological oncologists, given its most common primary location in the pelvis, though other sites have been described, such as head and neck. AA typically recurs locally, though dissemination in the abdominal cavity and overt metastatic disease has been reported. The differential diagnosis of these tumours includes fibromyxoma, sarcomas and other tumours, as noted above.
Two other translocations have been described in AA, both involving HMGA2. One case was described involving t(8;12) and overexpression of HMGA2 protein.10 In the other, the same translocation as the one imputed from the fusion described in this report was identified, but not the YAP1 partner to HMGA2.5
As is well recognised, HMGA2 is recurrently translocated in a variety of benign, mostly mesenchymal tumours, with over 20 partners described,11 but not YAP1 previously. HMGA2 is overexpressed in benign and malignant adipocytic tumours, leiomyomas and a variety of epithelial tumours as well; among normal cells, it is predominantly expressed by fibroblasts. It is part of the 12q amplicon that is many-fold amplified in well-differentiated–dedifferentiated liposarcoma.12 HMGA2 functions as a chromatin regulator, interacting with a large number of proteins at enhancers; its ability to bind to adenine-thymine (AT) rich sequences appears to be critical in its function. DNA-binding AT-hooks, three found in HMGA2, are all retained in the putative fusion protein.
YAP1 is part of the Hippo signalling pathway that dictates cell size and a number of other processes, including cell proliferation, cell death and cell migration.13 It binds to the SH3 domain of protein tyrosine kinase YES114 to effect least some of its functions, though it interacts with other proteins through its tryptophan-tryptophan (WW), glutamine-rich and transcriptional activation domains, all of which remain intact in the translocation product. Only the proline-rich N-terminal portion of the protein is deleted in the putative fusion protein.
It is unclear why AA is sensitive to oestrogen inhibition; that said, both leiomyomas and oestrogen receptor–expressing leiomyosarcomas of the uterus are sensitive to oestrogen antagonism.15 16 There are also not good clues to follow, given this or other HMGA2 translocations in benign and malignant tumours. We hypothesise that there may be unique features of fibroblasts in the pelvis or urogenital precursors that are uniquely impacted by an HMGA2 translocation; since these tumours are rare outside the pelvis, these and other similar data on this diagnosis point to site-specific differences between fibroblasts or their precursors.
Another important point demonstrated by this patient’s workup is that the appropriate workup of a soft tissue mass usually involves a core needle biopsy, which can reveal the nature of the lesion before a more aggressive procedure was done. Since the working diagnosis was Bartholin gland cyst, a biopsy was not performed, and the patient had attendant complications that might have been avoided with a more careful initial workup. Of even greater concern, even with a wider resection, the margins were still positive, indicating a higher risk of relapse expected than if an ‘R0’ microscopic margin negative surgery could have been achieved.
Given the rarity of the diagnosis, it would be useful to conduct a multicentre retrospective analysis of AA, designing fluorescence in situ hybridisation probes or conducting other molecular analyses of these tumours to better ascertain the clinical characteristics and molecular features of these locally aggressive and morbid neoplasms.
Learning points.
Aggressive angiomyxoma has a high local recurrence risk. It is sensitive to oestrogen antagonism.
Proper workup of a soft tissue mass that is growing and suspicious for sarcoma involves a core needle biopsy to provide histologic architecture that is not present on fine needle aspiration.
Translocations involving the high-mobility group AT-hook 2 (HMGA2) gene appear to be common in this class of tumours, in this case t(11;12)(q22.1;q14.3) high-mobility group AT-hook 2–yes-associated protein 1.
Yes-associated protein 1 is the first fusion partner for HGMA2 identified in aggressive angiomyxoma.
This report links the specific translocation to a radiological response of a patient to therapy.
Footnotes
Contributors: All authors contributed to data collection, analysis of the results and writing of the manuscript. RGM, M-yL and DCR were responsible for clinical aspects of patient care. All authors discussed the results and contributed to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: RGM has received consulting fees from Foundation Medicine within the last 5 years.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am J Surg Pathol 1983;7:463–75. [DOI] [PubMed] [Google Scholar]
- 2. Lee KA, Seo JW, Yoon NR, et al. Aggressive angiomyxoma of the vulva: A case report. Obstet Gynecol Sci 2014;57:164–7. 10.5468/ogs.2014.57.2.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sirasagi A, Arakeri S. Deep aggressive angiomyxoma of pelvic soft tissue: a rare case report. J Obstet Gynaecol India 2014;64:438–9. 10.1007/s13224-012-0263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han-Geurts IJ, van Geel AN, van Doorn L, et al. Aggressive angiomyxoma: multimodality treatments can avoid mutilating surgery. Eur J Surg Oncol 2006;32:1217–21. 10.1016/j.ejso.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 5. Micci F, Panagopoulos I, Bjerkehagen B, et al. Deregulation of HMGA2 in an aggressive angiomyxoma with t(11;12)(q23;q15). Virchows Arch 2006;448:838–42. 10.1007/s00428-006-0186-5 [DOI] [PubMed] [Google Scholar]
- 6. McCluggage WG, Jamieson T, Dobbs SP, et al. Aggressive angiomyxoma of the vulva: Dramatic response to gonadotropin-releasing hormone agonist therapy. Gynecol Oncol 2006;100:623–5. 10.1016/j.ygyno.2005.09.033 [DOI] [PubMed] [Google Scholar]
- 7. Im SW, Han SS. Treatment of aggressive angiomyxoma of the female perineum: Combined operative and hormone therapy. J Obstet Gynaecol 2016;36:819–21. 10.3109/01443615.2016.1157151 [DOI] [PubMed] [Google Scholar]
- 8. Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753–9. 10.1200/JCO.2006.07.3049 [DOI] [PubMed] [Google Scholar]
- 9. Gounder MM, Lefkowitz RA, Keohan ML, et al. Activity of Sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res 2011;17:4082–90. 10.1158/1078-0432.CCR-10-3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nucci MR, Weremowicz S, Neskey DM, et al. Chromosomal translocation t(8;12) induces aberrant HMGIC expression in aggressive angiomyxoma of the vulva. Genes Chromosomes Cancer 2001;32:172–6. 10.1002/gcc.1179 [DOI] [PubMed] [Google Scholar]
- 11. Wu J, Wei JJ. HMGA2 and high-grade serous ovarian carcinoma. J Mol Med 2013;91:1155–65. 10.1007/s00109-013-1055-8 [DOI] [PubMed] [Google Scholar]
- 12. Italiano A, Bianchini L, Gjernes E, et al. Clinical and biological significance of CDK4 amplification in well-differentiated and dedifferentiated liposarcomas. Clin Cancer Res 2009;15:5696–703. 10.1158/1078-0432.CCR-08-3185 [DOI] [PubMed] [Google Scholar]
- 13. Sebio A, Lenz HJ. Molecular Pathways: Hippo Signaling, a Critical Tumor Suppressor. Clin Cancer Res 2015;21:5002–7. 10.1158/1078-0432.CCR-15-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sudol M, Bork P, Einbond A, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem 1995;270:14733–41. 10.1074/jbc.270.24.14733 [DOI] [PubMed] [Google Scholar]
- 15. Takahashi K, Kawamura N, Tsujimura A, et al. Association of the shrinkage of uterine leiomyoma treated with GnRH agonist and deletion of long arm of chromosome 7. Int J Oncol 2001;18:1259–63. 10.3892/ijo.18.6.1259 [DOI] [PubMed] [Google Scholar]
- 16. O’Cearbhaill R, Zhou Q, Iasonos A, et al. Treatment of advanced uterine leiomyosarcoma with aromatase inhibitors. Gynecol Oncol 2010;116:424–9. 10.1016/j.ygyno.2009.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]


