Abstract
A 60-year-old man with a history of severe herpes simplex virus type 1 (HSV-1) encephalitis 2 years prior presented with acute onset of visual loss in the left eye. Dilated funduscopic examination showed retinitis and occlusive vasculitis with retinal necrosis. PCR of the vitreous fluid was positive for HSV-1, and he was diagnosed with acute retinal necrosis (ARN) due to HSV-1. The patient was treated with intravenous acyclovir and intravitreous foscarnet for 2 weeks, followed by high dose oral valacyclovir for 2 weeks. He was subsequently placed on planned life-long suppressive valacyclovir. His case demonstrates that acute visual loss concomitant with or subsequent to HSV-1 encephalitis warrants suspicion of ARN. Prompt therapy with effective antiviral medication is necessary to reduce the risk of sight-threatening complications. Chronic suppression with oral antiviral therapy after ARN is recommended to prevent involvement of the contralateral eye, though there is no consensus on the duration and dosage of antivirals.
Keywords: infectious diseases, ophthalmology
Background
Acute retinal necrosis (ARN), a rapidly progressive process, was first described in 1971. It mainly affects immunocompetent patients and is usually caused by herpes viruses. We encountered a patient with ARN due to HSV-1 who had HSV-1 encephalitis 2 years prior. While ARN occurring simultaneous with or subsequent to herpes simplex encephalitis has been reported, little is known regarding how long after encephalitis it may develop and how long such patients should be on chronic suppression to prevent involvement of the contralateral eye. We reviewed case reports of patients affected by both HSV encephalitis and ARN to better understand features such as their relative timing and the duration of chronic suppression used by providers.
Case presentation
A 60-year-old man presented with acute left sided visual loss. Two days prior, he had noted blurry vision. Over the next 2 days, his visual acuity worsened significantly. His medical history was notable only for HSV-1 encephalitis 2 years prior when he had developed sudden onset of headache, nausea, dizziness and altered mental status.
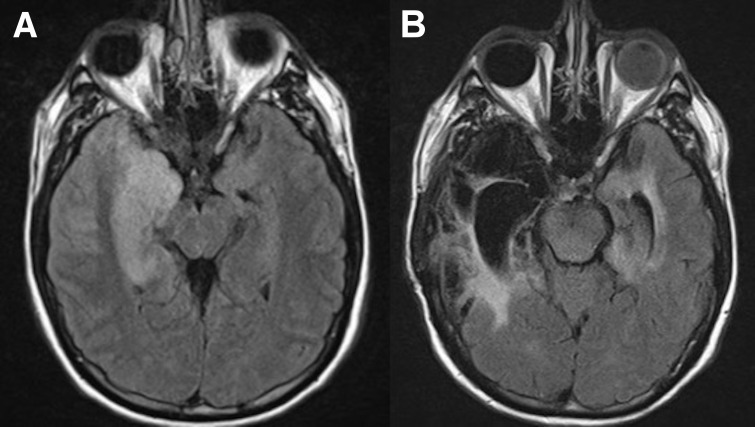
During his earlier hospitalisation for encephalitis, MRI of the brain revealed asymmetrical bilateral temporal lobe signal changes with involvement of the right hippocampus, amygdala and temporal pole with postcontrast enhancement (figure 1A). Also at the time of encephalitis, cerebrospinal fluid (CSF) showed moderate lymphocyte-predominant pleocytosis, normal glucose and moderately elevated total protein. PCR of CSF was positive for HSV-1. High-dose intravenous acyclovir was administered but he had progressive cerebral oedema that required hemicraniectomy and lobectomy. He completed 3 weeks of intravenous acyclovir and was discharged with moderate cognitive dysfunction. He was not placed on oral antiviral therapy for suppression/secondary prophylaxis and had been clinically stable until the current presentation.
Figure 1.
(A) MRI brain study showed asymmetrical bilateral temporal lobe signal changes with involvement of the right hippocampus, amygdala and temporal pole and postcontrast mild enhancement on the right side. (B) MRI brain study showed extensive areas of encephalomalacia predominantly involving the right temporal lobe, likely sequela of prior encephalitis.
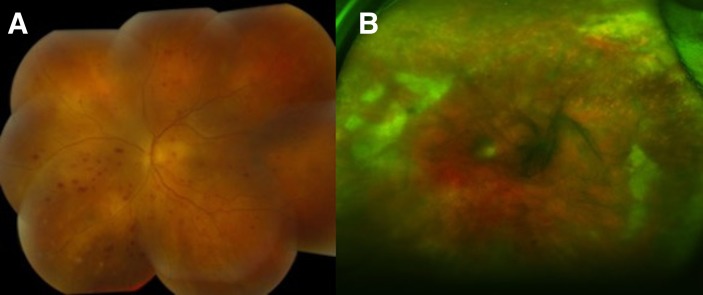
During the current hospitalisation for acute visual loss, physical examination revealed a well appearing man with normal vital signs. The left eye had an injected conjunctiva and dilated funduscopic examination showed retinal haemorrhage, occlusive vasculitis and ischaemic change (figures 2A,B and 3A,B). The right eye was unremarkable.
Figure 2.
(A) Fundus photography showed optic nerve oedema and multiple haemorrhage. (B) Fundus colour photo revealed vitreous haze with multiple white patchy ischaemic retinal lesions.
Figure 3.
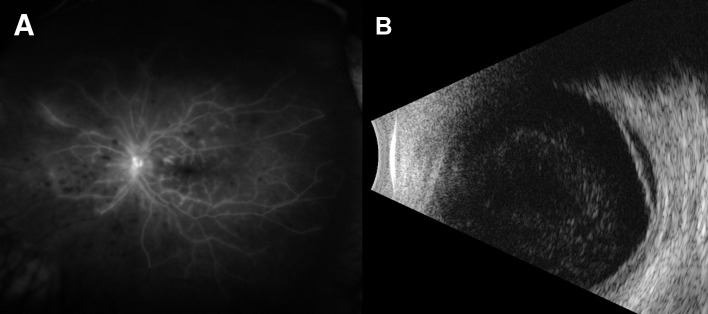
(A) Fluorescein angiography showed diffuse peripheral non-perfusion with late perivascular leakage posteriorly consistent with occlusive vasculitis. (B)B-scan showed retinal detachment.
Investigations
MRI of the brain with contrast showed extensive areas of encephalomalacia predominantly involving the right temporal lobe, likely sequelae of prior encephalitis (figure 1B). Negative laboratory tests from serum included antinuclear antibody, anti-neutrophil cytoplasmic antibody (ANCA), Toxoplasma IgM/IgG, QuantiFERON-TB gold, syphilis IgG, Lyme antibody and HLAB27 genotypes. Anterior chamber vitreous fluid PCR was positive for HSV-1 and negative for varicella zoster virus (VZV), cytomegalovirus (CMV) and Toxoplasma.
Differential diagnosis
Non-infectious causes considered before the return of the HSV PCR result included inflammatory and autoimmune conditions such as Behçet’s disease, sarcoidosis, lupus and ANCA-related vasculitis. Infectious agents considered included HSV, CMV, VZV, Borrelia burgdorferi, Mycobacterium tuberculosis, Treponema pallidum and Toxoplasma gondii.
Treatment
The patient was treated with 2 weeks of high-dose intravenous acyclovir (10 mg/kg/dose every 8 hours) and intravitreous foscarnet, followed by 2 weeks of high-dose oral valacyclovir (2 g three times per day). Patient was then placed on valacyclovir 1 g three times per day indefinitely to reduce the risk of involvement of the contralateral eye.
Outcome and follow-up
Left eye had only light perception at 2-month follow-up. Funduscopic exam of left eye revealed severe retinal scarring with retinal detachment centrally. At the 6-month assessment, the right eye remained unaffected and the left-sided retinal findings remained unchanged.
Discussion
ARN is a rapidly progressive process that typically involves one eye at its onset. Surveys in the United Kingdom revealed an annual ARN incidence of 0.5–0.63 cases per million people.1 2 Patients with ARN initially present with mild-to-moderate blurred vision, visual loss, red-eye, floaters, decreased peripheral vision, periorbital pain and photophobia. If untreated, ARN may involve the contralateral eye in up to 70% of patients.3 Contralateral involvement usually occurs within a few months but may occur years later.3 4
The diagnosis of ARN is made clinically. The American Uveitis Society established the following diagnostic criteria to diagnose ARN: (a) retinal necrosis with discrete borders, located in the periphery; (b) rapid progression in the absence of antiviral therapy; (c) circumferential spread; (d) occlusive vasculopathy and (e) prominent vitritis and/or anterior chamber inflammation.5 Identification of a virus is not required for the diagnosis, but viral identification by PCR is usually sought in practice. PCR is a highly specific and sensitive test and is useful for the detection of viruses in vitreous fluid.6 ARN is most commonly caused by VZV, followed by HSV-1 and HSV-2.7 CMV is a rare cause of ARN but must be considered in immunocompromised patients.
Although uncommon, other cases of ARN associated with HSV encephalitis have been reported. A search of English PubMed articles about patients diagnosed with both ARN and HSV-1 or HSV-2 encephalitis revealed 34 case reports that are summarised in table 1.8-35 Median age was 44.5 years (range of 23 days to 68 years old) and male gender was reported in 24/36 (67%) cases. Bilateral ARN at the onset was detected in eight cases (24%). Concurrent diagnosis of ARN and encephalitis was noted in 13/34 (38%) cases. For the 21 cases that developed ARN after the diagnosis of encephalitis, the time interval between the diagnosis of encephalitis and ARN ranged from 1 month to 30 years with a median time interval of 6 years. It is hypothesised that ARN occurring in a delayed fashion after herpes simplex encephalitis is due to reactivation of latent virus with anterograde axonal spread from the trigeminal ganglion to the retina. Interestingly, HSV encephalitis after ARN has also been reported.36 The Infectious Diseases Society of America Clinical Practice Guidelines for Encephalitis recommend high dose intravenous acyclovir for 14–21 days for herpes simplex encephalitis.37 While relapse rates as high as 5% have been reported, the guidelines do not comment on the benefit of suppression/secondary prophylaxis to prevent relapse, or specifically for delayed involvement of the retina. Our review of published case reports demonstrates visual outcomes of finger counting, light perception only, no light perception or retinal detachment in 18/36 (50%) cases at the end of treatment or at subsequent follow-up. The time points of final visual assessments varied between the case reports, so data are inadequate to know the ultimate prognosis for HSV ARN after encephalitis.
Table 1.
Cases of acute retinal necrosis after herpetic encephalitis
| Age | Sex | Laterality | Interval | HSV type | Antiviral therapy of acute phase | Duration | Chronic suppression | Duration | Visual outcome at the end of treatment or at follow-up | |
| (Year-old) | (oral) | |||||||||
| Bristow et al 9 | 59 | M | Unilateral | 1 month | 1 | Acyclovir(IV) | 2 weeks | Valacyclovir | 6 months | Counting fingers in the infected eye. Visual acuity 6/5 in the other eye |
| Gain et al 11 | 40 | M | Unilateral | Simultaneously | 1 | Acyclovir (IV) | 1 week | N/A | N/A | ‘Visual function was lost’ |
| Foscavir (IV) | 3 weeks | |||||||||
| Gaynor et al 12 | 45 | F | Unilateral | 6 years | N/A | Acyclovir (IV) | N/A | Acyclovir | N/A | ‘Visual acuity 20/40’ |
| Gupta et al 13 | 25 day | M | Unilateral | Simultaneously | 2 | Acyclovir (IV) | 3 weeks | Acyclovir | 6 weeks | N/A |
| (Twin 1) | ||||||||||
| 25 day | M | Bilateral | Simultaneously | 2 | Acyclovir(IV) | 5 days* | N/A | N/A | N/A | |
| (Twin 2) | ||||||||||
| Hirota et al 14 | 47 | M | Bilateral | Simultaneously | N/A | Acyclovir (IV) | N/A | N/A | N/A | ‘All sight was lost’ |
| Kamel et al 15 | 46 | M | Unilateral | 20 years | 1 | Acyclovir (IV) | N/A | N/A | N/A | ‘No light perception in the infected eye’ |
| Klein and Lefebvre16 | 67 | F | Unilateral | 3 months | 1 | Acyclovir (IV) | N/A | N/A | N/A | ‘Visual acuity 6/15–6/12’ |
| 3 years | N/A | None | None | None | None | |||||
| 12 years | 1 | Acyclovir (IV) | 2 weeks | Acyclovir | 5 months | |||||
| Kianersi et al 17 | 25 | M | Unilateral | 1 month | 2 | Acyclovir (IV) | 10 days | Acyclovir | N/A | ‘No retinal detachment in infected eye. No involvement in the other eye’ |
| Kim et al 18 | 57 | M | Unilateral | Simultaneously | 1 | Acyclovir (IV) | 2 weeks | N/A | N/A | ‘No light perception in the infected eye’ |
| Kychenthal et al 19 | 25 day | N/A | Unilateral | Simultaneously | 2 | Acyclovir (IV) | N/A | N/A | N/A | N/A |
| Landry et al 20 | 9 | F | Unilateral | 9 years | 2 | Acyclovir (IV) | 2 weeks | Valacyclovir | 1 year | ‘Visual acuity 20/60’ |
| Levinson et al21 | 16 | F | Unilateral | 16 years | N/A | Acyclovir (IV) | N/A | Acyclovir | N/A | ‘Small peripheral retinal detachment in the infected eye’ |
| Liang et al 22 | 44 | M | Bilateral | Simultaneously | 1 | Acyclovir (IV) | N/A | N/A | N/A | ‘Visual acuity was markedly improved’ |
| Maertzdorf et al 23 | 68 | M | Unilateral | 9 months | 1 | Acyclovir (IV) | N/A | Valacyclovir | N/A | ‘Visual acuity of 0.5’ |
| 64 | F | Unilateral | 9 days | 1 | Acyclovir (IV) | N/A | Valacyclovir | N/A | ‘Finger counting at 3 m’ | |
| Nolan et al 24 | 45 | M | Bilateral | 1.5 months | 2 | Acyclovir (IV) | 4 weeks | N/A | N/A | N/A |
| Ogura et al 25 | 55 | M | Bilateral | Simultaneously | 2 | Acyclovir (IV) | 3 weeks | Acyclovir | 4 weeks | ‘Finger counting in left eye and light perception in right eye’ |
| +intravitreous foscarnet | ||||||||||
| Okafor et al 8 | 30 | M | Unilateral | 30 years | N/A | Acyclovir (IV) | N/A | Valacyclovir | 1 year | ‘The infected eye became phthisical’. The other eye remained unaffected |
| 27 | M | Unilateral | 27 years | N/A | Acyclovir (IV) | N/A | Valacyclovir | 6 months | ‘Stable visual acuity’ | |
| 12 | M | Unilateral | 12 years | N/A | Acyclovir (IV) | N/A | Valacyclovir | N/A | ‘Light perception in the infected eye. The other eye remained unaffected’ | |
| Pavesio et al 26 | 27 | F | Unilateral | 7 years | N/A | Acyclovir (IV) | 1 week | Acyclovir | Indefinitely | ‘The other eye remained uninvolved’ |
| Acyclovir (oral) | 11 weeks | |||||||||
| 17 | M | Unilateral | 17 years | N/A | Acyclovir (IV) | 1 week | Acyclovir | Indefinitely | ‘Hand movement in the infected eye’ | |
| Acyclovir (oral) | 11 weeks | |||||||||
| Perry et al 27 | 64 | F | Bilateral | Simultaneously | 2 | Acyclovir (IV) | N/A | N/A | N/A | ‘No light perception in the infected eye’ |
| Preiser et al 28 | 55 | M | Unilateral | 6 years | N/A | Acyclovir (IV) | N/A | N/A | N/A | ‘Light perception in the infected eye’ |
| Rao et al 29 | 45 | M | Unilateral | 10 months | 1 | Acyclovir (IV) | 3 weeks | Valacyclovir | N/A | ‘Visual acuity 6/60 in the infected eye’ |
| Ren et al 35 | 23 day | M | Bilateral | Simultaneously | 2 | Acyclovir (IV) | N/A | Acyclovir | 6 months | ‘Visual evoked potential was normal and no difficulty in following light’ |
| +intravitreous ranibizumab | ||||||||||
| Schlingemann et al 30 | 28 | F | Unilateral | Simultaneously | 2 | Acyclovir (IV) | 2 weeks | Valacyclovir | 2 months | ‘Remained stable vision’ |
| Shahi10 | 40 | M | Unilateral | 20 years | N/A | Acyclovir (IV) | N/A | N/A | N/A | ‘Counting fingers in the infected eye. The other eye was not involved.’ |
| Curtis and Mandava33 | 3 | M | Unilateral | 1.5 year | 1 | Acyclovir (IV) | N/A | Acyclovir | N/A | N/A |
| Verma et al 31 | 50 | M | Unilateral | 3.5 years | N/A | Acyclovir (IV) | Lost follow-up | N/A | N/A | N/A |
| Yamamoto et al 34 | 63 | M | Unilateral | 1 month | N/A | Acyclovir (IV) | 2 weeks | N/A | N/A | ‘Retinal detachment and necrosis in the infected eye’ |
| Zhou et al 32 | 47 | F | Bilateral | Simultaneously | 1 | Acyclovir (IV) | 15 days | N/A | N/A | ‘Light perception in both eyes’ |
| Our case | 60 | M | Unilateral | 2 years | 1 | Acyclovir (IV) | 2 weeks | Valacyclovir | Indefinitely | ‘Light perception in the infected eye. The other eye remained unaffected.’ |
| High dose valacyclovir | 2 weeks |
*Patient expired.
HSV, herpes simplex virus; IV, intravenous.
High-dose intravenous acyclovir is the regimen of choice for acute ARN due to HSV. Regression of retinal lesions and reduction in contralateral eye involvement from 70% to 13% has been noted with intravenous acyclovir3compared with no antiviral therapy.38 Several studies reported success using oral antiviral therapy for ARN, but there are no studies that directly compare oral with intravenous therapy.6 39 Comparative studies that assessed the addition of intravitreal foscarnet found benefits of reduction in severe vision loss or retinal detachment with its use.6 40 41
After treatment of the acute phase of ARN, up to 3–6 months of chronic suppression with an active oral agent is usually recommended to reduce the risk of involvement of the contralateral eye.6 When there is subsequent involvement of the contralateral eye, it can occur as early as 5 weeks and as late as 19 years10 after the first ARN diagnosis. There is no guidance based on large-scale data to guide decisions on the duration and dose of oral suppression/preventive therapy. Some experts recommend lifelong antiviral prophylaxis given that the onset of contralateral disease can occur after decades, at least for patients with a history of neonatal HSV encephalitis.8 Our literature review revealed marked variation in the duration of oral chronic suppression used in individual cases (table 1), though most of our reviewed case reports were published before the recent treatment guidance provided by the American Academy of Ophthalmology.6 Lifelong chronic antiviral suppression was recommended to our patient since this was his second severe HSV-1 infection.
Visual outcome after ARN is often poor, owing to complications such as retinal detachment and ischaemic vasculopathy of the optic nerve or macula. In 1982 Fisher et al reported that 76% of 55 eyes affected by ARN had a final visual acuity of 20/400 or worse, and 75% of eyes developed retinal detachment.42 However, these patients did not receive any antiviral therapy as the aetiology was not known then. In 2017, Butler et al reported that 59% of eyes affected by ARN progressed to ≤20/200 visual acuity, half of which were light perception or non-light perception. They concluded the outcome of ARN in the past decade remains poor despite the use of aggressive antiviral therapy.43 The same author demonstrated that if the disease is arrested with less than 25% retinal involvement, the rate of retinal detachment is low. It is not clear whether the visual outcomes after ARN subsequent to HSV encephalitis are different from patients with ARN not associated with prior encephalitis.
Our report demonstrates that visual loss in a patient with historical or active HSV encephalitis warrants a high index of suspicion for ARN. Evaluation for and treatment of ARN in the early stage of illness is critical to reduce the risk of permanent visual impairment.
Learning points.
Acute retinal necrosis (ARN) is a rapidly progressive necrotising process, mainly affecting immunocompetent patients and usually caused by herpes viruses.
Acute visual loss occurring concurrent with or subsequent to herpes virus encephalitis warrants high degree of suspicion for ARN.
Prompt diagnosis and treatment of ARN is necessary to improve visual outcomes.
Intravitreal foscarnet combined with systemic antiviral therapy is more effective than systemic therapy alone for ARN.
Long term suppression with an antiviral agent is recommended to prevent involvement of the contralateral eye which may occur years after the initial insult though there is no consensus on the duration or dose.
Acknowledgments
The authors sincerely thank the Department of Ophthalmology at the University of Iowa Hospitals and Clinics for retina pictures (especially Dr Laith Kadasi, Dr Benjamin King, Dr Alison Bozung, Dr Alec Amram, Dr Sam Abbassi, Dr James Folk, and Dr Elliott Sohn).
Footnotes
Contributors: TK wrote the first draft of the manuscript. JS, PS and JM critically reviewed and revised the manuscript. All authors read and approved the final paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Muthiah MN, Michaelides M, Child CS, et al. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol 2007;91:1452–5. 10.1136/bjo.2007.114884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cochrane TF, Silvestri G, McDowell C, et al. Acute retinal necrosis in the United Kingdom: results of a prospective surveillance study. Eye 2012;26:370–8. 10.1038/eye.2011.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palay DA, Sternberg P, Davis J, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol 1991;112:250–5. 10.1016/S0002-9394(14)76725-X [DOI] [PubMed] [Google Scholar]
- 4. Tibbetts MD, Shah CP, Young LH, et al. Treatment of acute retinal necrosis. Ophthalmology 2010;117:818–24. 10.1016/j.ophtha.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 5. Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol 1994;117:663–7. [DOI] [PubMed] [Google Scholar]
- 6. Schoenberger SD, Kim SJ, Thorne JE, et al. Diagnosis and Treatment of Acute Retinal Necrosis: A Report by the American Academy of Ophthalmology. Ophthalmology 2017;124:382–92. 10.1016/j.ophtha.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 7. Lau CH, Missotten T, Salzmann J, et al. Acute retinal necrosis features, management, and outcomes. Ophthalmology 2007;114:756–62. 10.1016/j.ophtha.2006.08.037 [DOI] [PubMed] [Google Scholar]
- 8. Okafor K, Lu J, Thinda S, et al. Acute Retinal Necrosis Presenting in Developmentally-delayed Patients with Neonatal Encephalitis: A Case Series and Literature Review. Ocul Immunol Inflamm 2017;25:563–8. 10.3109/09273948.2016.1160131 [DOI] [PubMed] [Google Scholar]
- 9. Bristow EA, Cottrell DG, Pandit RJ. Bilateral acute retinal necrosis syndrome following herpes simplex type 1 encephalitis. Eye 2006;20:1327–30. 10.1038/sj.eye.6702196 [DOI] [PubMed] [Google Scholar]
- 10. Shahi SK. Acute retinal necrosis results in low vision in a young patient with a history of herpes simplex virus encephalitis. Clin Exp Optom 2017;100:208–13. 10.1111/cxo.12449 [DOI] [PubMed] [Google Scholar]
- 11. Gain P, Chiquet C, Thuret G, et al. Herpes simplex virus type 1 encephalitis associated with acute retinal necrosis syndrome in an immunocompetent patient. Acta Ophthalmol Scand 2002;80:546–9. 10.1034/j.1600-0420.2002.800517.x [DOI] [PubMed] [Google Scholar]
- 12. Gaynor BD, Wade NK, Cunningham ET. Herpes simplex virus type 1 associated acute retinal necrosis following encephalitis. Retina 2001;21:688–90. 10.1097/00006982-200112000-00028 [DOI] [PubMed] [Google Scholar]
- 13. Gupta A, Rani PK, Bagga B, et al. Bilateral herpes simplex-2 acute retinal necrosis with encephalitis in premature twins. J Aapos 2010;14:541–3. 10.1016/j.jaapos.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 14. Hirota K, Akimoto M, Katsura T. Bilateral acute retinal necrosis after herpetic meningitis. Clin Ophthalmol 2012;6:551–3. 10.2147/OPTH.S29966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamel OR, Galloway GD, Trew DR. Delayed onset acute retinal necrosis 20 years following herpetic encephalitis. Eye 2000;14 Pt 5(Pt 5):788–9. 10.1038/eye.2000.207 [DOI] [PubMed] [Google Scholar]
- 16. Klein A, Lefebvre P. Three consecutive episodes of acute retinal necrosis due to herpes simplex-1 over twelve years following herpetic encephalitis. Ocul Immunol Inflamm 2007;15:411–3. 10.1080/09273940701662510 [DOI] [PubMed] [Google Scholar]
- 17. Kianersi F, Masjedi A, Ghanbari H. Acute Retinal Necrosis after Herpetic Encephalitis. Case Rep Ophthalmol 2010;1:85–9. 10.1159/000321708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim SJ, Kang SW, Joo EY. An unusual case of herpes simplex viral encephalitis following acute retinal necrosis after administration of a systemic steroid. J Epilepsy Res 2012;2:21–4. 10.14581/jer.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kychenthal A,Coombes A, Greenwood J, et al. Bilateral acute retinal necrosis and herpes simplex type 2 encephalitis in a neonate. Br J Ophthalmol 2001;85:625f–625. 10.1136/bjo.85.5.625f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Landry ML, Mullangi P, Nee P, et al. Herpes simplex virus type 2 acute retinal necrosis 9 years after neonatal herpes. J Pediatr 2005;146:836–8. 10.1016/j.jpeds.2005.02.025 [DOI] [PubMed] [Google Scholar]
- 21. Levinson RD, Reidy R, Chiu MT. Acute retinal necrosis after neonatal herpes encephalitis. Br J Ophthalmol 1999;83:123a–4. 10.1136/bjo.83.1.123a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang ZG, Liu ZL, Sun XW, et al. Viral encephalitis complicated by acute retinal necrosis syndrome: A case report. Exp Ther Med 2015;10:465–7. 10.3892/etm.2015.2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maertzdorf J, Van der Lelij A, Baarsma GS, et al. Herpes simplex virus type 1 (HSV-1)--induced retinitis following herpes simplex encephalitis: indications for brain-to-eye transmission of HSV-1. Ann Neurol 2001;49:104–6. [DOI] [PubMed] [Google Scholar]
- 24. Nolan RC, Van Gessel H, Byrne M. An unusual complication of chemotherapy: herpes simplex meningoencephalitis and bilateral acute retinal necrosis. Clin Oncol 2004;16:81–2. 10.1016/j.clon.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 25. Ogura H, Fukae J, Kimura S, et al. Acyclovir resistant acute herpes simplex encephalitis associated with acute retinal necrosis: A case report and review of the literature. Rinsho Shinkeigaku 2017;57:230–3. 10.5692/clinicalneurol.cn-000959 [DOI] [PubMed] [Google Scholar]
- 26. Pavésio CE, Conrad DK, McCluskey PJ, et al. Delayed acute retinal necrosis after herpetic encephalitis. Br J Ophthalmol 1997;81:415a–6. 10.1136/bjo.81.5.415a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perry JD, Girkin CA, Miller NR, et al. Herpes simplex encephalitis and bilateral acute retinal necrosis syndrome after craniotomy. Am J Ophthalmol 1998;126:456–60. 10.1016/S0002-9394(98)00108-1 [DOI] [PubMed] [Google Scholar]
- 28. Preiser W, Doerr HW, Buxbaum S, et al. Acute retinal necrosis six years after herpes simplex encephalitis: an elusive immune deficit suggested by insufficient test sensitivity. J Med Virol 2004;73:250–5. 10.1002/jmv.20083 [DOI] [PubMed] [Google Scholar]
- 29. Rao V, Biswas J, Lingam G. Real-time polymerase chain reaction in acute retinal necrosis following encephalitis. Indian J Ophthalmol 2018;66:322–4. 10.4103/ijo.IJO_748_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schlingemann RO, Bruinenberg M, Wertheim-van Dillen P, et al. Twenty years' delay of fellow eye involvement in herpes simplex virus type 2-associated bilateral acute retinal necrosis syndrome. Am J Ophthalmol 1996;122:891–2. 10.1016/S0002-9394(14)70390-3 [DOI] [PubMed] [Google Scholar]
- 31. Verma L, Venkatesh P, Satpal G, et al. Bilateral necrotizing herpetic retinopathy three years after herpes simplex encephalitis following pulse corticosteroid treatment. Retina 1999;19:464–6. 10.1097/00006982-199919050-00023 [DOI] [PubMed] [Google Scholar]
- 32. Zhou C, Zhu L, Fang S. Fulminant bilateral acute retinal necrosis syndrome associated with viral encephalitis: A case report. Exp Ther Med 2016;12:2227–9. 10.3892/etm.2016.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Curtis TH, Mandava N. Acute retinal necrosis as a late sequela of herpes simplex type 1 encephalitis in a child. J Aapos 2007;11:509–10. 10.1016/j.jaapos.2007.02.010 [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto S, Nakao T, Kajiyama K. Acute retinal necrosis following herpes simplex encephalitis. Arch Neurol 2007;64:283 10.1001/archneur.64.2.283 [DOI] [PubMed] [Google Scholar]
- 35. Ren ZX, Xu F, Yao ZW, et al. Acute retinal necrosis in a neonate with HSV II encephalitis. Pediatr Neonatol 2018. 10.1016/j.pedneo.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 36. Miserocchi E, Iuliano L, Fogliato G, et al. Bilateral Acute Retinal Necrosis: Clinical Features and Outcomes in a Multicenter Study. Ocul Immunol Inflamm 2018:1–9. 10.1080/09273948.2018.1501494 [DOI] [PubMed] [Google Scholar]
- 37. Tunkel AR, Glaser CA, Bloch KC, et al. Infectious Diseases Society of America. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008;47:303–27. 10.1086/589747 [DOI] [PubMed] [Google Scholar]
- 38. Blumenkranz MS, Culbertson WW, Clarkson JG, et al. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology 1986;93:296–300. 10.1016/S0161-6420(86)33740-0 [DOI] [PubMed] [Google Scholar]
- 39. Taylor SR, Hamilton R, Hooper CY, et al. Valacyclovir in the treatment of acute retinal necrosis. BMC Ophthalmol 2012;12:48 10.1186/1471-2415-12-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong R, Pavesio CE, Laidlaw DA, et al. Acute retinal necrosis: the effects of intravitreal foscarnet and virus type on outcome. Ophthalmology 2010;117:556–60. 10.1016/j.ophtha.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 41. Yeh S, Suhler EB, Smith JR, et al. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome. Ophthalmic Surg Lasers Imaging Retina 2014;45:399–407. 10.3928/23258160-20140908-02 [DOI] [PubMed] [Google Scholar]
- 42. Fisher JP, Lewis ML, Blumenkranz M, et al. The acute retinal necrosis syndrome. Part 1: Clinical manifestations. Ophthalmology 1982;89:1309–16. [DOI] [PubMed] [Google Scholar]
- 43. Butler NJ, Moradi A, Salek SS, et al. Acute Retinal Necrosis: Presenting Characteristics and Clinical Outcomes in a Cohort of Polymerase Chain Reaction-Positive Patients. Am J Ophthalmol 2017;179:179–89. 10.1016/j.ajo.2017.05.006 [DOI] [PubMed] [Google Scholar]