Abstract
Necrotising autoimmune myopathy (NAM) is characterised by a common phenotype of profound symmetrical proximal muscle weakness, elevated creatine kinase levels, irritable myopathy on electromyography and histological findings of myocyte necrosis and regeneration without remarkable inflammation. NAM is associated with autoimmune antibodies including anti-3-hydroxy-3-methylglutaryl-coenzyme receptor, which is strongly associated with statin use. We report a case of statin-associated NAM with an atypical presentation of severe oropharyngeal dysphagia and no remarkable proximal muscle weakness at initial presentation but with rapid progression to severe quadriparesis in weeks. This case expands the spectrum of presentation patterns of this rare disease and highlights the need for a high index of suspicion in patients with a remote history of statin use.
Keywords: connective tissue disease, drugs: musculoskeletal and joint diseases, musculoskeletal syndromes
Background
Necrotising autoimmune myopathy (NAM) is a rare form of idiopathic inflammatory myopathy.1 2 Statin-associated NAM, a subtype has only been described in the literature in about 550 cases with an estimated incidence of two to three cases in 1 000 000 patients exposed to statins per year.3 We describe a case of statin-associated NAM presenting as severe oropharyngeal dysphagia and acutely progressing to severe symmetrical muscle weakness while receiving treatment with high-dose corticosteroids. This case aims to increase awareness of an atypical presentation pattern of this rare disease, highlights the need for a high index of suspicion in patients with a remote history of statin use and the importance of early recognition and having a low threshold to start aggressive immunosuppressive therapy.
Case presentation
A 71-year-old African-American man with a medical history of hypertension, peripheral artery disease and a remote history of simvastatin use, which was changed to high-dose atorvastatin and later discontinued due to abnormal liver function tests, presented with 2 weeks of dysphagia, dysphonia, hoarseness, excess salivation and a 2.7 kg weight loss. He described dysphagia to both solids and liquids with a feeling of food getting stuck in his throat. No myalgia was reported, but he mentioned he had mild fatigue and weakness which he attributed to his inability to eat.
On examination, the patient was normotensive with blood pressure of 126/70 mm Hg, heart rate of 60 bpm and a respiratory rate of 18/min. He had severe oropharyngeal dysphagia with pooled secretions in his oropharynx on swallow evaluation. Muscle strength was 5/5 in bilateral upper and lower extremities with normal gait.
Investigations
Given the nature of his symptoms, a mechanical obstruction due to a neoplasm was suspected. CT scan of his head, neck, chest, abdomen and pelvis were essentially negative except for peri-carinal lymph nodes, which were less than 1 cm. Upper gastrointestinal endoscopy revealed normal oesophagus and oesophagram could not be performed due to severe risk of aspiration. Flexible laryngoscopy showed normal vocal cords with no laryngeal masses or lesions. Non-contrast brain MRI performed to evaluate brainstem stroke was unremarkable, and there was also no evidence of a demyelinating disorder on the MRI although suspicion for this aetiology was low based on the patient’s presentation and isolated symptoms. Due to absence of mechanical causes of oropharygeal dysphagia and the patient’s atypical presentation, electromyography (EMG)/nerve conduction study was done. This test revealed evidence of myopathy with features of muscle membrane irritability suggestive of necrotising or inflammatory myopathy.
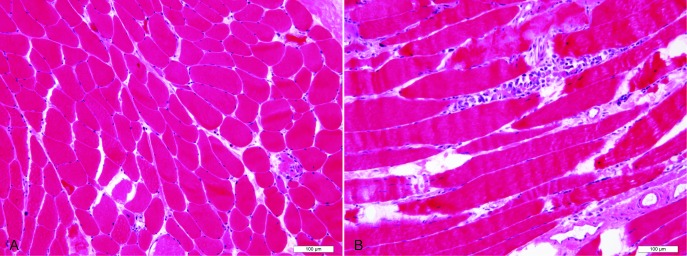
With this new finding, myositis workup was initiated. Pertinent positives were creatine kinase (CK) of 15 595 u/L (normal, 25 to 90), C-reactive protein of 9.76 mg/dL (normal, 0 to 0.5), urine myoglobin >12 700 mcg/L (normal, <28) and aldolase of 131.5 u/L (normal, 1 to 8 u/L). Of note, alanine aminotransferase, aspartate aminotransferase and lactate dehydrogenase were persistently elevated throughout his admission ranging from 266 to 581 u/L (normal, 5 to 35), 169 to 370 u/L (normal, 0 to 40) and 589 to 894 u/L (normal, 85 to 210), respectively. Enzyme-linked immunosorbent assay revealed anti- 3-hydroxy-3-methylglutaryl-coenzyme receptor (HMGCR) antibodies >200 units. Biopsy of the left quadriceps muscle showed active myopathic changes including fibre size variation, individual fibre necrosis and regenerating fibres without significant inflammatory nodule or rimmed vacuoles (figure 1). Immunohistochemical staining for the major histocompatibility complex class 1 showed membranous and cytoplasmic staining of the necrotising myopathic fibres with no significant background inflammation. Similar features were seen on with complement C5-9 staining. The left quadriceps muscle was chosen for muscle biopsy to avoid misinterpretation as the EMG was done on right side of the body.
Figure 1.
Quadriceps muscle biopsy (haematoxylin and eosin stain). (A) Transverse section showing fibre size variation with randomly admixed atrophic fibres and scattered necrotic fibres. (B) Longitudinal section showing a necrotic fibre with numerous intra-fibre macrophages. Scale bars: 100 micrometres.
Differential diagnosis
The differential diagnoses for the orophayngeal dysphagia were neoplasm involving the larynx or upper oesophagus, isolated brainstem lesions, myopathy including viral myositis, inclusion body myositis, polymyositis and necrotising autoimmune myopathy and myasthenia gravis. Mechanical causes including neoplasms were ruled out due to negative CT scans of the head, neck and chest. Brainstem lesion was ruled out due to negative brain MRI and laboratory results suggestive of alternative aetiology. Viral myositis was unlikely in this patient due to the atypical symptoms at presentation and the absence of viral illness prior to this presentation. Inclusion body myositis and polymyositis were ruled out based on the characteristic muscle biopsy findings, the high levels of HMGCR antibodies which are very specific for HMGCR-associated NAM and the absence of anti-Jo-1. Myasthenia gravis was ruled out in the setting of negative acetylcholine receptor antibodies and EMG findings not typical of myasthenia gravis.
Treatment
In view of the clinical symptoms and positive investigations, a diagnosis of NAM was made. The patient was started on prednisone 60 mg daily, and a percutaneous endoscopic gastrostomy tube was placed for enteral feeding pending improvement of oropharyngeal dysphagia.
Outcome and follow-up
He was subsequently discharged, but presented 3 weeks later with persistent oropharyngeal dysphagia, severe progressive muscle weakness and severe quadriparesis. On examination, muscle strength was 1/5 in the neck flexors and proximal upper and lower extremities, and 2/5 in distal upper and lower extremities. CK was elevated at 6329 u/L (normal, 25 to 90 u/L), but lower compared with the initial admission. He was admitted and treated with intravenous immunoglobulin (IVIG) at 1 g/kg/day for 2 days, pulse dose steroids (methylprednisolone, 1000 mg) daily for 3 days and mycophenolate 500 mg twice daily. Physical therapy was involved to improve functional mobility and some improvement in muscle weakness was noted while on admission. He was transferred to a subacute rehabilitation facility for physical therapy and placed on prednisone 60 mg daily, mycophenolate mofetil 500 mg twice daily, trimethoprim and sulfamethoxazole for pneumocystis carinii pneumonia prophylaxis and pantoprazole 40 mg daily with a plan for monthly IVIG and rheumatology follow-up as outpatient. Three weeks after discharge, we were notified that the patient passed away in the subacute rehabilitation facility. Cause of death is unknown.
Discussion
Idiopathic inflammatory myopathies (IIM) are heterogeneous acquired muscle diseases characterised by proximal muscle weakness, elevated muscle enzymes, irritable myopathy on EMG and characteristic histopathological features. IIM is currently classified into five main types based on the clinical and phenotype specific autoantibody group; dermatomyositis, polymyositis, inclusion body myositis, overlap myositis and NAM.3 4
NAM is associated with autoimmune antibodies directed against signal recognition particle (SRP) and HMGCR in two-thirds of patients, while a third of patients are antibody negative.4 5 NAM can be idiopathic, but has also been reported in association with paraneoplastic syndromes, connective tissue disease such as scleroderma, viral infections such as hepatitis C and HIV and in the setting of certain drug exposures such as statins.1
Anti-HMGCR-positive NAM is strongly associated with statin use, likely because statins upregulate HMGCR expression, a target of the immune-mediated injury in NAM. This may also explain the reason why the muscle injury persists even after the statin is discontinued.6 Genetic susceptibility and other unknown factors may also play a role, as not all patients taking a statin develop NAM.6 Unlike other statin-associated myopathies, NAM usually persists after discontinuation of statin or as in the present case, may present after discontinuation of statin.3 When onset follows discontinuation of statins, symptom onset has been reported 0.5 to 20 months following discontinuation of statin,1 7 which is similar to our patient who developed symptoms 13 months after stopping atorvastatin due to elevation in liver enzymes.
The common presentation of NAM is proximal muscle weakness and myalgia. Atypical presentations are relatively rare and include dysphagia (as in our patient), Raynaud’s phenomenon and interstitial lung disease.1 3 Symmetrical limb weakness in patients with anti-HMGCR antibody can develop acutely like in our patient or can be subacute to chronic. It is usually less severe and muscle strength ≤2/5 is not commonly observed.1 8 More severe muscle weakness is often reported in patients with SRP antibody myositis.1 8 Diagnosis can be challenging especially in the patients with an atypical presentation. In addition to the clinical symptoms and positive serology (anti-HMGCR or anti-SRP), patients typically have elevated creatine kinase, irritable myopathic features on electromyography such as spontaneous fibrillation potentials and positive sharp waves. MRI when done, shows widespread muscle oedema, with focal or diffuse abnormal signal in the trunk and limb muscles. Characteristic features on histology are myocyte necrosis and regeneration without significant inflammation.1 5 9 10 While histology is required to confirm diagnosis in the autoantibody negative NAM, specific histological features are not required to classify patients with autoantibody positive NAM.4
Best treatment options and long-term prognosis for NAM are incompletely defined. There are currently no randomised control trials to guide treatment protocols; thus recommendations are based on expert opinion from specialists experience, retrospective studies or case series. Initial treatment is usually with high-dose corticosteroids and majority of the patients require aggressive treatment with two or more immunotherapeutic agents including IVIG, methotrexate, mycophenolate mofetil, rituximab, azathioprine and occasionally plasmapheresis.1 9 11 Of note, our patient developed more severe symptoms after starting prednisone, requiring more aggressive immunosuppression. Studies have documented improvement in symptoms within the first 3 months of treatment and a high relapse rate when patients are weaned off corticosteroids or when immunotherapy is discontinued.1 11
NAM is rare and often misdiagnosed. Diagnosis may be challenging especially in patients presenting with atypical clinical features. Physicians should maintain a high index of suspicion for NAM in patients with a remote history of statins use discontinued for reasons other than myopathy and should consider early aggressive immunosuppressive therapy.
Learning points.
Oropharyngeal dysphagia is an uncommon first presentation for necrotising autoimmune myopathy (NAM).
A complete workup for other causes of oropharyngeal dysphagia should be performed while considering myopathy as a potential cause.
There should be a high index of suspicion for NAM in patients with a history of statin use.
Consider early aggressive immunosuppressive therapy in rapidly progressing cases of NAM, although additional information is required on best treatment protocols in these patients.
Acknowledgments
The authors are thankful to Manuel F Utset, MD, PhD (Pathologist, Department of Pathology, University of Illinois at Chicago) for his help with providing appropriate histological figures for this manuscript.
Footnotes
Contributors: All authors had access to the case and a role in writing the case report. OA: reviewed literature, wrote and edited the case summary, introduction and case discussion. MM: helped with planning, literature review and wrote the case presentation. NA: reviewed literature and edited the case report. BM: critically reviewed the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Basharat P, Christopher-Stine L. Immune-Mediated Necrotizing Myopathy: Update on Diagnosis and Management. Curr Rheumatol Rep 2015;17:72-015-0548-6 10.1007/s11926-015-0548-6 [DOI] [PubMed] [Google Scholar]
- 2. Orphanet. Immune-mediated necrotizing myopathy. 2014. Available at https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=206569 (Accessed Nov 19 2018).
- 3. Turrin M. Statins Immune-Mediated Necrotizing Myopathy. Clinical Management Issues 2018;12:77–88. [Google Scholar]
- 4. Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, et al. Classification and management of adult inflammatory myopathies. Lancet Neurol 2018;17:816–28. 10.1016/S1474-4422(18)30254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tiniakou E, Christopher-Stine L. Immune-mediated necrotizing myopathy associated with statins: history and recent developments. Curr Opin Rheumatol 2017;29:604–11. 10.1097/BOR.0000000000000438 [DOI] [PubMed] [Google Scholar]
- 6. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011;63:713–21. 10.1002/art.30156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grable-Esposito P, Katzberg HD, Greenberg SA, et al. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010;41:185–90. 10.1002/mus.21486 [DOI] [PubMed] [Google Scholar]
- 8. Watanabe Y, Suzuki S, Nishimura H, et al. Statins and myotoxic effects associated with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies: an observational study in Japan. Medicine 2015;94:e416 10.1097/MD.0000000000000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Essers D, Schäublin M, Kullak-Ublick GA, et al. Statin-associated immune-mediated necrotizing myopathy: a retrospective analysis of individual case safety reports from VigiBase. Eur J Clin Pharmacol 2019;75:409–16. 10.1007/s00228-018-2589-z [DOI] [PubMed] [Google Scholar]
- 10. Bergua C, Chiavelli H, Simon JP, et al. Immune-mediated necrotizing myopathy. Z Rheumatol 2016;75:151–6. 10.1007/s00393-015-0029-3 [DOI] [PubMed] [Google Scholar]
- 11. Hamann PD, Cooper RG, McHugh NJ, et al. Statin-induced necrotizing myositis - a discrete autoimmune entity within the "statin-induced myopathy spectrum". Autoimmun Rev 2013;12:1177–81. 10.1016/j.autrev.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]