Abstract
Retinal degenerative diseases are major causes of untreatable blindness worldwide and efficacious treatments for these diseases are sorely needed. A novel target for treatment of retinal disease is the transmembrane protein Sigma 1 Receptor (Sig1R). This enigmatic protein is an evolutionary isolate with no known homology to any other protein. Sig1R was originally thought to be an opioid receptor. That notion has been dispelled and more recent pharmacological and molecular studies suggest that it is a pluripotent modulator with a number of biological functions, many of which are relevant to retinal disease. This review provides an overview of the discovery of Sig1R and early pharmacologic studies that led to the cloning of the Sig1R gene and eventual elucidation of its crystal structure. Studies of Sig1R in the eye were not reported until the late 1990s, but since that time there has been increasing interest in the potential role of Sig1R as a target for retinal disease. Studies have focused on elucidating the mechanism(s) of Sig1R function in retina including calcium regulation, modulation of oxidative stress, ion channel regulation and molecular chaperone activity. Mechanistic studies have been performed in isolated retinal cells, such as Müller glial cells, microglial cells, optic nerve head astrocytes and retinal ganglion cells as well as in the intact retina. Several compelling studies have provided evidence of powerful in vivo neuroprotective effects against ganglion cell loss as well as photoreceptor cell loss. Also described are studies that have examined retinal structure/function in various models of retinal disease in which Sig1R is absent and reveal that these phenotypes are accelerated compared to retinas of animals that express Sig1R. The collective evidence from analysis of studies over the past 20 years is that Sig1R plays a key role in modulating retinal cellular stress and that it holds great promise as a target in retinal neurodegenerative disease.
1. Introduction
Sigma1 receptor (Sig1R) is an enigmatic molecule involved in a wide range of cellular functions. It is implicated in many diseases, the most prominent being neurodegenerative diseases, accompanied by death of neurons and loss of structural and functional integrity. Sig1R has been recognized increasingly as a novel target for treatment of neurodegenerations [Nguyen et al, 2015]. Given that many retinal diseases are neurodegenerative it is not surprising that Sig1R has been investigated in this tissue. This chapter focuses on our current understanding of the role of Sig1R in retina especially as a target in retinal disease. As a preface to that discussion, a brief overview is provided highlighting the initial discovery of the receptor, clarifying the confusion about its identity (especially the mistaken notion that it was an opioid receptor) and noting its role in other tissues. The balance of the chapter will focus on our understanding of Sig1R in retina.
1.1. Discovery of Sig1R and confusion about its identity
Sigma receptors were first described in 1976, when W.R. Martin and colleagues reported what they believed to be a new class of opioid receptor [Martin et al, 1976]. In this historic study, which has been cited more than 3,000 times, the investigators were evaluating SKF-10,047 (N-allylnormetazocine) and other benzomorphans in the morphine-dependent and non-dependent chronic spinal dog model. The study identified three syndromes, which were attributed to interaction of agonists with three distinguishable opioid receptors (mu, kappa and sigma). Morphine is the prototype agonist for the mu (μ) receptor, ketocyclazocine for the kappa receptor (κ) and SKF-10,047 for the sigma (σ) receptor. The naming of the receptors was derived from the first letter of the compound and was reflected as a Greek symbol, thus sigma (σ) for SKF-10,047. A limitation of Martin’s study was that racemic benzomorphans (i.e. both (+) and (-)-isomers of the compounds) were used in the experiments. Further investigations with enantiomerically pure probe compounds showed that the (+)-isomer of SKF-10,047 produces actions that were not sensitive to opioid antagonists [Vaupel 1983], whereas SKF-10,047 (-)-isomers were sensitive to these antagonists [Young and Khazan, 1984; Khazan et al, 1984]. Thus, Sig1R prefers (+)-benzomorphans, while true opioid receptors bind with high affinity only to (-)-enantiomers.
As the confusion over SKF-10,047 and the purported discovery of a new opioid receptor was dispelled, renewed interest in this receptor suggested that it possessed properties similar to a binding site for phencyclidine (PCP). For a brief period, several reports suggested that Sig1R was identical to the PCP binding site, however this site was eventually localized within the ionophore of the N-methyl-D-aspartate (NMDA) receptor [Mendelsohn et al, 1985; Sircar and Zukin, 1983; Sircar et al, 1986]. Notably, ligands that were selective for the NMDA receptor could only partially displace (+)-SKF-10,047 binding [Wong et al, 1988]. Thus, it became evident that (+)-SKF-10,047 bound to another site, in addition to the NMDA receptor ionophore, which ultimately was identified as Sig1R. For a number of years following these clarifications, Sig1R was defined as a “non-opioid, non-phencyclidine” binding site, because it’s cellular function was unknown.
1.2. Pharmacologic characterization of Sig1R
During the 1980s most of the studies defining properties of Sig1R used pharmacologic strategies. It was during this time period that Sig1R was definitively distinguished from other known receptors. Groundbreaking studies by Tsung-Ping Su confirmed that Sig1R was not an opioid receptor. He performed studies in guinea pig brain and showed that radiolabeled SKF-10,047 binding sites were not accessible to the opioid etorphine [Su, 1981; Su, 1982]. Su established that Sig1Rs had high affinity for (+)-benzomorphans including (+)-pentazocine, (+)-cyclazocine and dextrallorphan, whereas authentic opiates did not have affinity for these etrophine-inaccessible sites. Su demonstrated that Sig1R could bind propranolol (a β-adrenergic blocker), haloperiodol (an antipsychotic), and imipramine (an antidepressant) [Su, 1982]. William Tam and his colleague extended these studies to show that Sig1Rs bound a number of neuroleptic drugs including thioridazine, perphenaxine and chlorpromazine. They also found that H1 antihistamines could bind Sig1Rs [Tam and Cook, 1984].
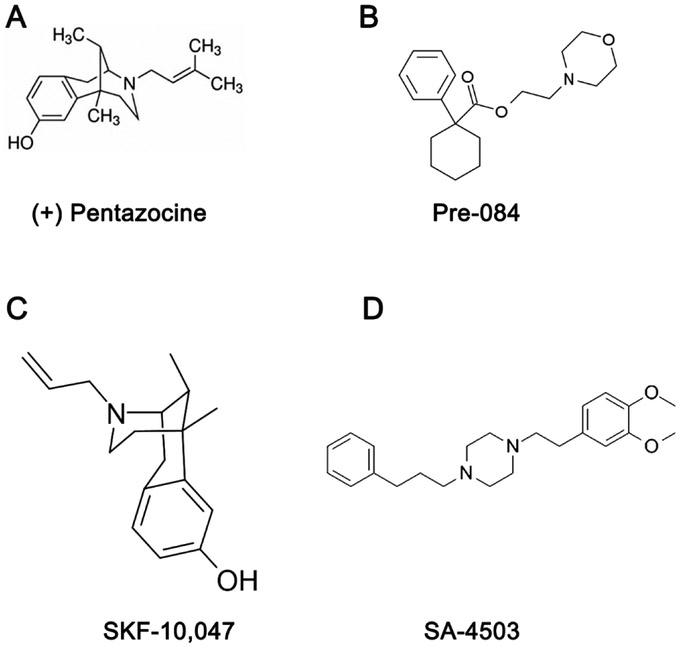
The field of pharmacologic characterization of Sig1R was advanced significantly with the establishment of selective, radiolabeled compounds. Several radioligands such as [3H]SKF-10,047, [3H]haloperidol and [3H](+)-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine (3PPP) provided some insights for Sig1R biology, however interpretation of results were problematic because oftentimes the compounds interacted with opioid, NMDA or dopamine receptors [Largent et al, 1984; Largent et al, 1986, Hjorth et al, 1981]. A major breakthrough in the field occurred with the synthesis and characterization of a tritiated guanidine derivative ([3H]di-otolylguanidine (DTG)), the first truly selective radioligand for Sig1R. Shortly thereafter, [3H]-(+)-pentazocine ((+)-PTZ) was identified as a potent and highly selective Sig1R radioligand [de Costa et al, 1989]. We have used (+)-PTZ extensively in our studies and its structure is shown in Fig. 1 along with the chemical structures of several other Sig1R ligands that have been used in retinal research.
Fig. 1. Chemical structure of several Sig1R ligands that have been used in retinal research.
(A) (+) pentazocine ((+)-PTZ) is a very high affinity Sig1R ligand (IC50 (nM) 2.34; K (nM) 1.62). It is frequently used in binding assays to establish that other compounds can bind to Sig1R (Lever et al, 2006). (B) Pre-084, a high affinity, selective Sig1R ligand (K; (nM) 2.2 nM). (C) SKF-10,047, selective Sig1R ligand (K; (nM) 597 nM). (D) SA-4503, high affinity, Sig1R ligand (IC50 (nM) l7.4; Ki (nM) 4.6).
1.3. Cloning of Sig1R
The discovery of (+)-PTZ as a highly selective ligand for Sig1R facilitated the transition from primarily pharmacologic studies to molecular and functional investigations. In 1996, members of the Glossmann laboratory cloned Sig1R from guinea pig liver [Hanner et al, 1996]. Classical biochemical techniques were employed that involved tracking (+)-PTZ binding activity and the use of degenerate oligonucleotide probes to clone the receptor for a cDNA library. The Sig1R cloned from guinea pig liver is 1857 bp and codes for a protein of 223 amino acids. The protein has an estimated molecular mass of 25.3 kDa. The deduced amino acid sequence was structurally unrelated to any known mammalian protein, although Sig1R was shown to share homology with fungal proteins involved in sterol synthesis. Following the initial report of cloning in guinea pig, Sig1R was cloned in the Ganapathy and Casellas laboratories from human [Kekuda et al, 1996, Jbilo et al, 1997], mouse [Seth et al, 1997], and rat [Seth et al, 1998].
1.4. Elucidation of the crystal structure of Sig1R
The crystal structure of Sig1R eluded investigators for many years. Hydrophobicity analysis initially suggested a hydrophobic segment predicting one transmembrane domain [Hanner et al, 1996], but subsequently a two-pass transmembrane model became more widely accepted [Aydar et al, 2002]. In 2016, a breakthrough discovery from the Kruse lab revealed the crystal structure of Sig1R to be a single-pass transmembrane protein with residues 32–223 forming a carboxy-terminal/cytosolic domain consisting of a β-barrel and two flanking α-helices [Alon et al, 2017b]. Interestingly, the protein crystallizes as an intimately associated triangular trimer with a transmembrane domain in each corner. The description of the approach to determine the crystal structure of Sig1R has been published [Schmidt et al, 2016]. To date, Sig1R remains an evolutionary isolate with no known homologous mammalian counterparts. A recent study using ascorbate peroxidase 2 (APEX2) evaluated the topology of Sig1R in the ER membrane. The APEX2 method of labeling proteins has utility in tagging the subcellular location of proteins because it retains robust peroxidase activity even when used under strong tissue fixation conditions. When evaluated with APEX2 at either the N-or the C-terminus of Sig1R, the N-terminus of the protein faces the cytosolic side of the ER and the C-terminus faces the lumen [Mavylutov et al, 2018]. It may seem peculiar and counterintuitive that the nomenclature for Sig1R includes the numerical “1” suggesting that there are other sigma receptors. Pharmacological studies had suggested a second member of the sigma family, however cloning of Sig1R in 1996 [Hanner et al, 1996] demonstrated that the pharmacologically similar receptor derived from an altogether different, unknown gene. Recent studies have solved the mystery revealing that the protein mistakenly termed “Sig2R” is actually TMEM97, an endoplasmic reticulum-resident transmembrane protein that regulates the sterol transporter Niemann-Pick disease Protein (NPC1) [Alon et al, 2017b].
The cloning of Sig1R launched the molecular era of investigation of Sig1R. This information, coupled with well-characterized pharmacologic tools, advanced our understanding of the tissue, cellular and subcellular distribution of Sig1R, its putative function, possible endogenous ligands and, as mentioned, above its crystal structure.
2. Studies of Sig 1R cellular distribution in ocular tissues
The first studies of tissue expression of Sig1R were reported in brain. A host of labs reported considerable levels of Sig1Rs in brainstem motor nuclei, cerebellum, basal ganglia, limbic regions, and hippocampus [Bouchard and Quirion, 1997; Largent et al, 1986; Seth et al, 2001]. Studies of other tissues revealed widespread distribution of Sig1Rs throughout the body including heart [Novakova et al, 1995], liver and kidney [Seth et al, 1998; Hellewell et al, 1994], spleen [Wolfe et al, 1997] and reproductive organs [Matsuno et al, 1993].
2.1. Studies of Sig1R in lacrimal gland
The earliest hint that Sig1Rs were expressed in tissues related to the visual system, came in the late 1980’s with autoradiographic detection of Sig1R in the lateral geniculate nucleus and superior colliculus [McLean and Weber, 1988]. Shortly thereafter, Schoenwald and colleagues used radiolabeled pharmacologic methods and demonstrated the presence of Sig1R in lacrimocytes isolated from the rabbit lacrimal gland [Schoenwald et al, 1993, Shirolkar et al, 1993]. These investigators proposed targeting Sig1R in the treatment of dry eye syndrome [Schoenwald et al, 1994]. They reported a study of human volunteers administered eyedrops containing the Sig1R ligand AF2975 (N,N-dimethyl-2-phenylethylamine HCl). Topical administration of AF2975 significantly decreased surface tension and stabilized tear film by increasing the concentration of proteins, including lipocalin, in human tears [Schoenwald et al, 1997].
2.2. Initial reports of Sig1R in retina
While the first studies targeting Sig1R as potentially beneficial for eye-related diseases were performed in the lacrimal gland, studies of Sig1R in retina followed quickly. The detection of Sig1R in lacrimocytes prompted Senda and colleagues to prepare retinal membranes from bovine eyes for Sig1R binding assays. Using [3H]-(+)-PTZ and [3H]-DTG, they demonstrated unequivocally the presence of Sig1R in retina [Senda et al, 1997]. Sig1R binding sites were subsequently demonstrated in human [Sharif and Xu, 1999a], rabbit and rat retina [Sharif and Xu, 1999b].
Senda’s group investigated whether administration of Sig1R ligands could protect retinal neurons from excitotoxicity. Excitotoxicity refers to an excessive activation of neuronal amino acid receptors; excitotoxicity, triggered by the amino acid glutamate, is a major pathogenic mechanism of neuron death [Mark et al, 2001]. Studies of primary rat cortical neuron cultures reported Sig1R-mediated neuroprotection against glutamate-induced death [DeCoster et al, 1995]. Senda and colleagues dissociated fetal rat retinas to yield a single cell suspension, which they cultured in media that enhanced viability of neurons, particularly amacrine cells. Amacrine cells are retinal interneurons that are synaptically active in the retinal inner plexiform layer and modulate visual information presented to ganglion cells. Exposure of these neuronal cultures to high levels of glutamate [500μM, 10 min], which acts through the NMDA receptor, led to cell death as detected by the trypan blue exclusion assay. Treatment with Sig1R ligands (+)-PTZ and SA4503(1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)-piperazine dihydrochloride) reduced neuronal toxicity in a dose-dependent manner [Senda et al, 1998]. The studies are noteworthy because although Sig1R is not a binding site within the NMDA receptor, the work clearly shows a relationship between Sig1R and NMDA activation and laid the foundation for future exploration of Sig1R, NMDA and calcium modulation in retina.
2.3. Molecular confirmation of Sig1R in mammalian retina
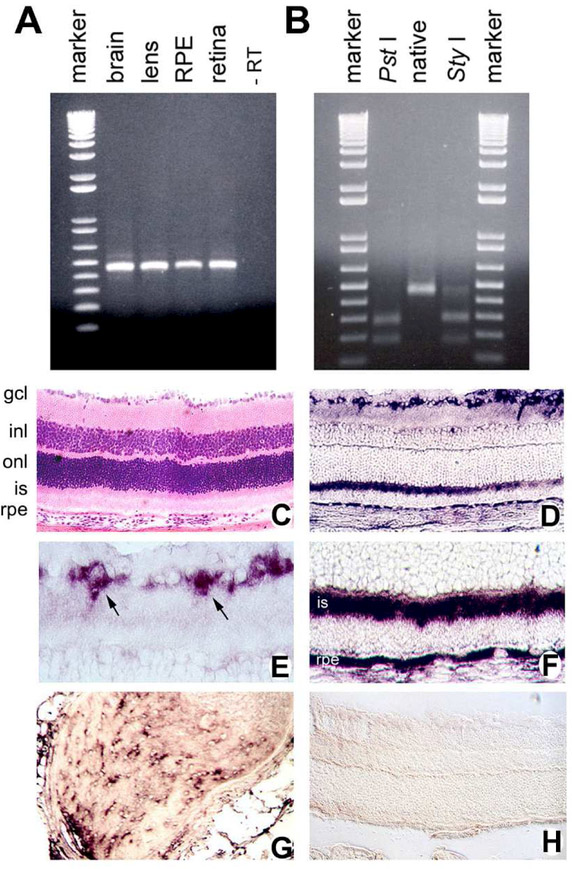
Aside from pharmacologic evidence that Sig1R was present in amacrine cells [Senda et al, 1998], there was no molecular evidence confirming its expression in retinal cells. Our colleague, Dr. Vadivel Ganapathy, had recently cloned Sig1R from mouse [Seth et al, 1997], thus affording an essential tool to characterize Sig1R at the molecular level. We extracted total RNA from mouse retina, lens, and RPE-choroid to examine whether Sig1R mRNA transcripts were present in these tissues; we used mouse brain as a positive control. Semi-quantitative RT-PCR amplified a product (465 bp) that was similar to that amplified from mouse brain [Ola et al, 2001]. The RT-PCR products from these tissues were gene-cleaned and subjected to restriction site analysis using two different enzymes (Pst I and Sty I). The restriction pattern with the two enzymes was identical for the RT-PCR products and was exactly as expected from the known restriction map of mouse Sig1R cDNA (Fig. 2A-B). We also demonstrated Sig1R expression in the rat Müller cell line (rMC-1) and the human RPE cell line (ARPE-19). To localize mRNA transcripts encoding Sig1R in retina and other eye tissues we prepared a digoxigenin-labeled mouse Sig1R-specific riboprobe and performed in situ hybridization in mouse ocular cryosections (Fig 2C-H). Sig1R mRNA transcripts were expressed abundantly in the retinal ganglion cell layer (Fig. 2E), in inner segments of photoreceptor cells and in RPE cells (Fig. 2F). There was expression of Sig1R mRNA transcripts also in the inner nuclear layer, though the signal was not as intense. Since we performed in situ hybridization using the whole mouse eye, we evaluated Sig1R mRNA transcripts in ocular tissues (in addition to retina) and noted abundant expression in the optic nerve head and the nerve itself (Fig. 2G), corneal epithelial cells, cells of the ciliary body and iris as well as lens epithelial cells. We concluded that Sig1R was expressed extensively in retina and other ocular tissues. Using an antibody generated against Sig1R by Dr. P. Casellas, we performed immunohistochemical investigation in the eye and detected Sig1R protein in a pattern similar to its gene expression [Ola et al, 2001]. These studies represented the first molecular evidence of Sig1R in retina. They were validated in rat retina several years later [Liu et al, 2010]. In rats, Sig1R was diffusely distributed in outer and inner plexiform layers, in many cell types of the inner nuclear layer and in the ganglion cell layer. Labeling of Sig1R was observed in horizontal cells, an interneuron that integrates input from multiple photoreceptor cells. The retinal cellular location of Sig1R was confirmed by investigators using radiolabeled pharmacologic approaches in rat and rabbit eyes [Wang et al, 2002]. In rat eyes ex vivo autoradiography using [11C]SA4503, a selective Sig1R radioligand, showed Sig1R accumulation in iris-ciliary body and retina. In this study the investigators determined that Sig1R was present on retinal ganglion cell axon terminals in the superior colliculus. The group confirmed their findings in rabbit eye using in vivo positron emission tomography (PET), again demonstrating high density in retina and iris-ciliary body [Wang et al, 2002]. The identification of Sig1R in retina set the stage for analysis of retinas of mice lacking Sig1R.
Fig. 2. Molecular confirmation of Sig1R expression in mouse retina.
(A) Total RNA was isolated from mouse brain (positive control), lens, RPE-choroid and neural retina. RT-PCR was carried out with primers specific for mouse Sig1R mRNA. As a negative control, the brain sample was run through the RT-PCR procedure without reverse transcriptase (-RT). (B) Restriction analysis of RT-PCR products. Shown here, the RT-PCR product from retina was gene cleaned and used for restriction analysis with PstI and StyI. The distribution of Sig1R-specific mRNA transcript in adult mouse retina was assessed by in situ hybridization. (C) Hematoxylin and eosin stained retinal section for comparison to adjacent retinal cryosections used for the in situ hybridization experiments. Several layers of the retina are indicated (gcl, ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer; is, inner segment; and rpe, retinal pigment epithelium). (D) Mouse retinal sections probed with the antisense digoxigenin-labeled Sig1R riboprobe showing positive reaction in ganglion cell, inner segment and RPE layers. The intense purple stain indicates a positive reaction. (E) Higher magnification of inner portion of retina in panel “D” showing intense labeling of ganglion cells (arrows). (F) Higher magnification of outer portion of retina in panel “D” showing intense labeling of inner segments and RPE layer. (G) Sig1R mRNA detected in optic nerve. (H) Mouse retina probed with sense (negative control) digoxigenin-labeled sigma receptor l riboprobe. No specific signal is detected with the sense probe. (Magnifications: C,D,H X200; E,F,GX400). (Figures adapted from Ola et al, 200l, with permission).
3. Late onset retinal degeneration in the Sig1R−/− mouse
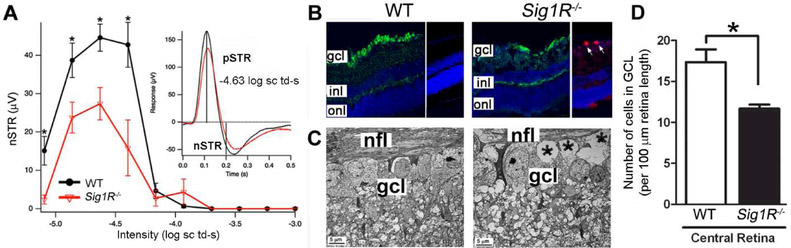
A global Sig1R knockout mouse was developed in the laboratory of Dr. Eric Zorrilla, Scripps Research Institute, LaJolla, CA in collaboration with the Mutant Mouse Resource Regional Center, University of California, Davis [Sabino et al, 2009]. Dr. Zorrilla used the mice to evaluate the role of Sig1R in modulating depressive-like, anxiety-like and motor behaviors. He generously provided founder heterozygous mice to our laboratory allowing us to establish a Sig1R−/− mouse colony. Assessment of intraocular pressure revealed no differences between mutant and wildtype mice and there were no differences observed in retinal vasculature of Sig1R−/− mice compared to age-matched controls. Electrophysiologic function was assessed at 5 and 18 weeks. At these young ages, the Sig1R−/− mice have a-wave amplitudes, implicit times and b-wave amplitudes that are similar to wildtype. Oscillatory potentials are normal as well. Systematic histologic evaluation of Sig1R−/− retinas indicate that Sig1R is not required for normal retinal development. That is, the retinal layers are intact during the first few months and are similar in thickness to wildtype. By 6 months, however, there are functional and histologic changes observed; indeed dying (TUNEL-positive) cells are detected in the optic nerve head. The comprehensive analysis of the Sig1R−/− mouse retinal phenotype is described in Ha et al [2011b]; key findings are summarized in Fig. 3. Over time, a late-onset retinal degeneration ensues in Sig1R−/− mice such that by one year there is loss of ganglion cell function (e.g. decreased negative scotopic threshold responses (nSTR) of the ERG (Fig. 3A)). Fig. 3B shows retinal cryosections stained to detect neurons in the ganglion cell layer (green fluorescence) or active caspase-3 (red fluorescence), a marker for apoptotic cells. There are fewer cells in the ganglion cell layer of Sig1R−/− mice compared to wildtype (Fig. 3B). Fig. 3C presents electron micrographs of wildtype and Sig1R−/− mice, the nerve fiber layer is labeled and just below it are the plump cell bodies in the ganglion cell layer of wildtype retinas. In Sig1R−/− mouse retinas there are areas of cell drop out (“*” denotes missing cells). The cell loss is worse in the central retina; the graph in Fig. 3D shows quantitative data of the number of cells in the ganglion cell layer in wildtype and Sig1R−/− mice. The remaining layers of the retina in Sig1R−/− mice do not differ from wildtype mice [Ha et al, 2011B]. The late-onset functional and structural dysfunction we observed in our comprehensive assessment of the Sig1R−/− retina suggests that Sig1R may have an important role in protecting the retina against stress. Sig1R may not be necessary for normal ocular/retinal development, but it appears to play a critical role in coping with long-term retinal cellular stress.
Fig. 3. Sig1R−/− mice develop a late-onset retinal degeneration characterized by loss of ganglion cells.
(A) Negative Scotopic threshold responses (nSTR) in 1-year-old Sig1R−/− mice. Electrophysiological analysis was performed under scotopic conditions over a range of dim flash intensities in mice (age 59 weeks). Data represent averaged values. (A, inset) The responses at - 4.6 log scotopic troland-seconds, with vertical lines at 200 and 110 ms indicating where nSTR and positive STR (pSTR) amplitudes were measured. *Significantly different from Sig1R−/− mice; p<0.05). (B) Immunohistochemical detection of neurofilament-light protein (NFL, green fluorescence) in retinal cryosections prepared from wildtype (WT) and Sig1R−/− mice. The ganglion cell layer (gcl) is uniform in WT, but is disrupted in retinas of Sig1R−/− mice. Inner nuclear layer (inl) and outer nuclear layer (onl) are preserved. (C) Ultrastructural analysis of retinas of WT and Sig1R−/− mice shows robust, healthy ganglion cells in WT, but areas of cellular dropout (denoted by “*”) in the Sig1R−/− mice. (D) Quantitative analysis of the number of cells in the gcl in the central retina of WT versus Sig1R−/− retina. *significantly different from wildtype, p<0.05. (Figures adapted from Ha et al, 2011, with permission).
4. Sig1R-mediated retinal neuroprotection in vitro and in vivo
4.1. In vitro studies in ganglion cells
To investigate whether activation of Sig1R could mitigate retinal neuronal death under excitotoxic conditions in vitro, we conducted studies in a cell line that was touted as a rat retinal ganglion cell line (RGC5). We exposed these cells to high levels of the excitotoxic amino acids glutamate [1mM] or homocysteine [1mM], which induced significant cell death (50%) within 24h exposure. The excitotoxic death was abrogated when cells were pre-and co-treated with (+)-PTZ [3μM and 10μM] [Martin et al, 2004]. A few years later Yorio’s group corroborated the sensitivity of RGC5 cells to high levels of glutamate and reported attenuation of apoptotic cell death using the Sig1R ligand SKF-10,047 [Tchedre and Yorio, 2008]. They demonstrated decreased Bax levels following Sig1R activation supporting a retinal neuroprotective role for Sig1R. The findings from studies using RGC5 cells were compelling, however, the investigators who developed the cell line later reported that it was contaminated [Krishnamoorthy et al, 2013]. Instead of being derived from ganglion cells, the investigators realized that the RGC5 cell line was actually the mouse cone photoreceptor cell line (661W) developed by the Al-Ubaidi group [Tan et al, 2004].
We established primary retinal ganglion cell culture in our lab to investigate Sig1R function in these neurons. We adapted a method described by Barres and colleagues, which used young post-natal rats and a two-step immunopanning procedure that first depleted macrophages from dissociated retinas followed by use of an antibody to Thy-1, a protein expressed by ganglion cells [Barres et al, 1988]. The method yields a highly purified population of ganglion cells. We adapted the method to isolate ganglion cells from P2-P4 mice [Dun et al, 2006]. Our standard protocol involves isolating ~12–16 retinas and incubating them in a buffer containing papain. After they are triturated, dissociated, and centrifuged, they are resuspended in a panning buffer that removes macrophages and microglial cells. The second immunopanning step uses purified rat anti-mouse Thy1.2 antibody, following which the ganglion cells are collected by centrifugation and resuspended in neurobasal medium enriched with insulin, transferrin, putrescine, BDNF (brain derived neurotrophic factor) and CNTF (ciliary neurotrophic factor). The isolation of retinal ganglion cells has provided a physiologically relevant paradigm for testing the neuroprotective effects of (+)-PTZ. To simulate excitotoxic insult, we exposed cells to glutamate or homocysteine. We used concentrations of glutamate and homocysteine that were several orders of magnitude less than had been used with the RGC5 cell line. We found that culturing the cells for 6 or 18 h in 10, 20, 25 or 50μM glutamate or 10, 25, 50 or 100μM homocysteine compromised viability severely, whereas pre-treatment (and co-treatment) with (+)-PTZ attenuated the cell death [Dun et al, 2007]. The cultured neurons extended neurites, which were highly sensitive to excitotoxins as evidenced by their shriveled and disrupted appearance. The neurites were preserved in cells treated with (+)-PTZ. In these studies, the stereoselective effect of (+)-PTZ for Sig1R was confirmed because experiments in which (-)-PTZ, the levo-isomeric form of pentazocine, was used revealed no neuroprotective effect on excitotoxin-induced ganglion cell death [Dun et al, 2007].
Yorio’s group also purified ganglion cells to study Sig1R, especially as related to calcium dynamics. Calcium is well known as an intracellular signaling molecule that regulates neuronal functions; dysregulation of calcium homeostasis is known to compromise neuronal integrity. Calcium homeostasis is maintained, in part, by ion channels that permit influx. Neurons express many of these channels including L-type voltage gated calcium channels, which play a major role in coupling neuronal activity to gene transcription. Excess calcium influx has been associated with deficits in synaptic plasticity and neuroinflammation [Navakkode et al, 2018]. Using primary rat ganglion cells Yorio’s laboratory made a key observation that Sig1R ligands (+)-SKF10,047 and (+)-PTZ could inhibit calcium ion influx through voltage gated calcium channels [Mueller et al, 2013]. They first demonstrated that the L-type voltage gated calcium channels were present in their primary cultures using immunoblotting methods. Subsequently, they used total internal reflection fluorescence microscopy with antibodies against Sig1R and L-type voltage gated calcium channels and found that Sig1Rs were distributed throughout the plasma membrane of the cell soma and on the proximal neurites of the primary ganglion cells. Using quantitative methods they determined that 50% of the L-type voltage gated calcium channels co-localize with Sig1R. They then used SKF-10,047 to evaluate effects on KCl-induced calcium ion influx in their purified ganglion cells. To image the calcium, they used fura-2-AM (an aminopolycarboxylic acid that is a ratiometric fluorescent dye, which binds to free intracellular calcium), and found that the calcium levels decreased upon exposure to the Sig1R ligand, whereas KCl activated the channels. Calcium signaling was potentiated when cells were treated with a Sig1R antagonist, BD1047 (N-[2-(3,4-Dichlorophenyl)ethyl]-N,N’,N’-trimethyl-1,2-ethanediamine. In a separate study, these researchers used co-immunoprecipitation methods and demonstrated a direct interaction between Sig1R and L-type voltage gated calcium channels on the plasma membrane [Tchedre et al, 2008, Mueller et al, 2013]. This direction of research for the field of Sig1R has received considerable attention as it may reflect a mechanism by which Sig1R mediates neuroprotection in retina. The findings are particularly interesting given earlier observations that Sig1R can, upon the stimulation of agonists or stress, translocate to the plasma membrane to interact with ion channels, receptors, and kinases [Su et al, 2010]. As is emphasized several times in this review, Sig1R has been localized to several cellular organelles. Its ability to interact with many different proteins in various cellular locations when either activated or under stressful conditions may be a key factor in explaining the beneficial properties of Sig1R.
4.2. In vivo studies: Sig1R neuroprotection
4.2.1. Ganglion cell death in diabetic retinopathy models
The detection of Sig1R in retinal ganglion cells prompted us to investigate its expression in the diabetic retina. The rationale for the investigation was that in the late 1990’s, a paradigm shift occurred in our understanding of how retina was impacted during diabetes. Dr. Tom Gardner and his team showed unequivocally that neurons, particularly ganglion cells, die in diabetic retinopathy [Barber et al, 1998]. While it is widely accepted today that diabetic retinopathy is a neurovascular disease [Lechner et al, 2017], the dogma in the field had been that diabetic retinopathy was primarily, if not solely, a vascular disease. The mechanism underlying ganglion cell death in diabetic retinopathy became an area of intense investigation. One line of research suggested that overstimulation of the N-methyl-D-aspartate (NMDA) subtype of glutamate receptor led to excessive levels of intracellular calcium triggering neuronal death [Lieth et al, 1998]. This observation was relevant to Sig1R because glutamate-induced amacrine cell death was reduced when cells were treated with the Sig1R ligand (+)-PTZ [Senda et al, 1998].
We investigated Sig1R-mediated neuroprotection in two murine models of diabetic retinopathy, the streptozotocin-induced model [Ola et al, 2002] and the Ins2Akita/+ mouse [Smith et al, 2008]. Our first experiments investigated whether Sig1R expression was altered in the diabetic retina [Ola et al, 2002]. We performed in situ hybridization using mRNA probes as well as immunohistochemistry and Western blotting using a polyclonal antibody generated in rabbit against the Sig1R peptide sequence SEVYYPGETVVHGPGEATDVEWG, which corresponds to residues 143-165 of rat Sig1R. We demonstrated the specificity of this antibody as described [Ola et al, 2002]. Our analyses revealed that Sig1R gene expression in both diabetic models was similar to control mice, both in location and quantity. Similarly, the pattern of Sig1R protein detection in retinal tissue did not differ between the diabetic versus the control mice; moreover the level of protein detected by Western blotting was similar [Ola et al, 2002; Smith et al, 2008]. The data suggested that retinal Sig1R was an available target for activation under diabetic conditions.
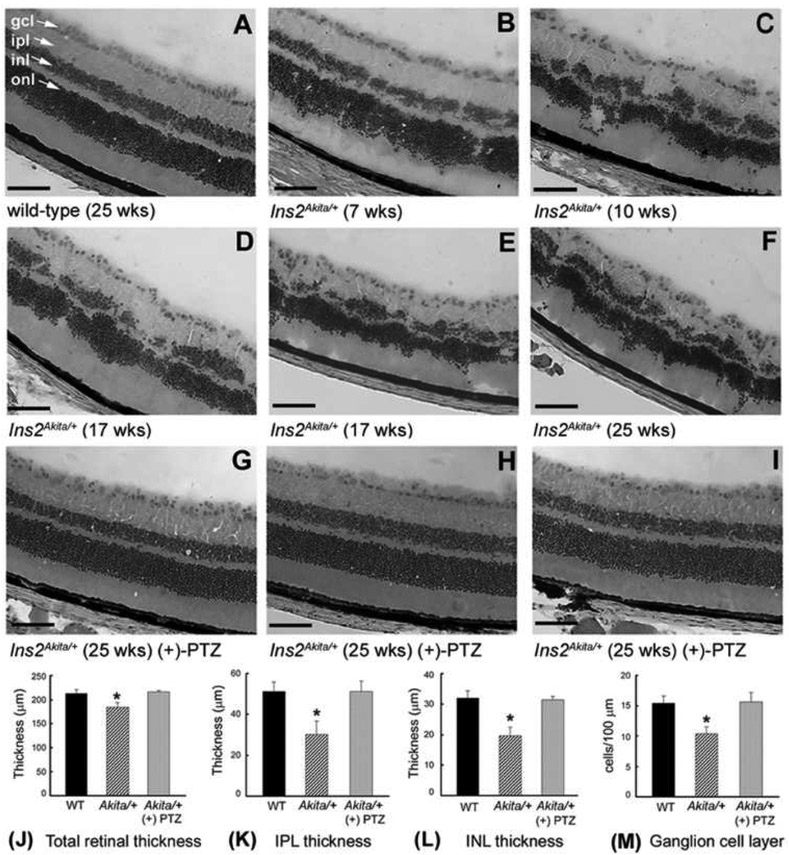
To evaluate retinal neuroprotective potential of targeting Sig1R against neuronal death in diabetes, we administered (+)-PTZ [0.5 mg/kg−1] intraperitoneally two times per week to Ins2Aklta/+ mice beginning at the onset of diabetes [Smith et al, 2008]. The injections continued for 22 weeks; mice were euthanized and eyes harvested for morphology at 25 weeks. The Ins2Akita/+ mouse harbors a point mutation in the Insulin 2 gene that renders the mice hyperglycemic and hypoinsulinemic by 3-4 weeks. The mice not only demonstrate a retinal vascular phenotype, characterized by acellular capillaries and increased vascular permeability, but also ~25% fewer cells in the ganglion cell layer and ~20-25% decrease in thickness of the inner plexiform layer constituting the synapses between bipolar and ganglion cells [Barber et al, 2005]. The mice demonstrate an irregular retinal architecture as well. Adhering to the treatment protocol of twice weekly (+)-PTZ injections, we observed a remarkable preservation of retinal architecture in Ins2Akita/+ mice. Fig. 4 presents the most salient data from this previously published study [Smith et al, 2008] and shows retinas of wildtype (C57Bl/6J) mice compared with the Ins2Akita/+ mouse over an age range of 25 weeks. Retinas of three Ins2Akita/+ mice that were injected with (+)-PTZ showed dramatic improvement of retinal architecture (Figs. 4G-I), which were representative of all Ins2Akita/+ mice treated in the study. Morphometric analysis confirmed the retinal preservation in mice treated with (+)-PTZ (Fig. 4K-L). The number of cells in the ganglion cell layer per 100pm length of retina in the untreated Ins2Akita/+ mice was ~20% less that of wildtype, whereas the (+)-PTZ-treated mice had approximately the same number of cells. Analysis of cell death using the TUNEL assay showed many apoptotic cells in the ganglion cell layer and inner nuclear layer of the untreated Ins2Akita/+ mice, but significantly fewer in the (+)-PTZ-treated Ins2Akita/+ mice [Smith et al, 2008].
Fig. 4. Preservation of retinal structure in Ins2Akita/+ mice administered (+)-PTZ.
Representative hematoxylin and eosin (H&E)-stained retinal cryosections of (A) wild-type mice: cells in the ganglion cell layer (gcl) are distributed evenly, nuclear layers are uniformly thick; (B-F) Ins2Akita/+ mice: inner nuclear layer (inl) becomes disrupted with age, gcl density is decreased; (G-I) Ins2Akita/+ mice treated with (+)-PTZ (0.5 mg/kg, 2X/wk i.p./22 weeks): marked preservation of retinal layers. (ipl = inner plexiform layer, inl = inner nuclear layer, onl = outer nuclear layer, magnification bar = 50 μm). Retinal sections were subjected to morphometric analysis: (J) total retinal thickness (K) IPL thickness (L) INL thickness (M) number of cell bodies in gcl per 100 μm length of retina. Data are means ± S.E. of measurements from retinas of 6 wildtype (WT, 12 eyes), 9 Ins2Akita/+ (18 eyes) and 8 Ins2Akita/+ treated with (+)-pentazocine (16 eyes). * Significantly different from WT and (+)-PTZ-treated mice (p<0.001). (Figure adapted from Smith et al, 2008, with permission).
4.2.2. Ganglion cell death in non-diabetic models
Sig1R-mediated retinal neuroprotection has been investigated in non-diabetic models as well, particularly in induced retinal degenerative models compromising ganglion cell viability and function. Notably, Bucolo and colleagues produced inner retinal dystrophy using an intravitreally-induced retinal ischemia-perfusion model in rats [Bucolo et al, 2006b]. Pretreatment of rats intraperitoneally with a novel adamantine derivative (A-methyladamantan-1-amine derivative, MR22, a highly selective Sig1R ligand) reversed deleterious biochemical changes including the increased lactate levels and decreased glucose and ATP production. The investigators evaluated retinal histology in the ischemia-perfusion model and noted that retinal architecture was preserved. Thus, a neuroprotective benefit of activating Sig1R was evident in two models of ganglion cell dysfunction, the ischemic-reperfused and the diabetic model [Bucolo et al, 2006b; Smith et al, 2008]. The Bucolo team also induced retinal dystrophy by administering amyloid beta (Αβ) intravitreally concomitant with administration of the Sig1R ligand Pre-084. In their Αβ injected rats, they observed increased levels of the proapoptotic protein Bax as well as JNK phosphorylation, which were abrogated in the rats pretreated with Pre-084 [Cantarella et al, 2007]. The investigators also examined the role of TRAIL (tumor necrosis factor related apoptosis inducing ligand), which is a mediator of Aβ toxicity. They found that TRAIL receptors were overexpressed in Aβ toxicity, but were diminished when Sig1R was activated using the compound Pre-084. The authors postulated that agonists for Sig1R constitute a therapeutic strategy to offset Aβ-induced retinal damage.
The studies described above ushered in the era of Sig1R as a potential target in retinal disease. The work demonstrated neuroprotection in vivo and provided clear evidence that ganglion cell death was attenuated when the Sig1R ligand was provided systemically rather than directly to the retina (e.g. via subretinal or intravitreal injection). The studies laid the foundation for future exploration of Sig1R as a treatment strategy for ganglion cell loss in glaucoma. Glaucoma, the leading cause of untreatable blindness, is characterized by loss of axons of ganglion cells that form the optic nerve [Fry et al, 2018].
Investigations of the potential neuroprotective role for Sig1R in glaucoma are underway in several laboratories. In the Bollinger laboratory, work is ongoing to optimize models for inducing glaucoma and testing effects of Sig1R agonists as well as mechanisms of ganglion cell death [Mysona et al, 2017]. This group is investigating whether Sig1R affords ganglion cell protection directly but also by modulating optic nerve head astrocytes, which are glial cells that maintain ganglion cell health. The Guo laboratory studies of the role of targeting Sig1R in alleviating ganglion cell death as well as photoreceptor cell death [Mavlyutov and Guo, 2017]. Recently, this team of investigators developed an intraocular drug delivery system using unimolecular micelle nanoparticles (unimNP) to prevent ganglion cell loss [Zhao L et al, 2017]. The unimPNs have a hydrophobic component that can encapsulate hydrophobic drugs and a polyethylene glycol component provides a hydrophilic shell allowing for dispersion in water. Their team loaded unimPN with dehydroepiandrosterone (DHEA). DHEA is an FDA-approved steroid with actions on a number of receptors including Sig1R, as well as androgen receptors, estrogen receptors and T-type calcium channels [Clark et al, 2018]. The study from the Guo group showed that packaging DHEA in the unimPN released it in vitro for over two months. In vivo studies using an NMDA-induced model of ganglion cell death showed that the DHEA-loaded unimNPs accumulated in the ganglion cell layer and preserved ganglion cells for 14 days [Zhao L et al, 2017]. This is an exciting direction for the field of Sig1R translational research as it may have applicability to treatments directed at glaucomatous pathology.
4.2.3. Light-induced photoreceptor degeneration model
The studies described above evaluated the role of Sig1R activation related to ganglion cell death. The finding that retinas of Sig1R−/− mice had a late-onset retinal dystrophy involving ganglion cells coupled with the observation that ganglion cell loss in the Ins2Akita/+ mouse could be attenuated following (+)-PTZ treatment raised the question: was Sig1R-mediated retinal protection limited to ganglion cell-related retinopathies or did it have potential for other retinal diseases? The first attempt to address this was in a light-induced retinopathy model. While light is essential to launch the visual transduction cascade, excessive light levels induce damage and death to photoreceptor cells [Wenzel et al, 2005; Organisciak and Vaughan, 2010]. Investigators in Hara’s lab subjected mice to intense light (8000 lux for 3 h), which reduced the thickness of the outer nuclear layer of photoreceptor cell nuclei by more than 50% within 5 days [Shimazawa et al, 2015]. The extremely rapid photoreceptor cell loss was attenuated somewhat when mice were administered SA4503 [500μM] intravitreally prior to the light insult. There was improvement in dark-adapted (scotopic) ERG function in SA4503-treated mice. This study provided proof-of-principle evidence that photoreceptor cell loss can be alleviated if Sig1R is activated prior to an induced insult and if administered directly to the target tissue (i.e. intravitreally). Whether improved photoreceptor function would occur if Sig1R was administered after the onset of disease and systemically rather than intravitreally was not known.
4.2.4. Genetic photoreceptor degeneration model
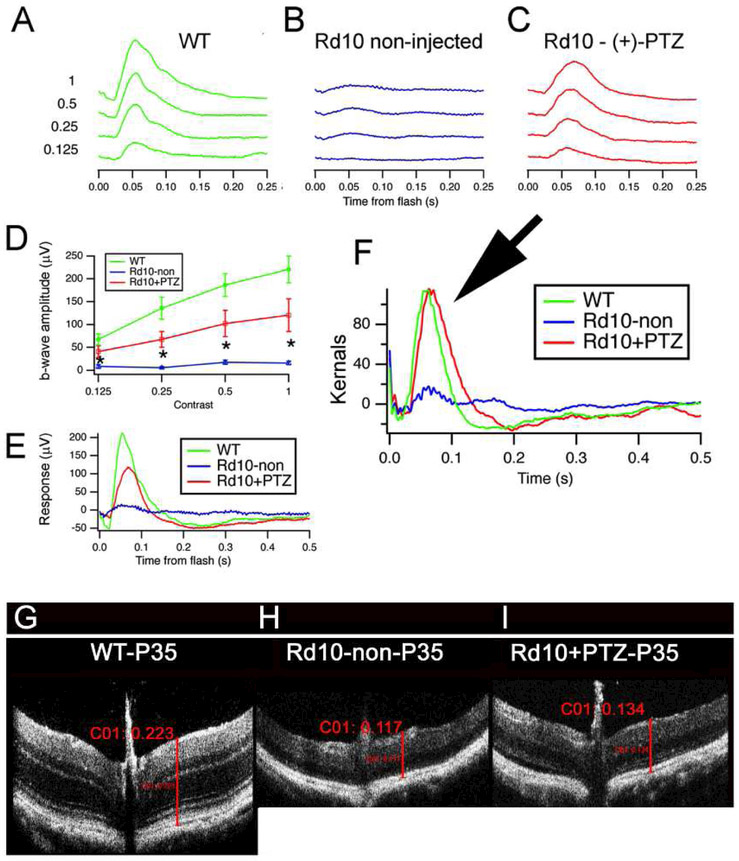
We assessed potential benefits of Sig1R activation in an inherited photoreceptor degeneration model, the Pde6brd10/J (rdl0) mouse, which carries a spontaneous missense point mutation in exon 13 of the beta-subunit of the rod cGMP phosphodiesterase (β-PDE) gene [Chang et al, 2002; Chang et al 2007]. The mouse loses rod photoreceptor cells beginning at P18; rod loss peaks at P25. Cone cell death follows. By P35 most cones are lost; cone function is minimal. Inner retinal cell loss ensues [Gargini et al, 2007]. Rd10 mice are a valuable model of retinal degeneration to evaluate rescue approaches for retinal diseases including retinitis pigmentosa [Guadagni et al, 2015]. To evaluate activation of Sig1R in this model, we administered (+)-PTZ (0.5 mg/kg) to rd10 mice systemically (via intraperitoneal route) beginning at P14; injections continued every other day through P42 [Wang et al, 2016b]; key data from this study are provided in Figs. 5–7. Mice were subjected to ERG analysis; photopic (cone) flash responses revealed significant improvement in rd10+PTZ-treated mice compared to rd10 non-treated animals. (+)-PTZ treatment appeared to rescue the rd10 cone pathway, a conclusion strengthened using a pseudorandom luminance noise test containing power at low temporal frequencies, providing a slower stimulus more similar to natural vision [Saul et al, 2016]. The kernels, or impulse response functions, illustrated in Fig. 5F suggest a strong rescue by (+)-PTZ, with similar amplitudes for wildtype and rd10+PTZ groups. The responses of rd10+PTZ treated mice are very similar to wildtype mice (arrow, Fig. 5F) versus the flat response of the non-treated rd10 mice. These results were noteworthy because intervention studies using rd10 mice often compare data for treated versus non-treated mutants, but much less often include data comparing to wildtype mice [Isiegas et al, 2016; Hanif et al, 2015; Barone et al, 2014; Drack et al, 2012]. Spectral domain-optical coherence tomography (SD-OCT) imaging of retinas in vivo revealed some preservation of retinal thickness in rd10+PTZ versus non-treated rd10 mice (Fig. 5I versus 5H).
Fig. 5. Photopic ERG responses and SD-OCT are improved significantly in rd10 mice administered (+)-PTZ.
Averaged photopic responses to 5 ms flashes at a series of intensities (log photopic troland-seconds) are provided for (A) wildtype (WT), (B) rdl0-non-, (C) rd10+PTZ-treated mice (age: P35). (D) Mean b-wave amplitudes of averaged photopic responses to 5 ms flashes above fixed pedestal luminance of 0.105 lumens (4 contrasts of the flash; contrast = (Flash - Pedestal)/Pedestal luminance). Data are mean ± SEM of 4 assays using eyes from 6–9 mice (*significantly different between rd10-non-injected and rd10+PTZ-treated, ^<0.005). (E) Averaged responses to photopic flash of contrast = 1 (replotted after superimposition). A second type of ERG test that uses ‘natural luminance noise’ stimuli was used to evaluate cone function. (F) Averaged kernels derived from responses to natural noise stimuli. Note the very similar responses of rd10+PTZ mice compared to WT (arrow), which are significantly greater than rd10-non-treated mice. Panels G-I: Representative SD-OCT data obtained from WT mice, rd10-non-, rd10+PTZ-treated mice at P35. Total retinal thickness averaged respectively, 223pm, 117pm, and 134pm for wildtype, rd10-non-treated, and rd10+PTZ-treated mice. (Figures adapted from Wang et al, 2016B, with permission).
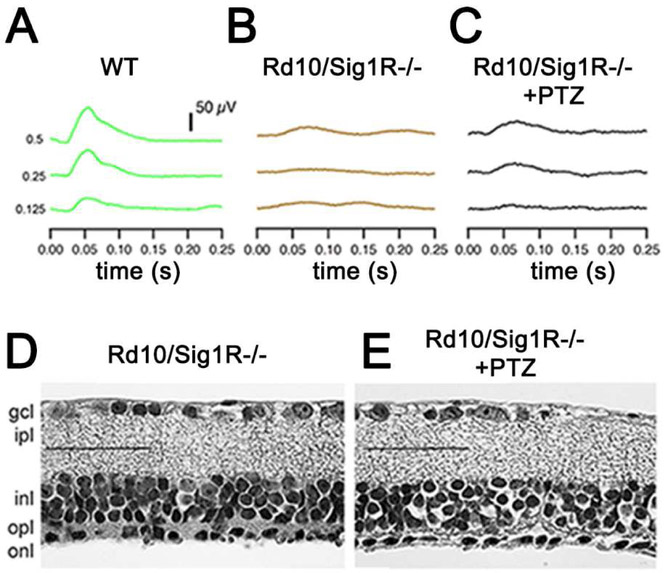
Fig. 7. PRCs are not preserved when rd10/Sig1KA mice are administered (+)-PTZ.
To evaluate whether Sig1R was required for (+)-PTZ to preserve cone PRCs, we established (rd10/Sig1R−/−) mice in our laboratory. The mice were administered (+)-PTZ following the same protocol as had been used in the rd10 mice and were subjected to functional and structural analyses. ERG of averaged photopic responses for (A) wildtype (WT), (B) (rd10/Sig1R−/−) non-treated, (C) (rd10/Sig1R−/−) (+)-PTZ. The WT responses were significantly greater than (rd10/Sig1R−/−) non- and (rd10/Sig1R−/−)(+)-PTZ injected (p<0.005)). Panels D and E: Retinal sections of rd10/Sig1R−/− non- and (+)-PTZ mice embedded in JB-4, stained with H&E (calibration bar: 50μm). The detachment of the retina is significant in both the (rd10/Sig1R−/−) non- and (rd10/Sig1R−/−)(+)-PTZ-treated animals, thus (+)-PTZ does not preserve retinal structure in the absence of Sig1R. (Figures adapted from Wang et al, 2016B, with permission).
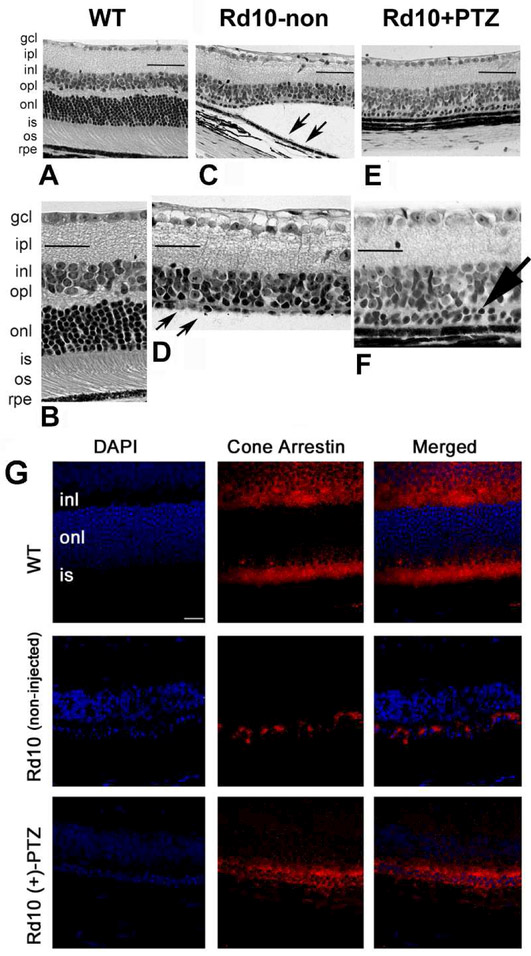
Retinal histologic photomicrographs from this study are shown in Fig 6. As described in detail in Wang et al, 2016b, wildtype retinas had the typical ~250pm thickness with ~10-12 rows of PRCs, rd10 mouse retinas were only half the thickness of wildtype with 0-1 row of PRCs (Fig. 6D), though the INL was intact. Retinal detachment was pronounced in rd10 mice (Fig. 6C). Regarding rd10+PTZ-treated mice, retinas were well-organized (Fig. 6E); there were discernible PRCs in the ONL (~2 rows, arrow, Fig. 6F) and detachment was attenuated. Cone preservation, detected by cone arrestin labeling (Fig. 6G), was robust in wildtype and was also observed in retinas of rd10+PTZ-injected mice, but was limited in non-treated rd10 mice. This study also revealed attenuation of gliosis and microglial activation when rd10 mice were treated with +PTZ. In aggregate, the aforementioned study [Wang et al, 2016b] provided evidence that systemic activation of Sig1R enhanced functional cone rescue. The study was conducted over a period of 6 weeks, and studies are now underway in our laboratory to determine whether the functional cone rescue can be maintained in the rd10 mice if (+)-PTZ treatment continues through 90, 120 and 180 days.
Fig. 6. Sig1R activation preserves cone PRCs in retinas of rd10 mice.
Rd10 mice were administered (+)-PTZ intraperitoneally from P14-P42. Retinal sections of P42 eyes embedded in JB-4 and stained with H&E (A, B) WT; (C, D) rd10-non-treated; (E, F) rd10+PTZ-treated mice. Note retinal detachment (arrows, C) and paucity of PRCs in the outer nuclear layer (onl) in rd10-non mice (arrows, D). Two rows of PRC nuclei remain in the onl of rd10+PTZ mice (large arrow, F). (G) Retinal cross-sections of cone-arrestin labeling (red fluorescence) in WT, rd10-non- and rd10+PTZ-treated mice. DAPI (blue fluorescence) labels nuclei. Calibration bar: 50 pm. (Figures adapted from Wang et al, 2016B, with permission).
Cone rescue in human diseases, such as retinitis pigmentosa, holds promise that an individual will retain ‘best visual acuity’ despite lack of functional rods. The findings of the study in rd10 mice were promising, but raised a key question: were (+)-PTZ-mediated neuroprotective effects in rd10 mice indeed due to Sig1R activation? To address this, we generated rd10 mice lacking Sig1 (rd10/Sig1R−/−), treated them with/without (+)-PTZ, subjected them to ERG, SD-OCT, histologic/morphometric analysis, and PNA immunodetection at P42 [Wang et al, 2016b]. ERG analysis showed robust photopic responses in wildtype mice (Fig. 7A) but minimal responses in rd10/Sig1R−/− mice regardless of (+)-PTZ treatment (Fig. 7B,C). Histological analysis showed no cone preservation in (+)-PTZ treated rd10/Sig1R−/− mice (Fig. 7D, E). These data suggest that Sig1R is required for (+)-PTZ-mediated cone rescue in rd10 mice.
4.3. Retinal dystrophy is accelerated in the absence of Sig1R
It is noteworthy that in several retinal degeneration models involving ganglion cells, the retinal phenotype worsened if Sig1R was absent. For example, Guo’s group showed that optic nerve crush accelerated ganglion cell loss in Sig1R−/− mice compared to wildtype [Mavlyutov et al, 2011]. In their study, they first labeled Sig1R binding sites with radioiodinated ligands and used autoradiography to label Sig1R-expressing cells. They observed an abundance of Sig1R particularly in ganglion cells and photoreceptor cells. In their experimental paradigm, they used intraorbital optic nerve crush in wildtype and Sig1R−/− mice; they used one eye for the crush and compared data with the fellow eye that was not subjected to optic nerve crush. One week following the optic nerve crush, they evaluated Nissl-stained retinal whole mounts. They observed ~14% reduction of ganglion cells in the wildtype mouse retinas following crush but ~32% reduction in the Sig1R−/− mouse retinas. The study was important as it demonstrated increased susceptibility of retinal ganglion cells to optic nerve crush in the absence of Sig1R - or conversely - it demonstrated a role for Sig1R in delaying crush-induced ganglion cell death. The study confirms Sig1R as a bona fide in vivo target to attenuate stress of ganglion cells.
The studies using the optic nerve crush model represented an acute injury leading to ganglion cell death. Other studies addressed the role of Sig1R in ganglion cells subjected to chronic stress. When Sig1R−/− mice were made diabetic by streptozotocin [Ha et al, 2012] or by crossing with Ins2Akita/+ mice [Wang et al, 2016a], the rate of ganglion cell loss accelerated and the functional loss, as detected by scotopic threshold responses (nSTR), worsened. In the streptozotocin model, diabetes was induced in wildtype or Sig1R−/− mice at 3 weeks and the retinas were examined 12 weeks later. The nSTRs were reduced in diabetic Sig1R−/− mice compared to all other mouse groups and the number of ganglion cells was reduced significantly. In additional experiments, ganglion cells were isolated from wildtype and Sig1R−/− mice and subjected to oxidative stress using xanthine:xanthine oxidase. When the cells were pre-treated with (+)-PTZ, there was significant attenuation of cell death in the cells harvested from wildtype mice. However, the ganglion cells isolated from the Sig1R−/− mice did not demonstrate improved survival upon exposure to oxidative stress when treated with (+)-PTZ demonstrating that the neuroprotective effects of (+)-PTZ require Sig1R [Ha et al, 2012].
We also investigated whether the absence of Sig1R would accelerate the retinal phenotype in Ins2Akita/+ mice [Wang et al, 2016a]. We found that there were significantly more dying cells in the ganglion cell layer of retinas of Ins2Akita/+ Sig1R−/− mice compared with wildtype or Ins2Akita/+ mice. The nerve fiber layer was also more disrupted as observed not only in fixed retinal histologic sections but also by SD-OCT performed in living mice. Thus, the absence of Sig1R in a model of chronic retinal ganglion cell loss worsens the phenotype just as occurs in an acute model of ganglion cell loss. The data suggest that Sig1R has an important role in preservation of retinal structure, especially during stress.
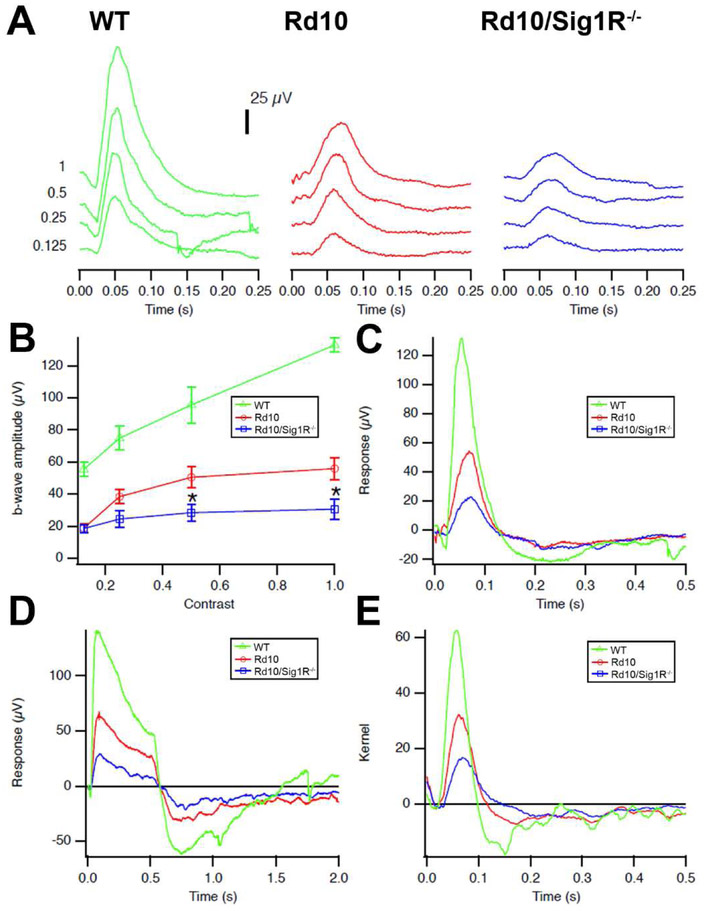
It was not clear whether acceleration of the retinal dystrophic phenotype in mice lacking Sig1R would be limited to models of ganglion cell loss, although ganglion cell loss is the key feature of the late-onset degeneration in Sig1R−/− mice. To evaluate whether there was an impact on photoreceptor cell loss in the absence of Sig1R, we utilized the rd10 mouse. Our studies showed that photoreceptor cell death is accelerated significantly when Sig1R is absent [Wang et al, 2017]. In a comprehensive study involving nearly 300 mice, we analyzed retinas over an age range of P15 through P42 [Wang et al, 2017]. Mice were subjected to in vivo functional analyses such as ERG and SD-OCT followed by morphometric analyses and immunofluorescent detection of gliosis, oxidative and ER stress. Using wildtype, rd10 and rd10/Sig1R−/− mice, we observed accelerated PRC loss and accelerated loss of cone function in the rd10/Sig1R−/− mice. Evidence of the accelerated degeneration is reflected in Fig. 8, which provides photopic ERG data for mice at P28. At this age, the rd10 mice have a detectable cone response, whereas the response is diminished markedly in rd10/Sig1R−/− mice. The diminished response to natural noise stimuli is quite evident in rd10/Sig1R−/− mice compared to rd10 mice (Fig. 8E). Additional OCT and histologic data are described in detail in Wang et al [2017]. There was also a notable increase in levels of ER and oxidative stress in retinas of rd10/Sig1R−/− mice versus rd10 mice [Wang et al, 2017]. Studies from the Guo lab published in 2017 also evaluated the consequences on the rd10 retina in the absence of Sig1R. The investigators reared rd10 and rd10/Sig1R−/− mice in dim light to decelerate the rapid rod and cone degeneration so that they could examine retinas a few weeks past the frank loss of rods and cones observed in ambient lighting conditions [Yang et al, 2017]. They reported that the retinal phenotype of rd10 mice worsened in the absence of Sig1R. However, an unexpected and paradoxical finding of their study was rod protection in three-week old rd10/Sig1R−/− mice, although at later stages the rods in these mice were lost. They theorized that the role of Sig1R in their experimental paradigm may be biphasic. Additional findings from this comprehensive study included increased levels of cleaved caspase3 (a marker of apoptosis) in rd10/Sig1R−/− mice compared to rd10/Sig1R−/− mice. In addition, markers of autophagy and ER stress, LC3II and CHOP were increased in the rd10 mice lacking Sig1R.
Fig 8. Photopic ERG and natural luminance noise test responses are diminished in rd10 mice lacking Sig1R.
(A) Photopic ERG traces. Averaged photopic responses to 5-ms flashes at a series of contrasts are provided for WT, Rd10, Rd10/Sig1R−/− mice at P28. (B). Mean b-wave amplitudes of averaged photopic responses to 5-ms flashes above a fixed pedestal luminance of 0.105 lumens [four contrasts of the flash; contrast = (flash-pedestal)/pedestal luminance]. Data are the mean ± SEM of four assays using eyes from six to nine mice. *, significantly different from the WT and rd10 groups, *p < 0.05. (C) Averaged responses to photopic flash of contrast =1 (replotted after superimposition). (D) Averaged responses to 0.5s long luminance steps are shown. The photopic negative response occurs after stimulus offset at 0.5s, and originates in part from RGCs. (E) Averaged kernels derived from responses to natural noise stimuli.
Taken collectively, the acceleration of retinal neuron loss when Sig1R is absent and the improvement in retinal function when Sig1R is targeted suggest that Sig1R is a modulator of stress in the retina. The mechanism(s) by which Sig1R mediates retinal neuroprotection is an area of intense investigation. Many of the investigations center on the interaction of Sig1R with various proteins. We will briefly describe initial studies performed in transformed non-retinal cell lines and cortical neurons, followed by studies that are more directly relevant to the retina.
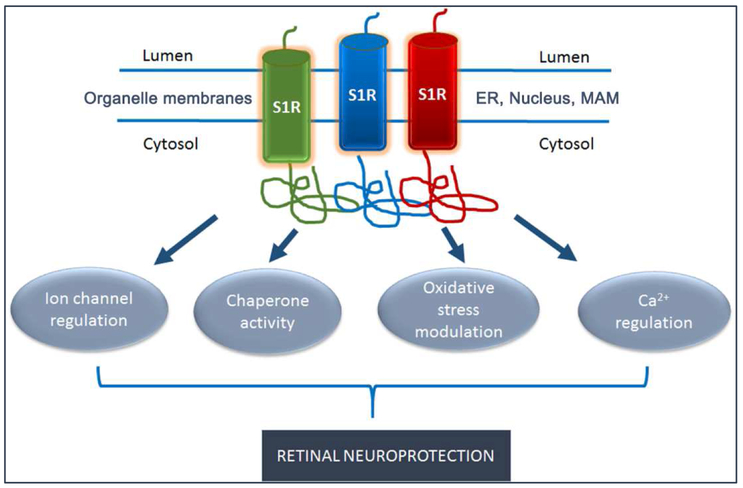
5. Biological role of Sig1R and mechanisms of retinal neuroprotection
5.1. Evidence that Sig1R is a ligand-operated molecular chaperone
In the early 2000’s, work from the laboratory of Hayashi and Su identified ankyrin as a protein that functioned coordinately with Sig1R. Ankyrins are cytoskeletal adaptor proteins present in the plasma membrane, endoplasmic reticulum (ER) membrane and Golgi complex. They control intracellular sorting of calcium homeostasis proteins by interacting with inositol 1,4,5-triphosphate receptors (IP3R). Using neuroblastoma-glioma hybrid cells (NG108), Hayashi and Su [2001] demonstrated that the trimeric protein complex (Sig1R, ankyrin B, IP3R3) could be co-immunoprecipitated by antibodies against any of the three proteins. They further established that Sig1R agonists dissociated ankyrin B from IP3R3. They also showed that Sig1R agonists (e.g. (+)-PTZ, pregnenolone sulfate, PRE084) elicited calcium signaling. Subsequently, these investigators reported that Sig1R forms unique cytoskeleton-associated ER-lipid droplet microdomains in NG108 cells [Hayashi and Su, 2003]. Lipid droplets are the site of storage for triglycerides and cholesteryl esters. These studies were extended in rat hippocampus primary cultures, a preparation that contains astrocytes and terminally differentiated oligodendrocytes. Oligodendrocytes are enriched in lipids, specifically galactosylceramides (GalCer) located on the ER; Sig1R resides in GalCer-containing microdomains, which may be important in oligodendrocyte differentiation [Hayashi and Su, 2004].
Later studies from this same group reported binding of Sig1R to BiP/GRP78, the master regulator of the ER stress response [Hayashi and Su, 2007]. (BiP/GRP78 is the abbreviation for Binding Protein/Glucose Regulated Protein (GRP) Mr 78kD). In studies using Chinese Hamster Ovary (CHO) cells, acute injury in the form of glucose deprivation or treatment with thapsigargin (a Ca2+-ATPase inhibitor) altered Sig1R-BiP binding. Under thapsigargin exposure Sig1R dissociated from BiP, whereas the glucose deprivation paradigm led to a transient increase in Sig1R-BiP binding [Hayashi and Su, 2007]. This study received considerable attention as it defined Sig1R as a molecular chaperone.
We were intrigued by the notion that Sig1R might be a molecular chaperone displaying differential responses under various types of stress. Given that oxidative stress is considered pathogenic in many retinal diseases, we analyzed Sig1R-BiP binding in a retinal neuronal cell line exposed to xanthine:xanthine oxidase, which induces oxidative stress. Sig1R-BiP binding increased following 6 h and 18 h of oxidative stress, but returned to baseline levels in the presence of (+)-PTZ [Ha et al, 2011a]. Whether this reflects an actual ‘chaperone’ function for Sig1R is less clear. As pointed out in a recent review from the Schnell group, further studies are required to confirm Sig1R as a molecular chaperone as few studies have appeared since Hayashi and Su first identified the activity [Ossa et al, 2017]. A number of important questions remain including whether the full-length receptor is required for function and whether the activity is ligand dependent.
Through various studies of protein-protein interaction, it was clear that Sig1R localized to the ER as well as the mitochondrial associated membrane (MAM), an interface of mitochondria and ER. If Sig1R played a key role in modulating ER stress, we reasoned that we should be able to detect alterations in proteins in the ER stress response pathway in retinas of Sig1R−/− mice. ER stress, which is implicated in a number of retinal diseases, reflects an imbalance between the cellular demand for ER function and ER capacity [Malhotra et al, 2007; Yoshida H., 2007; Ni and Lee, 2007]. When the influx of nascent unfolded polypeptides exceeds the folding and processing capacity of the ER, its normal physiological state is perturbed activating signaling pathways, termed the ER stress or unfolded protein response [Smith, 2018]. Three signaling pathways are involved in this stress response including (1) PERK (protein kinase-like ER kinase, pancreatic ER eukaryotic translation initiation factor (eIF)-2a kinase; official name: EIF2AK3), (2) IRE-1 (inositol-requiring protein 1; official name: ERN1), and (3) ATF6 (activating transcription factor 6).
When we evaluated levels of BiP/GRP78 and the three key effector proteins (PERK, IRE-1, ATF6) in whole retinas of Sig1R−/− mice, we did not detect altered expression at the gene or protein level over an age range of 4 days through 2 years. We were surprised by this finding, because we anticipated that if Sig1R plays a key role in modulating ER stress that at least some of these genes/proteins would be altered in retinas lacking Sig1R. We recognized that in analyzing the entire retina, which is composed of neurons, glial cells, and blood vessels, we might be masking ER stress gene changes that were present in only certain cell types. Since the Müller cell is the major glial cell in retina, responsible for maintaining the health and integrity of many retinal neurons [Reichenbach and Bringmann, 2013; Bringmann et al, 2009; Bringmann and Wiedemann, 2013], we chose to study ER stress and Sig1R activation in these cells.
We isolated Müller cells from retinas of wildtype and Sig1R−/− mice. In an initial study we observed slight increases in BiP/Grp78 in Sig1R−/− Müller cells compared to wildtype along with slight decreases in Perk, Ire1α and Atf4 [Ha et al, 2014]. In this set of experiments we detected a highly significant increase in Atf6 expression in the Sig1R−/− versus wildtype Müller cells, however subsequent repetitions of this experiment did not yield this result and we concluded that owing to the low copy number of the Atf6 gene, what we thought was a marked difference was likely artifactual. Thus, for both the Sig1R−/− retina and the isolated Müller cells, we did not observe a robust alteration of ER stress gene expression as anticipated. This curious finding not-with-standing, we did observe marked increase in ER stress genes in retinas of (rd10/Sig1R−/−) mice, which was greater than observed in (rd10/Sig1R−/−) mice [Wang et al, 2017].
Whether modulation of ER stress is a mechanism by which Sig1R mediates retinal neuroprotection thus remains unclear. This uncertainty prompted us to evaluate the retinal transcriptome of Sig1R−/− mice, searching for additional clues regarding Sig1R-mediated neuroprotection. We identified 76 genes that were either up- or down-regulated in Sig1R−/− compared to wildtype retinas [Ha et al. 2014]. Among these were several genes involved in oxidative stress, a common feature of many retinal diseases.
5.2. Evaluation of oxidative stress response in Sig1R−/−Müller cells
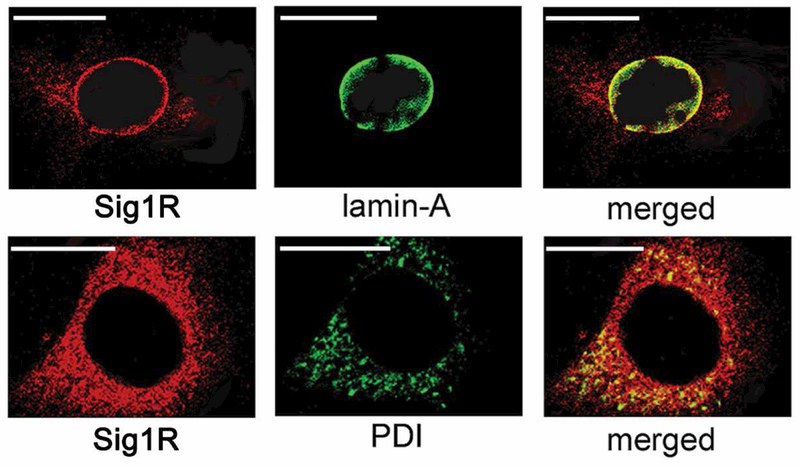
One of our earliest mechanistic studies of Sig1R-mediated neuroprotection was performed in mouse retinal M^ler cells and explored the subcellular location of Sig1R [Jiang et al, 2006]. We performed laser scanning confocal microscopy (LSCM) using an antibody against protein disulfide isomerase (PDI), an ER protein and confirmed Sig1R localization on the ER membrane. In addition, we used an antibody against the nuclear membrane (lamin A) and observed co-localization with Sig1R as well (Fig. 9). This provided strong evidence that Sig1R was a nuclear membrane protein in addition to an ER-mitochondrial protein. In subsequent studies from the Guo group, elegant immuno-electron microscopic studies demonstrated that Sig1R was present in the nuclear envelope of photoreceptor cells, bipolar cells and ganglion cells [Mavlyutov et al, 2015]. This same team of investigators has also used APEX2-enhanced electron microscopy and shown the Sig1R resides in the nucleoplasmic reticulum, a specialized nuclear compartment formed by nuclear envelope invagination [Mavlyutov et al, 2017]. Interestingly, in ganglion cells Sig1R was present also on the ER membrane. The investigators noted that the predominant localization of Sig1R in the nuclear envelope in these three major retinal neurons implicates a potential role of Sig1R in modulating nuclear activities. The significance of this finding was not apparent initially, but would become more relevant as we investigated the role of Sig1R and oxidative stress in retina.
Fig. 9. Subcellular localization of Sig1R in primary Müller cells.
Muller cells were isolated from mouse retina; their identity was verified by the presence of Müller cell markers (cellular retinaldehyde binding protein (CRALBP) and glutamine synthetase. The cells were subjected to double-labeling immunocytochemical analysis using a polyclonal antibody specific for σR1 (red fluorescence) and monoclonal antibodies (green fluorescence) that label the nuclear membrane (lamin-A) or the endoplasmic reticulum (PDI), respectively. Optical sectioning by confocal microscopy co-localized Sig1R with lamin-A (merged image) and with PDI (merged image). In the merged images, the orange signal is detected when the red and green fluorescence overlap indicative of colocalization. Calibration bar = 15 μm. (Figure adapted from Jiang et al, 2006, with permission).
We performed ligand binding assays in which we incubated Müller cell membranes with [3H](+)-PTZ in the presence/absence of various Sig1R inhibitors and in the presence/absence of donors of oxygen species (hydrogen peroxide and xanthine:xanthine oxidase) [Jiang et al, 2006]. There was a marked increase (3–4 fold) in binding of (+)-PTZ to Sig1R under oxidatively stressed conditions. Subsequently, there were several reports that Sig1R ligands could suppress production of reactive oxygen species (ROS) in several tissue types including lung and liver [Pal et al, 2012], cultured lens cells [Wang et al, 2012], retinal pigment epithelial cells [Bucolo et al, 2006a] and retinal neurons [Ha et al, 2011A]. Ruoho’s group transfected COS-7 cells with Sig1R and observed activation of antioxidant response elements followed by upregulation of genes encoding the antioxidant proteins NAD(P)H quinone oxidoreductase (NQO1) and superoxide dismutase (SOD1) [Pal et al, 2012].
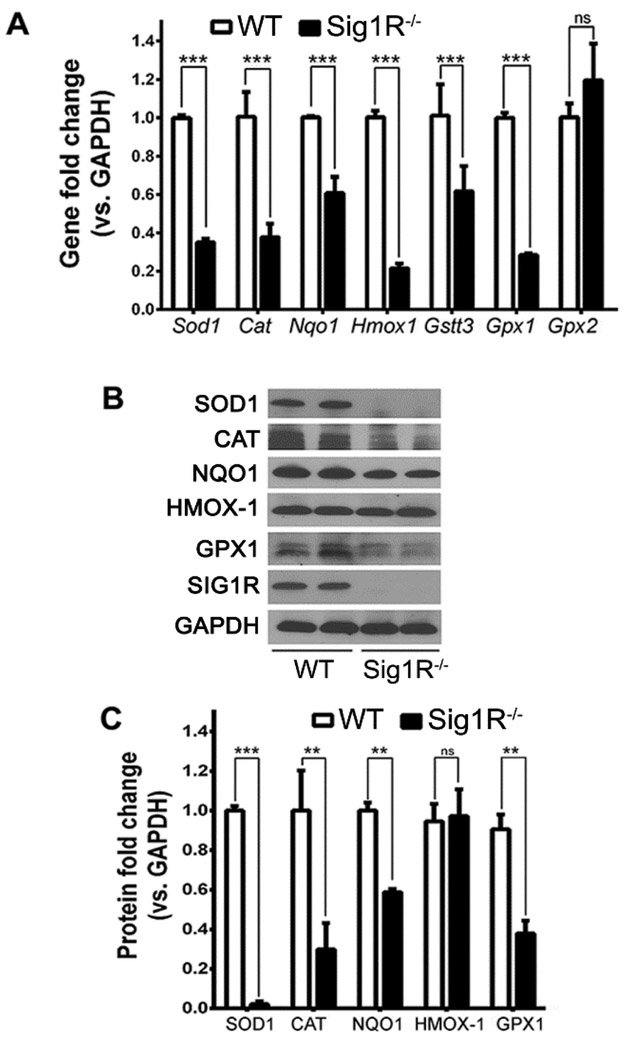
These observations prompted us to evaluate the oxidative stress response in Sig1R−/− Müller cells compared to wildtype. We first compared endogenous ROS levels. As anticipated there was minimal ROS in wildtype cells, however ROS levels were increased significantly in Sig1R−/− Müller cells - even though they had not been exposed to any oxidative insult [Wang et al, 2015]. We confirmed this by measuring ROS quantitatively (e.g. oxidative conversion of carboxyl-DCFH-DA to the highly fluorescent carboxyl-DCF) and observed ~50% increase in ROS in Sig1R−/− cells. We asked whether Sig1R is involved in regulating antioxidant balance by examining expression of genes encoding antioxidant proteins superoxide dismutase (SOD1), catalase (CAT), NQO1, heme oxygenase-1 (HMOX1), glutathione-S-transferase (GST), and glutathione peroxidases (GPX1, GPX2) using Sig1R−/− and wildtype Müller cells. qRT-PCR analysis showed that expression of these genes (except Gpx2) decreased significantly in Sig1R−/− Müller cells versus wildtype (Fig. 10A). Protein levels of SOD1, CAT, NQO1, GPX1 (though not HMOX-1) decreased in Sig1R−/− Müller cells compared to wildtype (Fig. 10B). Quantification of immunoblotting data is shown (Fig. 10C). Additional experiments were pursued to evaluate the cystine-glutamate exchanger (also called System xc-) in these cells. The exchanger, which takes cystine into cells in exchange for glutamate, is important for the synthesis of glutathione, a key retinal antioxidant. Our studies showed that the activity of the exchanger is diminished in Sig1R−/− Müller cells compared to wildtype; and the levels of xCT, a protein unique to this exchanger, are also diminished significantly when Sig1R is absent [Wang et al, 2015].
Fig. 10. Expression of antioxidant genes and proteins are decreased in primary Müller cells harvested from Sig1R−/− mice.
Müller cells were harvested from WT and Sig1R−/− mice following which RNA or protein was isolated. (A) qRT-PCR was performed to investigate expression Sod1, Cat, Nqo1, Hmox1, Gstt3, Gpx1, Gpx2. (B) Western blotting was performed to 57 evaluate protein levels of SOD1, CAT, NQO1, GPX1, HMOX-1. (C) Quantification of immunoblotting data (3 independent experiments). (Figures adapted from Wang et al, 2015, with permission).
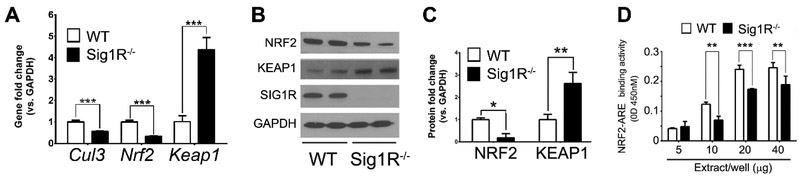
In aggregate, our data provided strong evidence for an attenuated response to oxidative stress in Müller cells harvested from mice lacking Sig1R. One common feature of the genes investigated in Figure 10 is that they contain an antioxidant response element (ARE), which is activated under stress by the protein NRF2 (nuclear factor erythroid 2-related factor 2). NRF2 is considered a “master regulator” of the antioxidant gene response as it modulates transcription of more than 500 antioxidant and cytoprotective genes [Sporn and Liby, 2012; Gorrini et al, 2013]. In the absence of overt cellular stress, NRF2 is retained at low levels in the cytoplasm by KEAP1 (kelch-like ECH-associated protein 1) and excess NRF2 is degraded by the proteasome. Under cellular stress, however, KEAP1 releases NRF2, which then translocates to the nucleus to activate AREs. Given the upregulation of NRF2-regulated antioxidant genes, we investigated expression of NRF2 and KEAP1 in Sig1R−/− Müller cells. Our retinal transcriptome analysis of Sig1R−/− retinas had revealed decreased Cullin 3 (Cul3) expression compared to wildtype [Ha et al, 2014] and Cul3 is an E3 ubiquitin ligase involved in proteasomal degradation of NRF2. As reported in Wang et al, 2015, we confirmed decreased Cul3 expression in Sig1R−/− Müller cells, a significant decrease (~60%) in Nrf2 expression in Sig1R−/− cells compared to wildtype and a marked increase in Keap1 expression (4.4±0.6 fold change in Sig1R−/− Müller cells versus wildtype) (Fig. 11A). Western blotting showed a similar change in NRF2 (decrease) and KEAP1 (increase) protein levels (Fig. 11B). Densitometry reflected ~80% decrease in NRF2 and ~50% increase in KEAP1 levels in Sig1R−/− Müller cells compared to wildtype (Fig. 11C). We then assessed NRF2 activation of AREs using a commercially available ARE oligonucleotide-based trans-activation assay. We performed this assay in nuclear extracts of Sig1R−/− and wildtype Müller cells and observed a significant decrease in NRF2 activity in the Sig1R−/− cells compared to wildtype (Fig. 11D). The data suggest an important role for Sig1R in modulating NRF2 transcriptional regulation of AREs of several antioxidant genes. To our knowledge these studies, conducted in retina, provided the first evidence that Sig1R plays a role in modulating NRF2. They set the stage to explore whether Sig1R-mediated rescue of cone PRCs, which we observed in the rd10 mouse, might occur at least, in part, via modulation of the NRF2-KEAP1 antioxidant pathway.
Fig. 11. Analysis of Nrf2 and Keapl (gene and protein) in WT and Sig1R/” Müller cells.
(A) Quantitative real-time RT-PCR analysis of Cul3, Nrf2, and Keapl mRNA from WT and Sig1R- ‘ Müller cells; data are mean ± SEM (4 independent experiments). (B) Representative immunoblotting data detecting NRF2, KEAP1 and SIG1R in proteins extracted from WT and Sig1R-- mouse Müller cells; GAPDH is the loading control. (C) Quantification of immunoblotting data (mean ± SEM; 3 independent experiments)). (D) NRF2/ARE binding activity performed in WT and Sig1R-- Müller cells. The data represent values obtained at 450nm (mean ± SEM, 3 independent experiments). (**p< 0.01, ***p<0.001.) (Figures adapted from Wang et al, 2015, with permission).
5.3. Sig1R activation attenuates retinal oxidative stress in vivo by modulating NRF2
We investigated whether Sig1R activation could attenuate oxidative stress in vivo by administering (+)-PTZ to rd10 mice beginning at P14 and continuing through P42 [Wang et al, 2016b]. In retina we examined lipid oxidation, protein carbonylation, superoxide levels, NRF2 protein levels, and expression of antioxidant genes regulated by NRF2. Retinal lipid oxidation quantified by measuring levels of malondialdehyde (MDA), a byproduct of lipid peroxidation, was reduced significantly in rd10+PTZ-treated retinas. Retinal protein oxidation, determined by measuring carbonyl content of proteins using 2,4-dinitrophenyl-hydrazine, was reduced significantly in rd10+PTZ-treated versus rd10-nontreated retinas and did not differ significantly from wildtype [Wang et al, 2016b]. The data suggested that retinal lipids and proteins are oxidized in rd10 retinas, whereas regular administration of (+)-PTZ attenuated this oxidative stress. Levels of NRF2 were normalized in the rd10+PTZ retinas as were levels of antioxidant genes. The finding that oxidative stress is a prominent feature of the rd10 retina is consistent with reported observations [Obolensky et al, 2011, Murakami et al, 2012; O’Driscoll et al, 2011; Oveson et al, 2011; Galbinur et al, 2009; Komeima et al, 2006; Xiong et al, 2015]. Our data support a role for Sig1R activation in reducing oxidative stress in vivo.
Our laboratory is continuing to explore the relationship between Sig1R and the NRF2 pathway. Using the rd10 retina, our published studies indicate that oxidative stress is attenuated when Sig1R is activated [Wang et al, 2016b]. This has considerable relevance to retinal diseases, especially RP. In the most common forms of RP, rods are lost preferentially and cone loss follows. It has been hypothesized that cone loss in RP is directly related to oxidative stress [Campochiaro and Mir, 2018]. Oxidative stress refers to an imbalance in the production or elimination of ROS. Rods constitute the majority of PRCs and consume 95% of the oxygen delivered to them by choroidal vessels. Unlike inner retinal blood vessels, choroidal vessels do not auto-regulate tissue oxygen levels. Thus, as rods die in RP, oxygen levels increase creating an oxidatively stressed environment for remaining cones. We believe that the improved cone function reported in rd10 mice treated with (+)-PTZ [Wang et al, 2016b] is due in large part to attenuation of oxidative stress. Studies are underway in our lab to evaluate comprehensively interactions between Sig1R and members of the NRF2 pathway and whether activation of Sig1R alters expression levels of NRF2-related proteins.
5.4. Other mechanisms of Sig1R neuroprotection in retina
Sig1R has many binding partners and likely mediates beneficial cellular phenomenon via a number of mechanisms. Recent reviews propose Sig1R as a pluripotent modulator with multiple functions in living systems [Su et al, 2016; Penke et al, 2018]. Related to retina, there have been a number of studies to understand the role of Sig1R in modulating survival of ganglion cells either directly or through effects on supporting glial cells such as astrocytes and microglia. Particularly intriguing are recent findings suggesting that Sig1R is able to differentially modulate the extracellular signal-regulated protein kinase (ERK1/2) in a cell-type specific manner. The ERK1/2 molecular signaling pathway regulates fundamental cellular processes including proliferation, differentiation and survival. The Bollinger lab has shown that (+)-PTZ reduces NMDA-induced murine ganglion cell death through a Sig1R-dependent mechanism that enhances activation of ERK1/2 [Zhao et al, 2016]. This finding agrees with previous work by the Yorio group showing the survival of purified retinal ganglion cells subjected to oxygen glucose deprivation is directly related to activation of the ERK1/2 pathway [Mueller et al, 2014].
Yet another avenue of investigation suggests that Sig1R regulates mitochondrial function including restoring mitochondrial membrane potential and cytochrome c oxidase activity. A study conducted by Ellis and colleagues exposed primary cultures of rat ganglion cells to oxygen and glucose deprivation. Adenoassociated viral vectors were used to increase expression of Sig1R, following which mitochondrial function was assessed [Ellis et al, 2017]. The investigators found that oxygen and glucose deprivation had a negative impact on mitochondrial function, which was improved in cells over-expressing Sig1R. Interestingly, in this study the investigators detected Sig1R on the nuclear membrane confirming earlier reports of Sig1R nuclear localization [Jiang et al, 2006; Mavlyutov et al, 2015]. Collectively, the data suggest a role for Sig1R in mitochondrial and nuclear activities.
Studies of Sig1R-mediated retinal neuroprotection have evaluated the role of Sig1R in retinal glial cells. Sig1R-mediated reductions in ERK1/2 activation are associated with outcomes typically associated with beneficial support of their neuronal partners. The Bollinger lab isolated astrocytes from the optic nerve head of rats and demonstrated that Sig1R activation by (+)-PTZ protected the astrocytes from oxidative stress-induced cell death and attenuated the level and duration of ERK activation [Zhao J et al, 2017]. They also showed that decreased ERK1/2 phosphorylation is a mechanism by which (+)-PTZ promotes survival. They inhibited phosphorylation of ERK1/2 in the optic nerve head astrocytes using U0126 and showed that cell death was reduced, demonstrating that ERK1/2 activation promotes survival of astrocytes under stressed conditions. They next showed that suppression of ERK1/2 activation occurs through Sig1R by using siRNA technology to diminish Sig1R expression. They found that astrocytes lacking Sig1R had a high baseline level of phosphorylated ERK and, in the absence of Sig1R, treatment with (+)-PTZ did not suppress the phosphorylation.
Investigations have been performed in primary retinal microglial cultures harvested from rat. Experiments demonstrated that activation of Sig1R suppressed ROS generation, reduced microglial inflammatory responses and suppressed activation of the ERK1/2 pathway [Zhao et al, 2014]. Researchers from Bollinger’s lab have also interrogated the possible relationship between Sig1R and brain derived neurotrophic factor (BDNF), which is critical for ganglion cell survival. Sig1R activation with (+)-PTZ increased optic nerve head astrocyte secretion of BDNF, though the mechanism of the increased BDNF secretion is not known [Mysona et al, 2018]. It is noteworthy that activation of Sig1R can inhibit osmotic swelling of Müller cell somata (induced by exposure to hypoosmotic solution), though not of bipolar cell somata. The authors of this study hypothesized that the neuroprotective effect of Sig1R activation in the retina is in part mediated by prevention of the cytotoxic swelling of retinal glial cells [Vogler et al, 2016].
The aforementioned experiments provide evidence of a role for Sig1R in retinal glial cells as well as retinal neurons. Studies have been performed in primary Müller cells, primary optic nerve head astrocytes and retinal microglial cells. In all cases, activation of Sig1R yielded beneficial effects, which could contribute significantly in survival of retinal neurons. In fact, we do not know whether the beneficial effects of administering Sig1R ligands in experimental models of retinal degeneration are efficacious due to direct effects on neurons or to secondary effects via glial cells. An approach to answering this question would be to use targeted knockdown of Sig1R in key cells and assess consequences following administration of Sig1R ligands. For example, in the rd10 mutant mouse, we do not whether cones were rescued due to a direct effect on these cells (as a consequence of (+)-PTZ administration) or to an indirect effect on glial cells, which secondarily spared the cones from death. To address this, Cre-Lox technology could be used and Sig1R could be eliminated in cone cells or in Müller cells and the effects of (+)-PTZ could be evaluated using functional and morphologic approaches similar to the study we reported previously [Wang et al, 2016B].
5.5. Evidence that miR-214-3p may regulate Sig1R
While a number of mechanistic studies of Sig1R have analyzed its potential interactions with other proteins, there have been very few studies about its relationship with microRNAs (miRNAs) [Bao et al, 2017; Bai et al, 2016]. miRNAs are small non-coding RNA molecules of ~22 nucleotides that bind to the 3’ untranslated region (UTR) sequence of target genes to negatively regulate gene expression. MiRNAs are highly conserved and are a vital evolutionary component of gene regulation [Hammond, 2015]. Aberrant miRNA expression has been implicated in neurodegenerative diseases, including retinal degenerative diseases, such as RP [Anasagasti et al, 2018]. We explored the potential relationship of miRNAs and Sig1R by using a commercially available PCR array to screen 84 common miRNAs in retinas of rd10 mice. Specifically, we investigated the miRNAs in retinas of (a) rd10/Sig1R−/− versus rd10/Sig1R−/− mice, and (b) rd10+PTZ versus rd10 mice. We found that 13 miRNAs were significantly increased in rd10/Sig1R−/− retinas, and were significantly decreased in rd10+PTZ-treated retinas. One of these miRNAs, miR-214-3p, is closely related to oxidative stress modulation. In unpublished work from our lab, we examined expression of miR-214-3p, which revealed significantly increased expression in (rd10/Sig1R−/−) versus rd10 retina (~5 fold higher) (Fig. 12A) and significantly decreased expression in (+)-PTZ-treated rd10 versus rd10 non-treated retina (~2 fold) (Fig. 12B). It is noteworthy that miR-214-3p is predicted to bind to Sig1R. We utilized three established miRNA target-prediction programs: (1) miRDB (http://mirdb.org/miRDB). (2) TargetScan (http://www.targetscan.org/), (3) miRanda (http://www.microrna.org/microrna/getGeneForm.do). In these data-bases, miR-214-3p is synchronously predicted as a potential miRNA to regulate Sig1R (Fig. 12C). 119–125 and 331337 of Sig1R 3’UTR were shown to be binding sites for mmu-miR-214-3p. As shown in Fig. 12C, the miR-214-3p from mouse (3’ strand) has the sequence GGACGAC, which binds CCUGCUG. The binding sequence, CCUGCUG in Sig1R, is highly conserved in human, rat and mouse specie (Fig. 12D), indicating a crucial requirement of Sig1R for this post-transcriptional regulation. To our knowledge, this information is the first evidence that Sig1R may interact with miRNAs in retinal disease. These preliminary data lay the ground work for future studies of novel mechanisms for Sig1R-mediated retinal protection.
Fig. 12. The relationship of miR-214-3p to Sig1R.
Using a commercially available miRNA PCR array (Qiagen) we screened retinas of (A) WT, rd10, and rd10/Sig1R−/− mice; and (B) WT, rd10, and (+)-PTZ-treated-rd10 mice, which revealed a significant difference in 13 miRNAs including miRNA-214-3p. (C) miR-214-3p is predicted as a potential miRNA to regulate Sig1R based on analysis using three miRNA target-prediction programs (TargetScan, miRDB and miRanda). (D) Conservation of Sig1R 3’UTR binding sites for miR-214-3p showing the binding sequence, CCUGCUG in Sig1R, is highly conserved in human, rat and mouse species.
6. Identification of endogenous ligands for Sig1R
As described above, Sig1R binds many proteins and considerable experimental evidence suggests that it modulates a number of cellular functions. Despite the progress that has been made in linking Sig1R with these functions, there is very limited information about endogenous molecules that bind to the receptor. Such information could be very useful in elucidating the primary biologic role of Sig1R within cells. While it is known that steroid hormones, such as progesterone, bind to Sig1R [Bouchard et al, 1995], they do not demonstrate agonist properties on Sig1R-regulated ion channels. Thus, the identification of an endogenous ligand has eluded the field for decades. Ruoho and colleagues reported N,N-dimethyltryptamine (DMT), as a putative endogenous Sig1R ligand [Fontanilla et al, 2009]. DMT acts as a hallucinogen, but its receptor target had been unclear. DMT was shown to bind to Sig1Rs and to inhibit voltage-gated sodium ion channels in primary cardiomyocytes and in heterologous cells that express Sig1Rs. Additionally, the investigators showed that DMT induced hypermobility in wildtype mice, but not in Sig1R−/− mice. While these data are compelling, other researchers have questioned DMT as an endogenous ligand because it binds so readily with a number of serotonergic receptors, all of which have greater affinity for DMT than does Sig1R. DMT induces head twitch response in wildtype, but not 5-HT2a knockout mice and 5-HT2a is a serotonin receptor sub-type [Keiser et al, 2009]. These studies from the Roth lab propose that 5-HT2a may be the primary target of DMT and Sig1R may be a secondary target. Efforts are ongoing to identify endogenous ligands for Sig1R. The current dogma is that there are several endogenous molecules that bind Sig1R including sphingosine, N,N-dimethyl sphingosine, DMT, dehydroepiandrosterone and progesterone [Chu and Ruoho, 2016], but the search for a high affinity, endogenous ligand continues. To date, there have been no studies performed in retina to identify endogenous ligands for Sig1R.
7. Future directions and conclusions
Retinal diseases constitute the major cause of unbeatable blindness worldwide, however there is considerable heterogeneity underlying retinal degenerative diseases. Thousands of mutations in more than 200 genes have been identified that lead to blindness in humans [Ran et al, 2014]. In developing therapeutic strategies to treat blindness, it may not be practical to target each genetic defect; however targeting common disease mechanisms holds promise for development of realistic therapies for individuals suffering from retinal disease. The discovery of a unique, though enigmatic, protein with apparently powerful neuroprotective properties offers a line of research to develop novel retinal therapeutic intervention strategies. While the history of Sig1R has been confusing, there is considerable evidence, from both in vitro and in vivo experiments, that targeting Sig1R may offer a retinal neuroprotective tool. The results of work in models of ganglion cell disruption and photoreceptor cell disease are encouraging for a number of retinopathies. New discoveries about the subcellular location of Sig1R have provided insights as to mechanism(s) by which activation of this target protects against retinal neuronal death. The diagram in Fig. 13 summarizes some of the theories of Sig1R-mediated neuroprotection including postulated roles in modulating oxidative stress, calcium, ion channels as well as it presumptive role as a molecular chaperone.
Fig. 13. Sig1R as a modulator of intracellular functions relevant to retinal disease.
Sig1R (SIR) is a single transmembrane domain that crystallizes as a trimer. It resides in ER, nuclear and the MAM. The bulk of the protein resides within the cytosol. Efforts to understand its mechanisms of retinal neuroprotection have identified functions associated with ion channel regulation, chaperone activity, oxidative stress modulation and regulation of calcium. (Abbreviations: Sigma 1 Receptor (SIR), endoplasmic reticulum (ER), mitochondrial associated membrane (MAM), calcium (Ca2+)
Despite the progress made, there is much to be done in the field of Sig1R biology especially related to neurodegenerative disorders and retinal degenerations. Definitive data regarding the mechanism(s) by which Sig1R is neuroprotective are sorely needed. It is not sufficient to establish that Sig1R co-localizes with another protein; it is essential to determine whether associations have biological significance. Understanding the precise role of Sig1R as a molecular chaperone in retina will be a fertile area of study. Some of the new technologies for examining Sig1R at extremely high resolution will be useful in this endeavor. Determining endogenous ligands for Sig1R has been confusing and somewhat ambiguous, but is key to understanding the biological role and represents another area ripe for intense investigation. Understanding whether Sig1R has different cellular functions depending upon cell type (e.g. retinal neurons versus retinal glial cells) is also an important area of investigation. Future work is required to determine whether compounds mentioned in this review, or novel compounds that are under development, can be brought to clinical trials in hopes of alleviating retinal disease. It is noteworthy that some Sig1R ligands, such as SA4503 (cutamesine) are currently in clinical trials for the treatment of stroke [Urfer et al, 2014]. Whether this compound would be efficacious in human retinal disease remains to be investigated. Our group is developing a study of cutamesine in the rd10 mouse model to determine whether effects are as promising (or even more promising) than the retinal neuroprotection afforded using (+)-PTZ. In addition to neuroprotective studies, the toxic effects of Sig1R stimulation need to be carefully investigated. Although no untoward effects have been reported to date in in vivo experimental models, this possibility cannot be overlooked. While we have concrete evidence that targeting Sig1R in retinal disease has had positive effects, a promising direction for the field would be exploration of combined therapies. In other words, targeting Sig1R may enhance the retinal milieu that could prove beneficial to success of other treatments, such as gene therapy or stem cell therapy, that are also being explored in the field. In summary, considerable progress has been made in our understanding of Sig1R and evidence suggests its potential in treatment of retinal diseases such as RP, diabetic retinopathy, glaucoma and others. It is hoped that the promising outcomes from in vivo experimental models and mechanistic studies will be translatable to treatments for humans suffering from severe retinal disease.
Acknowledgements
Omitted from this version to de-identify authors
List of abbreviations
- (+)-PTZ
(+)-pentazocine
- (NQO1)
NAD(P)H quinone oxidoreductase
- 3PPP
3-(3-hydroxyphenyl)-N-(1-propyl)piperidine
- 661W
cone photoreceptor cell line
- AF2975
(N,N-dimethyl-2-phenylethylamine HCl
- ARE
antioxidant response element
- ARPE-19
human RPE cell line
- ATF6
activating transcription factor 6
- ATP
Adenosine triphosphate
- Aβ
amyloid beta
- BAX
BCL-2 associated protein
- BDNF
brain derived neurotrophic factor
- BD1047
(N-[2-(3,4-Dichlorophenyl)ethyl]-N,N’,N’-trimethyl-1,2-ethanediamine), Sig1R antagonist
- BiP/GRP78
Binding Protein/Glucose Regulated Protein
- CHO
Chinese Hamster Ovary
- CNTF
ciliary neurotrophic factor
- COS-7 cells
monkey kidney fibroblast-like cell line
- Cul3
Cullin 3
- DTG
di-o-tolylguanidine
- DMT
dimethyltryptamine
- ER
Endoplasmic reticulum
- ERG
electroretinogram
- ERK1/2
extracellular signal-regulated protein kinase
- GalCer
galactosylceramides
- GCL
ganglion cell layer
- GFAP
glial fibrillary associated protein
- GPX
glutathione peroxidases
- GST
glutathione-S-transferase
- HMOX1
heme oxygenase-1
- Iba1
Ionized calcium binding adaptor molecule 1
- INL
inner nuclear layer
- Ins2Akita/+
Insulin2 mouse model of diabetes discovered at Akita University, Japan
- IP
intraperitoneal
- IP3R
1,4,5-triphosphate receptors
- IRE-1
inositol-requiring protein 1
- IS
inner segment
- JNK
c-Jun N-terminal kinases
- KEAP1
kelch-like ECH-associated protein 1
- LSCM
laser scanning confocal microscopy
- Lux
unit of illuminance, equal to one lumen per square meter
- MDA
malondialdehyde
- miRNA
micro-ribonucleic acid
- MR22
N-methyladamantan-1-amine
- NFL
nerve fiber layer
- NG108
neuroblastoma-glioma hybrid cells
- NMDA
N-methyl-D-aspartate
- NPC1
Niemann-Pick disease Protein (NPC1)
- NRF2
nuclear factor erythroid 2-related factor 2
- nSTR
negative scotopic threshold response
- ONL
outer nuclear layer
- PCP
phencyclidine
- Pde6brd10/J
beta-subunit of the rod cGMP phosphodiesterase (β-PDE)
- PDI
protein disulfide isomerase
- PERK
protein kinase-like ER kinase
- PNA
peanut agglutinin
- PRC
photoreceptor cell
- Pre-084
pregnenolone sulfate; (2-(4-Morpholinethyl) 1-phenylcyclohexanecarboxylate hydrochloride)
- Pst I, Sty I
restriction enzymes used in RT-PCR
- Rd10
shorthand reference to Pde6brd10/J mouse
- RGC-5
putative retinal ganglion cell line, later re-identified as the 661W cell line
- rMC1
rat Müller cell line
- ROS
reactive oxygen species
- RP
Retinitis pigmentosa
- RT-PCR
reverse transcribed polymerase chain reaction
- SA4503
1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)-piperazine dihydrochloride; cutamesine
- SD-OCT
spectral domain-optical coherence tomography
- Sig1R
Sigma 1 Receptor
- Sig1R−/−
genetic knockout mouse for Sig1R
- SKF-10,047
N-allylnormetazocine
- SOD1
superoxide dismutase
- TBARS
thiobarbituric acid
- TMTM97
transmembrane protein 97 (Sig2R)
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- UTR
untranslated region
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alon A, Schmidt H, Zheng S, Kruse AC. 2017a. Structural perspectives on Sigma-1 receptor function. Adv Exp Med Biol 964:5–13. [DOI] [PubMed] [Google Scholar]
- Alon A, Schmidt HR, Wood MD, Sahn JJ, Martin SF, Kruse AC. 2017b. Identification of the gene that codes for the σ(2) receptor. Proc Natl Acad Sci U S A. 114, 7160–7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anasagasti A, Ezquerra-Inchausti M, Barandika O, Munoz-Culla M, Caffarel MM, Otaegui D, Lopez de Munain A, Ruiz-Ederra J. 2018. Expression Profiling Analysis Reveals Key MicroRNA-mRNA Interactions in Early Retinal Degeneration in Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 59,2381–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. 2002. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 34, 399–410. [DOI] [PubMed] [Google Scholar]
- Bao Q, Zhao M, Chen L, Wang Y, Wu S, Wu W, Liu X. 2017. MicroRNA-297 promotes cardiomyocyte hypertrophy via targeting sigma-1 receptor. Life Sci 175,1–10. [DOI] [PubMed] [Google Scholar]
- Bai Y, Zhang Y, Hua J, Yang X, Zhang X, Duan M, Zhu X, Huang W, Chao J, Zhou R, Hu G, Yao H. 2016. Silencing microRNA-143 protects the integrity of the blood-brain barrier: implications for methamphetamine abuse. Sci Rep 6,35642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. 2005. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci 46, 2210–2218. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. 1998. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102,783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone I, Novelli E, Strettoi E. 2014. Long-term preservation of cone photoreceptors and visual acuity in rd10 mutant mice exposed to continuous environmental enrichment. Mol Vis 20,1545–1556. [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LL. 1988. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1,791–803. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Quirion R. 1997. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 76, 467–477. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Monnet F, Bergeron R, Roman F, Junien JL, de Montigny C, Debonnel G, Quirion R. 1995. In vivo modulation of sigma receptor sites by calcitonin gene-related peptide in the mouse and rat hippocampal formation: radioligand binding and electrophysiological studies. Eur J Neurosci 7:1952–1962. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. 2009. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res 28, 423–451. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Wiedemann P. 2012. Muller glial cells in retinal disease. Ophthalmologica. 227:1–19. [DOI] [PubMed] [Google Scholar]
- Bucolo C, Drago F, Lin LR, Reddy VN. 2006a. Sigma receptor ligands protect human retinal cells against oxidative stress. Neuroreport. 17, 287–291. [DOI] [PubMed] [Google Scholar]
- Bucolo C, Marrazzo A, Ronsisvalle S, Ronsisvalle G, Cuzzocrea S, Mazzon E, Caputi A, Drago F. 2006b. A novel adamantane derivative attenuates retinal ischemia-reperfusion damage in the rat retina through sigma1 receptors. Eur J Pharmacol 536, 200–203. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Mir TA. 2018. The mechanism of cone cell death in Retinitis Pigmentosa. Prog Retin Eye Res 62:24–37. [DOI] [PubMed] [Google Scholar]
- Cantarella G, Bucolo C, Di Benedetto G, Pezzino S, Lempereur L, Calvagna R, Clementi S, Pavone P, Fiore L, Bernardini R. 2007. Protective effects of the sigma agonist Pre-084 in the rat retina. Br J Ophthalmol 91,1382–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. 2002. Retinal degeneration mutants in the mouse. Vision Res 42, 517–525. [DOI] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, Phillips MJ, Stewart RE, Chaudhury R, Nickerson JM, Heckenlively JR, Boatright JH. 2007. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res 47, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu UB, Ruoho AE. 2016. Biochemical Pharmacology of the Sigma-1 Receptor. Mol Pharmacol 89:142–153. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Prough RA, Klinge CM. 2018. Mechanisms of Action of Dehydroepiandrosterone. Vitam Horm 108:29–73. [DOI] [PubMed] [Google Scholar]
- de Costa BR, Bowen WD, Hellewell SB, Walker JM, Thurkauf A, Jacobson AE, Rice KC. 1989. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett 251, 53–58. [DOI] [PubMed] [Google Scholar]
- DeCoster MA, Klette KL, Knight ES, Tortella FC. 1995. Sigma receptor-mediated neuroprotection against glutamate toxicity in primary rat neuronal cultures. Brain Res 671, 45–53. [DOI] [PubMed] [Google Scholar]
- Drack AV, Dumitrescu AV, Bhattarai S, Gratie D, Stone EM, Mullins R, Sheffield VC. 2012. TUDCA slows retinal degeneration in two different mouse models of retinitis pigmentosa and prevents obesity in Bardet-Biedl syndrome type 1 mice. Invest Ophthalmol Vis Sci 53,100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun Y, Mysona B, Van Ells T, Amarnath L, Ola MS, Ganapathy V, Smith SB. 2006. Expression of the cystine-glutamate exchanger (xc-) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 324,189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB. 2007. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci 48,4785–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DZ, Li L, Park Y, He S, Mueller B, Yorio T. 2017. Sigma-1 Receptor Regulates Mitochondrial Function in Glucose-and Oxygen-Deprived Retinal Ganglion Cells. Invest Ophthalmol Vis Sci 58;2755–2764. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. 2009. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 323,934–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry LE, Fahy E, Chrysostomou V, Hui F, Tang J, van Wijngaarden P, Petrou S, Crowston JG. 2018. The coma in glaucoma: Retinal ganglion cell dysfunction and recovery. Prog Retin Eye Res 65, 77–92. [DOI] [PubMed] [Google Scholar]
- Galbinur T, Obolensky A, Berenshtein E, Vinokur V, Chowers I, Chevion M, Banin E. 2009. Effect of para-aminobenzoic acid on the course of retinal degeneration in the rd10 mouse. J Ocul Pharmacol Ther 25, 475–482. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. 2007. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol 500, 222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrini C, Baniasadi PS, Harris IS, Silvester J, Inoue S, Snow B, Joshi PA, Wakeham A, Molyneux SD, Martin B, Bouwman P, Cescon DW, Elia AJ, Winterton-Perks Z, Cruickshank J, Brenner D, Tseng A, Musgrave M, Berman HK, Khokha R, Jonkers J, Mak TW, Gauthier ML. 2013. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J Exp Med 210, 1529–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagni V, Novelli E, Piano I, Gargini C, Strettoi E. 2015. Pharmacological approaches to retinitis pigmentosa: A laboratory perspective. Prog Retin Eye Res 48:62–81. [DOI] [PubMed] [Google Scholar]
- Ha Y, Dun Y, Thangaraju M, Duplantier J, Dong Z, Liu K, Ganapathy V, Smith SB. 2011A. Sigma receptor 1 modulates endoplasmic reticulum stress in retinal neurons. Invest Ophthalmol Vis Sci 52, 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Saul A, Tawfik A, Williams C, Bollinger K, Smith R, Tachikawa M, Zorrilla E, Ganapathy V, Smith SB. 2011B. Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (σR1). Invest Ophthalmol Vis Sci 52, 7749–7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Saul A, Tawfik A, Zorrilla EP, Ganapathy V, Smith SB. 2012. Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol Vis 18, 2860–2870. [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Shanmugam AK, Markand S, Zorrilla E, Ganapathy V, Smith SB. 2014. Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res 356,15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM. 2015. An overview of microRNAs. Adv Drug Deliv Rev 87,3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif AM, Lawson EC, Prunty M, Gogniat M, Aung MH, Chakraborty R, Boatright JH, Pardue MT. 2015. Neuroprotective effects of voluntary exercise in an inherited retinal degeneration mouse model. Invest Ophthalmol Vis Sci 56, 6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. 1996. purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 93, 8072–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. 2001. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 98, 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. 2003. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther 306, 718–725. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. 2004. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 101,14949–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131, 596–610. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. 1994. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol 268, 9–18. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Carlsson A, Wikstrom H, Lindberg P, Sanchez D, Hacksell U, Arvidssonc LE, Svensson U, Nilsson JL. 1981. 3-PPP, a new centrally acting DA-receptor agonist with selectivity for autoreceptors. Life Sci 28, 1225–1238. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Marinich-Madzarevich JA, Marchena M, Ruiz JM, Cano MJ, de la Villa P, Hernandez-Sanchez C, de la Rosa EJ, de Pablo F. 2016. Intravitreal injection of proinsulin-loaded microspheres delays photoreceptor cell death and vision loss in the rd10 mouse model of Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 57, 3610–3618. [DOI] [PubMed] [Google Scholar]
- Jbilo O, Vidal H, Paul R, De Nys N, Bensaid M, Silve S, Carayon P, Davi D, Galiegue S, Bourrie B, Guillemot JC, Ferrara P, Loison G, Maffrand JP, Le Fur G, Casellas P. 1997. Purification and characterization of the human SR 31747A-binding protein. A nuclear membrane protein related to yeast sterol isomerase. J Biol Chem 272, 27107–27115. [DOI] [PubMed] [Google Scholar]
- Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. 2006. Expression, subcellular localization, and regulation of sigma receptor in retinal Müller cells. Invest Ophthalmol Vis Sci 47,5576–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL. 2009. Predicting new molecular targets for known drugs. Nature. 462,175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. 1996. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem Biophys Res Commun 229, 553–558. [DOI] [PubMed] [Google Scholar]
- Khazan N, Young GA, El-Fakany EE, Hong O, Calligaro D. 1984. Sigma receptors mediated the psychotomimetic effects of N-allylnormetazocine (SKF-10,047), but not its opioid agonistic-antagonistic properties. Neuropharmacology. 23:983–987. [DOI] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L, Campochiaro PA. 2006. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 103, 11300–11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Clark AF, Daudt D, Vishwanatha JK, Yorio T. 2013. A forensic path to RGC-5 cell line identification: lessons learned. Invest Ophthalmol Vis Sci 54,5712–5719. [DOI] [PubMed] [Google Scholar]
- Largent BL, Gundlach AL, Snyder SH. 1984. Psychotomimetic opiate receptors labeled and visualized with (+)-[3H]3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. Proc Natl Acad Sci U S A. 81, 4983–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent BL, Gundlach AL, Snyder SH. 1986. Sigma receptors on NCB-20 hybrid neurotumor cells labeled with (+)[3H]SKF 10,047 and (+)[3H]3-PPP. Eur J Pharmacol 124,183–187. [DOI] [PubMed] [Google Scholar]
- Lechner J, O’Leary OE, Stitt AW. 2017. The pathology associated with diabetic retinopathy. Vision Res 139,7–14. [DOI] [PubMed] [Google Scholar]
- Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. 2006. Sigma1 and sigma2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 59,350–358. [DOI] [PubMed] [Google Scholar]
- Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. 1998. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 47, 815–820. [DOI] [PubMed] [Google Scholar]
- Liu LL, Wang L, Zhong YM, Yang XL. 2010. Expression of sigma receptor 1 mRNA and protein in rat retina. Neuroscience. 167,(4): 1151–9. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. 2007. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18,716–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM, Brown WD, Hacein-Bey L. 2001. Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. Am J Neuroradiol 22:1813–1824. [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Ola MS, Agarwal N, Ganapathy V, Smith SB. 2004. The sigma receptor ligand (+)-pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Brain Res Mol Brain Res 123, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. 1976. The effects of morphine- and nalorphine-like drugs in the non-dependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther 197,517–532. [PubMed] [Google Scholar]
- Matsuno K, Senda T, Mita S. 1993. Correlation between potentiation of neurogenic twitch contraction and benzomorphan sigma receptor binding potency in the mouse vas deferens. Eur J Pharmacol 231,451–457. [DOI] [PubMed] [Google Scholar]
- Mavlyutov TA, Chen X, Guo L, Yang J. 2018. APEX2-tagging of Sigma 1-receptor indicates subcellular protein topology with cytosolic N-terminus and ER luminal C-terminus. Protein Cell 9:733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein M, Guo LW. 2015. Subcellular localization of the sigma-1 receptor in retinal neurons - an electron microscopy study. Sci Rep 2, 10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Guo LW. 2017A. Peeking into Sigma-1 Receptor Functions Through the Retina. Adv Exp Med Biol 964, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Nickells RW, Guo LW. 2011. Accelerated retinal ganglion cell death in mice deficient in the Sigma-1 receptor. Mol Vis 17, 1034–1043. [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Yang H, Epstein ML, Ruoho AE, Yang J, Guo LW. 2017B. APEX2-enhanced electron microscopy distinguishes sigma-1 receptor localization in the nucleoplasmic reticulum. Oncotarget 8:51317–51330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S, Weber E. 1988. Autoradiographic visualization of haloperidol-sensitive sigma receptors in guinea-pig brain. Neuroscience. 25, 259–269. [DOI] [PubMed] [Google Scholar]
- Mendelsohn LG, Kalra V, Johnson BG, Kerchner GA. 1985. Sigma opioid receptor: characterization and co-identity with the phencyclidine receptor. J Pharmacol Exp Ther 233, 597–602. [PubMed] [Google Scholar]
- Mueller BH, Park Y, Daudt DR 3rd, Ma HY, Akopova I, Stankowska DL, Clark AF, Yorio T. 2013. Sigma-1 receptor stimulation attenuates calcium influx through activated L-type Voltage Gated Calcium Channels in purified retinal ganglion cells. Exp Eye Res 107, 21–31. [DOI] [PubMed] [Google Scholar]
- Mueller BH, Park Y, Ma HY, Dibas A, Ellis DZ, Clark AF, Yorio T. 2014. Sigma-1 receptor stimulation protects retinal ganglion cells from ischemia-like insult through the activation of extracellular-signal-regulated kinases 1/2. Exp Eye Res 128, 156–169. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Ikeda Y, Yoshida N, Notomi S, Hisatomi T, Oka S, De Luca G, Yonemitsu Y, Bignami M, Nakabeppu Y, Ishibashi T. 2012. MutT homolog-1 attenuates oxidative DNA damage and delays photoreceptor cell death in inherited retinal degeneration. Am J Pathol 181,1378–1386. [DOI] [PubMed] [Google Scholar]
- Mysona B, Kansara N, Zhao J, Bollinger K. 2017. The Role of Sigma 1 Receptor as a Neuroprotective Target in Glaucoma. Adv Exp Med Biol 964, 299–307. [DOI] [PubMed] [Google Scholar]
- Mysona BA, Zhao J, Smith S, Bollinger KE. 2018. Relationship between Sigma-1 receptor and BDNF in the visual system. Exp Eye Res 167,25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakkode S, Liu C, Soong TW. 2018. Altered function of neuronal L-type calcium channels in ageing and neuroinflammation: Implications in age-related synaptic dysfunction and cognitive decline. Ageing Res Rev 42,86–99. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Lucke-Wold BP, Mookerjee SA, Cavendish JZ, Robson MJ, Scandinaro AL, Matsumoto RR. 2015. Role of sigma-1 receptors in neurodegenerative diseases. J Pharmacol Sci 127, 17–29. [DOI] [PubMed] [Google Scholar]
- Ni M, Lee AS. 2007. ER chaperones in mammalian development and human diseases. FEBS Letters 581,3641–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova M, Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. 1995. Inotropic action of sigma receptor ligands in isolated cardiac myocytes from adult rats. Eur J Pharmacol 286,19–30. [DOI] [PubMed] [Google Scholar]
- Obolensky A, Berenshtein E, Lederman M, Bulvik B, Alper-Pinus R, Yaul R, Deleon E, Chowers I, Chevion M, Banin E. 2011. Zinc-desferrioxamine attenuates retinal degeneration in the rd10 mouse model of retinitis pigmentosa. Free Radic Biol Med 51, 1482–1491. [DOI] [PubMed] [Google Scholar]
- O’Driscoll C, Doonan F, Sanvicens N, Messeguer A, Cotter TG. 2011. A novel free radical scavenger rescues retinal cells in vivo. Exp Eye Res 93, 65–74. [DOI] [PubMed] [Google Scholar]
- Ola MS, Moore P, El-Sherbeny A, Roon P, Agarwal N, Sarthy VP, Casellas P, Ganapathy V, Smith SB. 2001. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Brain Res Mol Brain Res 95, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola MS, Moore P, Maddox D, El-Sherbeny A, Huang W, Roon P, Agarwal N, Ganapathy V, Smith SB. 2002. Analysis of sigma receptor (sigmaR1) expression in retinal ganglion cells cultured under hyperglycemic conditions and in diabetic mice. Brain Res Mol Brain Res 107, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisciak DT, Vaughan DK. 2010. Retinal light damage: mechanisms and protection. Prog Retin Eye Res 29,113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossa F, Schnell JR, Ortega-Roldan JL. 2017. A Review of the Human Sigma-1 Receptor Structure. Adv Exp Med Biol 964, 15–29. [DOI] [PubMed] [Google Scholar]
- Oveson BC, Iwase T, Hackett SF, Lee SY, Usui S, Sedlak TW, Snyder SH, Campochiaro PA, Sung JU. 2011. Constituents of bile, bilirubin and TUDCA, protect against oxidative stress-induced retinal degeneration. J Neurochem 116, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE 2012. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur.J.Pharmacol 682,12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke B, Fulop L, Szucs M, Frecska E. 2018. The Role of Sigma-1 Receptor, an Intracellular Chaperone in Neurodegenerative Diseases. Curr Neuropharmacol 16,97–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran X, Cai WJ, Huang XF, Liu Q, Lu F, Qu J, Wu J, Jin ZB. 2014. ‘RetinoGenetics’: a comprehensive mutation database for genes related to inherited retinal degeneration. Database (Oxford). pii: bau047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A. 2013. New functions of Müller cells. Glia. 61,651–678. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. 2009. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res 198,472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul AB, Still AE. 2016. Multifocal Electroretinography in the presence of temporal and spatial correlations and eye movements. Vision 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HR, Zheng S, Gurpinar E, Koehl A, Manglik A, Kruse AC. 2016. Crystal structure of the human σ1 receptor. Nature. 532, 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwald RD, Barfknecht CF, Shirolkar S, Xia E, Ignace CC. 1994. Identification of sigma receptors in lacrimocytes and their therapeutic implication in dry eye syndrome. Adv Exp Med Biol 350,141–146. [DOI] [PubMed] [Google Scholar]
- Schoenwald RD, Barfknecht CF, Xia E, Newton RE. 1993. The presence of sigma-receptors in the lacrimal gland. J Ocul Pharmacol 9, 125–139. [DOI] [PubMed] [Google Scholar]
- Schoenwald RD, Vidvauns S, Wurster DE, Barfknecht CF. 1997. Tear film stability of protein extracts from dry eye patients administered a sigma agonist. J Ocul Pharmacol Ther 13, 151–161. [DOI] [PubMed] [Google Scholar]
- Senda T, Matsuno K, Mita S. 1997. The presence of sigma receptor subtypes in bovine retinal membranes. Exp Eye Res 64, 857–860. [DOI] [PubMed] [Google Scholar]
- Senda T, Mita S, Kaneda K, Kikuchi M, Akaike A. 1998. Effect of SA4503, a novel sigmal receptor agonist, against glutamate neurotoxicity in cultured rat retinal neurons. Eur J Pharmacol 342, 105–111. [DOI] [PubMed] [Google Scholar]
- Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. 1998. Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem 70, 922–931. [DOI] [PubMed] [Google Scholar]
- Seth P, Ganapathy ME, Conway SJ, Bridges CD, Smith SB, Casellas P, Ganapathy V. 2001. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim Biophys Acta 1540:59–67. [DOI] [PubMed] [Google Scholar]
- Seth P, Leibach FH, Ganapathy V. 1997. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun 241,535–540. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Xu SX. 1999a. Human retina contains polyamine sensitive [3H]-ifenprodil binding sites: implications for neuroprotection? Br J Ophthalmol 83, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif NA, Xu SX. 1999b. Pharmacological characterization of [3H]-Ifenprodil binding to polyamine binding sites on rabbit and rat retinal homogenates: role in neuroprotection? J Ocul Pharmacol Ther 15, 271–281. [DOI] [PubMed] [Google Scholar]
- Shimazawa M, Sugitani S, Inoue Y, Tsuruma K, Hara H. 2015. Effect of a sigma-1 receptor agonist, cutamesine dihydrochloride (SA4503), on photoreceptor cell death against light-induced damage. Exp Eye Res 132, 64–72. [DOI] [PubMed] [Google Scholar]
- Shirolkar S, Schoenwald RD, Barfknecht CF, Xia E, Cheng B, Iwai Y, Ignace CC, Vidvauns S, Newton RE. 1993. Lacrimal secretion stimulants: sigma receptors and drug implications. J Ocul Pharmacol 9, 211–227. [DOI] [PubMed] [Google Scholar]
- Sircar R, Nichtenhauser R, Ieni JR, Zukin SR. 1986. Characterization and autoradiographic visualization of (+)-[3H]SKF10,047 binding in rat and mouse brain: further evidence for phencyclidine/”sigma opiate” receptor commonality. J Pharmacol Exp Ther 237, 681–688. [PubMed] [Google Scholar]
- Sircar R, Zukin SR. 1983. Characterization of specific sigma opiate/phencyclidine (PCP)-binding sites in the human brain. Life Sci 33 (Suppl 1), 259–262. [DOI] [PubMed] [Google Scholar]
- Smith SB, Duplantier J, Dun Y, Mysona B, Roon P, Martin PM, Ganapathy V. 2008. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci 49, 4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB 2018. Mechanisms of ER stress in retinal disease Ryan’s Retina (Sixth Edition) Editors: Schachat AP, Sadda S, Hinton DR, Wilkinson CP, Wiedemann P Elsevier, London: 596–605. [Google Scholar]
- Sporn MB, Liby KT. 2012. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 12,564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. 2010. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci 31, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Su TC, Nakamura Y, Tsai SY. 2016. The Sigma-1 Receptor as a Pluripotent Modulator in Living Systems. Trends Pharmacol Sci 37,262–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP. 1981. Psychotomimetic opioid binding: specific binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. Eur J Pharmacol 75, 81–22. [DOI] [PubMed] [Google Scholar]
- Su TP. 1982. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther 223, 284–290. [PubMed] [Google Scholar]
- Tam SW, Cook L. 1984. Sigma opiates and certain antipsychotic drugs mutually inhibit (+)-[3H] SKF 10,047 and [3H]haloperidol binding in guinea pig brain membranes. Proc Natl Acad Sci U S A. 81, 5618–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. 2004. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci 45,764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchedre KT, Yorio T. 2008. sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest Ophthalmol Vis Sci 49, 2577–2588. [DOI] [PubMed] [Google Scholar]
- Urfer R, Moebius HJ, Skoloudik D, Santamarina E, Sato W, Mita S, Muir KW; Cutamesine Stroke Recovery Study Group. 2014. Phase II trial of the Sigma-1 receptor agonist cutamesine (SA4503) for recovery enhancement after acute ischemic stroke. Stroke. 45:3304–3310. [DOI] [PubMed] [Google Scholar]
- Vaupel DB. 1983. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur J Pharmacol 92,269–274. [DOI] [PubMed] [Google Scholar]
- Vogler S, Winters H, Pannicke T, Wiedemann P, Reichenbach A, Bringmann A. 2016. Sigma-1 receptor activation inhibits osmotic swelling of rat retinal glial (Muller) cells by transactivation of glutamatergic and purinergic receptors. Neurosci Lett 610,13–18. [DOI] [PubMed] [Google Scholar]
- Wang J, Cui X, Roon P, Smith SB. 2016A. Role of Sigma 1 Receptor in Retinal Degeneration of the Ins2Akita/+ Murine Model of Diabetic Retinopathy. Invest Ophthalmol Vis Sci 57,2770–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Saul A, Cui X, Roon P, Smith SB. 2017. Absence of Sigma 1 Receptor accelerates photoreceptor cell death in a murine model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 58, 4545–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Saul A, Roon P, Smith SB. 2016B. Activation of the molecular chaperone, sigma 1 receptor, preserves cone function in a murine model of inherited retinal degeneration. Proc Natl Acad Sci U S A. 113, E3764–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shanmugam A, Markand S, Zorrilla E, Ganapathy V, Smith SB. 2015. Sigma 1 receptor regulates the oxidative stress response in primary retinal Müller glial cells via NRF2 signaling and system xc(−), the Na(+)-independent glutamate-cystine exchanger. Free Radic Biol Med 86, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Eldred JA, Sidaway P, Sanderson J, Smith AJ, Bowater RP, Reddan JR, Wormstone IM. 2012. Sigma 1 receptor stimulation protects against oxidative damage through suppression of the ER stress responses in the human lens. Mech Ageing Dev 133, 665–674. [DOI] [PubMed] [Google Scholar]
- Wang WF, Ishiwata K, Kiyosawa M, Kawamura K, Oda K, Kobayashi T, Matsuno K, Mochizuki M. 2002. Visualization of sigma1 receptors in eyes by ex vivo autoradiography and in vivo positron emission tomography. Exp Eye Res 75,723–730. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Reme CE. 2005. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res 24:275–306. [DOI] [PubMed] [Google Scholar]
- Wolfe SA Jr, Ha BK, Whitlock BB, Saini P. 1997. Differential localization of three distinct binding sites for sigma receptor ligands in rat spleen. J Neuroimmunol 72, 45–58. [DOI] [PubMed] [Google Scholar]
- Wong EH, Knight AR, Woodruff GN. 1988. [3H]MK-801 labels a site on the N-methyl-D-aspartate receptor channel complex in rat brain membranes. J Neurochem 50, 274–281. [DOI] [PubMed] [Google Scholar]
- Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. 2015. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest 125, 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Fu Y, Liu X, Shahi PK, Mavlyutov TA, Li J, Yao A, Guo SZ, Pattnaik BR, Guo LW. 2017. Role of the sigma-1 receptor chaperone in rod and cone photoreceptor degenerations in a mouse model of retinitis pigmentosa. Mol Neurodegener 12,68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H 2007. ER stress and diseases. FEBS J 274,630–658. [DOI] [PubMed] [Google Scholar]
- Young GA, Khazan N. 1984. Differential neuropharmacological effects of mu, kappa and sigma opioid agonists on cortical EEG power spectra in the rat. Stereospecificity and naloxone antagonism. Neuropharmacology. 23, 1161–1165. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ha Y, Liou GI, Gonsalvez GB, Smith SB, Bollinger KE. 2014. Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest Ophthalmol Vis Sci 55, 3375–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Mysona BA, Qureshi A, Kim L, Fields T, Gonsalvez GB, Smith SB, Bollinger KE. 2016. (+)-Pentazocine Reduces NMDA-Induced Murine Retinal Ganglion Cell Death Through a σR1-Dependent Mechanism. Invest Ophthalmol Vis Sci 57,453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Mysona BA, Wang J, Gonsalvez GB, Smith SB, Bollinger KE. 2017. Sigma 1 receptor regulates ERK activation and promotes survival of optic nerve head astrocytes. PLoS One. 12(9):e0184421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen G, Li J, Fu Y, Mavlyutov TA, Yao A, Nickells RW, Gong S, Guo LW. 2017. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J Control Release. 247:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]