Abstract
Background
Despite the availability of effective surveillance for colorectal cancer with colonoscopy, relatively few at-risk individuals utilize this option. Colon cancer chemoprevention might be a more acceptable alternative. Some epidemiologic studies have suggested that statins may have chemopreventive effects without the risks of nonsteroidal anti-inflammatory drugs, but other epidemiologic studies have found no effect of statins.
Methods
We aimed to evaluate the efficacy of atorvastatin in inducing apoptosis in vitro, in preventing polyp formation in the min mouse, and in preventing tumor growth in nude mice.
Results
Atorvastatin rapidly induces apoptosis in the HCT116 colon cancer cell line in vitro, and this effect is reversible with mevalonate and geranylgeranyl pyrophosphate, but less so by farnesyl pyrophosphate. Atorvastatin chow was ineffective in reducing polyp formation in the min mouse model, with no significant effect on polyp number. Atorvastatin was effective in significantly slowing the growth of HCT116 colon cancer cell xenografts in nude mice (p = 0.008). Further, this reduction is due to increased levels of apoptosis.
Conclusions
Atorvastatin can induce apoptosis in vitro, through mevalonate and prenylation pathways. Atorvastatin, while not effective in preventing polyp formation in the min mouse model, was very effective in slowing tumor growth in a nude mouse model. Consistent with in vitro findings, increased apoptosis accounted for decreased tumor growth. Statins may have benefit in cancer by slowing tumor growth, rather than preventing tumor initiation.
Keywords: Colon cancer, Chemoprevention, Statins, Apoptosis
Introduction
Colorectal cancer is the third most common cancer in the United States, and the second most common cause of cancer-related mortality [1]. While adoption of colonoscopy as a screening and prevention tool has reduced the incidence and stage of colorectal cancer in the United States, colonoscopy is not embraced by patients, with adherence rates to any form of colorectal cancer screening of 30–55% in eligible patients [2, 3]. In spite of colonoscopy screening, 50% of patients still present with stage III disease [4]. Therefore, other forms of prevention are needed.
Statins, 3-hydroxy-3-methylglutaryl coenzyme-A (HMG CoA) reductase inhibitors, are widely used as lipid-lowering agents. Multiple epidemiologic studies have found associations between statin use and a decreased incidence of colorectal cancer. Recent analyses of cohort data find decreases in the incidence of colorectal cancer ranging from 35 to 47% [5, 6]. However, other retrospective studies have not found this effect, which may be due to limited sample size, young subjects with low event rates, short follow-up, low levels of statin exposure, confounding indications and medications, and differences in endpoint analysis [7–10]. Meta-analyses of randomized controlled trials, though protected from treatment bias, have limited power due to short study duration, low cancer event rates, and the timing of statin use [11].
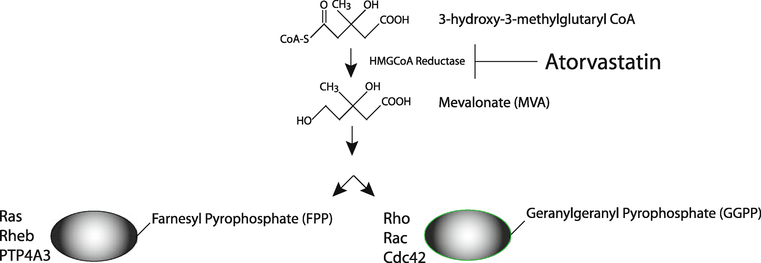
The mechanism of action of statins suggests that they might function to prevent neoplasia and potentially, oncogenesis. As inhibitors of the HMG-CoA reductase pathway (See Fig. 1), statins affect the production of mevalonate. Statins also inhibit the generation of other products further downstream in the mevalonate pathway, including the generation of isoprenoids. Isoprenoids are long hydrophobic molecules including farnesyl and geranylgeranyl groups, which perform membrane anchoring functions for the Ras/Rho superfamily. Statins act by inhibiting prenylation, or the modification of G-proteins with these isoprenoid intermediates, thus preventing the anchoring of Ras/Rho/RAB/RAC/RAP to the cell membrane. Such prenylation is believed to be a crucial step in the apoptotic, angiogenic, and inflammatory effects of statins [12]. Early in vitro experiments with lovastatin demonstrated the induction of apoptosis in common colon cancer cell lines [13]. Interestingly, mevalonate and geranylgeranylpyrophosphate (but not farnesylpyrophosphate) reversed lovastatin-induced apoptosis. These investigators further demonstrated that lovastatin in combination with sulindac reduced the number of aberrant crypt foci in an in vivo rat azoxymethane colon carcinogenesis model. More recent evidence suggesting a role for statins in the chemoprevention of colorectal cancer reveals that BMP2, which is a member of the bone morphogenic protein family, may be a key target involved in tumor sensitivity to the lipid-lowering agents [14].
Fig. 1.
HMG-CoA reductase pathway. The relationship between statins, mevalonate, geranylgeranylpyrophosphate, and farnesylpyrophosphate is illustrated. Statins, including atorvastatin inhibit HMG-CoA reductase, mevalonate synthesis, and downstream products including isoprenoids and Ras/Rho anchoring
This study will explore the relationship between statins and apoptosis. In the first part of the study, we employ cell culture to demonstrate that the highly potent statin, atorvastatin, induces PARP cleavage in several colon cancer cell lines, and rapid apoptosis of the HCT116 line. The epidemiologic studies briefly reviewed above reveal a potential benefit in the chemoprevention of colon cancer. We therefore tested the effects of atorvastatin as a single chemopreventive agent in two mouse models. We used the MIN+/− (multiple intestinal neoplasia) mouse, an established model of murine bowel polyposis, to test the effect of atorvastatin on the formation of intestinal polyps. This model is the murine counterpart to humans with familial adenomatous polyposis (FAP), though the MIN+/− phenotype produces small bowel, rather than colonic polyps. We also separately evaluated the effect of atorvastatin on the engraftment and growth of colon tumors. This was performed by xenografting human HCT116 colon cancer cells into nude mice and randomizing them to atorvastatin chow vs. placebo chow.
Methods
Materials
All cell culture reagents were purchased from Invitrogen (San Diego, CA). HCT-116 cells were obtained from ATCC (Rockville, MD). All other reagents were purchased from Sigma (St. Louis, MO) unless otherwise indicated.
Diets
Atorvastatin calcium was a gift from Pfizer (New York, NY). As previous chemopreventive studies used a dosage of 250 ppm, and this was nontoxic [15], we designed a dosing scheme in which the mice would consume 222 ppm atorvastatin in the modified chow. The diet was formulated by Harlan Teklad (Madison, WI), as a modification of their Rodent chow formula 2018 to contain 222 mg atorvastatin/ kg of chow. Based on typical consumption of 4.5 g/chow/ day, the mice ingested ~0.25 mg atorvastatin/kg body weight/day. They were allowed ad libitum access to both chow and water. Control mice in each experiment consumed unmodified Harlan Teklad Rodent chow formula 2018.
Methods
Cell Lines
The colon cancer cell line, HCT116, was obtained from the American Type Culture collection (ATCC, Manassas, VA), and maintained in McCoy’s 5A media, with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) at 37°C in a humidified atmosphere of 5% CO2. Cultures were periodically screened for mycoplasma contamination with the VenorGeM Mycoplasma Detection Kit (Sigma, St. Louis, MO). Cells were fed three times weekly and subcultured at 80% confluence. All experiments were performed using cells in mid-logarithmic phase. For apoptosis experiments, 1 × 105 cells per well were plated in 6-well plates.
Morphology Assays
To determine the affect of atorvastatin, mevalonate, FPP (farnesyl pyrophosphate), and GGPP (geranylgeranyl pyrophosphate) on the cell line, HCT116, we examined the morphology of the cell line post treatment. These chemicals affect the prenylation of important proteins in carcinogenesis, including Ras and Rho, as seen in Fig. 1. All images are 4009 (Kodak, Rochester, NY).
Immunoblotting
Immunoblotting was utilized for the detection of cleaved PARP. Cell lysates were prepared and separated by SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes (Amersham Biosciences, Piscataway, NJ). Membranes were blocked in 5% milk solution for 1 h at room temperature or overnight at 4°C. Cleaved PARP (asp214; Cell Signaling Technology, Danvers, MA) was detected by incubating the transferred membrane overnight at 4°C with rabbit anti-human polyclonal antibody (Zymed, San Francisco, CA) at 1: 5,000 dilution in 5% milk/TBST solution. As a loading control, a mouse antibody for GAPDH (Chemicon, Temecula, CA) was used to detect this housekeeping protein after a stripping procedure. Secondary antibody anti-rabbit IgG + HRP (cleaved PARP) or anti-mouse (GAPDH; Amersham, Piscataway, NJ) was incubated for 1 h at room temperature and the signal was detected by the ECL detection system (Pierce, Rockford, IL). Autoradiographs were scanned and quantitated using Image J analysis software (NIH, Bethesda, MD).
TUNEL Assay (HCT-116 cells)
1 × 106 cells HCT-116 were trypsinized, washed twice with ice-cold PBS, fixed for 30 min on ice in 1% fresh paraformaldehyde, Samples were washed once with ice-cold PBS, resuspended in an excess volume of 70% ethanol, and stored at −20°C overnight. To remove excess ethanol, the cells were washed twice with ice-cold PBS prior to performing the TUNEL assay.
An in situ Cell Death Detection (TUNEL) assay (Roche Indianapolis, IN) was performed in accordance with the manufacturer’s instructions. Briefly, cells were labeled with 100 μl of labeling solution for 60 min at 37°C in the dark and washed twice with PBS. Negative controls (TUNEL reaction without labeling solution or labeling solution without terminal deoxynucleotidyl transferase) and a positive control (HCT-116 cells treated with 15 U of DNaseI) were included in each TUNEL analysis. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) at the University of Michigan Flow Cytometry Core Facility (Ann Arbor, MI). WINMDI version 2.8 (The Scripps Institute, Flow Cytometry Core Facility, La Jolla, CA) was used to generate histograms and analyze apoptosis.
Caspase 3/7 Assay
Caspase-3 and −7 activities were determined by Caspase-Glo 3/7 Assay (Promega, Madison, WI) in accordance with the manufacturer’s instructions. HCT-116 cells were plated on a 96-well plate at 1 × 105 cells per well. Twenty-four hours post-treatment, 100 μl of caspase 3/7 reagent was added to each well, mixed and incubated 1 h at room temperature. Luminescence was determined using a PerkinElmer Victor3 luminometer (PerkinElmer, Shelton, CT).
TUNEL Assay (Fluorescence)
Paraffin sections of each HCT 116 initiated tumor xenograft were dewaxed and deparaffinized and rehydrated through a graded series of ethanol and water. Slides were then placed in 0.1 M citrate buffer pH6.0 and permeabilized by exposure to 6 min of microwave irradiation (350 W). A known positive sample and two negative control slides were included in the run. Staining was performed using a commercially available apoptosis kit (In Situ Cell Death Detection Kit, Roche) following the manufacturer’s instructions. TUNEL reaction mixture was incubated on the slides for 1 h at 37°C, with a negative control slides receiving labeling mixture devoid of TdT enzyme. After three washes in 1× PBS, slides were coverslipped using Prolong Gold with DAPI (Molecular Probes, Eugene, OR). Detection was observed as a fluorescent green signal contributed by FITC and photographed by a blinded observer (JEC), taking four non-overlapping images/slide (1009). ImageJ was used to quantify the number of fluorescent cells/section/treatment, indicative of TUNEL activity.
Breeding and Genotyping
We employed the following breeding strategy: male MIN+/− mice and female wild-type C57BL/6 J were obtained from the Jackson Laboratories (Bar Harbor, ME). The mice were maintained under conventional housing conditions (ULAM approval protocol #8715).
Pups were weaned at 21 days and were subjected to genotyping. The offspring were genotyped by using tail sample DNA extraction (Qiagen, Valencia, CA). PCR to determine either MIN+/− or wild-type status was determined. PCR primers to determine the status were: 5′-GCC ATCCCTTCACGTTAG-3′; 5′-TTCCACTTTGGCATAAGG-3′ in a 10-μl reaction including 1.5 mmol/l magnesium acetate, 50 mmol/l KCl, TRIS HCl 10 mmol/l buffer, 50 pmol/ldNTPs (Roche Laboratories, Nutley, NJ), 20–30 pmol primers, TAQ polymerase (5 U/μl; Roche Laboratories), with ~100 ng DNA at 94°C for 1 min, 55°C for 1 min; 72°C for 1 min, 40 cycles; 70°C for 7-min extension (Stratagene RoboCycler, LaJolla, CA). The resulting product was visualized on a 2% agarose gel with ethidium bromide staining. Mice were then randomly divided to the ingestion of atorvastatin (222 mg/kg chow or regular chow; Harlan Teklad, Madison, WI). At 16–20 weeks of age the mice were euthanized. The entire small bowel and colon were then harvested and placed into the Swiss roll configuration prior to fixation(10%neutralbufferedformalin).Once fixed, the harvested tissues underwent H&E staining prior to presentation to our pathologist for analysis.
HCT-116 Xenografts
A total of 50,000 wild-type HCT-116 cells (ATCC, Manassas, VA) were injected subcutaneously into the flanks of athymic nu/nu mice (Charles River). The cells were suspended in Matrigel (Becton–Dickinson) in a 1:1 ratio such that the total volume injected was 100 μl. Mice were permitted ad libitum regular vs. atorvastatin (222 mg/kg) chow (Harlan Teklad, Madison, WI).
Statistics
In the min mouse model, total polyp number was enumerated for each Swiss roll by our pathologist (KE). The total polyp number was modeled with a multivariate mixed-effect model, treating genotype and treatment as fixed effects, with father and cage number as random effects. The PROC GLIMMIX in SAS 9.1 software package (Cary, NC) was used for the analysis. Estimates of each fixed effect were calculated.
In the HCT116-nude mouse xenograft model, Student’s t-test was used to compare the mean tumor size and TUNEL analysis between standard chow and atorvastatin chow groups of mice.
Results
Atorvastatin Induces Apoptosis in HCT116 Colon Cancer Cells
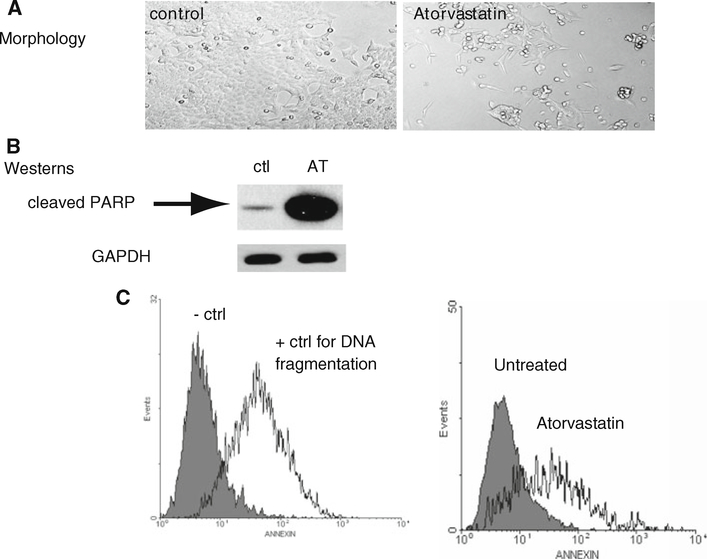
The colon cancer cell line, HCT-116, was incubated in the presence of atorvastatin (100 μm) for 24 h. Evaluation of cell morphology demonstrated dramatic cell loss in the atorvastatin treated cultures. (Fig. 2a). As cleaved PARP (polyADP-ribose polymerase) is a marker of DNA breaks, which are an early event in the apoptotic pathway, we then proceeded to immunoblotting for PARP. In the untreated condition, very little cleaved PARP was present, while in the presence of atorvastatin, increased PARP cleavage was noted (Fig. 2b). As measures of events later in the apoptotic cascade, the TUNEL (terminal deoxynucleotidyl transferase) assay was completed under the three conditions (Fig. 2c) and flow cytometry for annexin V was measured. Atorvastatin induced both increased DNA fragmentation and increased externalization of annexin (Fig. 2c, panel 2).
Fig. 2.
Atorvastatin increases the expression of PARP and DNA fragmentation. a Light microscopy of HCT-116 cells. Untreated (control) cells compared to cells treated with 100 μm atorvastatin for 24 h. Photomicrographs of live HCT 116 cells were taken with Leica DMIRB inverted microscope at 400× magnification. b Western-blot analysis of cleaved PARP expression in HCT-116 cells. (Ctl) untreated HCT-116 cells. (AT) HCT-116 cells treated with 100 μm atorvastatin for 24 h. c TUNEL assay with FACScan analysis of apoptosis. Representative histograms are shown. 1 × 106 HCT-116 cells were treated with 100 μm atorvastatin for 24 h. TUNEL assay controls. Untreated HCT 116 cells (negative Ctl) vs. HCT-116 cells treated with (15 U) DNaseI (positive control for apoptosis). Untreated HCT 116 cells vs. HCT 116 cells treated for 24 h with 100 μm atorvastatin (n = 3 trials)
Mevalonate and GGPP, but Not FPP, Reverse Atorvastatin-Induced Apoptosis
The colon cancer cell line, HCT-116, was incubated in the presence of the HMG-CoA reductase inhibitor, atorvastatin (100 μm). Either mevalonate (MVA, 100 μm), geranylgeranylpyrophosphate (GGPP, 10 μm) or farnesylpyrophosphate (FPP, 10 μm) was added to the atorvastatin-containing cultures. Microscopy reveals that the addition of mevalonate or geranylgeranylpyrophosphate to the atorvastatin-containing cultures was able to decrease cell loss to baseline (untreated) levels while the addition of FPP was not able to completely compensate for the effects of atorvastatin (Fig. 3a). These results are corroborated by the expression of cleaved PARP protein, as detected by immunoblotting and caspase 3/7 activity. The expression of cleaved PARP is greatest in the cultures containing atorvastatin alone. However, the addition of mevalonate or GGPP significantly decreases the amount of PARP (Fig. 3b, lanes 3 and 4). The cultures containing FPP (Fig. 3b, lane 5) reveal signal intensities nearly reaching the level of the atorvastatin alone cultures. Similarly, caspase 3/7 activity was highest in atorvastatin treated cultures (Fig. 3c) while mevalonate or GGPP significantly decreased caspase 3/7 activity. FPP-treated cultures had higher caspase 3/7 activity than either mevalonate or GGPP.
Fig. 3.
Mevalonate and GGPP (but not FPP) are able to reverse atorvastatin-induced apoptosis. Light microscopy of HCT-116 cells. Photomicrographs of live HCT-116 cells were taken with Leica DMIRB inverted microscope at 400× magnification. (control) Untreated HCT-116 cells. (atorvastatin) HCT-116 cells treated for 24 h with 100 μm atorvastatin. (AT + MVA) HCT-116 cells treated for 24 h with 100 μm atorvastatin and 100 μm mevalonate. (AT + GGPP) HCT-116 cells treated for 24 h with 100 μm atorvastatin and 10 μm geranylgeranylpyrophosphate. (AT + FPP) HCT-116 cells treated for 24 h with 100 μm atorvastatin and 10 μm farnesylpyrophosphate. a Representative Western-blot of cleaved PARP expression in HCT-116 cells with GAPDH protein expression as a loading control. Untreated cells (lane 1, Ctl) compared to cells treated with 100 μm atorvastatin (lane 2, AT), 100 μm atorvastatin and 100 μm mevalonate (lane 3, AT + MVA), 100 μm atorvastatin and 10 μm geranylgeranylpyrophosphate (lane 4, AT + GGPP), 100 μm atorvastatin and 10 μm farnesylpyrophosphate (lane 5, AT + FPP). b Caspase 3/7 activity in HCT-116 cells after 24 h treatment with 100 μm atorvastatin (AT), 100 μm atorvastatin and 100 μm mevalonate (AT + MVA), 10 μm geranylgeranylpyrophosphate (AT + GGPP), or 10 μm farnesylpyrophosphate (AT + FPP) compared to untreated cells (control). Results are from seven independent experiments
In the MIN+/− Mouse Model, Atorvastatin Had No Effect on Polyp Number
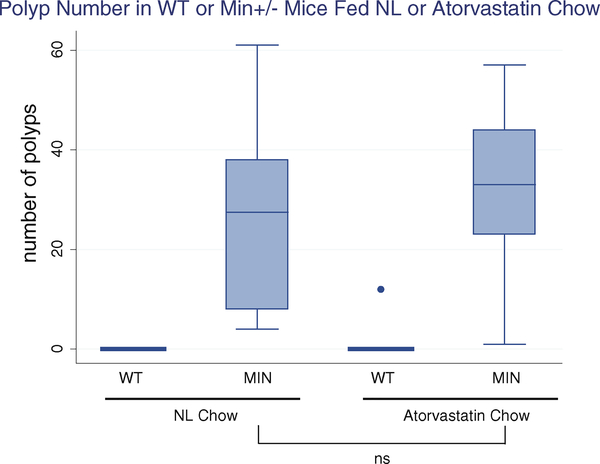
All comparisons were controlled for genotype and father (who carried the MIN+/− gene). In MIN+/− mice, the average number of small-bowel polyps was 30.17 more than in wild-type mice (95% CI: 20.05–40.31). On average, the mice maintained on the atorvastatin diet, after controlling for genotype, exhibited a small average increase in polyps (+4.73, 95% CI: −6.49 to 15.94), which was not statistically significant, compared to the mice on standard chow (Table 1 and Fig. 4).
Table 1.
Atorvastatin has no effect on the number of polyps in MIN+/− mice
| Estimate | SE | t value | Lower CL | Upper CL | |
|---|---|---|---|---|---|
| Atorvastatin | 4.73 | 5.35 | 0.88 | −6.4886 | 15.9436 |
| Genotype | 30.18 | 4.93 | 6.12 | 20.05 | 40.30 |
CL confidence limit
Fig. 4.
Atorvastatin does not inhibit polyp formation in MIN+/− mice. Number of polyps in WT and MIN+/− mice fed normal chow (left panel) compared to WT and MIN+/− fed atorvastatin chow (right panel). MIN+/− mice fed atorvastatin chow had a slight but not statistically significant increase in polyp number (+4.73, p = 0.34)
Atorvastatin Decreases HCT-116 Tumor Volume in a Xenograft Model of Tumorigenesis
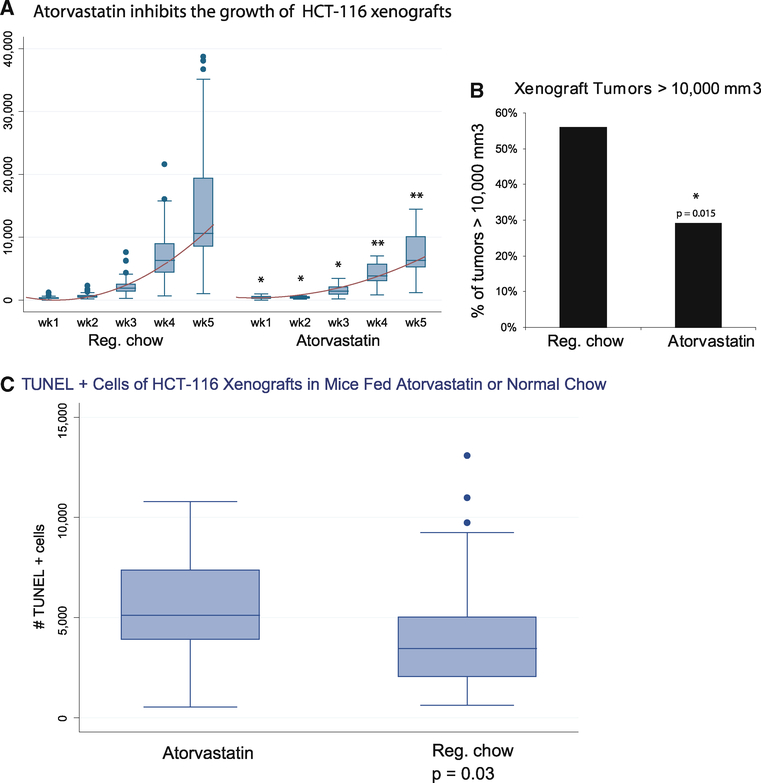
A total of 50,000 wild-type HCT-116 cells were implanted as subcutaneous xenografts into the flanks of athymic nude mice. Based on power analyses and differences in the variation in tumor growth in a pilot experiment, 48 mice were placed in the control group on normal chow, while 24 mice received the chow containing atorvastatin. These mice were observed weekly for serial growth of tumors, which were measured by the same individual (MJH) using calipers. Measurements for these tumors were calculated using the formula: 4/3πb2a in which a is the longest, and b is the shortest dimension. Though there was 100% tumor engraftment, and the normal chow and atorvastatin animals developed similar-sized tumors by day 7 post-injection (310 vs. 402 mm3, p = 0.07), by day 14 the tumor volume in the normal chow group doubled from 310 to 631 mm3. Significantly, the atorvastatin tumor volume remained unchanged (399 mm3, p = 0.008).
Tumor growth rates from normal chow and atorvastatinfed mice were fitted to an exponential growth curve using regression analysis with tumor size as the dependent variable and (days)2 as the independent variable. The coefficient for (days)2 was 12.7 (95% CI = 11.2–14.2) in the normal chow group and 6.32 (95% CI = 5.5–7.2) in the atorvastatin group. While maintenance of these mice on atorvastatin did not fully inhibit growth, there was a significant sustained decrement in growth throughout the period of observation (p = 0.001), culminating in a twofold decrease in final tumor size of the atorvastatin group compared to the normal chow (7,120 mm3 vs. 14,249 mm3, p = 0.0009; Fig. 5a). In addition, the atorvastatin cohort had a lower percentage of large (>10,000 mm3) tumors compared to mice fed normal chow (Fig. 5b).
Fig. 5.
Atorvastatin inhibits growth of HCT-116 xenografts. Fifty thousand HCT116 cells were placed into the subcutaneous flanks of athymic nu/nu mice. a Tumor dimensions of xenograft tumors from 48 control (standard chow) and 24 atorvastatintreated mice were measured weekly using calipers. Volumes were calculated using the formula 4/3πb2a, where b = shorter dimension.* p<0.1; ** p<0.01. b Percentage of mice with large(>10,000 mm3) tumors by day 34 post-injection. Mice fed atorvastatin chow (Atorvastatin) had a lower percentage (29 vs. 56%, p = 0.015) large tumors compared to mice fed normal chow (Reg. chow). c Atorvastatin inhibits growth of HCT-116 xenografts due to increased apoptosis. TUNEL analysis of tumor xenograft sections reveal increased TUNEL activity in the tumors of mice on atorvastatin chow vs. normal chow. p = 0.03
To compare the level of apoptosis between the mice maintained on atorvastatin chow and the control group maintained on normal chow, the TUNEL assay was applied to the paraffin-embedded tissue sections. Consistent with the decrement in tumor growth seen in the mice maintained on atorvastatin, analysis of TUNEL activity revealed increases in TUNEL in this group compared to those mice maintained on normal chow (Fig. 5c, p = 0.03).
Discussion
While significant advances have been made in the treatment of colon cancer, effective preventive strategies, including screening colonoscopy, have been limited by poor adherence to screening in clinical practice. This makes chemoprevention of colorectal cancer an appealing alternative. However, previous large-scale chemopreventive trials with cox-2 inhibitors were discontinued due to an increased incidence of cardiac events [16]. Epidemiologic studies of statins as chemopreventive agents for colon cancer have had mixed results. These data demonstrate that atorvastatin fails to reduce polyp formation in the min mouse model, but it can inhibit the growth of colon cancer cell line xenografts. This finding may help explain some of the widely disparate results of epidemiologic studies, some of which have found up to 47% reductions in colon cancer incidence [6], and others which have found no effect in large datasets [9].
The limitations of this study include those of the APCMin model, which include a strikingly different phenotype from human FAP, and a lack of metastatic cancer development. There are also limitations to the nude mouse xenograft model, including the use of immunocompromised host mice, a heterotopic tumor location (without the contribution of the tumor microenvironment), and the use of established human malignancies in mice. Despite these limitations, it is striking that atorvastatin is not able to reduce polyp number in the APC Min model, nor tumor cell engraftment in the nude mouse, but is able to slow tumor growth in nude mice (Fig. 5). Our findings using the APCMin model are in contrast to those of Swamy [17] (who documented a substantial decrement in intestinal polyp growth using a dosage of 100 ppm atorvastatin. In the study of Swamy, their murine diet was modified to a fat content of 12%, while normal chow contains 5%. Further, they generated their own modified chow weekly, In contrast, we used a commercially formulated chow based on a standard diet which contained medical grade atorvastatin and is substantially lower in fat (5.8% fat vs. 12%). While our data reveal intestinal polyp counts in the same range as Swamy’s group, atorvastatin, however, did not produce a significant difference in polyp number in our study.
Other preclinical studies include the work of Reddy et al. [15], which employs azoxymethane chemical induction of colon cancer in rats. These studies revealed that atorvastatin reduced not only the incidence of colon cancer but also the multiplicity of these lesions. Notably, the COX-2 inhibitor celecoxib exhibited the most marked inhibition when given alone. Impressively, the addition of celecoxib to atorvastatin significantly decreased the incidence and multiplicity of adenocarcinomas, to about 71 and 90%, respectively. Further, the addition of aspirin to atorvastatin suppressed the incidence of adenocarcinomas by 67% and the multiplicity by 64%. As in our studies, the administration of atorvastatin significantly increased apoptosis, both in vitro and in vivo.
The finding that mevalonate and geranylgeranylpyrophosphate reverse the effects of atorvastatin, while farnesyl pyrophosphate does not, suggests that Ras (but not Rho) may be critical to statin-induced apoptosis. While up to 60% of colorectal cancers are known to have mutations in p53 [18, 19], other mechanisms for colorectal oncogenesis include loss of tumor suppressors, epigenetic alterations, and mutations in other proto-oncogenes. Mutations in k-ras are found in 40% of colorectal cancer [20, 21]. While the HCT 116 cell line is wild-type for p53, this cell line has a classic codon 13 ras mutation [22]. In patients with colorectal cancer, those with mutations in k-ras are resistant to therapies targeted against epidermal growth factor receptor [23]. With the demonstrated susceptibility of a ras-mutated colon cancer cell line to atorvastatin found in this study, in the dose range achievable in humans, statins may be useful as an adjuvant agent for tumors that are resistant to other therapies due to ras mutations.
Acknowledgments
We are grateful to Duyen Dang, MD and John Kao, MD, for careful reading of the manuscript. We acknowledge Marda Jorgenson and the Cells and Tissue Analysis Core at the University of Florida for the TUNEL staining. EHH was supported by NCI K08 91975 and is currently supported by the Eli and Edythe Broad Foundation. JEC is supported by the Regeneration Project (University of Florida) and the Broad Foundation.
Contributor Information
Emina H. Huang, Department of Surgery, University of Florida, Gainesville, FL, USA
Laura A. Johnson, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA
Kathryn Eaton, Department of Comparative Pathology, University of Michigan, Ann Arbor, MI, USA.
Mark J. Hynes, Department of Surgery, University of Michigan, Ann Arbor, MI, USA
Joseph E. Carpentino, Department of Surgery, University of Florida, Gainesville, FL, USA
Peter D. R. Higgins, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- 2.Sewitch MJ, et al. Adherence to colorectal cancer screening guidelines in Canada. BMC Gastroenterol. 2007;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeazel MW, et al. Colorectal cancer screening adherence in a general population. Cancer Epidemiol Biomarkers Prev. 2004;13: 654–657. [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer facts and figures. 2008; a–70.
- 5.Farwell WR, et al. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100: 134–139. [DOI] [PubMed] [Google Scholar]
- 6.Poynter JN, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. [DOI] [PubMed] [Google Scholar]
- 7.Blais L, et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–2368. [DOI] [PubMed] [Google Scholar]
- 8.Friis S, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–647. [DOI] [PubMed] [Google Scholar]
- 9.Kaye JA, Jick H. Statin use and cancer risk in the general practice research database. Br J Cancer. 2004;90:635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khurana V, et al. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest. 2007;131:1282–1288. [DOI] [PubMed] [Google Scholar]
- 11.Bhuket TP, Higgins PD. Drug insight: statins and gastrointestinal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:552–562. [DOI] [PubMed] [Google Scholar]
- 12.Demierre MF, et al. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal B, et al. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res. 1999;5:2223–2229. [PubMed] [Google Scholar]
- 14.Kodach LL, et al. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134:1332–1341. [DOI] [PubMed] [Google Scholar]
- 15.Reddy BS, et al. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66:4542–4546. [DOI] [PubMed] [Google Scholar]
- 16.Couzin J Drug safety. Withdrawal of Vioxx casts a shadow overCOX-2 inhibitors. Science. 2004;306:384–385. [DOI] [PubMed] [Google Scholar]
- 17.Swamy MV, et al. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCM in mice. Cancer Res. 2006;66:7370–7377. [DOI] [PubMed] [Google Scholar]
- 18.Baker SJ, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989;244:217–221. [DOI] [PubMed] [Google Scholar]
- 19.Baker SJ, et al. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990;50:7717–7722. [PubMed] [Google Scholar]
- 20.Bos JL, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. [DOI] [PubMed] [Google Scholar]
- 21.Normanno N, et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–527. [DOI] [PubMed] [Google Scholar]
- 22.Schroy PC 3rd, et al. Detection of p21ras mutations in colorectal adenomas and carcinomas by enzyme-linked immunosorbent assay. Cancer. 1995;76:201–209. [DOI] [PubMed] [Google Scholar]
- 23.Benvenuti S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. [DOI] [PubMed] [Google Scholar]