Abstract
Objectives—
To determine whether contrast-enhanced sonographic quantitative perfusion parameters can detect bowel wall fibrosis in the setting of mixed inflammatory and fibrotic lesions in a Crohn disease animal model.
Methods—
This study was approved by the institutional Committee on the Use and Care of Animals. Multiple (range, 1–5) 2,4,6-trinitrobenzenesulfonic acid–ethanol enemas were used to create intestinal inflammatory lesions with variable fibrosis in female Lewis rats. Low–mechanical index contrast-enhanced sonography was performed 3 days after the final enema using a 0.2-mL bolus of sulfur hexafluoride microbubbles injected through a tail vein. Contrast-enhanced sonographic data were analyzed with software that converts video data into echo-power (linearized) data. Colorectal lesions were scored for histopathologic inflammation and fibrosis; bowel wall collagen was quantified by Western blotting. The Spearman correlation was used to assess associations between contrast-enhanced sonographic quantitative parameters and bowel wall collagen; the Kruskal-Wallis test was used to compare continuous results between histopathologic groups.
Results—
Thirty-one animals were included in our analysis. Animals were placed into 3 histopathologic cohorts: (1) severe bowel wall inflammation/minimal or no fibrosis (n = 11); (2) severe bowel wall inflammation/moderate fibrosis (n = 9); and (3) severe bowel wall inflammation/severe fibrosis (n = 11). Western blotting showed a significant difference in bowel wall collagen between histopathologic cohorts (P = .0001). There was no correlation between any contrast-enhanced sonographic quantitative parameter and bowel wall collagen (P > .05). There was no difference between histopathologic cohorts for any contrast-enhanced sonographic quantitative parameter (P > .05).
Conclusions—
Contrast-enhanced sonographic quantitative perfusion parameters failed to effectively detect bowel wall fibrosis in the setting of superimposed inflammation in a Crohn disease animal model.
Keywords: animal model, contrast-enhanced sonography, Crohn disease, gastrointestinal ultrasound, fibrosis, inflammation, microbubble contrast agent
Crohn disease is a chronic inflammatory condition that affects the human gastrointestinal tracts of both children and adults and is due to immune system dysregulation.1,2 Patients with this disorder commonly have periods of disease exacerbation due to worsening intestinal inflammation. In some patients, however, an exacerbation of symptoms may be due to the presence of a stricture.3 Strictures are segments of narrowed bowel lumen with variable degrees of obstruction that are most often due to a combination of both inflammation and fibrosis.4 Pathologic bowel wall fibrosis is the result of aberrant healing secondary to recurrent bouts of intestinal inflammation and associated damage.3 The detection of bowel wall fibrosis is paramount, as areas of intestinal narrowing that are primary inflammatory may be sufficiently managed with medical (eg, anti-inflammatory or immunosuppressive) therapy, whereas strictures containing considerable fibrosis require mechanical treatments, such as intestinal resection, stricturoplasty, or endoscopic balloon dilation.3,5
Unfortunately, the detection and measurement of intestinal fibrosis using conventional radiologic tests is difficult. A study by Adler et al6 showed that whereas computed tomography can effectively detect active bowel wall inflammation, it performs suboptimally when trying to predict intestinal fibrosis. Although magnetic resonance imaging offers greater soft tissue contrast resolution compared to computed tomography, recent studies suggest that this modality can only effectively detect severe transmural intestinal fibrosis based on the presence of delayed contrast enhancement7 or substantial luminal dilatation proximal to an area of narrowing.8
A few small preliminary studies have assessed a variety of ultrasound technologies for the detection of bowel wall fibrosis in the setting of Crohn disease. Kim et al9 and Stidham et al10 showed that ultrasound strain elastography can be used to detect bowel wall fibrosis in a Crohn disease rodent model, whereas Dillman et al11,12 showed that ultrasound shear wave elastography may be useful for detecting bowel wall fibrosis in both rodents and ex vivo human Crohn disease specimens. A small number of investigators have attempted to use contrast-enhanced sonography to detect fibrosis in human Crohn disease strictures. Nylund et al13 concluded that fibrotic strictures were associated with reduced blood flow and blood volume based on contrast-enhanced sonographic assessment. Quaia et al14 also concluded that fibrotic strictures could be differentiated from inflamed bowel segments based on contrast-enhanced sonographic time-versus-signal intensity areas under the curve.
The purpose of our study was to further evaluate the ability of contrast-enhanced sonography using an intravascular microbubble contrast agent to detect the presence of intestinal fibrosis in a Crohn disease animal model. Specifically, we wanted to determine whether a variety of contrast-enhanced sonographic quantitative perfusion parameters derived from time-versus-signal intensity curves can be used to detect the presence of bowel wall fibrosis in mixed inflammatory and fibrotic lesions.
Materials and Methods
This investigation was approved by the institutional Committee on the Use and Care of Animals. The previously described 2,4,6-trinitrobenzenesulfonic acid (TNBS)–ethanol enema Crohn disease rat model was used to create intestinal inflammatory lesions with variable amounts of bowel wall fibrosis.15,16 In this chemically induced colitis model, ethanol is used to disrupt the integrity of the mucosa of the distal colon and rectum, whereas TNBS serves as a hapten, producing a vigorous immunologic inflammatory reaction in the bowel wall consisting of neutrophils, lymphocytes, activated mast cells, macrophages, and fibroblasts. A single TNBS-ethanol enema causes acute colorectal inflammation that peaks in 2 to 3 days and resolves in about 1 week. Repetitive weekly TNBS-ethanol treatments cause repeated cycles of intestinal inflammation and healing that effectively produce acute-on-chronic inflammation and transmural bowel wall fibrosis in 4 to 6 weeks in a manner similar to human Crohn disease.9,11
Thirty-six female Lewis rats were divided into 5 groups. Group 1 animals (n = 12) received a single TNBS-ethanol enema in an attempt to create colorectal inflammatory lesions without fibrosis; these animals underwent contrast-enhanced sonography 3 days after the enema procedure. Group 2, 3, 4, and 5 animals (n = 6, respectively) received 2, 3, 4, and 5 weekly enemas, respectively, in an attempt to create intestinal inflammatory lesions with variable amounts of associated bowel wall fibrosis. Again, contrast-enhanced sonography was performed 3 days after the final enema. Group 5 animals were expected to develop intestinal inflammatory lesions with associated severe transmural fibrosis. All enemas were delivered through a 5-F pediatric feeding tube while the animals were transiently anesthetized with inhaled isoflurane.
Low–mechanical index (0.08) contrast-enhanced sonography was performed with Cadence contrast pulse sequencing technology (Acuson 3000 ultrasound system and 9L4 linear high-frequency transducer; Siemens Medical Solutions USA, Inc, Mountain View, CA) and a sulfur hexafluoride microbubble contrast agent (SonoVue; Bracco Diagnostics, Inc, Milan, Italy) injected through a peripheral 24-gauge catheter placed within a lateral tail vein. Contrast-enhanced sonography using Cadence contrast pulse sequencing allows the detection of strong non-linear fundamental and high-order harmonic signals that are predominantly from the intravascular contrast agent.17
The microbubble contrast agent was hand injected by “bolus” technique and a 1-mL syringe, with 0.2 mL of the contrast agent injected over 1 second, followed immediately by a 0.5 mL of a normal saline flush injected over 5 seconds. Sixty-second video clips were recorded in the area of greatest colorectal wall thickening as depicted by grayscale sonography (Figure 1), starting at the initiation of contrast agent injection. Contrast-enhanced sonography was performed in the transverse plane (Figure 2).
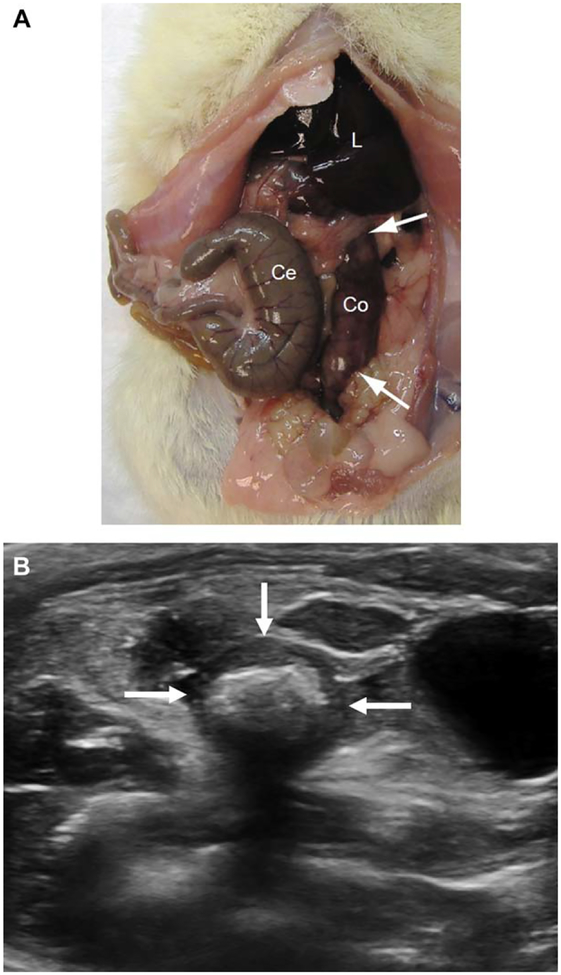
Figure 1.
A, Appearance of a euthanized rat abdomen after a single TNBS-ethanol enema. The cecum (Ce) appears normal, whereas the descending colon (Co) is thick-walled and inflamed. The colon appears very erythematous between the arrows. L indicates liver. B, Transverse grayscale sonogram the rat pelvis showing marked wall thickening of the rectosigmoid colon (arrows) due to a combination of inflammation and fibrosis. Echogenic shadowing material in the bowel lumen is fecal material.The anechoic fluid-filled structure to the left of the colon is the urinary bladder.
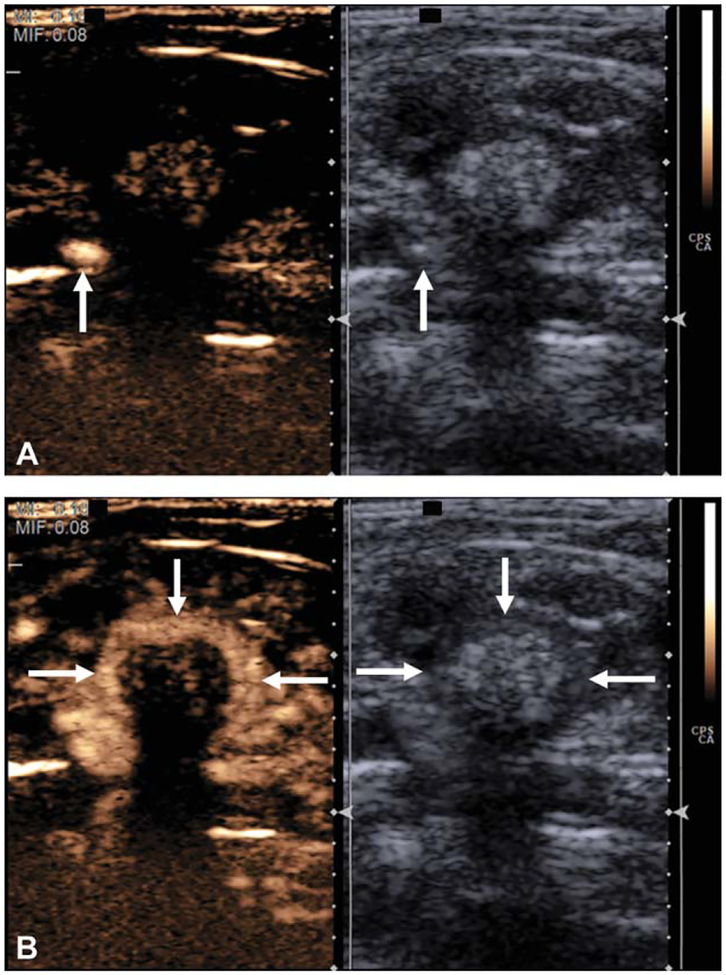
Figure 2.
A, Grayscale and contrast-specific sonograms through an abnormal rat colon immediately after contrast agent injection and before bowel wall enhancement. Contrast material is present in a large pelvic vein (arrows). B, Grayscale and contrast-specific images a few seconds later showing avid enhancement of the bowel wall (arrows).
Digital Imaging and Communications in Medicine contrast-enhanced sonographic video clips were analyzed with VueBOX version 6.0 software (Bracco Suisse SA, Plan-les-Ouates, Switzerland). This software package converts Digital Imaging and Communications in Medicine video data into echo-power (linearized) data, a quantity that is directly proportional to the instantaneous concentration of the contrast agent at each location on the image. Numerous quantitative perfusion parameters (time [seconds], amplitude [arbitrary units], rate [arbitrary units], and area under the curve [arbitrary units] based) were generated from sonographic time-versus-signal intensity (enhancement) curves to provide estimates of local perfusion, as shown in Figure 3. All video clips were processed by a single author. For each contrast-enhanced sonographic clip, a region of interest was placed within the anterior wall (from approximately the 9- to 10- to 2- to 3-o’clock locations) of the bowel segment of interest, extending from the inner mucosa to the outer serosa (Figure 4). Color parametric maps for contrast-enhanced sonographic quantitative perfusion parameters also were generated, as also shown in Figure 4.
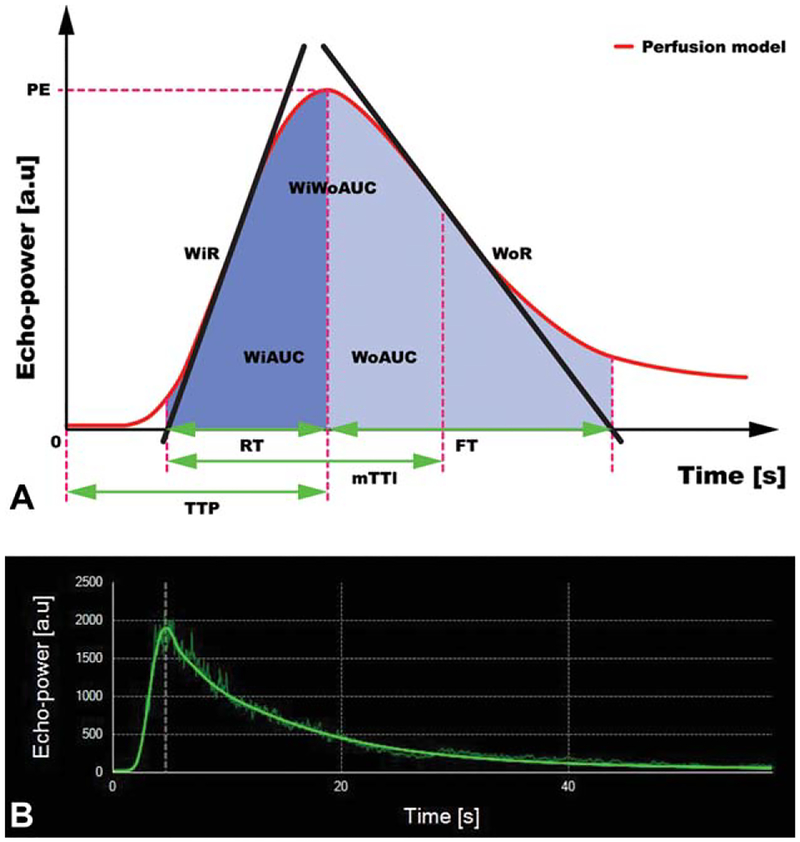
Figure 3.
A, Schematic of a time-versus-signal intensity curve using the bolus contrast-enhanced sonographic technique. Numerous quantitative parameters can be derived from this curve. FT indicates fall time; mTTl, mean local transit time; PE, peak enhancement; RT, rise time; TTP, time to peak; WiAUC, wash-in area under the curve; WiR, wash-in rate; WiWoAUC, wash-in + wash-out area under the curve; WoAUC, wash-out area under the curve; and WoR, wash-out rate. Modified from http://vuebox.bracco.ch/. B, Time-versus-signal intensity curve from a distal rat colon showing a linearized signal and fitted curve (solid green line); a.u. indicates arbitrary units.
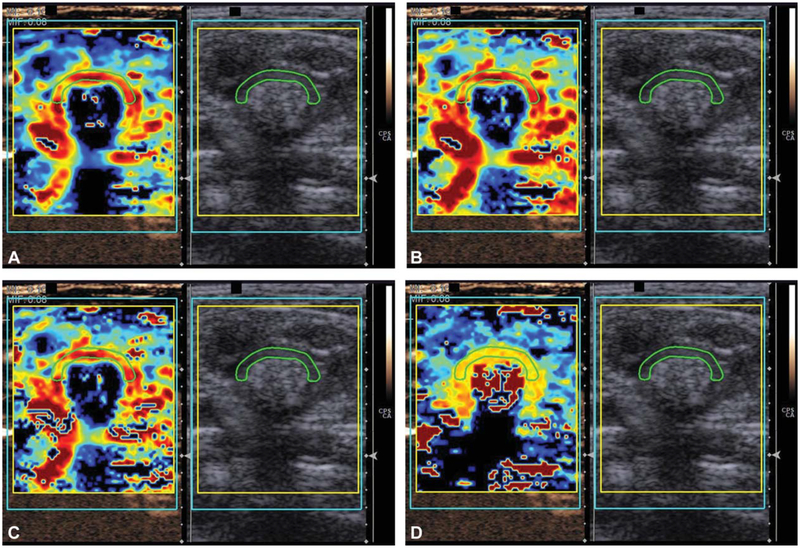
Figure 4.
Contrast-enhanced sonographically derived quantitative parameters can be expressed as color parametric maps. A, Peak enhancement. B, Wash-in area under the curve. C, Combined wash-in and wash-out area under the curve. D, Time to peak enhancement.
Colorectal Histologic Scoring
After contrast-enhanced sonography, animals were immediately euthanized, and colorectal tissues were dissected free. Full-thickness bowel wall tissue was sampled from the area of greatest colorectal wall thickening and processed by standard histologic methods. A board-certified gastrointestinal pathologist reviewed hematoxylin-eosin–as well as Masson trichrome–stained slides to document the amounts of bowel wall histopathologic inflammation and fibrosis using Likert-like scales (Table 1), while blinded to the contrast-enhanced sonographic results. Animals were then placed into new cohorts for our final analyses based on histopathologic scoring.
Table 1.
Histologic Grading of Bowel Wall Inflammation and Fibrosis
| Score | Description |
|---|---|
| Inflammation | |
| 0 | No inflammation |
| 1 (mild) | Few neutrophils in mucosa/submucosa; no transmural injury or necrosis |
| 2 (moderate) | Many neutrophils in mucosa/submucosa; no transmural injury or necrosis |
| 3 (severe) | Neutrophils in all layers of bowel wall; transmural injury or necrosis |
| Fibrosis | |
| 0 | No architectural distortion; negative Masson trichrome staining |
| 1 (mild) | No architectural distortion; positive Masson trichrome staining in <50% bowel wall |
| 2 (moderate) | No architectural distortion; positive Masson trichrome staining in >50% bowel wall |
| 3 (severe) | Architectural distortion of muscularis propria; positive Masson trichrome staining in all layers of bowel wall |
Bowel wall collagen protein was isolated from the sampled bowel wall specimens, quantified by Western blotting using a rabbit polyclonal antibody against type I collagen (Rockland, Gilbertsville, PA), and normalized to glyceraldehyde-3-phosphate dehydrogenase.10 Auto-radiographs were digitally scanned and quantified with ImageJ analysis software.18
Statistical Analysis
As the number of animals in each histopathologic cohort was relatively small, medians were used to summarize continuous data, and nonparametric statistical testing was performed. The Spearman rank correlation was used to assess the relationships between contrast-enhanced sonographic quantitative parameters and bowel wall collagen. The Kruskal-Wallis test (nonparametric version of analysis of variance) was used to compare continuous data (medians) between 3 or more groups. Pair-wise comparisons were performed using the Dunn multiple-comparisons test. P < .05 was considered statistically significant for all inference testing. Statistical analysis was performed with Prism 6 software (GraphPad Software, Inc, La Jolla, CA).
As our study was small and pilot in nature, we chose not to adjust our significance level (α) to account for the multiplicity of contrast-enhanced sonographic quantitative parameters assessed. An a priori power calculation showed that we had at least 80% power to detect a correlation of 0.5 or greater between contrast-enhanced sonographic quantitative parameters and bowel wall collagen. Any statistically significant correlation of less than 0.5 that we might have been underpowered to detect was thought to be unlikely to be clinically important.
Results
Thirty-one animals were included in our final analysis, which yielded diagnostic contrast-enhanced sonographic time-versus-signal intensity data (VueBOX quality of fit, >90%). Five animals were excluded from the analysis because of nondiagnostic contrast-enhanced sonographic video clips; these animals had considerable intestinal peristalsis, which resulted in poor curve fitting to the linearized sonographic data and, thus, likely inaccurate estimates of contrast-enhanced sonographic quantitative perfusion parameters.
Bowel Wall Histopathologic Scoring and Collagen Content
After histopathologic assessment of sampled colorectal tissues, animals were placed into 3 cohorts: a cohort with severe transmural bowel wall inflammation (inflammation score of 3) and minimal fibrosis (fibrosis score of 0 or 1; n = 11); a cohort with severe transmural bowel wall inflammation (inflammation score of 3) and moderate fibrosis (fibrosis score of 2; n = 9); and a cohort with severe transmural bowel wall inflammation (inflammation score of 3) and severe transmural fibrosis (fibrosis score of 3; n = 11). The Western blot analysis revealed a significant difference in median normalized bowel wall collagen between histopathologic cohorts (cohorts 1 versus 2 versus 3: 0.005 versus 0.43 versus 0.23; P = .0001). There were significant differences in bowel wall collagen between cohorts 1 and 2 (P = .0005) and cohorts 1 and 3 (P = .0008) but not between cohorts 2 and 3 (P > .99).
Correlation Between Contrast-Enhanced Sonographic Quantitative Perfusion Parameters Versus Bowel Wall Collagen
Spearman rank correlation was used to assess the relationship between the numerous contrast-enhanced sonographic quantitative perfusion parameters evaluated and bowel wall collagen. No statistically significant correlation was identified. Correlation coefficients and P values are presented in Table 2.
Table 2.
Correlation Between Contrast-Enhanced Sonographic Quantitative Perfusion Parameters and Bowel Wall Collagen
| Parameter | ρa | Pa |
|---|---|---|
| Peak enhancement, a.u. | 0.10 | .62 |
| Wash-in AUC, a.u. | 0.05 | .80 |
| Rise time, s | −0.33 | .08 |
| Mean transit time, s | −0.17 | .39 |
| Time to peak, s | −0.36 | .06 |
| Wash-in rate, a.u. | 0.19 | .33 |
| Wash-in perfusion index, a.u. | 0.11 | .57 |
| Wash-out AUC, a.u. | −0.03 | .86 |
| Wash-in + wash-out AUC, a.u. | −0.01 | .96 |
| Fall time, s | −0.28 | .15 |
| Wash-out rate, a.u. | 0.22 | .25 |
AUC indicates area under the curve; and a.u., arbitrary units.
Spearman rank correlation.
Contrast-Enhanced Sonographic Quantitative Perfusion Parameters Versus Histopathologic Cohort
The Kruskal-Wallis test was used to compare contrast-enhanced sonographic quantitative perfusion parameter medians for each histopathologic cohort. No significant difference was identified between cohorts for any parameter. Medians and P values are presented in Table 3.
Table 3.
Contrast-Enhanced Sonographic Quantitative Perfusion Parameter Medians by Histopathologic Cohort
| Parameter | Cohort 1 (n = 11) | Cohort 2 (n = 9) | Cohort 3 (n = 11) | Pa |
|---|---|---|---|---|
| Peak enhancement, a.u. | 61.8 | 117.0 | 119.6 | .34 |
| Wash-in AUC, a.u. | 128.0 | 169.5 | 130.1 | .31 |
| Rise time, s | 2.9 | 1.8 | 1.9 | .07 |
| Mean transit time, s | 12.1 | 11.5 | 8.9 | .09 |
| Time to peak, s | 3.7 | 2.6 | 2.8 | .07 |
| Wash-in rate, a.u. | 29.8 | 71.0 | 94.0 | .20 |
| Wash-in perfusion index, a.u. | 40.4 | 77.1 | 75.5 | .28 |
| Wash-out AUC, a.u. | 364.4 | 512.1 | 266.2 | .09 |
| Wash-in + wash-out AUC, a.u. | 513.7 | 681.5 | 374.1 | .13 |
| Fall time, s | 8.7 | 4.5 | 4.2 | .07 |
| Wash-out rate, a.u. | 7.2 | 16.8 | 30.4 | .16 |
AUC indicates area under the curve; and a.u., arbitrary units.
Kruskal-Wallis test.
Discussion
Although numerous studies have demonstrated that contrast-enhanced sonography can be used to identify and quantify bowel wall inflammation in the setting of Crohn disease,19–21 our results suggest that contrast-enhanced sonographic quantitative perfusion parameters cannot effectively detect the presence of bowel wall fibrosis when considerable intestinal inflammation is also present. This key limitation is important to recognize, as human Crohn disease strictures can be histopathologically mixed, containing both inflammation and fibrosis.7–9 Unfortunately, this particular clinical setting (histopathologically mixed strictures) is common and may represent most resected Crohn disease strictures in both children and adults based on these recent publications.
Microbubble contrast agents used for contrast-enhanced sonography remain strictly intravascular and do not have an extravascular (or interstitial) phase. Therefore, the degree and pattern of bowel wall enhancement observed on contrast-enhanced sonography are principally perfusion related. Accordingly, it is likely that in the setting of mixed inflammatory and fibrotic Crohn disease lesions, contrast-enhanced sonographic quantitative perfusion parameters are predominantly influenced by the amount of bowel wall inflammation, regardless of the amount of superimposed fibrosis. This situation is different than with intravenously administered iodinated contrast agents used for computed tomographic enterography and conventional linear and macrocyclic gadolinium chelates used for magnetic resonance enterography, which have both intravascular and extravascular phases. Although early bowel wall enhancement is driven by perfusion and the amount of the intravascular contrast agent for these particular contrast materials, the contrast agent also can enter the interstitium of the bowel wall over time and accumulate/wash-out at different rates based on the presence of fibrosis, as documented by Rimola et al.7
Our results are in contradistinction to 2 prior studies that assessed contrast-enhanced sonography in human patients with Crohn disease. Nylund et al13concluded that fibrotic strictures were associated with reduced blood flow and blood volume based on contrast-enhanced sonographic assessment, whereas Quaia et al14 concluded that fibrotic strictures could be differentiated from inflamed bowel segments based on contrast-enhanced sonographic time-versus-signal intensity areas under the curve. However, a review of the methods used by Nylund et al13 showed that surgically resected intestine was available for histopathologic assessment and to serve as a reference standard for only about half of their patients. Additionally, whereas resected bowel specimens were scored for the amount of histopathologic bowel wall fibrosis, there was no specific mention of histopathologic scoring of inflammation or how many resected fibrotic strictures had superimposed inflammation (and to what degree). The reference standard in the study by Quaia et al14 was endoscopic deep mucosal biopsy, which provides a limited assessment of the bowel wall, as it fails to reflect the status of most of the bowl wall depth with regard to inflammation and fibrosis. In their study, they classified patients into 2 discrete categories: those with inflammatory strictures and those with fibrotic strictures. Their study failed to account for the possibility of mixed lesions containing both inflammation and fibrosis, which again appear to be quite common on the basis of recent reports.
Our study had limitations. First, it was performed in a Crohn disease animal model as opposed to human patients with Crohn disease. However, this model can be considered advantageous in some ways, and it allows reproducible scientific methods and effective access to full-thickness bowel wall histopathologic findings as a reference standard to compare our contrast-enhanced sonographic results against. Second, all colorectal lesions that were created by the TNBS-ethanol enema model contained severe transmural inflammation; we did not specifically assess the ability of contrast-enhanced sonography to detect fibrosis in the absence of inflammation. Again, such strictures seem to be uncommon according to recently published reports. Finally, the number of animals used in our study was relatively small. When performing animal studies and determining the animals to be subjected to experimentation, it is important to balance the ability to detect a statistically significant result with what would be a clinically relevant result as well as with ethical concerns. On the basis of our results, we believe that our study contained a sufficient number of animals to support our conclusions.
In conclusion, contrast-enhanced sonographic quantitative perfusion parameters failed to reliably detect bowel wall fibrosis in the setting of superimposed inflammation in a Crohn disease animal model. Likewise, there was no significant correlation between any contrast-enhanced sonographic quantitative perfusion parameter and the amount of bowel wall collagen, as determined by the Western blot technique. It is very likely that these parameters are primarily influenced by bowel wall inflammation, as microbubble contrast agents are confined to the intravascular compartment. Our results bring into question the ability of contrast-enhanced sonography to detect and quantify bowel wall fibrosis most human Crohn disease strictures, which commonly contain both substantial inflammation and fibrosis.
Acknowledgments
This research was partly funded by unrestricted investigator-initiated grants from Bracco Diagnostics, Inc, and Siemens Medical Solutions USA, Inc (J.R.D.).
Abbreviations
- TNBS
2,4,6-trinitrobenzenesulfonic acid
References
- 1.Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr 2015; 169:1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009; 361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut 2013; 62: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker ME, Hara AK, Platt JF, Maglinte DD, Fletcher JG. CT enterography for Crohn’s disease: optimal technique and imaging issues. Abdom Imaging 2015; 40:938–952. [DOI] [PubMed] [Google Scholar]
- 5.Malgras B, Pautrat K, Dray X, et al. Multidisciplinary management of gastrointestinal fibrotic stenosis in Crohn’s disease. Dig Dis Sci 2015; 60:1152–1168. [DOI] [PubMed] [Google Scholar]
- 6.Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis 2012; 18: 849–856. [DOI] [PubMed] [Google Scholar]
- 7.Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015; 110:432–440. [DOI] [PubMed] [Google Scholar]
- 8.Barkmeier DT, Dillman JR, Al-Hawary M, et al. MR enterography-histology comparison in resected pediatric small bowel Crohn disease strictures: can imaging predict fibrosis? Pediatr Radiol 2016; 46:498–507. [DOI] [PubMed] [Google Scholar]
- 9.Kim K, Johnson LA, Jia C, et al. Noninvasive ultrasound elasticity imaging (UEI) of Crohn’s disease: animal model. Ultrasound Med Biol 2008; 34:902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stidham RW, Xu J, Johnson LA, et al. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology 2011; 141:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillman JR, Stidham RW, Higgins PD, Moons DS, Johnson LA, Rubin JM. US elastography-derived shear wave velocity helps distinguish acutely inflamed from fibrotic bowel in a Crohn disease animal model. Radiology 2013; 267:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillman JR, Stidham RW, Higgins PD, et al. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med 2014; 33:2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nylund K, Jirik R, Mezl M, et al. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with Crohn’s disease. Ultrasound Med Biol 2013; 39:1197–1206. [DOI] [PubMed] [Google Scholar]
- 14.Quaia E, De Paoli L, Stocca T, Cabibbo B, Casagrande F, Cova MA. The value of small bowel wall contrast enhancement after sulfur hexa-fluoride–filled microbubble injection to differentiate inflammatory from fibrotic strictures in patients with Crohn’s disease. Ultrasound Med Biol 2012; 38:1324–1332. [DOI] [PubMed] [Google Scholar]
- 15.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989; 96:795–803. [PubMed] [Google Scholar]
- 16.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007; 2:541–546. [DOI] [PubMed] [Google Scholar]
- 17.Phillips P, Gardner E. Contrast-agent detection and quantification. Eur Radiol 2004; 14(suppl 8):P4–P10. [PubMed] [Google Scholar]
- 18.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Franco A, Di Veronica A, Armuzzi A, et al. Ileal Crohn disease: mural microvascularity quantified with contrast-enhanced US correlates with disease activity. Radiology 2012; 262:680–688. [DOI] [PubMed] [Google Scholar]
- 20.Girlich C, Schacherer D, Jung EM, Schreyer A, Büttner R. Comparison between a clinical activity index (Harvey-Bradshaw-Index), laboratory inflammation markers and quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound in Crohn’s disease. Eur J Radiol 2012; 81:1105–1109. [DOI] [PubMed] [Google Scholar]
- 21.Ripollés T, Martínez MJ, Paredes JM, Blanc E, Flors L, Delgado F. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 2009; 253:241–248. [DOI] [PubMed] [Google Scholar]