Abstract
The chemical synthesis of phosphoramidite derivatives of all four 5´-deoxy-5´-thioribonucleosides is described. These phosphoramidites contained either trityl (A, G, C and U), dimethoxytrityl (A, G) or tert-butyldisulfanyl (G) as the 5´-S protecting group. The application of several of these phosphoramidites for solid-phase synthesis of oligoribonucleotides containing a 2´-O-photocaged 5´-S-phosphorothiolate linkage or 5´-thiol labeled RNAs is also further investigated.
Graphical Abstract

INTRODUCTION
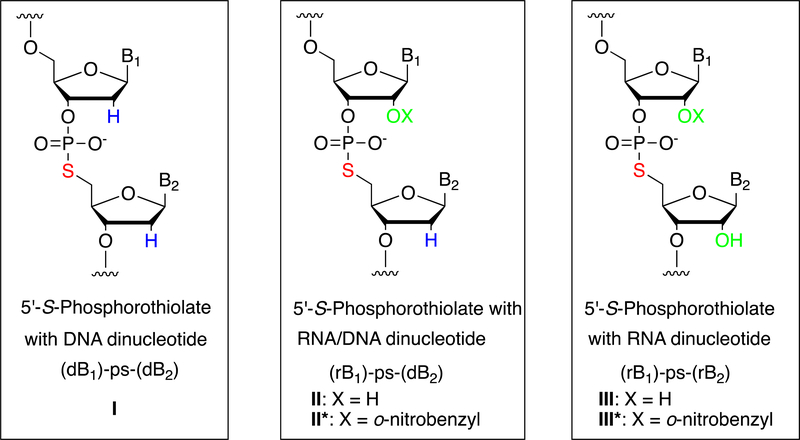
The 5´-S-phosphorothiolate (5´-PS) oligonucleotides (Figure 1) including oligodeoxynucleotides (ODNs) and oligoribonucleotides (ORNs) containing a 5´-S-phosphorothiolate linkage have received much attention due to their potential therapeutic applications and as biological probes to investigate the catalytic mechanisms of protein and RNA enzymes.1,2 The linear ODNs containing a site-specific 5´-S-phosphorothiolate DNA linkage have been prepared by solid-phase synthesis through coupling to 2´-deoxy-5´-thionucleoside phosphoramidites.3,4 These 5´-PS ODNs (Figure 1, I) have been used as suicide substrates for the studies of DNA site-specific recombinases,5–8 and DNA Topoisomerase.5,9–14 Additionally cyclic 5´-PS ODNs have been chemically synthesized and their stability and structural conformations were investigated.15–20 The cyclic d(GA-ps-GA) and d(A-ps-G) molecules containing a 5´-PS linkage were investigated as antiviral agents16 and Ricin Toxin A-chain inhibitors.21 The ODNs containing a RNA/DNA dinucleotide 5´-PS linkage (Figure 1, II) have been prepared by solid-phase synthesis with coupling to 2´-deoxy-5´-thionucleoside phosphoramidites and has been used to investigate the catalytic mechanism of the hammerhead ribozyme.22–26 Oligonucleotides containing photocaged RNA/DNA dinucleotide 5′-PS linkage (Figure 1, II*) have been synthesized with coupling to 2´-deoxy-5´-thioguanosine phosphoramidite, followed by coupling to 2´-photocaged cytidine phosphoramidite, and used to reveal the general acid mechanism of HDV ribozyme catalyzed reaction.27 For the preparation of photocaged RNA oligonucleotides containing a 5´-PS linkage (Figure 1, III) we reported a general and efficient approach by a two-step enzymatic ligation method starting from a caged 5´-PS dinucleotide (rB1)-ps-(rB2).28 These caged RNAs have proven to be valuable substrates to investigate the mechanisms of the Varkud Satellite (VS) and hairpin ribozyme catalyzed reactions.29,30 In order to develop an efficient synthesis of ORNs containing a 2´-O-photocaged 5´-S-phosphorothiolate linkage (Figure 1, III), here we report the synthesis of 5´-S-tritylthio-3´-O-ribonucleoside phosphoramidites and their incorporation into ORNs containing a 5´-PS linkage by phosphoramidite chemistry.
Figure 1.
Structures of oligonucleotides containing a site-specific 5´-S-phosphorothiolate linkage. I: ODN with a 5´-PS DNA dinucleotide (dB1)-ps-(dB2); II: ODN with 5´-PS RNA/DNA dinucleotide (rB1)-ps-(dB2); III: ORN with a 5´-PS RNA dinucleotide (rB1)-ps-(rB2).
RESULTS AND DISCUSSION
1. Synthesis of 5´-thio-2´-O-TBS guanosine derivatives
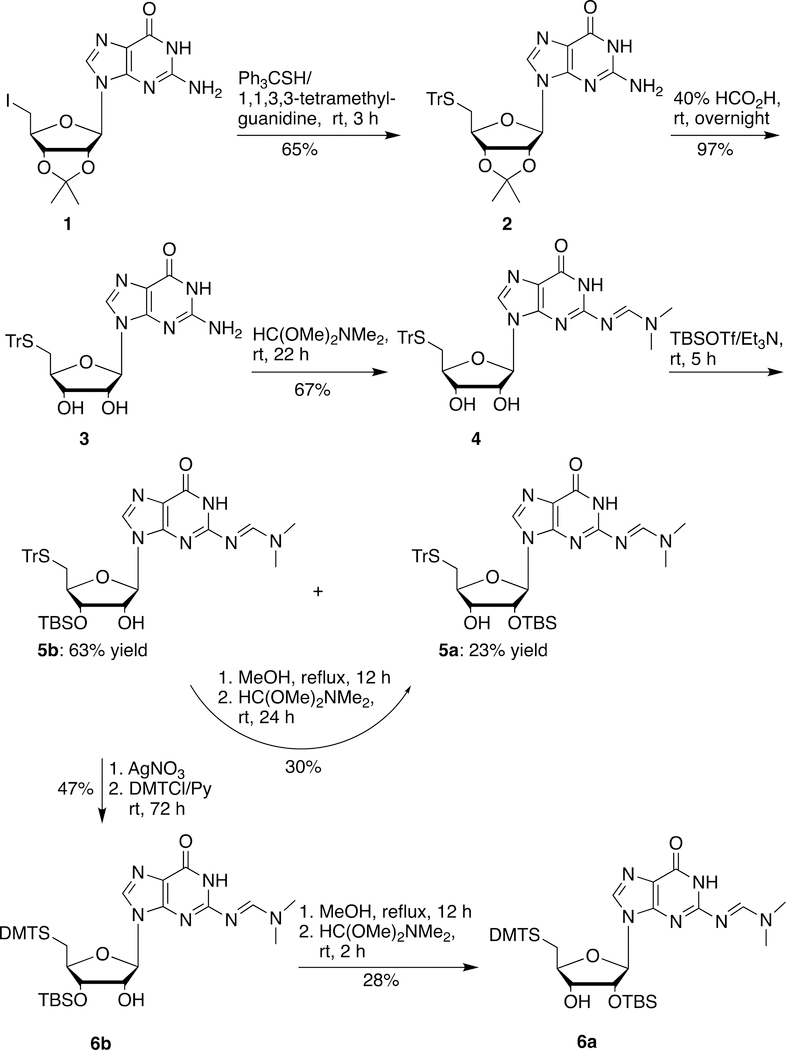
The DMT and trityl groups have been successfully utilized for the protection of 5´-thiol group during the preparation and incorporation of 2´-deoxy-5´-thionucleoside phosphoramidites into ODNs containing a 5´-PS DNA dinucleotide (dB1)-ps-(dB2) (Figure 1, I) by solid phase synthesis.3,4 Accordingly we chose trityl and DMT as protective groups for the synthesis of 5´-thio-3´-O-ribonucleoside phosphoramidites. 5´-Iodo-5´-deoxy-2´,3´-O-isopropylideneguanosine (1) could be prepared by iodination of 2´,3´-O-isopropylideneguanosine.31 Substitution of 1 with tritylthiol yielded 5´-tritylthio-5´-deoxy-2´,3´-O-isopropylideneguanosine (2) in 65% yield (Scheme 1). Treatment of 2 with 40% formic acid removed the isopropylidene group and generated 5´-tritylthio-5´-deoxyguanosine (3) in 97% yield. Protection of the 2-NH2 group of 3 with N,N-dimethylformamide dimethyl acetal generated 4 in 67% yield. Silylation of 4 with TBSOTf in the presence of triethylamine yielded the corresponding desired 2´-O-TBS isomer 5a in 23% yield along with the undesired 3´-O-TBS isomer 5b in 63% yield. Fortunately, the undesired 5´-tritylthio-3´-O-TBS isomer 5b could be partially isomerized to the 2´-O-TBS isomer 5a (30% yield) in refluxing methanol. It could also be converted into the 5´-DMT-thio-2´-O-TBS derivative 6a in four steps (13% overall yield) as shown in Scheme 1.
Scheme 1.
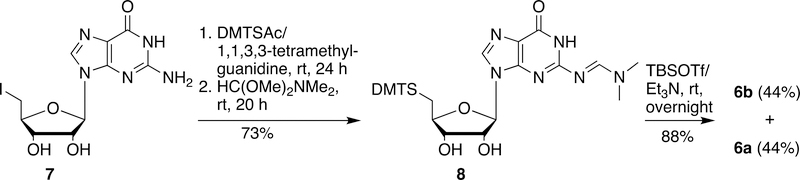
Starting from guanosine, a straightforward method for the synthesis of 6a was developed (Scheme 2). 5´-Iodo-5´-deoxyguanosine (7) prepared from guanosine by the reaction with iodine/Ph3P32 could be converted into N2-dimethyaminomethylene-5´-DMT-thioguanosine (8) in two steps (73% yield). Silylation of 8 with TBSOTf in the presence of triethylamine yielded the corresponding desired 2´-O-TBS derivative 6a (44% yield) along with the undesired 3´-O-TBS derivative 6b (44% yield).
Scheme 2.
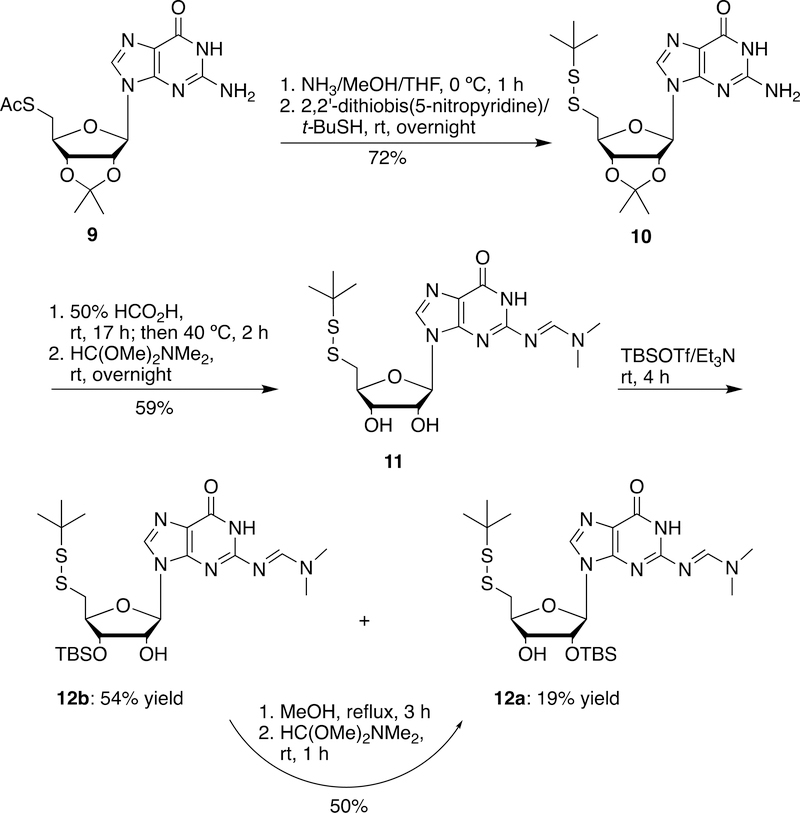
In addition to the synthesis of 5´-S-tritylthio- and 5´-DMT-thio-2´-O-TBS-5´-deoxyguanosine derivatives (5a and 6a), we also developed an efficient method to synthesize 5´-tert-butyldisulfanyl-2´-O-TBS guanosine derivative (Scheme 3). Substitution of 5´-iodo-5´-deoxy-2´,3´-O-isopropylideneguanosine (1) with potassium thioacetate yielded the corresponding 5´-acetylthio derivative (9).28 After removal of the acetyl group and subsequent reaction with tert-butylthiol in the presence of 2,2’-dithiobis(5-nitropyridine), compound 9 was converted into the 5´-tert-butyldisulfanyl guanosine derivative (10) in 72% yield. Treatment of 10 with 50% formic acid removed the isopropylidene group. Protection of the 2-NH2 group of guanosine with N,N-dimethylformamide dimethyl acetal generated 11 in 59% yield. Silylation of 11 with TBSOTf in the presence of triethylamine yielded the corresponding desired 5´-tert-butyldisulfanyl-2´-O-TBS derivative 12a (19% yield) along with the undesired 5´-disulfide-3´-O-TBS derivative 12b (54% yield). Similarly, the undesired 5´-disulfide-3´-O-TBS isomer 12b could be partially isomerized to the 5´-disulfide-2´-O-TBS isomer 12a (50% yield) in refluxing methanol.
Scheme 3.
2. Synthesis of 5´-thio-2´-O-TBS adenosine derivatives
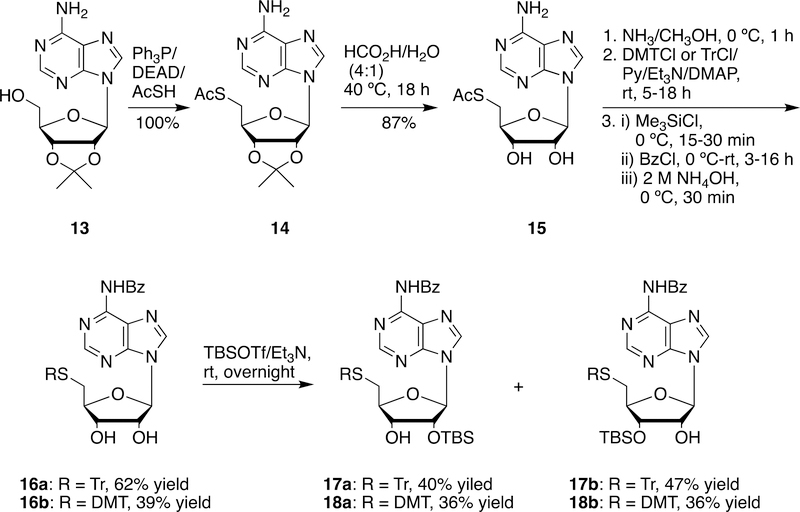
5´-Acetylthio-5´-deoxy-2´,3´-O-isopropylideneadenosine (14) was prepared from commercially available 2´,3´-O-isopropylideneadenosine (13) and thioacetic acid under Mitsunobu conditions in quantitative yield according to Pignot et al.’s procedure (Scheme 4).33 Treatment of 14 with 80% formic acid at 40 ºC for 18 hours yielded 5´-acetylthio-5´-deoxyadenosine (15) in 87% yield. Compound 15 could be converted to the 5´-tritylthioadenosine derivative (16a) and the 5´-DMT-thioadenosine derivative (16b) in three steps (deacetylation, 5´-S-trityl protection and N6-protection) with 62%, and 39% yields, respectively. Silylation of 16a and 16b with TBSOTf yielded the corresponding desired 2´-O-TBS derivatives 17a and 18a in 36–40% yields along with the undesired 3´-O-TBS derivatives 17b and 18b in 36–47% yields (Scheme 4).
Scheme 4.
3. Synthesis of 5´-thio-2´-O-TBS cytidine derivative
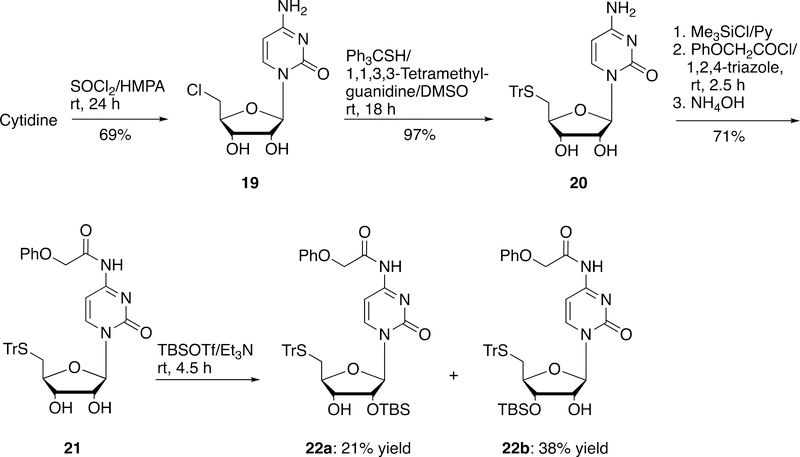
5´-Chloro-5´-deoxycytidine (19) could be prepared by the direct halogenation of cytidine with SOCl2 in 69% yield (Scheme 5).34 SN2 reaction of 19 with triphenylmethanethiol under basic condition generated 5´-tritylthio-5´-deoxycytidine (20) in 97% yield. Transient protection of the cytosine amino group of 20 with phenoxyacetyl group gave compound 21 in 71% yield.35 Silylation of 21 with TBSOTf yielded the corresponding desired 2´-O-TBS derivatives 22a in 21% yield along with the undesired 3´-O-TBS derivatives 22b in 38% yield.
Scheme 5.
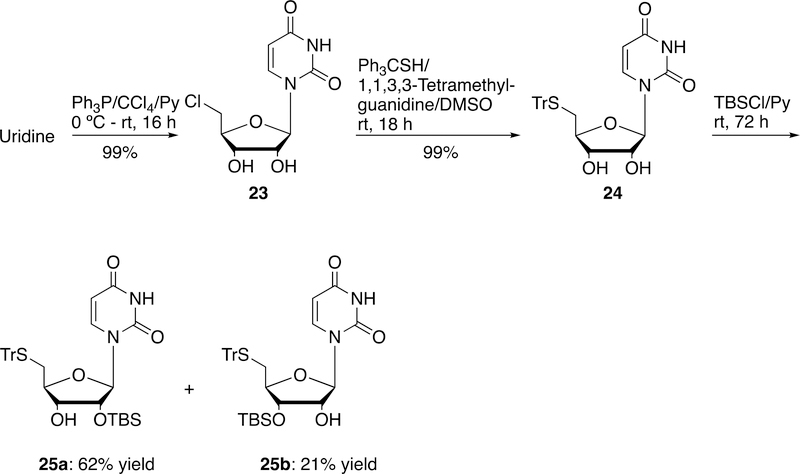
4. Synthesis of 5´-thio-2´-O-TBS uridine derivative
5´-Chloro-5´-deoxyuridine (23) could be prepared by the 5´-halogenation of uridine with carbon tetrachloride and triphenylphosphine in pyridine in 99% yield (Scheme 6).36 SN2 reaction of 23 with triphenylmethanethiol under basic condition generated 5′-tritylthio-5′-deoxyuridine (24) in 99% yield. Unfortunately, attempts to prepare the 2´-O-TBS derivatives 25a by the reaction of 24 with TBSOTf failed. However, silylation of 24 with TBSCl in pyridine successfully gave the corresponding desired 2′-O-TBS derivatives 25a in 62% yield along with the undesired 3′-O-TBS derivatives 25b in 21% yield.
Scheme 6.
The RF value of 2´-O-TBS isomer on TLC is higher than that of 3´-O-TBS isomer. The structure was further confirmed by the COSY spectra (See supporting information for the cosy of 17a and 17b).
5. Synthesis of 5´-thio-3´-O-ribonucleoside phosphoramidites
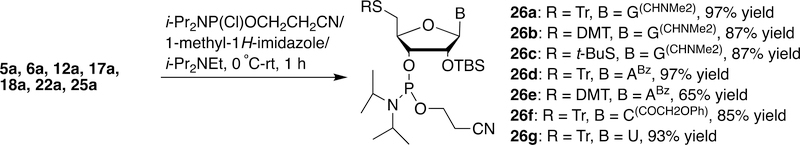
3´-Phosphitylation of the 5´-thio 2´-O-TBS-ribonucleosides (5a, 6a, 12a, 17a, 18a, 22a and 25a) generated the corresponding 5´-thio-2´-O-TBS-3´-O-ribonucleoside phosphoramidites 26a-26g in 65–97% yields (Scheme 7).
Scheme 7.
6. Solid-phase synthesis
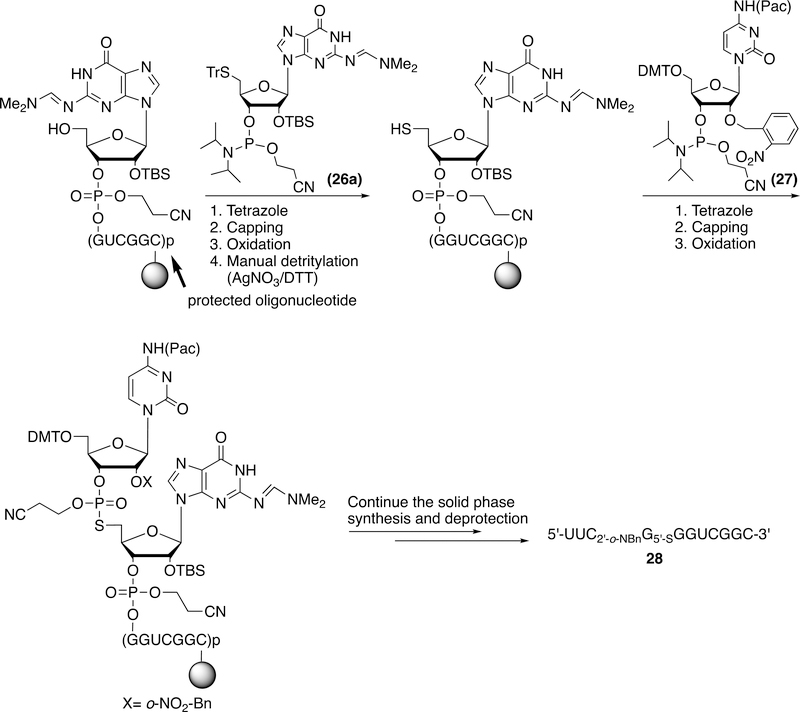
As an example for application of these 5´-thio-3´-O-ribonucleoside phosphoramidites, we utilized both 5´-tritylthio-3´-O-guanosine phosphoramidites 26a and 2´-photocaged cytidine phosphoramidite 2737 to prepare an 11 mer ORN containing a 5´-PS linkage: 5′-UUC2’-o-NBnG5’-SGGUCGGC-3′ (28) successfully (Scheme 8). We modified the protocol to include double coupling for modified phosphoramidites. After standard solid phase synthesis to the residue immediately preceding the 5´-thionucleoside and subsequent double coupling to 26a, the 5´-trityl group was removed by the treatment of aqueous silver nitrate. After further double coupling to phosphoramidite 27, the synthesis was continued for the rest of the designed RNA. The synthesized RNA was deprotected and purified by dPAGE. The desired RNA containing a PS linkage (28) was obtained and the structure was confirmed by the MALDI-TOF MS. Attempts to extend this solid-phase synthetic method to prepare ORN containing an internal 5´-PS linkage (5´-GCGCG2’-o-NBnA5’-SAGGGCGUC-3´) for the mechanism studies of VS catalyzed reaction was unsuccessful. No desired length of RNA containing a 5´-PS linkage was isolated. We surmised that the failed synthesis could reflect inefficient removal of the 5´-S protecting group or inefficient coupling of the 5´-SH to the 2´-photocaged guanosine phosphoamidite. To investigate further, we carried out an additional synthesis of an oligonucleotide containing the sequence: 5´-UUUUG2’-o-NBnU5’-SUUUU-3´. After careful analysis of trityl yields during synthesis and HPLC and MS data of the resulting crude product mixture, we determined that coupling of the 5´-thiol group to the 2´-O-photocaged phosphoramidite occurred with poor efficiency. The trityl yield decreased to about 30% after coupling between 5´-tritylthioU-CE and 2´-O-photocaged G-CE phosphoramidite. The reverse phase HPLC profile of the crude synthesis showed that two major products, 5´-TrSUUUUU-3´ and 5´-HSUUUUU-3´, (confirmed by MS), form in a ratio of 54:46, indicating that the silver nitrate mediated detritylation occurred with only about 50% efficiency. Although the apparent trityl yield suggested that the modified phosphoramidites possibly couple with 30% efficiency, we could detect no desired 5´-UUUUG2’-o-NBnU5’-SUUUU-3´. We have also attempted to make other RNAs containing 5´-PS linkages including 5´-GGCAAGGAGGUAAAAAUGUA2’-o-NBnG5’-SAAAAACAAU-3´ (30 mer), 5´-ACGUU2’-o-NBnA5’-SACGU-3´ (10 mer), and 5´-GCCGUC2’-o-NBnC5’-SCCCG-3´ (11 mer). However, according to MALDI-TOF MS, only 5´-GCCGUC2’-o-NBnC5’-SCCCG-3´ containing a C2’-o-NBnC5’-S linkage (28a) formed in low yield. We conclude that at present only the 2´-O-photocaged cytidine phosphoramidite 27 couples to the 5´-thiol group with sufficient yield to give the corresponding RNA containing PS linkage. Further work will be needed to identify strategies to improve 5´-thiol coupling efficiencies for the 2´-O-photocaged phosphoramidites of G, A, and U.
Scheme 8.
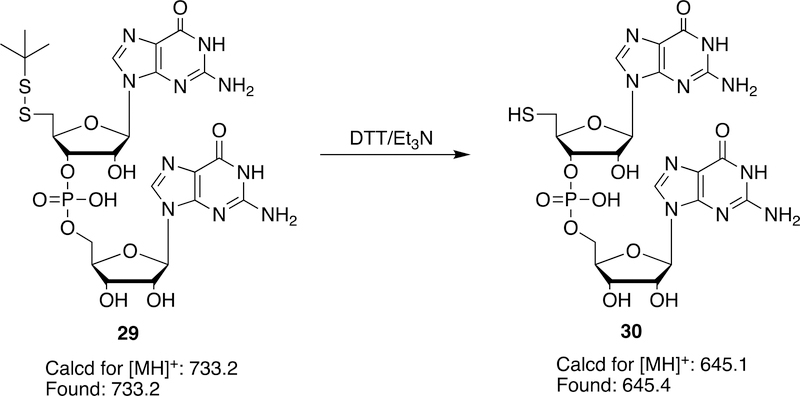
We also used 26c to prepare the (5´-tert-butyldisulfanyl)-GG dinucleotide 29 by solid phase synthesis. Although the tert-butyldisulfanyl group of 29 is quite stable, as expected, the disulfide bond of 29 can be efficiently broken by DTT/Et3N overnight to give free 5′-HS-GG dinucleotide (30) (Scheme 9). 5´-Thiol-tagged oligonucleotides have been received much attention due to their efficiency to conjugate to important biomolecules or nanoparticles through reaction with an α,β-unsaturated carbonyl groups or thiol group. They can easily react with cysteines in proteins to form disulfide bonds and or bind to gold nanoparticles.38–42
Scheme 9.
Conclusion
We have developed synthetic methods to prepare all of four 5´-thio-3´-O-ribonucleoside (guanosine, adenosine, cytidine and uridine) phosphoramidites. These phosphoramidites are useful for synthesis of RNAs that contain a 5´-thiol terminus. In addition, although coupling to the 5´-S occurs with low efficiency in some cases, the phosphoramidites enable synthesis of short RNAs containing and internal PS linkage. It is complementary to our enzymatic two ligation step method for the construction of long RNAs containing an internal 5´-PS PS linkage.28
Experimental
5´-Deoxy-2´,3´-O-isopropylidene-5´-tritylthioguanosine (2)
To the solution of 5´-deoxy-5´-iodo-2´,3´-O-isopropylideneguanosine (1)31 (1.55 g, 3.58 mmol) and Ph3CSH (1.09 g, 3.94 mmol) in DMF (15 mL), 1,1,3,3-tetramethylguanidine (454 mg, 3.94 mmol) was added. The mixture was stirred at room temperature for 3 h. TLC indicated the reaction was complete. The solvent was removed, the residue was isolated by silica gel chromatography, eluting with 5% methanol in chloroform to give 2 as a white foam: 1.35 g (65% yield). 1H NMR (DMSO-d6) δ 10.70 (brs, 1H), 7.80 (s, 1H), 7.35–7.20 (m, 15H), 6.50 (brs, 2H), 5.91 (s, 1H), 5.20 (m, 1H), 4.89 (dd, 1H, J = 3.2, 6.0 Hz), 3.77 (m, 1H), 2.41 (d, 2H, J = 6.52 Hz), 1.43 (s, 3H), 1.25 (s, 3H); 13C NMR (DMSO-d6) δ 156.8, 153.6, 150.4, 144.2, 136.5, 129.1, 128.0, 126.8, 117.0, 113.1, 88.2, 85.3, 83.6, 83.3, 66.2, 34.6, 26.9, 25.3; HRMS (TOF, ESI/APCI) calcd for C32H32N5O4S [MH+] 582.2175, found 582.2169.
5´-Deoxy-5´-tritylthioguanosine (3)
5´-Deoxy-2´,3´-O-isopropylidene-5´-tritylthioguanosine (2) (0.390 g, 0.67 mmol) was treated with 60% HCO2H (50 mL) at room temperature overnight, TLC indicated that the reaction was not complete. The reaction was then heated at 40 ºC for 4 h, which resulted in successful reaction completion as indicated by TLC. The solvent was removed and the residue was co-evaporated with ethanol until no formic acid was left. The residue was purified by silica gel chromatography, eluting with 10% methanol in chloroform to give 3 as a white foam: 0.351 g (97% yield). 1H NMR (DMSO-d6) δ 10.59 (brs, 1H), 7.85 (s, 1H), 7.34–7.23 (m, 15H), 6.48 (brs, 2H), 5.60 (d, 1H, J = 5.6 Hz), 5.47 (d, 1H, J = 5.6 Hz), 5.15 (d, 1H, J = 5.2 Hz), 4.45 (m, 1H), 3.85 (m, 1H), 3.62 (m, 1H), 2.55 (dd, 1H, J = 5.9, 12.8 Hz), 2.35 (dd, 1H, J = 7.6, 12.8 Hz); 13C NMR (DMSO-d6) δ 156.8, 153.8, 151.5, 144.4, 135.7, 129.2, 128.1, 126.9, 116.7, 86.4, 82.5, 72.70, 72.66, 66.4, 34.6; HRMS (TOF, ESI/APCI) calcd for C29H28N5O4S [MH+] 542.1862, found 542.1854.
5´-Deoxy-N2-[(dimethylamino)methylene]-5´-tritylthioguanosine (4)
The mixture of 5´-deoxy-5´-tritylthioguanosine (3) (1.989 g, 3.67 mmol) and N,N-dimethylformamide dimethyl acetal (4.87 mL, 36.7 mmol) in a mixed solvent of anhydrous dichloromethane (30 mL) and anhydrous methanol (30 mL) was stirred at room temperature for 22 h. TLC indicated reaction was complete. The solvent was removed, and the residue was purified by silica gel chromatography, eluting with 10% methanol in chloroform to give 4 as a white foam: 1.47 g (67% yield). 1H NMR (DMSO-d6) δ 8.52 (brs, 1H), 7.98 (s, 1H), 7.33–7.20 (m, 16H), 5.71 (d, 1H, J = 5.5 Hz), 5.46 (d, 1H, J = 5.9 Hz), 5.23 (d, 1H, J = 5.3 Hz), 4.51 (dd, 1H, J = 5.5, 11.0 Hz), 3.96 (dd, 1H, J = 5.0, 9.5 Hz), 3.61 (m, 1H), 3.11 (s, 3H), 3.03 (s, 3H), 2.59 (dd, 1H, J = 5.8, 12.8 Hz), 2.36 (dd, 1H, J = 7.8, 12.8 Hz); 13C NMR (DMSO-d6) δ 157.9, 157.6, 157.3, 150.0, 144.4, 137.1, 129.2, 128.1, 126.9, 119.8, 86.9, 82.5, 73.0, 72.7, 66.3, 40.7, 34.7, 34.6; HRMS (TOF, ESI/APCI) calcd for C32H33N6O4S [MH+] 597.2284, found 597.2272.
2´-O-tert-Butyldimethylsilyl-5´-deoxy-N2-[(dimethylamino)methylene]-5´-tritylthioguanosine (5a) and 3´-O-tert-Butyldimethylsilyl-5´-deoxy-N2-[(dimethylamino)methylene]-5´-tritylthioguanosine (5b)
To the solution of 5´-deoxy-N2-[(dimethylamino)methylene]-5´-tritylthioguanosine (4) (0.744 g, 1.25 mmol) in dichloromethane (35 mL) at 0 ºC was added triethylamine (0.87 mL, 6.2 mmol), followed by the slow addition of TBSOTf (0.64 mL, 2.79 mmol). The mixture was stirred at room temperature for 4 h. TLC showed the reaction was complete. The mixture was washed with saturated aqueous sodium bicarbonate, brine. The solvent was removed and the residue was isolated by silica gel chromatography, eluting with 2% methanol in chloroform to give 2´-O-TBS isomer 5a (the up spot on TLC): 0.202 g (23% yield) and 3´-O-TBS isomer 5b (the bottom spot on TLC): 0.562 g (63% yield) as white foams.
5a: 1H NMR (CDCl3/TMS) δ 9.66 (brs, 1H), 8.55 (s, 1H), 7.67 (s, 1H), 7.45–7.20 (m, 15H), 5.78 (d, 1H, J = 4.4 Hz), 4.50 (m, 1H), 3.95 (m, 1H), 3.88 (m, 1H), 3.11 (s, 3H), 3.09 (s, 3H), 2.75 (dd, 1H, J = 5.2, 13.0 Hz), 2.59 (d, 1H, J = 5.6 Hz), 2.52 (dd, 1H, J = 6.4, 13.0 Hz), 0.84 (s, 9H), −0.03 (s, 3H), −0.12 (s, 3H); 13C NMR (CDCl3) δ 158.3, 158.0, 156.9, 150.2, 144.5, 136.4, 129.6, 128.1, 126.9, 120.8, 88.1, 87.8, 82.6, 75.9, 67.1, 41.4, 35.3, 34.6, 25.7, 18.0, −4.8, −5.1; HRMS (TOF, ESI/APCI) calcd for C38H47N6O4SSi [MH+] 711.3149, found 711.3138.
5b: 1H NMR (CDCl3/TMS) δ 9.15 (brs, 1H), 8.55 (s, 1H), 7.74 (s, 1H), 7.45–7.20 (m, 15H), 5.87 (d, 1H, J = 5.0 Hz), 4.42 (m, 1H), 4.09 (m, 1H), 4.00 (m, 1H), 3.24 (d, 1H, J = 6.0 Hz), 3.12 (s, 3H), 3.09 (s, 3H), 2.65 (dd, 1H, J = 5.5, 12.7 Hz), 2.44 (dd, 1H, J = 6.4, 12.7 Hz), 0.90 (s, 9H), 0.08 (s, 3H), 0.03 (s, 3H); 13C NMR (CDCl3) δ 158.2, 158.1, 156.8, 150.3, 144.4, 136.6, 129.6, 128.1, 127.0, 120.7, 88.5, 83.2, 74.4, 74.3, 67.1, 41.5, 35.2, 34.6, 25.8, 18.1, −4.6; HRMS (TOF, ESI/APCI) calcd for C38H47N6O4SSi [MH+] 711.3149, found 711.3139.
Conversion of 5b to 5a
The solution of 5b (0.787 g, 1.10 mmol) in methanol (30 mL) was heated to reflux for 12 h. After it was cooled down, the mixture was treated with N,N-dimethylformamide dimethyl acetal (1.47 mL, 11.0 mmol) and stirred at room temperature for 24 h. The solvent was removed, the residue was isolated by silica gel chromatography, eluting with 2% methanol in chloroform to give 5a as a white foam (the up spot on TLC): 0.237 g (30% yield) along with the recovered starting 5b as a white foam (bottom TLC): 0.550 g (70% yield).
3´-O-tert-Butyldimethylsilyl-5´-deoxy-N2-[(dimethylamino)methylene]-5´-dimethoxytritylthioguanosine (6b)
To the solution of 5b (0.578 g, 0.81 mmol) in THF/MeOH (3:1, 16 mL) was added a solution of AgNO3 (275 mg, 2.0 eq.) in water (1.0 mL) and MeOH (5.0 mL). After 2 min, the solids were recovered by centrifugation. To the pellet was added THF/MeOH (1:1, 40 mL) and DTT (500 mg), and the mixture was stirred for 5 min. The yellow solid was removed by filtration through a short silica gel column, which was washed with THF/MeOH (1:1). The solution was evaporated under reduced pressure and the residue was washed with water. The solid was recovered by filtration through a pad of sand. The product was eluted from sand by washing with THF. The solvent was removed by rotary evaporation and co-evaporated with toluene. The residue was dissolved into pyridine (15 mL). To the resulting solution, DMTCl (549 mg, 1.62 mmol) and DMAP (198 mg, 1.62 mmol) were added. The mixture was stirred at room temperature for 72 h. The solvent was removed, the residue was isolated by silica gel chromatography, eluting with 3% methanol in chloroform to give 6b as a pale-yellow foam: 0.297 g (47% yield). 1H NMR (CDCl3/TMS) δ 9.87 (brs, 1H), 8.48 (s, 1H), 7.73 (s, 1H), 7.40–7.15 (m, 10H), 6.79 (d, 4H, J = 8.4 Hz), 5.85 (d, 1H, J = 4.8 Hz), 4.54 (m, 1H), 4.09 (m, 1H), 3.98 (m, 1H), 3.77 (s, 6H), 3.07 (s, 3H), 3.02 (s, 3H), 2.68 (dd, 1H, J = 5.2, 12.8 Hz), 2.44 (dd, 1H, J = 6.4, 12.8 Hz), 0.89 (s, 9H), 0.08 (s, 3H), 0.03 (s, 3H); 13C NMR (CDCl3) δ 158.1, 158.0, 157.9, 150.2, 144.9, 136.58, 136.56, 130.5, 129.2, 127.8, 126.6, 120.4, 113.9, 88.3, 83.2, 74.1, 66.0, 55.1, 41.3, 35.0, 34.5, 25.6, 17.9, −4.78, −4.79; HRMS (TOF, ESI/APCI) calcd for C40H51N6O6SSi [MH+] 771.3360, found 771.3355.
2´-O-tert-Butyldimethylsilyl-5´-deoxy-N2-[(dimethylamino)methylene]-5´-dimethoxytritylthioguanosine (6a)
The solution of 6b (0.179 g, 1.10 mmol) in methanol (10 mL) was heated to reflux overnight. After it was cooled down, the mixture was treated with N,N-dimethylformamide dimethyl acetal (0.477 mL, 3.59 mmol) and stirred at room temperature for 2 h. The solvent was removed, the residue was isolated by silica gel chromatography, eluting with 3% methanol in chloroform to give 2´-O-TBS guanosine derivative 6a (the up spot on TLC): 51 mg (28% yield) and to recover the starting material 6b (the low spot on TLC): 84 mg (47% yield). 6a: 1H NMR (CDCl3/TMS) δ 9.79 (brs, 1H), 8.55 (s, 1H), 7.70 (s, 1H), 7.40–7.15 (m, 10H), 6.79 (d, 4H, J = 8.5 Hz), 5.79 (d, 1H, J = 5.0 Hz), 4.52 (t, 1H, J = 5.0 Hz), 3.98 (m, 1H), 3.94 (m, 1H), 3.78 (s, 6H), 3.11 (s, 3H), 3.09 (s, 3H), 2.75 (dd, 1H, J = 5.5, 13.0 Hz), 2.64 (brs, 1H), 2.54 (dd, 1H, J = 6.0, 13.0 Hz), 0.84 (s, 9H), −0.03 (s, 3H), −0.12 (s, 3H); 13C NMR (CDCl3) δ 158.1, 157.9, 156.8, 150.1, 145.0, 136.72, 136.70, 136.3, 130.6, 129.3, 127.9, 126.7, 120.7, 113.2, 88.0, 82.7, 75.8, 73.0, 66.1, 55.2, 41.2, 35.2, 34.6, 25.5, 17.9, −4.92, −5.22; HRMS (TOF, ESI/APCI) calcd C40H51N6O6SSi [MH+] 771.3360, found 771.3369.
5´-Deoxy-N2-[(dimethylamino)methylene]-5´-dimethoxytritylthioguanosine (8)
5´-Dimethoxytrityl thioacetate (DMTSAc) (10.0 mmol) was prepared quantitatively by the reaction of DMTCl (3.38 g, 10.0 mmol) with potassium thioacetate (2.28 g, 20.0 mmol) in anhydrous dichloromethane (50 mL) at room temperature for 1 h. The solvent was removed and the residue was then purified by silica gel chromatography, eluting with 10% ethyl acetate in hexane. 1H NMR (CDCl3) δ 7.46 (brs, 5H), 7.38 (m, 4H), 7.02 (m, 4H), 4.00 (s, 6H), 2.46 (s, 3H); 13C NMR (CDCl3) δ 193.7, 158.3, 144.3, 136.2, 130.8, 129.6, 127.7, 127.0, 113.0, 69.7, 55.1, 30.5.
To the solution of 5´-deoxy-5´-iodoguanosine (7)32 (2.83 g, 7.2 mmol) and DMTSAc (3.27 g, 8.6 mmol) in DMF (50 mL), 1,1,3,3-tetramethylguanidine (1.35 mL, 10.75 mmol) was added. The mixture was stirred at room temperature for 24 h. DMF was removed under reduced pressure, and the residue was dissolved into dichloromethane. The solution was washed with water, saturated aqueous NaHCO3 and brine. The solvent was removed and the residue was isolated by silica gel chromatography, eluting with 5–15% methanol in chloroform to give 5´-deoxy-5´-dimethoxytritylthioguanosine. The mixture of 5´-deoxy-5´-dimethoxytritylthioguanosine and N,N-dimethylformamide dimethyl acetal (11.4 mL, 85.6 mmol) in a mixed solvent of anhydrous dichloromethane (50 mL) and anhydrous methanol (50 mL) was stirred at room temperature for 20 h. TLC showed the reaction was complete. The solvent was removed, the residue was purified by silica gel chromatography, eluting with 10% methanol in chloroform to give 8 as a pale-yellow foam: 3.44 g (73% yield for two steps). 1H NMR (DMSO-d6) δ 11.3 (s, 1H), 8.52 (brs, 1H), 7.99 (s, 1H), 7.27 (m, 5H), 7.19 (m, 4H), 6.85 (m, 4H), 5.74 (d, 1H, J = 8.0 Hz), 5.46 (d, 1H, J = 4.0 Hz), 5.23 (d, 1H, J = 4.0 Hz), 4.52 (m, 1H), 3.98 (m, 1H), 3.73 (s, 6H), 3.67 (m, 1H), 3.11 (s, 3H), 3.03 (s, 3H), 2.61 (dd, 1H, J = 4.0, 12.0 Hz), 2.39 (m, 1H); 13C NMR (DMSO-d6) δ 158.2, 158.03, 157.96, 157.6, 150.4, 145.5, 137.4, 136.88, 136.85, 130.6, 129.4, 128.3, 127.0, 120.2, 113.6, 87.2, 82.9, 73.4, 73.1, 65.7, 55.4, 41.0, 35.0; HRMS (TOF, APCI) calcd for C32H33N6O4S [MCl−] 691.2111, found 691.2100.
Synthesis of 6a and 6b
Both 6a (0.959 g, 44% yield) and 6b (0.953 g, 44% yield) were prepared by the reaction of 8 (1.837 g, 2.80 mmol) with TBSOTf (1.47 mL, 6.4 mmol) in presence of triethylamine (1.95 mL, 14.0 mmol) in dichloromethane (50 mL) according to the procedure for the synthesis of 5a and 5b.
5´-tert-Butyldisulfanyl-5´-deoxy-2´,3´-O-isopropylideneguanosine (10)
Under argon a solution of 5´-aceylthio-5´-deoxy-2´,3´-O-isopropylideneguanosine (9)28 (0.902 g, 2.36 mmol) in a mixed solvent of THF and methanol (90 mL, v/v: 1/1) was saturated with ammonia at 0 ºC for 30 min. The solution was stirred at 0 ºC for additional 30 min. The solvent was removed, and the residue was dried over vacuum. Under argon the dried residue was dissolved into anhydrous DMF (50 mL). To the resulting solution 2,2’-dithiobis(5-nitropyridine) (805 mg, 2.60 mmol) and 2-methyl-2-propanethiol (2.66 mL, 2.36 mmol) were added and the mixture was stirred at room temperature overnight. The solvent was removed, and the residue was isolated by silica gel chromatography, eluting with 5–10% methanol in chloroform to give 10 as a white foam: 0.729 g (72% yield). 1H NMR (DMSO-d6) δ 10.78 (brs, 1H), 7.84 (s, 1H), 6.58 (brs, 2H), 5.97 (d, 1H, J = 1.6 Hz), 5.31 (dd, 1H, J = 6.4, 1.6 Hz), 5.06 (dd, 1H, J = 6.4, 3.2 Hz), 4.24 (m, 1H), 3.00 (dd, 1H, J = 6.8, 13.6 Hz), 2.90 (dd, 1H, J = 7.2, 13.6 Hz), 1.47 (s, 3H), 1.29 (s, 3H), 1.19 (s, 9H); 13C NMR (DMSO-d6) δ 157.2, 153.9, 150.8, 137.0, 117.2, 113.5, 89.1, 85.9, 83.9, 83.5, 48.1, 42.8, 29.7, 27.1, 25.4; HRMS (TOF, ESI/APCI) calcd for C17H26N5O4S2 [MH+] 428.1421, found 428.1431.
5´-tert-Butyldisulfanyl-5´-deoxy-N2-[(dimethylamino)methylene]guanosine (11)
5´-tert-Butyldisulfanyl-5´-deoxy-2′,3′-O-isopropylideneguanosine (10) (0.665 g, 1.55 mmol) was treated with 50% HCO2H (30 mL) at room temperature for 17 h and then heated to 40 ºC for 2 h. The solvent was removed, the residue was dissolved into methanol, then basified with triethylamine. The solvent was removed, and the residue was co-evaporated with toluene to remove the trace amount of formic acid and then dried over vacuum. The dried residue was dissolved in methanol (20 mL). To the resulting solution N,N-dimethylformamide dimethyl acetal (2.06 mL, 15.5 mmol) was added and the mixture was stirred at room temperature overnight. The solvent was removed, and the residue was isolated by silica gel chromatography, eluting with 15% methanol in chloroform to give 11 as a white foam: 0.407 g (59% yield). 1H NMR (DMSO-d6) δ 11.33 (brs, 1H), 8.52 (s, 1H), 7.99 (s, 1H), 5.77 (d, 1H, J = 4.8 Hz), 5.56 (brs, 1H), 5.46 (brs, 1H), 4.62 (m, 1H), 4.13 (m, 1H), 4.05 (m, 1H), 3.13–3.00 (m, 2H), 3.14 (s, 3H), 3.01 (s, 3H), 1.25 (s, 9H); 13C NMR (DMSO-d6) δ 158.3, 158.1, 157.6, 150.4, 137.8, 120.0, 87.3, 83.0, 73.2, 72.6, 48.1, 43.6, 41.1, 35.0, 29.8; HRMS (TOF, ESI/APCI) calcd for C17H27N6O4S2 [MH+] 443.1530, found 443.1532.
2´-O-tert-Butyldimethylsilyl-5´-tert-butyldisulfanyl-5´-deoxy-N2-[(dimethylamino)methylene]guanosine (12a) and 3´-O-tert-Butyldimethylsilyl-5´-tert-butyldisulfanyl-5´-deoxy-N2-[(dimethylamino)methylene]guanosine (12b)
To the solution of 11 (0.448 g, 1.01 mmol) in dichloromethane (35 mL) at 0 ºC was added triethylamine (0.70 mL, 5.1 mmol), followed by the slow addition of TBSOTf (0.46 mL, 2.02 mmol). The mixture was warmed up and stirred at room temperature for 4 h. TLC showed the reaction was complete. The mixture was washed with saturated sodium bicarbonate, brine. The solvent was removed, the residue was isolated by silica gel chromatography, eluting with 2–5% methanol in chloroform to give 2´-O-TBS isomer 12a (the up spot on TLC): 0.105 g (19% yield) and 3´-O-TBS isomer 12b (the low spot on TLC): 0.304 g (54% yield) as white foams.
12a: 1H NMR (CDCl3/TMS) δ 10.04 (brs, 1H), 8.58 (s, 1H), 7.77 (s, 1H), 5.83 (d, 1H, J = 4.0 Hz), 4.70 (m, 1H), 4.31 (m, 2H), 3.30–3.05 (m, 2H), 3.17 (s, 3H), 3.11 (s, 3H), 2.99 (brs, 1H), 1.32 (s, 9H), 0.83 (s, 9H), −0.03 (s, 3H), −0.14 (s, 3H); 13C NMR (CDCl3) δ 158.2, 157.9, 156.8, 150.2, 136.5, 120.7, 87.8, 82.9, 75.3, 72.3, 48.2, 44.2, 41.3, 35.2, 29.7, 25.5, 17.8, −5.1, −5.3; HRMS (TOF, ESI/APCI) calcd for C23H41N6O4S2Si [MH+] 557.2395, found 557.2401.
12b: 1H NMR (CDCl3/TMS) δ 9.95 (brs, 1H), 8.46 (s, 1H), 7.66 (s, 1H), 5.82 (d, 1H, J = 6.0 Hz), 4.79 (m, 1H), 4.43 (m, 1H), 4.25 (m, 2H), 3.20–2.90 (m, 2H), 3.13 (s, 3H), 3.02 (s, 3H), 1.28 (s, 9H), 0.93 (s, 9H), 0.18 (s, 3H), 0.17 (s, 3H); 13C NMR (CDCl3) δ 158.1, 156.7, 150.2, 137.2, 120.7, 88.7, 83.8, 73.7, 73.4, 48.2, 43.6, 41.5, 35.2, 29.8, 25.9, 18.2, −4.50, −4.54; HRMS (TOF, ESI/APCI) calcd for C23H41N6O4S2Si [MH+] 557.2395, found 557.2396.
Conversion of 12b to 12a
The solution of 3´-O-tert-butyldimethylsilyl-5´-tert-butyldisulfanyl-5´-deoxy-N2-[(dimethylamino)methylene]guanosine (12b) (0.165 g, 0.296 mmol) in methanol (10 mL) was heated to reflux for 3 h. After it was cooled down, the mixture was treated with N,N-dimethylformamide dimethyl acetal (0.395 mL, 2.96 mmol) and stirred at room temperature overnight. The solvent was removed, the residue was isolated by silica gel chromatography, eluting with 2% methanol in chloroform to give 12a (the up spot on TLC): 82.5 mg (50% yield) along with the recovered starting 12b (the low spot on TLC): 70.5 mg (43% yield).
5´-Acetylthio-5´-deoxy-2´,3´-O-isopropylideneadenosine (14)
Under argon to a solution of triphenylphosphane (7.55 g, 28.8 mmol) in anhydrous THF (40 mL) at 0 ºC, diethyl azodicarboxylate (4.53 mL, 28.8 mmol) was added over 5 min. After stirring for 30 min, 2´,3´-O-isopropylideneadenosine (13) (4.00 g, 13.0 mmol) was added and stirring was continued for 30 min. To the resulted yellow suspension, a solution of thioacetic acid (2.06 mL, 28.8 mmol) in THF (5.0 mL) was slowly added and stirring was continued for another 1 h at 0 ºC. During this time, the yellow suspension cleared, and an orange solution was obtained. The solvent was removed under reduced pressure and the residue was purified by silica gel chromatography, eluting with chloroform/THF (4:1, v/v), followed by 5–10% CH3OH in chloroform to give 1433 as an oil: 4.76 g (100% yield). 1H NMR (CDCl3/TMS) δ 8.35 (s, 1H), 7.96 (s, 1H), 7.34 (brs, 2H), 6.12 (d, 1H, J = 1.8 Hz), 5.55 (dd, 1H, J = 6.3, 1.9 Hz), 5.01 (dd, 1H, J = 6.3, 3.0 Hz), 4.36 (m, 1H), 3.30 (dd, 1H, J = 13.8, 7.3 Hz), 3.19 (dd, 1H, J = 13.8, 6.6 Hz), 2.35 (s, 3H), 1.60 (s, 3H), 1.39 (s, 3H); 13C NMR (CDCl3) δ 194.4, 156.0, 152.9, 148.8, 139.6, 120.0, 114.2, 90.6, 85.9, 84.0, 83.5, 31.1, 30.4, 26.9, 25.2.
5´-Acetylthio-5´-deoxyadenosine (15)
To the flask containing 5´-acetylthio-5´-deoxy-2´,3´-O-isopropylideneadenosine (14) (1.10 g, 3.00 mmol), a mixture of formic acid (10 mL) and water (2.5 mL) was added. The reaction mixture was heated and stirred at 40 ºC for 18 h. TLC showed the reaction was complete. The solvent was removed under reduced pressure, and the trace amount of formic acid was removed by co-evaporating with ethanol. The product was purified by silica gel chromatography, eluting with 10% methanol in chloroform to give 15:33 0.844 g (87% yield). 1H NMR (DMSO-d6) δ 8.36 (s, 1H), 8.17 (s, 1H), 7.33 (brs, 2H), 5.89 (d, 1H, J = 5.8 Hz), 5.56 (d, 1H, J = 5.4 Hz), 5.42 (d, 1H, J = 3.9 Hz), 4.80 (m, 1H), 4.11 (m, 1H), 3.93 (m, 1H), 3.36 (dd, 1H, J = 13.8, 5.7 Hz), 3.18 (dd, 1H, J = 13.8, 7.5 Hz), 2.35 (s, 3H); 13C NMR (DMSO-d6) δ 194.9, 156.1, 152.7, 149.4, 140.0, 119.2, 87.5, 82.9, 72.63, 72.56, 31.3, 30.5.
N6-Benzoyl-5´-deoxy-5´-tritylthioadenosine (16a)
Under argon 5´-acetylthio-5´-deoxyadenosine (15) (1.122 g, 3.45 mmol) in anhydrous methanol (60 mL) was saturated with ammonia at 0 ºC for 30 min and kept at 0 ºC for additional 30 min. TLC showed the reaction was complete. The solvent was removed, and the residue was dried over vacuum for 15 min. Under argon the dried residue was dissolved into dry pyridine (40 mL). To this solution, DMAP (42 mg, 0.35 mmol), triethylamine (1.34 mL, 9.66 mmol) and trityl chloride (2.32 g, 8.30 mmol) were added. After the reaction mixture was stirred at room temperature for 18 h, TLC showed reaction was complete. To this reaction mixture trimethylsilyl chloride (2.53 mL, 20.0 mmol) was slowly added and stirred at 0 ºC for 15 min. Benzoyl chloride (2.32 mL, 20.0 mmol) was then added and the mixture was stirred at room temperature for 16 h. The reaction mixture was chilled in an ice bath, and added cold water (8 mL), followed after 5 min by concentrated aqueous ammonia (8 mL). After 30 min, the solvent was removed, the residue was dissolved into dichloromethane, washed with water, saturated NaHCO3 and brine. The solvent was removed, the residue was purified by silica gel chromatography, eluting with 3% methanol in chloroform to give 16a as a yellow foam: 1.349 g (62% yield). 1H NMR (CDCl3/TMS) δ 9.53 (brs, 1H), 8.43 (s, 1H), 8.11 (s, 1H), 7.91 (d, 2H, J = 7.5 Hz), 7.50–7.10 (m, 18H), 5.94 (d, 1H, J = 5.5 Hz), 4.72 (m, 1H), 4.08 (m, 1H), 4.04 (m, 1H), 2.66 (dd, 1H, J = 5.9, 12.7 Hz), 2.50 (dd, 1H, J = 6.0, 12.7 Hz); 13C NMR (CDCl3) δ 165.1, 152.0, 151.0, 148.9, 144.2, 142.1, 133.0, 132.8, 129.4, 128.6, 127.9, 126.3, 122.6, 88.9, 83.6, 74.2, 73.1, 66.8, 34.5; HRMS (TOF, ESI/APCI) calcd for C36H32N5O4S [MH+] 630.2175, found 630.2196.
N6-Benzoyl-5´-deoxy-5´-dimethoxytritylthioadenosine (16b)
Under argon 5´-acetylthio-5´-deoxyadenosine (15) (1.06 g, 3.26 mmol) in anhydrous methanol (40 mL) was saturated with ammonia at 0 ºC for 30 min and kept at 0 ºC for additional 30 min. The solvent was removed, and the residue was dried over vacuum for 15 min to give white foam. Under argon the dried residue was dissolved into dry pyridine (30 mL). To this solution, DMAP (40 mg, 0.33 mmol), triethylamine (0.64 mL, 4.6 mmol) and DMTCl (1.33 g, 3.92 mmol) were added. After the reaction mixture was stirred at room temperature for 5 h, TLC showed reaction was complete. To this reaction mixture at 0 ºC trimethylsilyl chloride (2.06 mL, 16.3 mmol) was slowly added and stirred for 30 min. Benzoyl chloride (1.89 mL, 16.3 mmol) was then added and the mixture was stirred at room temperature for 3 h. The reaction mixture was chilled in an ice bath, and added cold water (5 mL), followed after 5 min by concentrated aqueous ammonia (5 mL). After 30 min, the solvent was removed, the residue was dissolved into dichloromethane, washed with water, saturated NaHCO3 and brine. The solvent was removed, the residue was purified by silica gel chromatography, eluting with 3% methanol in chloroform to give 16b as a yellow foam: 0.875 g (39% yield). 1H NMR (CDCl3/TMS) δ 9.55 (brs, 1H), 8.45 (s, 1H), 8.14 (s, 1H), 7.92 (d, 2H, J = 7.5 Hz), 7.50–7.10 (m, 12H), 6.76 (d, 4H, J = 9.0 Hz), 5.96 (d, 1H, J = 5.5 Hz), 4.74 (m, 1H), 4.12 (m, 1H), 4.09 (m, 1H), 3.71 (s, 6H), 2.68 (dd, 1H, J = 6.0, 12.5 Hz), 2.52 (dd, 1H, J = 6.0, 12.5 Hz); 13C NMR (CDCl3) δ 165.0, 158.0, 152.0, 151.0, 148.9, 144.9, 142.1, 136.6, 133.0, 132.8, 130.5, 129.2, 128.6, 128.2, 127.8, 126.6, 122.5, 113.1, 88.9, 83.7, 74.2, 73.1, 65.9, 55.1, 34.6; HRMS (TOF, ESI/APCI) calcd for C38H35N5O6SNa [MNa+] 712.2206, found 712.2197.
N6-Benzoyl-2´-O-tert-butyldimethylsilyl-5´-deoxy-5´-tritylthioadenosine (17a) and N6-Benzoyl-3´-O-tert-butyldimethylsilyl-5´-deoxy-5´-tritylthioadenosine (17b)
To N6-benzoyl-5´-deoxy-5´-tritylthioadenosine (16a) (579 mg, 0.92 mmol) in dichloromethane (20 mL) was added triethylamine (0.64 mL, 4.6 mmol), followed by TBSOTf (250 μL, 1.10 mmol). The mixture was stirred at room temperature for 1 h. TLC showed reaction was not complete. To the mixture was added additional TBSOTf (125 μL, 0.55 mmol), and the mixture was stirred at room temperature overnight. The reaction was quenched with methanol (1 mL). The mixture was washed with saturated sodium bicarbonate, brine. The solvent was removed, the residue was isolated by silica gel chromatography, eluting with 1% methanol in chloroform to give 2´-O-TBS isomer 17a (the up spot on TLC): 0.276 g (40% yield) and 3´-O-TBS isomer 17b (the low spot on TLC): 0.319 g (47% yield).
17a: 1H NMR (CDCl3/TMS) δ 9.14 (brs, 1H), 8.73 (s, 1H), 8.09 (s, 1H), 8.03 (d, 2H, J = 7.6 Hz), 7.60 (t, 1H, J = 7.3 Hz), 7.52 (t, 2H, J = 7.5 Hz), 7.45–7.20 (m, 15H), 5.87 (d, 1H, J = 4.6 Hz), 4.93 (m, 1H), 4.04 (m, 1H), 3.90 (m, 1H), 2.80 (dd, 1H, J = 6.0, 12.8 Hz), 2.61 (dd, 1H, J = 6.7, 12.8 Hz), 2.57 (d, 1H, J = 5.1 Hz), 0.83 (s, 9H), −0.053 (s, 3H), −0.17 (s, 3H); 13C NMR (CDCl3) δ 164.5, 152.7, 151.2, 149.6, 144.3, 142.0, 133.6, 132.8, 129.5, 128.8, 127.9, 127.8, 126.8, 123.5, 89.5, 83.3, 74.6, 73.1, 67.0, 34.2, 25.5, 17.8, −5.0, −5.2; HRMS (TOF, ESI/APCI) calcd for C42H45N5O4SSiNa [MNa+] 766.2854, found 766.2891.
17b: 1H NMR (CDCl3/TMS) δ 9.58 (brs, 1H), 8.61 (s, 1H), 8.13 (s, 1H), 7.98 (d, 2H, J = 7.3 Hz), 7.50–7.15 (m, 18H), 5.93 (d, 1H, J = 4.6 Hz), 4.68 (m, 1H), 4.26 (m, 1H), 4.04 (m, 1H), 3.47 (d, 1H, J = 5.6 Hz), 2.65 (dd, 1H, J = 5.4, 12.5 Hz), 2.55 (dd, 1H, J = 8.6, 12.5 Hz), 0.89 (s, 9H), 0.070 (s, 3H), 0.036 (s, 3H); 13C NMR (CDCl3) δ 164.7, 152.1, 151.2, 149.5, 144.1, 142.1, 133.4, 132.4, 129.3, 128.5, 127.8, 126.6, 123.6, 89.4, 83.6, 74.1, 73.9, 66.8, 34.2, 25.5, 17.8, −4.77, −4.96; HRMS (TOF, ESI/APCI) calcd for C42H45N5O4SSiNa [MNa+] 766.2854, found 766.2833.
N6-Benzoyl-2´-O-tert-butyldimethylsilyl-5´-deoxy-5´-dimethoxytritylthioadenosine (18a) and N6-Benzoyl-3´-O-tert-butyldimethylsilyl-5´-deoxy-5´-dimethoxytritylthioadenosine (18b)
To the solution of N6-benzoyl-5´-deoxy-5´-dimethoxytritylthioadenosine (16b) (837 mg, 1.21 mmol) in dichloromethane (25 mL) at 0 ºC was added triethylamine (0.84 mL, 6.1 mmol), followed by the slow addition of TBSOTf (306 μL, 1.33 mmol). The mixture was stirred at room temperature overnight. TLC showed reaction was not complete. To the mixture was added additional TBSOTf (278 μL, 1.21 mmol), and the mixture was stirred at room temperature for 1 h. TLC showed reaction was almost complete. The reaction was quenched with methanol (1.0 mL). The mixture was washed with saturated sodium bicarbonate, brine. The solvent was removed, the residue was isolated by silica gel chromatography, eluting with 1% methanol in chloroform to give 2´-O-TBS isomer 18a (the up spot on TLC): 0.351 g (36% yield) and 3´-O-TBS isomer 18b (the low spot on TLC): 0.352 g (36% yield).
18a: 1H NMR (CDCl3/TMS) δ 9.17 (brs, 1H), 8.72 (s, 1H), 8.10 (s, 1H), 8.02 (d, 2H, J = 7.3 Hz), 7.58 (t, 1H, J = 7.3 Hz), 7.48 (t, 2H, J = 7.2 Hz), 7.40–7.18 (m, 9H), 6.78 (m, 4H), 5.88 (d, 1H, J = 4.7 Hz), 4.93 (m, 1H), 3.95 (m, 1H), 3.76 (m, 6H), 2.78 (dd, 1H, J = 6.0, 12.8 Hz), 2.59 (dd, 1H, J = 6.6, 12.8 Hz), 0.81 (s, 9H), −0.068 (s, 3H), −0.19 (s, 3H); 13C NMR (CDCl3) δ 164.4, 157.9, 152.5, 151.1, 149.5, 144.8, 141.9, 136.6, 136.5, 133.4, 132.6, 130.4, 129.2, 128.6, 127.7, 127.6, 126.5, 123.3, 113.0, 89.3, 83.3, 74.5, 73.0, 65.9, 55.0, 34.2, 25.3, 17.6, −5.2, −5.4; HRMS (TOF, ESI/APCI) calcd for C44H49N5O6SSiNa [MNa+] 826.3071, found 826.3066.
18b: 1H NMR (CDCl3/TMS) δ 9.45 (brs, 1H), 8.69 (s, 1H), 8.19 (s, 1H), 8.02 (d, 2H, J = 7.4 Hz), 7.56 (t, 1H, J = 7.2 Hz), 7.49 (t, 2H, J = 7.7 Hz), 7.39 (d, 1H, J = 7.6 Hz), 7.35–7.15 (m, 7H), 6.79 (d, 4H, J = 8.8 Hz), 5.94 (d, 1H, J = 4.8 Hz), 4.67 (m, 1H), 4.28 (m, 1H), 4.05 (m, 1H), 3.77 (m, 6H), 3.21 (brs, 1H), 2.66 (dd, 1H, J = 5.4, 12.7 Hz), 2.56 (dd, 1H, J = 6.8, 12.7 Hz), 0.90 (s, 9H), 0.08 (s, 3H), 0.04 (s, 3H); 13C NMR (CDCl3) δ 164.9, 158.2, 152.5, 151.2, 149.7, 145.0, 142.0, 136.8, 133.7, 132.9, 130.7, 129.4, 128.9, 128.04, 127.99, 126.8, 123.8, 113.3, 89.8, 84.0, 74.38, 74.36, 66.2, 55.3, 34.6, 25.8, 18.1, −4.5, −4.8; HRMS (TOF, ESI/APCI) calcd for C44H49N5O6SSiNa [MNa+] 826.3071, found 826.3062.
5´-Chloro-5´-deoxycytidine (19)
According to the procedure described by Kikugawa and Ichino,34 5´-chloro-5´-deoxycytidine (3.74 g, 69% yield) was prepared by the direct halogenation of cytidine (5.00 g, 20.6 mmol) with thionyl chloride (8.60 mL, 118 mmol) in HMPA (6 mL) at room temperature for 24 h. The product 19 was recrystallized from hot water and obtained as white needle crystals. 1H NMR (DMSO-d6) δ 7.59 (d, 1H, J = 7.6 Hz), 7.28 (brs, 1H), 7.18 (brs, 1H), 5.81 (d, 1H, J = 4.8 Hz), 5.76 (d, 1H, J = 7.6 Hz), 5.43 (d, 1H, J = 5.6 Hz), 5.31 (d, 1H, J = 5.2 Hz), 4.03 (m, 1H), 3.96 (m, 1H), 3.91 (m, 1H), 3.87 (m, 1H), 3.79 (dd, 1H, J = 11.6, 6.0 Hz); 13C NMR (DMSO-d6) δ 165.7, 155.5, 141.7, 94.7, 89.6, 82.5, 73.1, 70.9, 45.2.
5´-Tritylthio-5´-deoxycytidine (20)
To the solution of 5´-chloro-5´-deoxycytidine (19) (3.72 g, 15.3 mmol) and Ph3CSH (7.69 g, 27.8 mmol) in DMSO (50 mL), 1,1,3,3-tetramethylguanidine (1.94 g, 16.8 mmol) was added. After the reaction mixture was stirred at room temperature for 18 h, water (100 mL) was then added. The precipitated solid was collected by filtration and further purified by silica gel chromatography, eluting with 8% methanol in chloroform to give 20 as a white foam: 7.48 g (97% yield). 1H NMR (DMSO-d6) δ 7.45 (d, 1H, J = 7.5 Hz), 7.33 (m, 15H), 5.76 (d, 1H, J = 7.5 Hz), 5.67 (d, 1H, J = 4.5 Hz), 5.33 (d, 1H, J = 5.5 Hz), 5.07 (d, 1H, J = 5.5 Hz), 3.91 (m, 1H), 3.68–3.53 (m, 2H), 2.45 (dd, 1H, J = 4.8, 12.8 Hz), 2.45 (dd, 1H, J = 7.6, 12.8 Hz); 13C NMR (DMSO-d6) δ 165.5, 155.3, 144.4, 141.8, 129.2, 128.2, 127.0, 94.6, 90.0, 81.4, 73.0, 72.7, 66.3, 34.6; HRMS (TOF, ESI) calcd for C28H27N3O4SNa [MNa+] 524.1620, found 524.1615.
N-Phenoxyacetyl-5´-tritylthio-5´-deoxycytidine (21)
Under argon 5´-tritylthio-5´-deoxycytidine (20) (7.48 g, 14.9 mmol) was suspended in dry pyridine (90 mL). Chlorotrimethylsilane (15.2 mL) was added to the suspension at 0 ºC. After the mixture was stirred at room temperature for 45 min, a solution of phenoxyacetyl chloride (3.09 mL, 22.4 mmol) and 1,2,4-triazole (1.55 g, 22.4 mmol) in pyridine-acetonitrile (60 mL, 1:1) was slowly added. After the mixture was stirred at room temperature for 2.5 h, the reaction was quenched by addition of H2O (15 mL). After stirring for 5 min, concentrated aqueous NH4OH (6.7 mL) was added at 0 ºC, and the mixture was stirred for 30 min. The solution was concentrated to remove pyridine and the residue was redissolved into water (20 mL), extracted with dichloromethane (3 × 40 mL). The organic layers were combined and evaporated, the residue was then purified by silica gel chromatography, eluting with 5 % MeOH in CH2Cl2 to give 21 (6.68 g, 71% yield) as a yellow foam. 1H NMR (CDCl3/TMS) δ 9.35 (brs, 1H), 8.05 (d, 1H, J = 7.6 Hz), 7.54 (d, 1H, J = 7.6 Hz), 7.45–6.91 (m, 20H), 5.68 (d, 1H, J = 4.8 Hz), 4.65 (s, 2H), 4.20 (m, 1H), 4.00 (m, 1H), 3.86 (dd, 1H, J = 3.6, 5.2 Hz), 2.56 (dd, 1H, J = 4.8, 12.8 Hz), 2.33 (dd, 1H, J = 7.2, 12.8 Hz); 13C NMR (CDCl3) δ 168.6, 161.7, 156.69, 156.66, 144.6, 144.3, 130.0, 129.6, 128.2, 127.1, 122.7, 114.7, 96.9, 93.4, 84.6, 76.6, 73.7, 67.4, 67.2, 34.6; HRMS (TOF, ESI) calcd for C36H34N3O6S [MH+] 636.2168, found 636.2167.
2´-O-tert-Butyldimethylsilyl-N4-phenoxyacetyl-5´-tritylthio-5´-deoxycytidine (22a) and 3´-O-tert-Butyldimethylsilyl-N4-phenoxyacetyl-5´-tritylthio-5´-deoxycytidine (22b)
To the solution of 21 (1.00 g, 1.57 mmol) in dichloromethane (35 mL) at 0 ºC was added triethylamine (1.09 mL, 7.85 mmol), followed by the slow addition of TBSOTf (0.54 mL, 2.36 mmol). The mixture was warmed up and stirred at room temperature for 3 h. TLC showed reaction was not complete. To the mixture were added additional triethylamine (1.09 mL, 7.85 mmol) and TBSOTf (0.54 mL, 2.36 mmol), and the mixture was stirred at room temperature for 1.5 h. TLC showed the reaction was complete. The mixture was neutralized with 1 N HCl, washed with brine and dried over MgSO4. The solvent was removed, and the residue was isolated by silica gel chromatography, eluting with 25–50% ethyl acetate in hexane to give 2′-O-TBS isomer 22a (the up spot on TLC): 0.256 g (21% yield) and 3′-O-TBS isomer 22b (the low spot on TLC): 0.448 g (38% yield).
22a: 1H NMR (CDCl3/TMS) δ 9.54 (brs, 1H), 7.79 (d, 1H, J = 7.6 Hz), 7.50–6.80 (m, 21H), 5.64 (s, 1H), 4.65 (s, 2H), 4.15 (d, 1H, J = 4.8 Hz), 3.84 (m, 1H), 3.58 (m, 1H), 2.77 (dd, 1H, J = 3.6, 13.2 Hz), 2.49 (dd, 1H, J = 8.0, 13.2 Hz), 2.25 (d, 1H, J = 9.6 Hz), 0.91 (s, 9H), 0.23 (s, 3H), 0.12 (s, 3H); 13C NMR (CDCl3) δ 168.7, 161.7, 156.7, 154.8 144.6, 144.4, 129.9, 129.6, 128.1, 127.0, 122.6, 114.6, 96.3, 92.2, 82.3, 76.0, 72.8, 67.4, 67.2, 34.4, 25.9, 18.1, −4.3, −5.4; HRMS (TOF, ESI) calcd for C42H48N3O6SSi [MH+] 750.3033, found 750.3023.
22b: 1H NMR (CDCl3/TMS) δ 9.54 (brs, 1H), 7.97 (d, 1H, J = 7.6 Hz), 7.50–6.80 (m, 21H), 5.73 (d, 1H, J = 3.6 Hz), 4.64 (s, 2H), 4.05–3.95 (m, 2H), 3.88 (m, 1H), 3.42 (brs, 1H), 2.52 (dd, 1H, J = 4.4, 13.2 Hz), 2.39 (dd, 1H, J = 7.6, 12.8 Hz), 2.25 (d, 1H, J = 9.6 Hz), 0.83 (s, 9H), 0.001 (s, 3H), −0.05 (s, 3H); 13C NMR (CDCl3) δ 168.8, 161.8, 156.8, 155.6, 145.4, 144.4, 130.0, 129.6, 128.2, 127.1, 122.6, 114.7, 96.7, 93.1, 83.4, 75.7, 74.4, 67.5, 34.6, 25.9, 18.2, −4.6, −4.7; HRMS (TOF, ESI) calcd for C42H48N3O6SSi [MH+] 750.3033, found 750.3028.
5´-Chloro-5´-deoxyuridine (23)
According to the procedure described by Anisuzzaman and Whistler,36 5´-chloro-5´-deoxyuridine (1.30 g, 99% yield) was prepared by the 5´-halogenation of uridine (1.22 g, 5.00 mmol) with carbon tetrachloride (0.48 mL, 5.0 mmol) and triphenylphosphane (2.62 g, 10.0 mmol) in dry pyridine (50 mL) at room temperature for 16 h. The reaction was quenched with methanol (10 mL), the solvent was removed and the residue was isolated by silica gel chromatography, eluting with 5% methanol in dichloromethane to give product as a white foam. 1H NMR (CD3OD/TMS) 7.73 (d, 1H, J = 8.0 Hz), 5.90 (d, 1H, J = 4.8 Hz), 5.79 (d, 1H, J = 8.0 Hz), 4.26 (m, 1H), 4.24–4.15 (m, 2H), 3.93 (dd, 1H, J = 12.4, 3.6 Hz), 3.85 (dd, 1H, J = 12.4, 4.4 Hz); 13C NMR (CD3OD) δ 165.9, 152.2, 142.2, 103.0, 90.8, 84.1, 74.7, 71.8, 45.4.
5´-Tritylthio-5´-deoxyuridine (24)
To the solution of 5´-chloro-5´-deoxyuridine (23) (1.30 g, 4.95 mmol) and Ph3CSH (2.07 g, 7.50 mmol) in DMSO (15 mL), 1,1,3,3-tetramethylguanidine (633 mg, 5.50 mmol) was added. After the reaction mixture was stirred at room temperature overnight, water (30 mL) was added. The precipitated solid was collected by filtration and further purified by silica gel chromatography, eluting with 5% methanol in chloroform to give 24: 2.47 g (99% yield) as a white foam. 1H NMR (CDCl3/TMS) δ 10.65 (brs, 1H), 7.40 (d, 6H, J = 7.6 Hz), 7.34 (d, 1H, J = 8.0 Hz), 7.26 (t, 6H, J = 7.6 Hz), 7.18 (t, 3H, J = 7.2 Hz), 5.77 (d, 1H, J = 4.4 Hz), 5.65 (d, 1H, J = 8.0 Hz), 4.07 (m, 1H), 3.93 (m, 1H), 3.85 (m, 1H), 2.62 (dd, 1H, J = 4.0, 12.4 Hz), 2.42 (dd, 1H, J = 7.2, 12.8 Hz); 13C NMR (CDCl3) δ 163.5, 150.5, 144.0, 139.9, 129.1, 127.7, 126.5, 102.1, 89.6, 82.0, 73.7, 72.1, 66.5, 34.4; HRMS (TOF, ESI) calcd for C28H26N2O5SNa [MNa+] 525.1460, found 525.1455.
2´-O-tert-Butyldimethylsilyl-5´-tritylthio-5´-deoxyuridine (25a) and 3´-O-tert-Butyldimethylsilyl −5´-tritylthio-5´-deoxyuridine (25b)
To the solution of 24 (503 mg, 1.00 mmol) in dry pyridine (2 mL) at room temperature, TBSCl (453 mg, 3.0 mmol) was added. The mixture was stirred at room temperature for 72 h. The solvent was removed, the residue was dissolved into dichloromethane, washed with saturated aqueous sodium bicarbonate, brine and dried over MgSO4. The solution was removed, and the residue was isolated by silica gel chromatography, eluting with 25% ethyl acetate in hexane to give 2´-O-TBS isomer: 25a as a white foam (the up spot on TLC): 0.385 g (62% yield) and 3´-O-TBS isomer: 25b (the low spot on TLC): 0.135 g (21% yield).
25a: 1H NMR (CDCl3/TMS) δ 9.99 (brs, 1H), 7.46 (m, 6H), 7.36 (d, 1H, J = 8.0 Hz), 7.30 (m, 6H), 7.23 (m, 3H), 5.76 (d, 1H, J = 7.2 Hz), 5.71 (d, J = 3.2 Hz), 4.17 (dd, 1H, J = 3.6, 5.2 Hz), 3.81 (dd, 1H, J = 6.4, 10.4 Hz), 3.70 (t, 1H, J = 5.6 Hz), 2.72 (dd, 1H, J = 4.4, 13.2 Hz), 2.51 (dd, 1H, J = 7.0, 13.2 Hz), 0.91 (s, 9H), 0.14 (s, 3H), 0.10 (s, 3H); 13C NMR (CDCl3) δ 163.7, 150.2, 144.3, 140.0, 129.6, 128.0, 126.9, 102.7, 90.2, 82.4, 75.3, 72.6, 67.1, 34.4, 25.7, 18.0, −4.7, −5.2; HRMS (TOF, ESI) calcd for C34H40N2O5SSiNa [MNa+] 639.2325, found 639.2329.
25b: 1H NMR (CDCl3/TMS) δ 9.85 (brs, 1H), 7.45–7.35 (m, 7H), 7.29 (m, 6H), 7.25 (m, 3H), 5.75 (d, 1H, J = 8.0 Hz), 5.67 (d, 1H, J = 4.4 Hz), 4.10 (m, 1H), 4.00 (m, 1H), 3.94 (m, 1H), 2.54 (dd, 1H, J = 4.0, 12.8 Hz), 2.44 (dd, 1H, J = 6.8, 12.8 Hz), 0.87 (s, 9H), 0.04 (s, 3H), −0.03 (s, 3H); 13C NMR (CDCl3) δ 163.6, 150.5, 144.3, 141.1, 129.5, 128.1, 127.0, 102.7, 91.6, 83.0, 74.1, 73.8, 67.0, 34.6, 25.8, 18.0, −4.6, −4.8; HRMS (TOF, ESI) calcd for C34H40N2O5SSiNa [MNa+] 639.2325, found 639.2322.
3´-O-tert-Butyldimethylsilyl-5´-deoxy-N2-[(dimethylamino)methylene]-5´-tritylthioguanosine 3´-N,N-Diisopropyl(cyanoethyl)phosphoramidite (26a)
To the solution of 5a (0.298 g, 0.42 mmol) and i-Pr2NEt (365 μL, 2.10 mmol) in anhydrous dichloromethane (15 mL) at 0 ºC, ClP(NPr-i2)OCH2CH2CN (187 μL, 0.84 mmol) was added, followed by the addition of 1-methylimidazole (18 μL, 0.22 mmol). After the reaction mixture was stirred at room temperature for 1 h, the reaction was quenched with methanol (1.0 mL). The solvent was removed, the residue was purified by silica gel chromatography, eluting with 2% CH3COCH3 in CH2Cl2 containing 0.5% Et3N to give product 26a as white foam: 0.372 g (97% yield, >95% purity). 1H NMR (CD3CN) δ 9.80 (brs, 1H), 8.53 (s, 0.5H), 8.52 (s, 0.5H), 7.67 (s, 1H), 7.45–7.20 (m, 15H), 5.78 (m, 1H), 4.75 (m, 1H), 4.40–3.20 (m, 6H), 3.04 (m, 6H), 2.80–2.40 (m, 4H), 0.78 (m, 9H), −0.01−-0.20 (m, 6H); 31P NMR (CD3CN) δ 152.3, 151.6; HRMS (TOF, ESI/APCI) calcd for C47H64N8O5PSSi [MH+] 911.4227, found 911.4206.
3´-O-tert-Butyldimethylsilyl-5´-deoxy-N2-[(dimethylamino)methylene]-5´-dimethoxytritylthioguanosine 3´-N,N-Diisopropyl(cyanoethyl)phosphoramidite (26b)
To the solution of 6a (50 mg, 0.065 mmol) and i-Pr2NEt (87 μL, 0.50 mmol) in anhydrous dichloromethane (5 mL) at 0 ºC, ClP(NPr-i2)OCH2CH2CN (45 μL, 0.20 mmol) was added, followed by the addition of 1-methylimidazole (2.6 μL, 0.03 mmol). After the reaction mixture was stirred at room temperature for 1 h, the reaction was quenched with methanol (1.0 mL). The solvent was removed, the residue was purified by silica gel chromatography, eluting with 2% CH3COCH3 in CH2Cl2 containing 0.5% Et3N to give product 26b as white foam: 55 mg (87% yield, >95% purity). 1H NMR (CD3CN) δ 9.20 (brs, 1H), 8.52 (s, 0.6H), 8.51 (s, 0.4H), 7.65 (s, 1H), 7.35–7.20 (m, 9H), 6.83 (m, 4H), 5.78 (m, 1H), 4.75 (m, 1H), 4.40–3.20 (m, 6H), 3.76 (s, 6H), 3.04 (m, 6H), 2.80–2.40 (m, 4H), 0.79 (m, 9H), −0.01−-0.20 (m, 6H); 31P NMR (CD3CN) δ 150.1, 149.3; HRMS (TOF, ESI/APCI) calcd for C49H68N8O7PSSi [MH+] 971.4439, found 971.4442.
3´-O-tert-Butyldimethylsilyl-5´-tert-butyldisulfanyl-5´-deoxy-N2-[(dimethylamino)methylene]guanosine 3´-N,N-Diisopropyl(cyanoethyl)phosphoramidite (26c)
To the solution of 12a (84 mg, 0.15 mmol) and i-Pr2NEt (196 μL, 1.13 mmol) in anhydrous dichloromethane (5 mL) at 0 ºC, ClP(NPr-i2)OCH2CH2CN (100 μL, 0.45 mmol) was added, followed by the addition of 1-methylimidazole (5.6 μL, 0.07 mmol). After the reaction mixture was stirred at room temperature for 1 h, the reaction was quenched with methanol (1.0 mL). The solvent was removed, the residue was purified by silica gel chromatography, eluting with 1% CH3OH in CH2Cl2 containing 0.5% Et3N to give product 26c:100 mg (87% yield, 95% purity) as a white foam. 1H NMR (CD3CN) δ 9.83 (brs, 1H), 8.57 (s, 1H), 7.76 (s, 1H), 5.85 (m, 1H), 4.92 (m, 1H), 4.60–3.20 (m, 6H), 3.18–3.07 (m, 6H), 2.80–2.60 (m, 4H), 1.34 (s, 4.9H), 1.32 (s, 5.1H), 0.78 (m, 9H), −0.01−-0.20 (m, 6H); 31P NMR (CD3CN) δ 150.6, 149.4; HRMS (TOF, ESI/APCI) calcd for C32H58N8O5PS2Si [MH+] 757.3473, found 757.3479.
N6-Benzoyl-2´-O-tert-butyldimethylsilyl-5´-deoxy-5´-tritylthioadenosine 3´-N,N-Diisopropyl(cyanoethyl)phosphoramidite (26d)
To the solution of 17a (0.142 g, 0.19 mmol) and i-Pr2NEt (330 μL, 1.90 mmol) in anhydrous dichloromethane (10 mL) at 0 ºC, ClP(NPr-i2)OCH2CH2CN (170 μL, 0.76 mmol) was added, followed by the addition of 1-methylimidazole (8.0 μL, 0.10 mmol). The reaction mixture was stirred at room temperature for 1 h. TLC showed the reaction was complete. The reaction was quenched with methanol (1.5 mL). The solvent was removed, the residue was purified by silica gel chromatography, eluting with 2% CH3COCH3 in CH2Cl2 containing 0.5% Et3N to give 26d as white foam: 0.174 g (97% yield, >95% purity). 1H NMR (CD3CN) δ 9.50 (brs, 1H), 8.54 (s, 0.4H), 8.53 (s, 0.6H), 8.22 (s, 1H), 8.00 (d, 2H, J = 7.5Hz), 7.70–7.20 (m, 18H), 5.92 (m, 1H), 5.11–4.90 (m, 1H), 4.40–3.20 (m, 6H), 2.85–2.40 (m, 4H), 0.73 (m, 9H), −0.04−-0.25 (m, 6H); 31P NMR (CD3CN) δ 152.8, 151.4; HRMS (TOF, ESI/APCI) calcd for C51H63N7O5PSSi [MH+] 944.4118, found 944.4119.
N6-Benzoyl-2´-O-tert-butyldimethylsilyl-5´-deoxy-5´-dimethoxytritylthioadenosine 3´-N,N-Diisopropyl(cyanoethyl)phosphoramidite (26e)
To the solution of 18a (0.100 g, 0.124 mmol) and i-Pr2NEt (109 μL, 0.625 mmol) in anhydrous dichloromethane (10 mL) at 0 ºC, ClP(NPr-i2)OCH2CH2CN (55 μL, 0.25 mmol) was added, followed by the addition of 1-methylimidazole (5.3 μL, 0.067 mmol). The reaction mixture was stirred at room temperature for 1 h. TLC showed the reaction was complete. The reaction was quenched with methanol (1.0 mL). The solvent was removed, the residue was purified by silica gel chromatography, eluting with 2% CH3COCH3 in CH2Cl2 containing 0.5% Et3N to give 26e as white foam: 81 mg (65% yield, >95% purity). δ 9.30 (brs, 1H), 8.55 (m, 1H), 8.22 (s, 1H), 8.00 (d, 2H, J = 7.5Hz), 7.70–7.20 (m, 12H), 6.82 (m, 4H), 5.92 (m, 1H), 5.11–4.95 (m, 1H), 4.40–3.20 (m, 6H), 3.75 (s, 6H), 2.85–2.45 (m, 4H), 0.77 (m, 9H), −0.04−-0.20 (m, 6H); 31P NMR (CD3CN) δ 153.5, 152.1; HRMS (TOF, ESI/APCI) calcd for C53H67N7O7PSSi [MH+] 1004.4330, found 1004.4323.
N4-Phenoxyacetyl-2´-O-tert-butyldimethylsilyl-5´-deoxy-5´-tritylthiocytidine 3´-N,N-Diisopropyl(cyanoethyl)phosphoramidite (26f)
To the solution of 22a (317 mg, 0.42 mmol) and i-Pr2NEt (365 μL, 2.1 mmol) in anhydrous dichloromethane (10 mL) at 0 ºC, ClP(NPr-i2)OCH2CH2CN (187 μL, 0.84 mmol) was added, followed by the addition of 1-methylimidazole (18 μL, 0.22 mmol). The reaction mixture was stirred at room temperature for 1 h. TLC showed the reaction was complete. The reaction was quenched with methanol (1.0 mL). The solvent was removed, the residue was purified by silica gel chromatography, eluting with 2% acetone in CH2Cl2 containing 0.2% Et3N to give 26f as white foam: 340 mg (85% yield, >95% purity). δ 10.21 (brs, 1H), 7.81 (m, 1H), 7.50–6.90 (m, 21H), 5.61 (m, 1H), 4.81 (s, 2H), 4.40–4.25 (m, 1H), 4.20–4.00 (m, 1H), 3.80–3.40 (m, 5H), 2.85–2.30 (m, 4H), 1.15–0.98 (m, 12H), 0.91 (m, 9H), 0.16–0.05 (m, 6H); 31P NMR (CD3CN) δ 150.4, 149.0; HRMS (TOF, APCI) calcd for C51H65N5O7PSSi [MH+] 950.4112, found 950.4109
2´-O-tert-Butyldimethylsilyl-5´-deoxy-5´-tritylthiouridine 3´-N,N-Diisopropyl(cyanoethyl)phosphoramidite (26g)
To the solution of 25a (155 mg, 0.25 mmol) and i-Pr2NEt (218 μL, 1.25 mmol) in anhydrous dichloromethane (5.0 mL) at 0 ºC, ClP(NPr-i2)OCH2CH2CN (112 μL, 0.50 mmol) was added, followed by the addition of 1-methylimidazole (10.0 μL, 0.125 mmol). The reaction mixture was stirred at room temperature for 1 h. TLC showed the reaction was complete. The reaction was quenched with methanol (1.0 mL). The solvent was removed, the residue was purified by silica gel chromatography, eluting with 4% acetone in CH2Cl2 containing 0.5% Et3N to give 26g as white foam: 190 mg (93% yield, >95% purity). δ 7.50–7.20 (m, 18H), 5.80–5.60 (m, 2H), 4.40–3.40 (m, 7H), 2.85–2.30 (m, 4H), 1.30–0.98 (m, 12H), 0.89 (m, 9H), 0.15–0.06 (m, 6H); 31P NMR (CD3CN) δ 149.1, 148.7; HRMS (TOF, APCI) calcd for C43H58N4O6PSSi [MH+] 817.3584, found 817.3583.
Synthesis of Olig 5´-UUC2’-o-NBnG5’-SGGUCGGC-3´ (28) and 5´-GCCGUC2’-o-NBnC5’-SCCCG-3´ (28a)
The standard RNA 1 μmol protocol was modified for the double coupling to phosphoramidite 26a (75 mg in 1.0 mL CH3CN). After coupling to 26a, oxidation and capping, the column was removed from instrument, washed with water, and treated with aqueous AgNO3 (50 mM, 3 mL) for 60 min in dark. The column was rinsed thoroughly with water, treated with DTT (50 mM, 3 mL) for 15 min. The column was washed with acetonitrile, dried over vacuum desiccator for 15 min. After double coupling to phosphoramidite 27 (75 mg in 0.75 mL CH3CN), the synthesis was continued to finish the rest of synthesis. After the solid support was treated with concentrated ammonium hydroxide/ethanol (3:1, v/v) at 55 ºC for 2–4 h and desilylation with triethylamine trihydrofluoride at 65 ºC for 25 min, dPAGE gel purification gave desired RNA 28 (~20 nmol). MALDI-TOF MS calcd: 3649.0, found: 3648.7. Similarly oligo 5´-GCCGUC2’-o-NBnC5’-SCCCG-3´ (28a) (~30 nmol) could be prepared from phosphoramdites 26f and 27. MALDI-TOF MS calcd: 3568.1, found: 3568.2.
Synthesis of 5′-HS-GG dinucleotide (30)
After standard detritylation of i-Pr-Pac-G-RNA-CPG column with 3% TCA in dichloromethane, it was manually coupled to 26C (33 mg in 0.30 mL dry acetonitrile) with activator (0.50 mL, 0.45 M tetrazole in acetonitrile) at room temperature for 20 min, then manually oxidized with 10% tert-BuOOH in decane/acetonitrile (1:5, v/v) (0.5 mL) for 1 min. Deprotection was achieved by treatment of the solid support with concentrated ammonium hydroxide/ethanol (3:1, v/v) at 55 ºC for 2 h, followed by desilylation with triethylamine trihydrofluoride at 65 ºC for 25 min.43 The solvent was removed, the residue was extracted with water, rinsed with chloroform (3 × 0.3 mL). The crude 5´-t-BuS-SGG dinuleotide (29) was purified by Sep-Pack C18 column and further purified by RP HPLC. MALDI-TOF MS calcd for M+: 733.16, Found: 733.23; Treatment of 5´-t-ButylSS-GG dinuleotide with DTT in the presence of triethylamine yielded the corresponding 5´-HS-GG (30). MALDI-TOF MS calcd for M+: 645.12; Found: 645.44.
Supplementary Material
Acknowledgement
We thank Mr. Saurja Dasgupta and Dr. Sandip A. Shelke for helpful discussions and critical comments on the manuscript. This work was supported by an N.I.H. grant to J.A.P. (1R01AI081987).
Footnotes
Supporting Information
1H NMR and 31P NMR of phosphoramidites 26a −26g, MALDI-TOF MS of 28, 28a, 29 and 30, 1H NMR and 13C NMR spectra of all other new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Gaynor JW; Cosstick R Curr. Org. Chem 2008, 12, 291–308. [Google Scholar]
- (2).Li N-S; Frederiksen JK; Piccirilli JA Acc Chem Res 2011, 44, 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jahn-Hofmann K; Engels JW Helv. Chim. Acta 2004, 87, 2812–2828. [Google Scholar]
- (4).Mag M; Lüking S; Engels JW Nucleic Acids Res 1991, 19, 1437–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Burgin AB; Huizenga BN; Nash HA Nucleic Acids Res 1995, 23, 2973–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Burgin AB; Nash HA Curr Biol 1995, 5, 1312–1321. [DOI] [PubMed] [Google Scholar]
- (7).Luetke KH; Zhao BP; Sadowski PD Nucleic Acids Res 1997, 25, 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ghosh K; Lau C-K; Gupta K; Van Duyne GD Nat Chem Biol 2005, 1, 275–282. [DOI] [PubMed] [Google Scholar]
- (9).Interthal H; Quigley PM; Hol WGJ; Champoux JJ J Biol Chem 2004, 279, 2984–2992. [DOI] [PubMed] [Google Scholar]
- (10).Deneke J; Burgin AB; Wilson SL; Chaconas G J Biol Chem 2004, 279, 53699–53706. [DOI] [PubMed] [Google Scholar]
- (11).Krogh BO; Shuman S Mol Cell 2000, 5, 1035–1041. [DOI] [PubMed] [Google Scholar]
- (12).Krogh BO; Cheng C; Burgin A; Shuman S Virology 1999, 264, 441–451. [DOI] [PubMed] [Google Scholar]
- (13).Hwang Y; Park M; Fischer WH; Burgin A; Bushman F Virology 1999, 262, 479–491. [DOI] [PubMed] [Google Scholar]
- (14).Hwang Y; Burgin A; Bushman F J Biol Chem 1999, 274, 9160–9168. [DOI] [PubMed] [Google Scholar]
- (15).Smietana M; Kool ET Angew Chem Int Ed Engl 2002, 41, 3704–3707. [DOI] [PubMed] [Google Scholar]
- (16).Smietana M; Johnson RB; Wang QM; Kool ET Chem. Eur. J 2004, 10, 173–181. [DOI] [PubMed] [Google Scholar]
- (17).Herrlein MK; Letsinger RL Angew. Chem. Int. Ed 1997, 36, 599–601. [Google Scholar]
- (18).Herrlein MK; Nelson JS; Letsinger RL J. Am. Chem. Soc 1995, 117, 10151–10152. [Google Scholar]
- (19).Miller GP; Kool ET Org Lett 2002, 4, 3599–3601. [DOI] [PubMed] [Google Scholar]
- (20).Xu Y; Kool ET Tetrahedron Lett 1997, 38, 5595–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sturm MB; Roday S; Schramm VL J Am Chem Soc 2007, 129, 5544–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kuimelis RG; McLaughlin LW Bioorg Med Chem 1997, 5, 1051–1061. [DOI] [PubMed] [Google Scholar]
- (23).Kuimelis RG; McLaughlin LW Biochemistry 1996, 35, 5308–5317. [DOI] [PubMed] [Google Scholar]
- (24).Kuimelis RG; Mclaughlin LW J. Am. Chem. Soc 1995, 117, 11019–11020. [Google Scholar]
- (25).Zhou DM; Usman N; Wincott FE; Matulic-Adamic J; Orita M; Zhang LH; Komiyama M; Kumar PKR; Taira KJ Am. Chem. Soc 1996, 118, 5862–5866. [Google Scholar]
- (26).Thomas JM; Perrin DM J Am Chem Soc 2009, 131, 1135–1143. [DOI] [PubMed] [Google Scholar]
- (27).Das SR; Piccirilli JA Nat Chem Biol 2005, 1, 45–52. [DOI] [PubMed] [Google Scholar]
- (28).Li N-S; Frederiksen JK; Koo SC; Lu J; Wilson TJ; Lilley DMJ; Piccirilli JA Nucleic Acids Res 2011, 39, e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kath-Schorr S; Wilson TJ; Li N-S; Lu J; Piccirilli JA; Lilley DMJ J Am Chem Soc 2012, 134, 16717–16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wilson TJ; Li N-S; Lu J; Frederiksen JK; Piccirilli JA; Lilley DMJ Proc Natl Acad Sci U S A 2010, 107, 11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Zhang B; Cui Z; Sun L Org Lett 2001, 3, 275–278. [DOI] [PubMed] [Google Scholar]
- (32).Kim IH; Shin S; Jeong YJ; Hah SS Tetrahedron Lett. 2010, 51, 3446–3448. [Google Scholar]
- (33).Pignot M; Pljevaljcic G; Weinhold E Eur. J. Org. Chem 2000, 549–555. [Google Scholar]
- (34).Kikugawa K; Ichino M Tetrahedron Lett 1971, 87–90. [DOI] [PubMed] [Google Scholar]
- (35).Singh KK; Nahar P Synth. Commun 1995, 25, 1997–2003. [Google Scholar]
- (36).Kashem A; Anisuzzaman M; Whistler RL Carbohydr Res 1978, 61, 511–518. [Google Scholar]
- (37).Lu J; Koo SC; Li N-S; Piccirilli JA Nucleosides Nucleotides Nucleic Acids 2015, 34, 114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Lee J-S; Green JJ; Love KT; Sunshine J; Langer R; Anderson DG Nano Lett 2009, 9, 2402–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Vázquez-Dorbatt V; Tolstyka ZP; Chang C-W; Maynard HD Biomacromolecules 2009, 10, 2207–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Giljohann DA; Seferos DS; Prigodich AE; Patel PC; Mirkin CA J Am Chem Soc 2009, 131, 2072–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Heredia KL; Nguyen TH; Chang C-W; Bulmus V; Davis TP; Maynard HD Chem Commun (Camb) 2008, 3245–3247. [DOI] [PubMed] [Google Scholar]
- (42).York AW; Huang F; McCormick CL Biomacromolecules 2010, 11, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wincott F; DiRenzo A; Shaffer C; Grimm S; Tracz D; Workman C; Sweedler D; Gonzalez C; Scaringe S; Usman N Nucleic Acids Res 1995, 23, 2677–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.