INTRODUCTION
Sleep is central to a healthy childhood, and sleep-disordered breathing (SDB)—the disruption of normal respiratory patterns and ventilation during sleep—is implicated in several behavioral and physical health issues. Although we now have strong evidence that chronic, unchecked obstructive sleep apnea (OSA) can lead to hypertension, cardiovascular disease, metabolic disorders, obesity, and neuropsychiatric and developmental issues, the full scope of the effect of SDB on health remains underappreciated by many clinicians (Table 1).
Table 1: Disease States Associated with or Complicated by Chronic Sleep-Disordered Breathing.
| System | Disease State |
|---|---|
| Cardiovascular | Hypertension |
| Pulmonary hypertension, cor pulmonale | |
| Pulmonary | Bronchopulmonary dysplasia |
| Hematologic | Sickle cell disease |
| Nutrition & metabolic | Obesity |
| Insulin resistance | |
| Failure to thrive (malnutrition) | |
| Neuropsychiatric | Attention-deficit/hyperactivity disorder |
| Major depressive disorder | |
| Developmental delay |
Sleep is central to a healthy childhood, and sleep-disordered breathing (SDB)—the disruption of normal respiratory patterns and ventilation during sleep—is implicated in several behavioral and physical health issues. Although we now have strong evidence that chronic, unchecked obstructive sleep apnea (OSA) can lead to hypertension, cardiovascular disease, metabolic disorders, obesity, and neuropsychiatric and developmental issues, the full scope of the effect of SDB on health remains underappreciated by many clinicians (Table 1).
Sleep research has rapidly expanded and evolved in the past few decades. Of recent note, chronobiologists Hall, Rosbash, and Young received the 2017 Nobel prize in medicine for their work on the cell’s internal clock. While the biological role of sleep remains mysterious, as famed researcher Allan Rechtschaffen once remarked, “If sleep does not serve an absolutely vital function, then it is the biggest mistake the evolutionary process has ever made.” Intriguing new research has begun to suggest concrete mechanisms by which sleep maintains health, including the diurnal clearance of toxic central nervous system metabolites. (1) In another striking example of the central role of sleep on health, recent research suggests that telomere length, a marker of a chromosome’s viability and a proxy for aging, is significantly shortened in children with chronically insufficient sleep. (2)
Sleep-disordered breathing is common, with the American Academy of Pediatrics (AAP) Subcommittee on Pediatric Sleep estimating that 1.2% to 5.7% of children are affected by OSA alone. (3) It is generally acknowledged that this may be an underestimate of the true prevalence, and it is widely believed among sleep experts that the incidence of OSA in the pediatric and adolescent populations is increasing in the United States as a consequence of the childhood obesity epidemic. Affected children are at risk for wide-ranging direct health consequences from sequelae of OSA, including the impact of insufficient sleep on the development or exacerbation of chronic disease. Beyond the physical and mental health effects of SDB on the child, disrupted or disordered sleep can be a major psychosocial stressor for children and their families. (4) As such, effective screening for SDB in the general pediatric and adolescent medicine clinics can have a significant effect on the health and well-being of children and their families. The general pediatrician should feel empowered to identify SDB in their patients (Table 2).
Table 2: American Academy of Pediatrics 2012 Guideline Recommendations for the Diagnosis and Treatment of OSA in Children and Adolescents 3.
| 1. All children/adolescents should be screened for snoring. |
| 2. Polysomnography should be performed in children/adolescents with snoring and symptoms/signs of OSA; if polysomnography is not available, then alternative diagnostic tests or referral to a specialist for more extensive evaluation may be considered. |
| 3. Adenotonsillectomy is recommended as the first-line treatment of patients with adenotonsillar hypertrophy. |
| 4. High-risk patients should be monitored as inpatients postoperatively. |
| 5. Patients should be reevaluated postoperatively to determine whether further treatment is required. Objective testing should be performed in patients who are high risk or have persistent symptoms/signs of OSA after therapy. |
| 6. Continuous positive airway pressure is recommended as treatment if adenotonsillectomy is not performed or if OSA persists postoperatively. |
| 7. Weight loss is recommended in addition to other therapy in patients who are overweight or obese. |
| 8. Intranasal corticosteroids are an option for children with mild OSA in whom adenotonsillectomy is contraindicated or for mild postoperative OSA. |
OSA = obstructive sleep apnea
OBSTRUCTIVE SLEEP APNEA
Introduction
A variety of physiologic changes occur during the normal transition to sleep, including relaxation of upper airway tone and a decrease in respiratory tidal volumes. (5) However, in patients with OSA, airflow is severely reduced or even entirely blocked during sleep by an abnormality of the upper airway anatomy, most commonly adenotonsillar hypertrophy. Obstructive sleep apnea in the pediatric population is defined as either frank obstruction or evidence of hypopnea or obstructive hypoventilation on a sleep study, occurring in the context of a history of snoring, labored breathing during sleep, daytime sleepiness, or learning and behavioral issues (Tables 3 and 4). As a consequence of these obstructive events and subsequent arousals, children and adolescents with OSA may have reduced sleep quality, resulting in behavioral issues, poor school performance, or daytime sleepiness.
Table 3: Diagnostic Criteria of Pediatric Obstructive Sleep Apnea.
| A. The presence of ≥1 of the following: | 1. Snoring |
| 2. Labored, paradoxical, or obstructed breathing during the child’s sleep | |
| 3. Sleepiness, hyperactivity, behavioral problems, or learning problems | |
| B. Polysomnography demonstrates 1 or both of the following: | 1. One or more obstructive apneas, mixed apneas, or hypopneas per hour of sleep, OR |
| 2. A pattern of obstructive hypoventilation, defined as ≥25% of total sleep time with hypercapnia (PaCO2 > 50 mmHg) in association with ≥1 of the following: snoring, flattening of the inspiratory pressure waveform, or paradoxical thoracoabdominal motion |
Criteria A and B must be met
Reprinted with permission from The International Classification of Sleep Disorders, 3rd Ed.
Table 4: The spectrum of Pediatric Obstructive Sleep-Disordered Breathing.
| Primary snoring | Habitual snoring > 3 nights/week without apneas, hypopneas, frequent arousals, or gas exchange abnormalities (prevalence, 7.45%) |
| Upper airway resistance syndrome | Snoring, increased work of breathing, and frequent arousals without recognizable obstructive events or gas-exchange abnormalities |
| Obstructive hypoventilation | Snoring plus elevated end-expiratory carbon dioxide partial pressure in the absence of recognizable obstructive events |
| OSA syndrome | Recurrent events of partial complete upper airway obstruction (hypopneas, obstructive or mixed apneas) with disruption of normal oxygenation, ventilation, and sleep patterns (prevalence, 1% to 5%) |
OSA = obstructive sleep apnea
Courtesy of Catherine Kier, MD, FAAP, FCCP, DABSM, AE-C of Stony Brook Children’s Hospital
An understanding of the adverse health effects of and therapies for the management of OSA have greatly evolved since Guilleminault, Eldridge, and Dement first described the pediatric OSA syndrome in the early 1970s. In this early report, 2 of 8 patients in the case series had marked OSA with severe comorbid hypertension, which resolved after tracheostomy. (6)(7)(8) Fortunately, in current practice, most cases of even extreme OSA are amenable to less invasive forms of therapy. There is now ample evidence of both adverse cardiac and autonomic effects of chronic OSA, including correlations with systolic and diastolic hypertension, tachycardia, and increased heart rate variability. (3) A variety of psychological and neuropsychiatric studies have clearly linked OSA to learning and attention deficits and behavioral issues. This is in contrast to adults with OSA, where the main symptom is daytime sleepiness. Happily, emerging evidence suggests that some of these impairments may be mitigated by effective OSA treatment. (3)
Today, in conjunction with adenotonsillectomy (AT) and other interventions, a variety of sophisticated devices for the delivery of positive airway pressure (PAP) permit effective management of OSA. However, significant discrepancies in the delivery of care to these patients remain, and there is evidence that OSA may disproportionately affect children from socioeconomically disadvantaged backgrounds, even after correcting for previously established risk factors such as obesity and prematurity. (9)
Clinical Features of OSA in Pediatric Patients
History.
A focused sleep history, guided by an understanding of the most common sleep disorders of childhood, can be a quick and high-yield addition to the pediatrics clinic visit. Generally, such a history will start with general questions regarding the parents’ or child’s perception of their sleep quality, followed by more specific questions regarding the nature of any reported sleep disturbance.
Parents will commonly not mention concerns about their child’s sleep issues if not asked directly. When prompted, parents will often report snoring in their children but will frequently qualify this observation as occurring exclusively when their child is congested or with an upper respiratory tract infection. Stertor (heavy inspiratory snoring) with nasal congestion is quite common in practice and, in and of itself, does not mandate a sleep study. However, further probing questions should be asked to ascertain whether the child has any other signs of sleep disturbance, particularly signs of respiratory difficulty or apnea during sleep, and daytime concerns about school performance, behavior, focus, and sleepiness. Patients with snore concerning for OSA will generally have symptoms of audible snore 3 or more nights a week outside of the context of upper respiratory tract infections.
When parents are able to describe the concerning nocturnal breathing patterns in detail, they may describe snoring punctuated by episodes of frank obstruction or pauses in the child’s breathing. Older children and adolescents may describe waking from sleep due to cough or with a panicked, choking sensation. Parents may observe visibly labored breathing accompanying pronounced snoring, including stertor, gasps, retractions, or apnea. These witnessed overt apneas are often described as “choking.” However, in many children, OSA manifests as hypopneas or obstructive hypo-ventilation, which may not be obvious to parents. Indeed, hypopneas are quite characteristic of pediatric SDB, which is in distinct contrast to adults with OSA, who often demonstrate frank obstruction.
There may be other subtle signs of symptomatic OSA. Children may habitually sleep in positions that minimize obstruction, including with several pillows, or perhaps prone or with their neck hyperextended. (3) Older children with clinically significant OSA may present with daytime sleepiness as a core concern. The Epworth Sleepiness Scale for Children and Adolescents is a helpful quantitative and trackable assessment of daytime sleepiness. (10)(11) In addition to daytime sleepiness, the clinician should also screen for behavioral and learning issues, particularly regarding classroom performance. Children with marked nocturnal hypoventilation may report morning headache, which is related to carbon dioxide (CO2) retention. Nocturnal secondary enuresis, defined as nighttime incontinence, which emerges after at least 6 months of continence, is also suggestive of SDB. (12)
A child’s medical history may also inform the pediatrician’s focused sleep assessment. Obstructive sleep apnea is prevalent in children with a history of prematurity. Children with chronic lung diseases, including bronchopulmonary dysplasia, are more likely to have comorbid SDB. (13) Children with neuromuscular diseases are intrinsically at risk for nocturnal hypoventilation and decreased airway tone. (5) (14) Patients with syndromes associated with macroglossia (eg, trisomy 21 and Beckwith-Wiedemann syndrome), micrognathia (eg, Pierre-Robin sequence), or palatal abnormalities may be anatomically predisposed to upper airway obstruction. (15)(16) In children with severe gastroesophageal reflux or aerodigestive issues, reflux and subclinical aspiration during sleep may mimic the “choking” some-times observed with obstructive events and may contribute to lung disease and nocturnal hypoxemia. Patients with a history of sickle cell disease are at increased risk for disordered nocturnal breathing and are uniquely susceptible to nighttime oxygen desaturations attendant to severe OSA. (17) Any pediatric sleep history also should include screening for safe sleep practices, particularly for infants.
Physical Examination.
The physical examination of the patient with suspected OSA is most effective when informed by an understanding of the myriad ways in which subtle abnormalities of a child’s dentition, mandible, tongue, adenoid tissue and tonsils, and hard and soft palate interplay to contribute to an upper airway obstruction. When asleep, the muscles suspending the soft palate relax, as does the tongue. When the patient sleeps supine, these tissues may largely obstruct the upper airway. Ideally, the oropharyngeal examination should note both the Mallampati classification and the tonsillar size, as both are associated with severity of OSA (Fig 1). (18) The presence of tonsillar hypertrophy should be assessed by using a standard Brodsky scale from 0 to 4, wherein a score of 0 indicates the surgically induced absence of all tonsillar tissue and 4 indicates extension of the tonsils to the midline (so-called kissing tonsils). A high-arched palate may distort the architecture of the associated oropharynx and cause reduced nasal airflow. (5) Retrognathia or micrognathia reduces the space wherein otherwise normally sized tongue and soft tissues rest in the oropharynx. Malocclusion of the teeth may act in a similar way, forcing the jaw posteriorly and functionally reducing space in the mouth. The nasal passages should be assessed for polyps or allergic rhinitis, which can exacerbate chronic nasal congestion. The adenoidal tissue, a midline aggregation of lymphoid tissue in the upper oropharynx, is generally not observable by standard clinical examination and is best assessed via flexible nasopharyngoscopy.
Figure 1: Mallampati score and Friedman tonsil scale.
The Mallampati score, while principally intended to help estimate the relative difficulty of intubation in pre-operative patients, is also useful in assessing the contribution of the upper airway anatomy on obstructive sleep breathing. The Friedman tonsil scale is useful for describing the size of tonsils in children, with a score of 0 representing surgically removed tonsils, and a size of 4 describing large tonsils that meet at the midline. Image courtesy of Namrita Jain, MD.
Truncal obesity, redundant neck tissue, and other signs of metabolic syndrome or insulin resistance, such as acanthosis nigricans, suggest obesity-related OSA and comorbid metabolic disease. (19) Conversely, in very young infants OSA is often associated with failure to thrive, as the work of breathing required to overcome the obstruction can quickly burn calories. The thoracic cage and sternum may be visually examined for signs of pectus excavatum or carinatum, or other malformations. These changes in thoracic structure may impinge on the mechanics of ventilation and predispose a patient to obstructive hypoventilation. Signs of neuromuscular disease, such as decreased tone, Gower sign, and calf hypertrophy, should be assessed. Clubbing of the fingertips, which is often related to chronic hypoxemia and hypoventilation, may indicate underlying lung disease. In patients with established, long-term PAP use, examination of the nasal bridge for erythema, ulceration, or deformation is important to ensure appropriate mask fit. Blood pressure screening should be included in clinic triage vitals, and hypertension should be investigated when found.
Diagnosis of OSA
Basic Polysomnography.
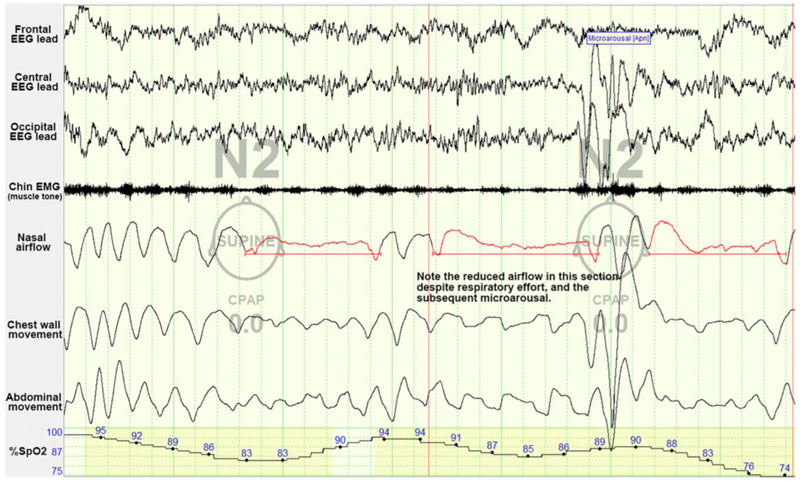
The in-laboratory sleep study, or polysomnography (PSG), is the gold standard in the diagnosis of SDB in children. The PSG informs the care of patients with clinically suspected SDB. It is most effective when paired with the clinician’s history and physical examination to guide the supervising sleep physician and sleep technologists in designing and interpreting the study. On PSG, obstructive apneic events in children are defined as a decrease of airflow (as measured by a nasal flow detector) by 90% or more despite a respiratory effort for the duration of 2 breaths (Table 3, Fig 2). Hypopneas are lower-magnitude diminutions of airflow, between 30% and 90%, which are nevertheless associated with oxygen desaturations or arousal. (20)
Figure 2: Example of obstructive sleep apnea as seen on polysomnography.
In this example of 2 obstructive sleep apnea events, note the dampening of airflow tracings with ongoing evidence of respiratory effort as seen in the thoracic and abdominal tracings. CPAP= continuous positive airway pressure, EEG=electroencephalogram, EMG=electromyography. Image courtesy of Ellen Grealish, RPSGT, R.EEG T.
A full pediatric sleep study in an American Academy of Sleep Medicine–accredited laboratory includes monitoring of several physiologic parameters throughout the course of an evening. In very young infants, “nap” sleep studies during the day may also be adequate. For a typical sleep study, the child and parent arrive at the sleep laboratory in the early evening. After the child acclimates to the sleep room, various leads, bands, and nasal flow sensors for data collection are attached to the patient. The room is designed to be cool and quiet, in emulation of an ideal sleeping arrangement. Parents are generally encouraged to stay overnight with young children on a nearby cot or sleeper chair. The child is monitored throughout the evening by a certified sleep technician, who observes the tracings from 1 or more patients in a central control room. Should the patient exhibit severe desaturations, prolonged hypercapnia, worrisome electrocardiographic tracings, or evidence of previously unidentified seizure activity, the sleep technician is trained to identify these issues and consult the on-call supervising sleep physician for guidance in intervention. In some cases, a “split” sleep study is conducted in which half the night is used for a standard diagnostic sleep study followed by the addition of a therapeutic intervention, typically PAP, for the remainder of the evening, which is aimed at titrating the new therapy and demonstrating efficacy. Generally, an initial sleep study should not be used for the initiation of mask-delivered PAP, particularly in very young children who are likely to be frightened by the modality and the unfamiliarity with the sleep laboratory environment. Although standard practice in the adult population, the use of in-home sleep studies is not recommended in the diagnosis of OSA in patients younger than 18 years of age, according to the most recent American Academy of Sleep Medicine position statement. (21) In-laboratory PSG remains the standard of care for these patients. Home oximetry is likely not sufficient for the diagnosis of OSA but may be informative in settings in which full PSG is unavailable. (22)
The resultant sleep study is staged by a technologist, a process wherein the various phases of sleep are identified and respiratory events such as obstructions are noted. A sleep physician then interprets the study, generating a summary report and communication with the ordering provider, describing the general quality and architecture of the patient’s sleep. The resultant apnea-hypopnea index (AHI) reflects a combination of obstructive events, central apneas, and hypopneas and is reported as an average number of events per hour (see Table 5 for typical pediatric cutoff values). Of note, even a low AHI can cause significant impairment in children; the developing child is exquisitely sensitive to OSA.
Table 5: Classification of Sleep Apnea Severity in Children Based on the AHI.
| AHI, No. of Events Per Hour | ||
|---|---|---|
| Severity | Pediatric | Adult |
| Normal | ≤1 | ≤5 |
| Mild | 1 to ≤5 | 5 to ≤15 |
| Moderate | >5 to ≤10 | >15 to ≤30 |
| Severe | >10 | >30 |
AHI=apnea-hypopnea index.
Adapted with permission from Dehlink E, Tan H-L. Update on paediatric obstructive sleep apnoea. J Thorac Dis. 2016;8(2):224–235.
Drug-Induced Sleep Endoscopy.
Drug-induced sleep endoscopy is performed by specialists who fully understand the upper airway anatomy dynamics contributing to OSA in the sleeping patient and is particularly useful in those with persistently elevated AHI after AT. (23) Sleep endoscopy serves to define the level of the obstruction and may reveal sleep state–dependent laryngomalacia or palatine collapse, facilitating more targeted intervention from the otolaryngologist. Successful sleep endoscopy requires precise titration of anesthesia from an experienced anesthesiologist because the goal is emulation of the sleep state without obscuring the patient’s actual upper airway dynamics by inducing too deep a sleep state.
Imaging and Laboratory Studies.
In some cases of suspected OSA it may be beneficial to obtain lateral neck films to demonstrate upper airway narrowing. In patients with complex craniofacial abnormalities, computed tomography with three-dimensional reconstruction can be a powerful tool for oromaxillofacial surgical consultants in staging and planning interventions. (24)(25) Although there is currently a great deal of research in identifying effective biomarkers for OSA, there are presently no clinically available laboratory tests that are sensitive and specific for the disorder. (26) An early-morning blood gas may be informative in screening for nocturnal CO2 retention. Similarly, in clinical settings equipped with end-tidal or transcutaneous CO2 monitoring, this can be helpful in cases where chronic hypoventilation is a clinical concern, including in obesity-related hypoventilation or respiratory insufficiency due to neuromuscular disease. In rare cases in which a previously undiagnosed neuromuscular disorder or syndrome is suspected, referral to a specialist for targeted genetic screening should be considered. Generally, pulmonary function testing is not indicated for the evaluation of SDB; however, full pulmonary function tests can provide important information when there is a concern for underlying restrictive lung disease or a diffusion defect that may exacerbate OSA.
Treatment of OSA
Lifestyle Modification.
Pediatric obesity, a worsening public health issue in the United States, is an independent risk factor for OSA. For patients in whom obesity is considered a likely driver of obstructive SDB, a trial of lifestyle and dietary modification is often worthwhile, and the aid of a nutritionist can be particularly helpful in these cases. It is fairly common to see improvement in the AHI with 5% to 10% weight loss. (19) Furthermore, longer sleep duration is associated with improved measures of healthy bodyweight, and so optimal control of OSA could help patients to lose weight. (27)
Noninvasive PAP.
Positive airway pressure has long been a mainstay of noninvasive management in OSA, with the first successful use in children as a tracheostomy-sparing modality described by Guilleminault et al in 1986. (28) Continuous PAP (CPAP) delivers a single set pressure to patients. This pressure is optimally set during a CPAP titration study in the sleep laboratory, wherein pressure is gradually up-titrated until resolution of the obstructive or hypopneic events is observed in real-time. Bi-level PAP (Bi-level) provides different inspiratory and expiratory airway pressures, which may be better tolerated in patients because the lower expiratory PAP permits easier exhalation. Modern CPAP devices may be equipped with an expiratory pressure release mode, which emulates Bi-level by dropping the CPAP pressure by some amount (typically 2–3 cmH2O) during exhalation. Good mask fit is crucial to delivering appropriate pressures in CPAP and to fostering long-term patient adherence, and mask leak is recorded by most modern units for use in troubleshooting. Another important comfort consideration in children includes the use of a “ramp” with modern PAP units. A pressure ramp instructs the CPAP or Bi-level unit to slowly increase pressure over time so that the patient does not experience the full force of the PAP device until they are fully asleep. PAP is generally delivered via nasal or oronasal masks in children; however, very small infants may require the use of a next-generation nasal cannula because standard pediatric masks may be too large. Non-adherence to PAP is a common complication in both pediatric and adolescent patients. Behavioral therapy may be beneficial in acclimating patients to the mask in young children and in fostering long-term adherence. (29)
Pharmacologic Management.
There are presently no Food and Drug Administration (FDA) –approved medications for the treatment of OSA in children. Montelukast, a leukotriene receptor antagonist used as an adjunctive therapy in persistent asthma, may have some benefit in OSA in which tissue inflammation (particularly of the adenoid tissue) contributes to the obstruction. In select patients, nasal corticosteroids combined with montelukast may be helpful by similarly reducing tissue inflammation and swelling in the nasal passages and adenoids, which can contribute to airflow resistance. (30) In some patients with extreme PAP mask intolerance, which may be due to sensory aversions or claustrophobia, judicious and careful use of anxiolytics or mild sedatives may be warranted, as mask aversion is a major driver of PAP nonadherence in the pediatric population. (31) Some patients with severe SDB and nocturnal hypoventilation may benefit from supplemental oxygen therapy. Although oxygen does not alleviate the mechanical ventilatory problem inherent in OSA, it may serve to minimize nocturnal hypoxemia and its effects on intellectual development and cardiopulmonary health.
Surgical Management.
Adenotonsillectomy is the first-line treatment in the management of OSA in children with adenotonsillar hypertrophy. The Childhood Adenotonsillectomy Trial demonstrated improvement in PSG metrics, as well as behavioral and quality of life secondary outcomes, in both obese and non-obese children with PSG-diagnosed OSA without prolonged oxyhemoglobin desaturation. (32) Although no surgical procedure is without risks, AT is generally safe and well-tolerated, even in obese pediatric patients. (33)
One important consideration to counsel parents about when referring for AT is the possibility of reaccumulation of adenoidal tissue, which occurs in 1.3% to 26% of patients and may require subsequent surgical revision. (34) Patients with severe OSA before AT, or with persistent comorbid obesity, may benefit from follow-up PSG to confirm resolution of OSA. (3) Residual OSA, albeit to a lesser degree, persists after AT in up to 50% of patients; vigilance for recurrence of symptoms is recommended. (35) Patients who are at particular risk for residual OSA after AT include those with craniofacial abnormalities and Mallampati class III and IV airways.
There are unique management considerations in certain patient populations. The AAP guidelines suggest that children with cardiac abnormalities, specifically left or right ventricular hypertrophy, be monitored inpatient postoperatively because they have an increased risk of respiratory complication after AT. (3) Tongue debulking is occasionally used in patients with significant macroglossia related to syndromes such as Beckwith-Wiedemann syndrome and trisomy 21; however, this procedure is avoided when possible due to the highly vascular nature of the tongue base and the potential for surgical complication. Mandibular distraction osteogenesis is a relatively invasive but highly efficacious procedure for patients with retrognathia or micrognathia, wherein the mandible is surgically fractured and affixed to an internal device that permits gradual distraction of the mandible at a rate that facilitates bone remodeling. Recent advances in this technique have permitted full internalization of a motorized distraction device in select patients, obviating the need for external pins. (36) Young infants with upper airway abnormalities may benefit from supraglottoplasty. Hypoglossal nerve stimulation is a promising intervention, currently under investigation in children, which alleviates airway obstruction due to tongue malposition by applying low-voltage electric pulses to the hypoglossal nerve in concert with diaphragmatic excursion, activating the muscles that move the tongue forward. (37) Tracheostomy is rarely indicated in the management of OSA but may be useful in patients with severe OSA associated with failure to thrive in very young infants with severe laryngomalacia, in select patients with neurologic disorders, or as a temporizing measure in patients undergoing complicated craniofacial or upper airway interventions. Treatment-emergent central apnea is a rare and generally transient complication of successful OSA therapies, particularly those surgical interventions that quickly alleviate long-standing obstruction (Table 6).
Table 6: Therapies for OSA.
| Therapy | Indications |
|---|---|
| Adenotonsillectomy | Adenoidal or tonsillar hypertrophy, with strong clinical evidence of OSA or a definitive sleep study. Of note, adenoidal tissue may reaccumulate after adenotonsillectomy. |
| CPAP / Bi-level | PAP is the commonest non-invasive treatment for OSA, providing the additional pressure needed to help the patient overcome an upper airway obstruction. |
| Tracheostomy | Principally indicated in those cases where a patient is failing to thrive or has otherwise been exceptionally refractory to less-intensive OSA therapy. Tracheostomy may be helpful in cases of severe craniofacial abnormalities or neurologic issues. |
| Mandibular distraction | Surgical separation of the mandible is paired with an implanted mechanical distraction device, which permits the controlled separation of opposing bone at a rate which promotes bone remodeling and, thus, gradual extension of the mandible, resulting in a lengthening of the lower jaw and that yields less crowding of the tongue and associated soft tissue in the oropharynx. |
| Palatal expansion and distraction osteogenesis maxillary expansion | Use of these orthodontic devices expands the hard palate, correcting associated narrowing of the nasal passages. |
| Hypoglossal nerve stimulator | Presently in the investigational stage in children, these devices supply a titratable electric stimulus to the hypoglossal nerve while the patient is asleep, inducing the tongue to push forward and alleviate airway obstruction. This device is actuated remotely by the parents each evening at bedtime so as not to cause disruption to speech or comfort during waking hours. |
| Montelukast and intranasal corticosteroids | Leukotriene receptor antagonists, used principally as an adjunctive therapy to intranasal corticosteroids, likely improves nasal airflow by reducing the inflammatory bulk of the nasal turbinates and adenoidal tissue. |
Bi-level=bilevel positive airway pressure, CPAP=continuous positive airway pressure, OSA=obstructive sleep apnea, PAP=positive airway pressure
CENTRAL SLEEP APNEA
The physiology of respiratory control is complex, involving integration of multiple afferent signals from both the pons and the peripheral chemoreceptors and mechanoreceptors, at the ventral and dorsal respiratory groups and the solitary tract of the medulla, which subsequently drives respiration. (38) Children with central sleep apnea (CSA) may have some fundamental maladaptation to the transition from waking control of respiration to sleep. Central sleep apnea is a cessation or diminution of airflow due to dysregulation of the central respiratory drive. In newborns and infants, CSA is often related to immaturity or dysmaturity of the brain’s control of respiration, and apnea of prematurity is a common indication for sleep consultation in the NICU. The relationship of immature control of breathing to BRUEs (brief, resolved, unexplained events, formerly “ALTE”) and sudden infant death syndrome remains unclear. Apart from the relatively common idiopathic and transient causes of CSA in very young children, rare causes of CSA that must be excluded include congenital central hypoventilation syndrome (CCHS) and brainstem abnormalities such as Chiari malformation.
History and Physical Examination
A history of pauses in breathing while asleep is a frequent presenting concern for parents with young infants. Irregular breathing patterns are the rule rather than the exception in the first several months of life. (39) Specifically, periodic breathing, which is a typical neonatal respiratory pattern consisting of 5- to 10-second pauses in breathing followed by 10 to 15 seconds of compensatory tachypnea, is quite common in infancy and is a normal feature of rapid eye movement phase sleep in infants until up to 2 months after term gestational age. (20) However, when parental report of unusual breathing in an infant is complicated or otherwise not reassuringly typical for an infant, it may be difficult to distinguish true CSA from periodic breathing based purely on the history.
A PSG should be pursued in cases in which the history is notable for apnea, which is apparent to parents as being especially prolonged, or associated with any frank color change or other signs of respiratory distress. Apneas in excess of 20 seconds are typically indicative of CSA of prematurity. Similar to patients with OSA, patients with CSA may have signs or be able to report morning headache. Older patients may describe a sensation of “drowning” with arousals from CSA on awakening from sleep. A variety of syndromes, notably Rett syndrome, are associated with CSA. Patients with congenital central hypoventilation syndrome typically present in infancy and may demonstrate absence of respiratory distress despite hypoxemia and hypercapnia during cyanotic episodes.
In infants with suspected CSA, the physical examination should include an assessment of the infant’s tone. In older children, the physical examination of the patient with suspected CSA is targeted toward excluding actionable intracranial or brainstem abnormalities, which may be physically impinging on the centers of respiratory control in the medulla and pons. A basic fundal examination for papilledema, although potentially challenging in the pediatric patient, can be informative. The remainder of the standard sleep examination, as described in the OSA section previously herein, should be considered as warranted by the patient’s presentation.
Diagnosis
PSG Findings.
In children, CSA is formally defined as the reduction of airflow by 90% or more for at least 20 seconds; for 2 breath cycles if associated with an arousal, a 3% or greater decrease in arterial oxygen saturation, or a decrease in heart rate to less than 50 beats/min for at least 5 seconds; or a heart rate less than 60 beats/min for 15 seconds in infants (Table 7, Fig 3). The hallmark of CSA is that there is no respiratory effort during these events. (20) Although not standard practice, a CO2 stimulation challenge may be useful in differentiating atypical periodic breathing or other-wise idiopathic central apneas in young infants from the intractable chemosensory dysfunction seen in CCHS. (40)
Table 7: Diagnostic Criteria of Primary Central Sleep Apnea of Prematurity.
| A. Apnea or cyanosis is noted by an observer, or an episode of sleep-related central apnea, desaturation, or bradycardia is detected by hospital monitoring in the postnatal period. | |
| B. The infant has a conceptional age < 37 weeks at the time of onset of symptoms. | |
| C. Polysomnography or alternative monitoring such as a hospital or home apnea monitoring shows either: | 1. Recurrent prolonged (>20-second duration) central apneas 2. Periodic breathing for >5% of total sleep time |
| D. The disorder is not better explained by another sleep disorder, medical or neurological disorder, or medication. | |
Criteria A-D must be met.
Reprinted with permission from The International Classification of Sleep Disorders, 3rd Ed.
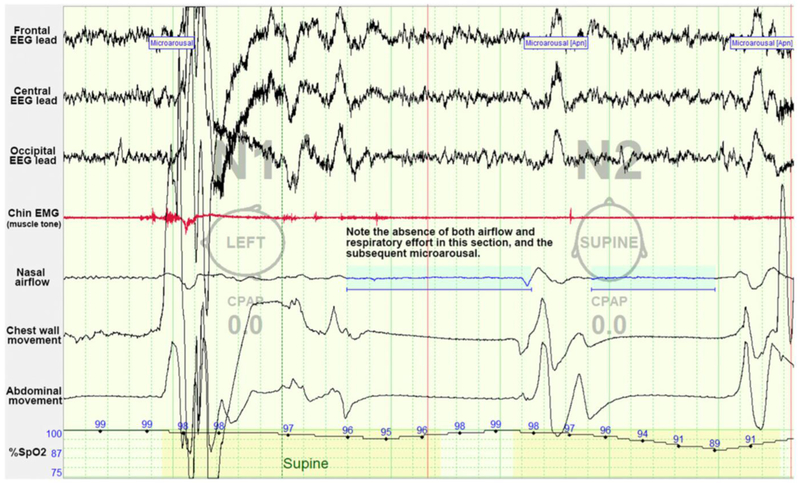
Figure 3: Example of central sleep apnea as seen on polysomnography.
In this example of central sleep apnea, there is an absence of both airflow and respiratory effort. These central events are followed by arousals. CPAP=continuous positive airway pressure, EEG=electroencephalogram, EMG=electromyography. Image courtesy of Ellen Grealish, RPSGT, R.EEG T.
Laboratory Tests and Imaging.
In infants in the first few months after birth with classical periodic breathing or brief episodes of irregular breathing pattern without abnormal neurologic examination findings, no further evaluation may be needed. Premature infants with signs of especially persistent or frequent episodes of apnea of prematurity may benefit from assessment of serum partial pressure of carbon dioxide with a blood gas test. In patients with severe and refractory CSA in the neonatal period, CCHA—a syndrome of dysregulated physiologic response to hypoxemia and hypercarbia resulting in hypoventilation while asleep—should be excluded by genetic testing for PHOX2B mutation. In older children with CSA of unknown cause, magnetic resonance imaging of the head and neck is an important imaging modality for excluding Chiari malformation and other brainstem issues.
Management.
Apnea of prematurity is often quite responsive to oral caffeine, and studies of this intervention reassuringly suggest that treated infants may experience modest neuroprotective benefits. (41)(42) Caffeine therapy is generally not indicated for idiopathic CSA of infancy that persists past 37 weeks’ corrected gestational age. Patients with idiopathic CSA may benefit from the use of the carbonic anhydrase inhibitor acetazolamide, which increases respiratory drive by shifting the partial pressure of CO2 threshold. (43)(44) Even in the absence of overt nocturnal hypoxemia, supplemental oxygen works in some patients by stabilizing or reducing the responsiveness of the peripheral chemoreceptors; however, significant sleep fragmentation often persists in patients treated with oxygen alone. (44) Patients with refractory CSA or CCHS require management with nocturnal PAP, generally Bi-level with a
backup respiratory rate or an assist-control ventilator mode. Patients with evidence of Chiari I malformation, wherein the brain stem and associated center of respiratory control is compressed, benefit from urgent neurosurgical evaluation for consideration of surgical decompression.
SUMMARY.
Based on strong research evidence and expert consensus, all children and adolescents should be screened for snoring and other signs and symptoms of sleep-disordered breathing. (3)
Based on some research evidence as well as expert consensus, adenotonsillectomy is the first-line therapy for children and adolescents with obstructive sleep apnea in the context of adenotonsillar hypertrophy. (32)
Based on expert consensus, in-laboratory sleep studies remain the gold standard for the diagnosis of sleep-disordered breathing in patients younger than 18 years. (21)
Content Specifications:
Plan an appropriate evaluation for obstructive sleep apnea
Plan appropriate management of obstructive sleep apnea
Recognize complications associated with obstructive sleep apnea
Objectives.
After completing this article, readers should be able to:
Recognize the signs and symptoms of obstructive sleep apnea in pediatric and adolescent patients.
Understand the role of in-laboratory sleep studies in the evaluation of pediatric patients with suspected sleep-disordered breathing.
Weigh the indications, benefits, and risks of various therapies for obstructive sleep apnea.
Have a general understanding of, and maintain an appropriate index of suspicion for, comorbid central sleep apnea when a patient presents with signs of sleep-disordered breathing.
Practice Gaps.
An estimated 1 to 5% of children have sleep-disordered breathing related to obstructive sleep apnea, with a smaller proportion of children having central or mixed sleep apnea. Improved screening for sleep-disordered breathing in the general pediatrics clinic, coupled with effective management strategies, has the potential to have wide-ranging benefits on the patient’s long-term health and development.
Abbreviations
- AAP
American Academy of Pediatrics
- AHI
apnea-hypopnea index
- AT
adenotonsillectomy
- Bi-level
bilevel positive airway pressure
- CPAP
Continuous positive airway pressure
- CSA
Central sleep apnea
- FDA
Food and Drug Administration
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PSG
polysomnography
- SDB
sleep-disordered breathing
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to disclose.
Financial Disclosure:
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Xie L, Kang H, Xu Q, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James S, McLanahan S, Brooks-Gunn J, et al. Sleep Duration and Telomere Length in Children. The Journal of Pediatrics. June 2017. doi: 10.1016/j.jpeds.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. PEDIATRICS. 2012;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 4.Diagnosis Bhargava S. and Management of Common Sleep Problems in Children. Pediatrics in Review. 2011;32(3):91–99. doi: 10.1542/pir.32-3-91. [DOI] [PubMed] [Google Scholar]
- 5.Guilleminault C, Lee JH, Chan A. Pediatric Obstructive Sleep Apnea Syndrome. Arch Pediatr Adolesc Med. 2005;159(8):775–785. doi: 10.1001/archpedi.159.8.775. [DOI] [PubMed] [Google Scholar]
- 6.Guilleminault C, Eldridge F, Dement WC. Insomnia, narcolepsy, and sleep apneas. Bull Physiopathol Respir (Nancy). 1972;8(5):1127–1138. [PubMed] [Google Scholar]
- 7.Huon L-KA, Guilleminault C. A Succinct History of Sleep Medicine. Adv Otorhinolaryngol. 2017;80:1–6. doi: 10.1159/000470486. [DOI] [PubMed] [Google Scholar]
- 8.Guilleminault C, Eldridge FL, Simmons FB, Dement WC. Sleep apnea in eight children. PEDIATRICS. 1976;58(1):23–30. [PubMed] [Google Scholar]
- 9.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. The Journal of Pediatrics. 2006;149(3):342–347. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 10.Johns MW. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Janssen KC, Phillipson S, O’Connor J, Johns MW. Validation of the Epworth Sleepiness Scale for Children and Adolescents using Rasch analysis. Sleep Med. 2017;33:30–35. doi: 10.1016/j.sleep.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Bediwy AS, El-Mitwalli A, Zaher AA, Belal T, Saleh ABM. Sleep apnea in children with refractory monosymptomatic nocturnal enuresis. Nature and Science of Sleep. 2014;6:37–42. doi: 10.2147/NSS.S59317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. The Journal of Pediatrics. 2003;142(4):383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 14.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. The Lancet Neurology. 2010;9(2):177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 15.Skotko BG, Macklin EA, Muselli M, et al. A predictive model for obstructive sleep apnea and Down syndrome. Am J Med Genet. 2017;173(4):889–896. doi: 10.1002/ajmg.a.38137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cielo CM, Marcus CL. Obstructive sleep apnoea in children with craniofacial syndromes. Paediatric Respiratory Reviews. 2015;16(3):189–196. doi: 10.1016/j.prrv.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaleyias J, Mostofi N, Grant M, et al. Severity of obstructive sleep apnea in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30(9):659–665. doi: 10.1097/MPH.0b013e31817eb7ef. [DOI] [PubMed] [Google Scholar]
- 18.Myers KA, Mrkobrada M, Simel DL. Does This Patient Have Obstructive Sleep Apnea?: The Rational Clinical Examination Systematic Review. JAMA. 2013;310(7):731–741. doi: 10.1001/jama.2013.276185. [DOI] [PubMed] [Google Scholar]
- 19.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J Clin Sleep Med. 2011;7(3):268–273. doi: 10.5664/JCSM.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry RB, Brooks R, Gamaldo CE, Harding SM. The AASM manual for the scoring of sleep and associated events. 2012.
- 21.Kirk V, Baughn J, D’Andrea L, et al. American Academy of Sleep Medicine Position Paper for the Use of a Home Sleep Apnea Test for the Diagnosis of OSA in Children. J Clin Sleep Med. 2017;13(10):1199–1203. doi: 10.5664/jcsm.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirk VG, Bohn SG, Flemons WW, Remmers JE. Comparison of Home Oximetry Monitoring With Laboratory Polysomnography in Children*. Chest. 2003;124(5):1702–1708. doi: 10.1378/chest.124.5.1702. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox LJ, Bergeron M, Reghunathan S, Ishman SL. An updated review of pediatric drug-induced sleep endoscopy. Laryngoscope Investigative Otolaryngology. 2017;2(6):423–431. doi: 10.1002/lio2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh GD, Ran BD, Ben-David R, Cohen T, Tamir C. System and method for three-dimensional airway reconstruction, assessment and analysis. Eur J Clin Nutr. 2010;56(12):1155–1161. doi: 10.1038/sj.ejcn.1601465. [DOI] [Google Scholar]
- 25.Arens R, McDonough JM. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am Thoracic Soc. 2001. [DOI] [PubMed] [Google Scholar]
- 26.McGrath B, Lerman J. Pediatric sleep-disordered breathing: an update on diagnostic testing. Curr Opin Anaesthesiol. 2017;30(3):357–361. doi: 10.1097/ACO.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 27.Cespedes EM, Hu FB, Redline S, et al. Chronic insufficient sleep and diet quality: Contributors to childhood obesity. Obesity. 2015;24(1):184–190. doi: 10.1002/oby.21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilleminault C, Nino-Murcia G, Heldt G, Baldwin R, Hutchinson D. Alternative treatment to tracheostomy in obstructive sleep apnea syndrome: nasal continuous positive airway pressure in young children. PEDIATRICS. 1986;78(5):797–802. [PubMed] [Google Scholar]
- 29.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: Clinical and empiric insights for developing CPAP adherence interventions. Sleep Medicine Reviews. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. PEDIATRICS. 2008;122(1):e149–e155. doi: 10.1542/peds.2007-3398. [DOI] [PubMed] [Google Scholar]
- 31.Marcus CL, Rosen G, Ward SLD, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. PEDIATRICS. 2006;117(3):e442–e451. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 32.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavin JM, Shah RK. Postoperative complications in obese children undergoing adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 2015;79(10):1732–1735. doi: 10.1016/j.ijporl.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 34.Kim SY, Lee WH, Rhee CS, Lee CH, Kim JW. Regrowth of the adenoids after coblation adenoidectomy: Cephalometric analysis. The Laryngoscope. 2013;123(10):2567–2572. doi: 10.1002/lary.23984. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 36.Abramson ZR, Susarla SM, Lawler ME, Peacock ZS, Troulis MJ, Kaban LB. Effects of Mandibular Distraction Osteogenesis on Three-Dimensional Airway Anatomy in Children With Congenital Micrognathia. YJOMS. 2013;71(1):90–97. doi: 10.1016/j.joms.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Diercks GR, Wentland C, Keamy D, et al. Hypoglossal Nerve Stimulation in Adolescents With Down Syndrome and Obstructive Sleep Apnea. JAMA Otolaryngol Head Neck Surg. 2017;144(1):37–42. doi: 10.1001/jamaoto.2017.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez AB, Patil SP. Pathophysiology of central sleep apneas. Sleep Breath. 2016;20(2):467–482. doi: 10.1007/s11325-015-1290-z. [DOI] [PubMed] [Google Scholar]
- 39.Parmelee A, Stern E, Harris M. Maturation of Respiration in Prematures and Young Infants. Neuropediatrics. 2008;3(03):294–304. doi: 10.1055/s-0028-1091768. [DOI] [PubMed] [Google Scholar]
- 40.Gozal D Congenital central hypoventilation syndrome: An update. Pediatr Pulmonol. 1998;26(4):273–282. doi:. [DOI] [PubMed] [Google Scholar]
- 41.Doyle LW, Schmidt B, Anderson PJ, et al. Reduction in Developmental Coordination Disorder with Neonatal Caffeine Therapy. The Journal of Pediatrics. 2014;165(2):356–359.e2. doi: 10.1016/j.jpeds.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt B Methylxanthine Therapy for Apnea of Prematurity: Evaluation of Treatment Benefits and Risks at Age 5 Years in the International Caffeine for Apnea of Prematurity (CAP) Trial. Neonatology. 2005;88(3):208–213. doi: 10.1159/000087584. [DOI] [PubMed] [Google Scholar]
- 43.White DP. Central sleep apnea. Improvement with acetazolamide therapy. Archives of Internal Medicine. 1982;142(10):1816–1819. doi: 10.1001/archinte.142.10.1816. [DOI] [PubMed] [Google Scholar]
- 44.Thomas RJ. Alternative approaches to treatment of Central Sleep Apnea. Sleep Medicine Clinics. 2014;9(1):87–104. doi: 10.1016/j.jsmc.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dehlink E, Tan H-L. Update on paediatric obstructive sleep apnoea. J Thorac Dis. 2016;8(2):224–235. doi: 10.3978/j.issn.2072-1439.2015.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]