Abstract
A 14-year-old boy with familial Li-Fraumeni syndrome presented with diplopia. Brain MRI revealed a right temporoparietal rim-enhancing mass. Following surgical resection and diagnosis of a gigantocellular-type glioblastoma multiforme (GBM), his family wished to avoid cytotoxic chemotherapy given the amplified risk of secondary malignancy. As such, we performed whole exome and transcriptome sequencing, which revealed germline TP53 and somatic TSC2 mutations. On completion of adjuvant radiotherapy, he was started on maintenance therapy with everolimus per recommendations from our multi-institutional brain tumour precision medicine tumour board. He has achieved a complete remission with resolution of visual symptoms and remains on everolimus therapy with concurrent electromagnetic field therapy, now 33 months from diagnosis. Our data highlight the benefit of precision medicine in children with GBM and offer insight into a targetable pathway that may be involved in similar cases.
Keywords: paediatric oncology, neuro-oncology, cancer intervention, CNS cancer
Background
Glioblastoma multiforme (GBM) is a rare and aggressive brain tumour, characterised as a WHO histologic grade IV tumour of glial origin.1 The vast majority of cases of GBM are observed in adults.2 However, high-grade gliomas (HGGs) in general are the second most common malignant brain tumour type in the paediatric population, with an incidence of 0.8 per 100 000 for patients under the age of 19 years.3 Standard treatment for GBM at any age includes a combination of surgery, chemotherapy and radiation therapy.4 One-year overall survival for paediatric patients with HGG is estimated at 72%, and 1-year event-free survival is estimated at 49%, based on recent data from a cohort of 108 patients with anaplastic astrocytoma or glioblastoma; furthermore, 3-year overall survival is estimated at 28%, and 3-year event-free survival is estimated at 22%.5
A histological subtype known as giant-cell GBM, characterised by the presence of multinucleated giant cells, abundant eosinophilic cytoplasm and a dense reticulin fibre network, has been well described.1 6–8 This histology is also believed to have a temporal lobe predilection.6 Giant-cell GBM only accounts for approximately 2%–5% of all GBM cases but appears to be more common in children and confers a superior, but still dismal, prognosis and life expectancy.7 Another similar histological subtype, gigantocellular glioblastoma multiforme, characterised predominantly by bizarre, multinucleated giant cells, has been reported less frequently.9
Paediatric HGGs exhibit unique molecular genetics, and their phenotypes differ from their adult counterparts, despite histological similarities.10 These differences, taken together with the poor prognosis that is little changed over the past decade, motivate exploration of alternate treatment modalities to improve survival outcomes for paediatric patients with HGG in the future.11 12 The need for personalised, targeted treatment options for patients has led to the development of precision oncology with small molecular inhibitors directed at targets identified by tumour genomic sequencing. Here, we present an adolescent patient with a gigantocellular GBM and discuss our experience with targeted therapy.
Case presentation
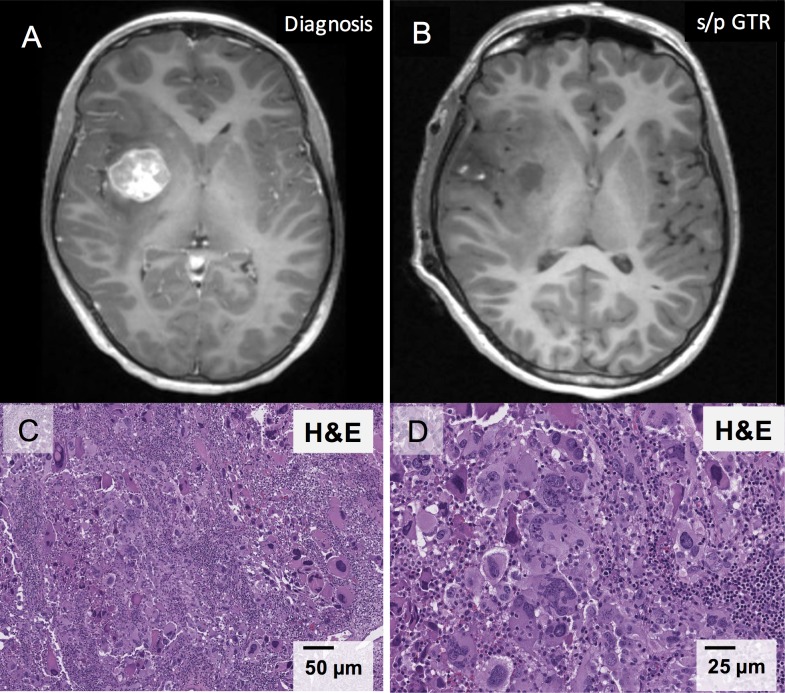
A 14-year-old previously healthy man presented with diplopia. Brain MRI (figure 1A) revealed a rim-enhancing right temporoparietal mass measuring 2.7×2.5×3.2 cm with significant oedema. He underwent gross total resection of the mass (figure 1B) and had near-immediate resolution of his diplopia. Pathology (figure 1C,D) revealed a glial neoplasm with gigantocellular morphology, no appreciable reticulin staining (not shown) and a Ki67 proliferation rate that approached 60% focally, favouring a diagnosis of GCG over pleomorphic xanthroastrocytoma. There was faint nuclear positivity for p53 and retained nuclear ATRX staining. Giant cells were weak to negative for glial fibrillary acidic protein (GFAP), while smaller, intermixed tumour cells were strongly positive for GFAP. The tumour was negative for BRAF V600E mutation by PCR. Total spine MRI 1 week postoperatively was negative for metastatic disease.
Figure 1.
Gigantocellular glioblastoma imaging and pathology. (A) Brain MRI, axial image, T1 series postcontrast, illustrating right temporoparietal tumour prior to initial resection. (B) Brain MRI, axial image, T1 series postcontrast, showing the tumour bed following gross total resection. (C) H&E stain of the tumour at ×20 magnification reveals large cells with glassy eosinophilic cytoplasm and frequent multinucleation, bizarre nuclei and intranuclear inclusions. Numerous mitoses and significant lymphocytic infiltrates are also observed. (D) H&E stain at ×40 magnification shows multinucleated tumour ‘giant cells’ with atypical mitoses. GTR, gross total resection; s/p, status/post.
This patient’s family history included a deceased father with leiomyosarcoma and a paternal grandfather with leiomyosarcoma and colorectal cancer. Both the patient and his brother were found to have a 5′UTR_EX1 germline deletion in p53—the same as was identified in their father—confirming a diagnosis of familial Li-Fraumeni syndrome (LFS). Our central nervous system (CNS) tumour board recommended adjuvant radiation with consideration of adjuvant cytotoxic chemotherapy, according to Children’s Oncology Group protocol ACNS0423. However, the patient’s father had expressed his wish against the use of chemotherapy should either of his sons receive a cancer diagnosis. Given this wish, combined with the increased risk of second malignancy in the setting of LFS, he received 60.72 GyE of conformal proton radiotherapy to the right temporal region without concurrent or adjuvant chemotherapy.
We then performed whole exome (tumour and germline DNA) and transcriptome (tumour RNA) sequencing on his tumour to explore potential targeted therapy options. Sequencing revealed an R611W missense mutation in TSC2, homozygous deletion of PAK1 and copy-neutral loss of heterozygosity of TP53 in the tumour, as well as germline loss of exons 1 and 2 of TP53 (table 1). Additionally, the tumour was PTEN and IDH wild type. Results of his tumour sequencing were discussed in a CNS precision medicine tumour board teleconferenced with clinicians at multiple children’s hospitals, which recommended everolimus, an mTOR inhibitor.
Table 1.
Integrative sequencing results
| Mutation class | Gene/aberration |
| Copy number variation | Copy gain of chr1, 7, 9, 18 and X Copy loss of chr19 and 22 Homozygous deletion of PAK1 |
| Somatic mutations | TSC2 (p.R611W), unknown significance |
| Gene fusions | No significant gene fusions |
| Outlier expression | N/A |
| Germline variants for disclosure | TP53, deletion of exons 1 and 2 |
| Pathogens | N/A |
N/A, not applicable.
Outcome and follow-up
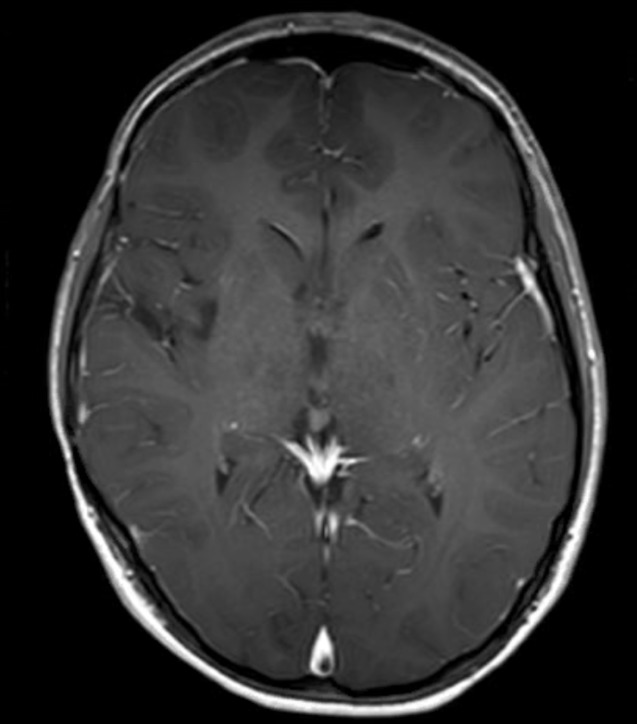
Five months after completion of radiation therapy, everolimus was initiated at 7.5 mg/day (patient weight=60.3 kg) and increased to 10 mg/day 4 months later. In addition, the patient was also approved for adjuvant electromagnetic field therapy with Optune (18 hours daily), based on the family’s interest and promising results in adult GBM.13 The patient maintained complete remission and has survived 33 months since diagnosis (25 months on treatment with everolimus), with excellent quality of life and no adverse effects from everolimus other than occasional mouth sores. The patient’s most recent brain MRI is shown in figure 2.
Figure 2.
MRI on maintenance everolimus brain MRI, axial image, T1 series postcontrast, taken 32 months after diagnosis of the patient’s glioblastoma multiforme and after 24 months of maintenance therapy with everolimus.
Discussion
The giant-cell variant of GBM confers a similar, but slightly improved, dismal prognosis to the classic GBM histology, with a 5-year overall survival of 12.3% versus 3.4%.7 A gigantocellular variant of GBM has also been reported, with one small paediatric series reporting a longer but not statistically significant difference in mean survival time for patients with gigantocellular GBM compared with patients with classic GBM.9
The mutations observed in this patient’s GCG included germline TP53 in the setting of familial LFS, as well as a somatic mutation in TSC2. TP53 is a tumour suppressor gene that codes for the protein p53, which has multiple roles in genomic stability and antineoplastic progression.14 LFS is a genetic disorder classically caused by germline mutations in TP53 (75% of patients), and it is known to increase susceptibility to a wide array of cancers, including HGG.10 15 Pollack et al reported in a series of HGG cases (anaplastic astrocytoma and GBM) that TP53 mutations were observed in 40.5% of tumours, further affirming this association.16
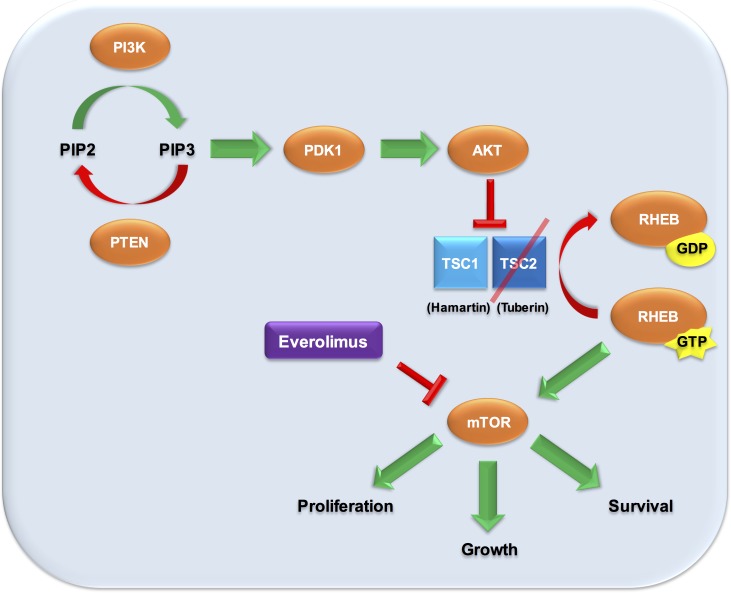
TSC1 and TSC2 are two tumour suppressor genes implicated in tuberous sclerosis complex (TSC), a syndrome characterised by widespread growth of benign and malignant tumours in the brain (particularly subependymal giant-cell astrocytomas (SEGAs)), heart and kidneys, as well as by seizures, skin findings and intellectual disability. The gene products, hamartin and tuberin, respectively, function as cell cycle inhibitors within the PI3K/AKT/mTOR pathway (figure 3), and the TSC2 R611W point mutation observed here has also been previously reported.17 While most TSC-associated brain tumours are WHO grade I SEGAs, a limited number of cases of TSC-associated GBM have been previously reported.18
Figure 3.
mTOR inhibition for TSC2 mutant-driven oncogenesis loss of function of tuberin, the gene product of TSC2, prevents inhibition of downstream signals promoting cellular proliferation and oncogenesis. Downstream inhibition of the PI3K/AKT/mTOR pathway can be achieved with everolimus, an mTOR inhibitor. This is hypothesised to be the driving mutant in the presented patient’s tumour. Original illustration by authors AHZ and CK.
The identification of a TSC2 mutation in this patient’s tumour led to an actionable change in management with the selection of everolimus, after consideration of multiple factors as a part of our institution’s CNS Targeted Agent Prediction programme.19 Everolimus inhibits the oncogenic pathway activated by a TSC2 mutation, there was positive preclinical data, and paediatric phase I and CNS clinical data were available.20 21 Additionally, CNS penetration with everolimus is promising. In animal models, everolimus achieves a brain:plasma concentration ratio of 1.5%, and a brain concentration above the IC50 for GBM cell lines for 12–24 hours postdose.22 While everolimus is a relatively large molecule, the moderate lipophilicity and inhibitory effects on P-glycoprotein also result in accumulation in the CNS over time.22
In summary, we have presented a paediatric patient with a GCG and associated TP53 and TSC2 mutations who remains in complete remission 33 months after diagnosis, with surgical resection, radiation therapy, everolimus and electromagnetic therapy. We are hopeful that this treatment approach for a poor prognosis tumour will contribute to a better outcome for this patient and will further endorse the value of personalised, targeted therapy for patients with GBM in the future.
Learning points.
Precision oncology is a needed area of research for personalised treatment of paediatric patients with cancer.
We demonstrate here a successful outcome for a paediatric patient with glioblastoma treated with a targeted agent selected through results of tumour sequencing.
Everolimus has good central nervous system penetration and may be efficacious in targeting the cellular pathway implicated by a TSC2 driving mutation.
Acknowledgments
The authors thank the Michigan Center for Translational Pathology for whole exome and transcriptome tumour sequencing analysis through the Peds-MiOncoSeq programme. Lastly, the authors thank Bernard L Marini, PharmD, and Patricia L Robertson, MD, for their contributions to the manuscript and for serving as scientific advisors. The authors also thank the patient and his family.
Footnotes
Contributors: AHZ: investigation, methodology, writing—original draft preparation, and writing—review and editing. KAM: resources and writing—review and editing. RM: writing—review and editing, and funding acquisition. CK: conceptualisation, supervision, writing—review and editing, project administration and funding acquisition.
Funding: CK is supported by National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke grant K08-NS099427-01, the UM Department of Pediatrics Chad Carr Pediatric Brain Tumor Center, the Chad Tough Foundation, the Catching Up with Jack Foundation, the Prayers from Maria Foundation, and the U CAN-CER VIVE Foundation. The University of Michigan Peds-MiOncoSeq study was supported by grant 1UM1HG006508 from the NIH Clinical Sequencing Exploratory Research Award (private investigator: Arul Chinnaiyan, coinvestigator: Rajen Mody).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803–20. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 2. Georgiu C, MihuŢ E, Raus I, et al. Pediatric glioblastoma with giant cells and "supratentorial" primitive neuroectodermal component - case report and review of the literature. Rom J Morphol Embryol 2015;56:1165–71. [PubMed] [Google Scholar]
- 3. Sturm D, Bender S, Jones DT, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer 2014;14:92–107. 10.1038/nrc3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 5. Jakacki RI, Cohen KJ, Buxton A, et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol 2016;18:1442–50. 10.1093/neuonc/now038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Margetts JC, Kalyan-Raman UP. Giant-celled glioblastoma of brain. A clinico-pathological and radiological study of ten cases (including immunohistochemistry and ultrastructure). Cancer 1989;63:524–31. [DOI] [PubMed] [Google Scholar]
- 7. Kozak KR, Moody JS. Giant cell glioblastoma: a glioblastoma subtype with distinct epidemiology and superior prognosis. Neuro Oncol 2009;11:833–41. 10.1215/15228517-2008-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karremann M, Butenhoff S, Rausche U, et al. Pediatric giant cell glioblastoma: New insights into a rare tumor entity. Neuro Oncol 2009;11:323–9. 10.1215/15228517-2008-099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikitović M, Stanić D, Pekmezović T, et al. Pediatric glioblastoma: a single institution experience. Childs Nerv Syst 2016;32:97–103. 10.1007/s00381-015-2945-6 [DOI] [PubMed] [Google Scholar]
- 10. Braunstein S, Raleigh D, Bindra R, et al. Pediatric high-grade glioma: current molecular landscape and therapeutic approaches. J Neurooncol 2017;134:541–9. 10.1007/s11060-017-2393-0 [DOI] [PubMed] [Google Scholar]
- 11. Jones C, Karajannis MA, Jones DTW, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol 2017;19:153–61. 10.1093/neuonc/now101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chamdine O, Gajjar A. Molecular characteristics of pediatric high-grade gliomas. CNS Oncol 2014;3:433–43. 10.2217/cns.14.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stupp R, Taillibert S, Kanner AA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015;314:2535–43. 10.1001/jama.2015.16669 [DOI] [PubMed] [Google Scholar]
- 14. Kim S, An SS, Ss A. Role of p53 isoforms and aggregations in cancer. Medicine 2016;95:e3993 10.1097/MD.0000000000003993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guha T, Malkin D. Inherited TP53 Mutations and the Li–Fraumeni Syndrome. Cold Spring Harb Perspect Med 2017;7:a026187 10.1101/cshperspect.a026187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children’s Cancer Group 945 cohort. J Neurosurg 2006;105(5 Suppl):418–24. 10.3171/ped.2006.105.5.418 [DOI] [PubMed] [Google Scholar]
- 17. Nellist M, Verhaaf B, Goedbloed MA, et al. TSC2 missense mutations inhibit tuberin phosphorylation and prevent formation of the tuberin-hamartin complex. Hum Mol Genet 2001;10:2889–98. 10.1093/hmg/10.25.2889 [DOI] [PubMed] [Google Scholar]
- 18. Reyes D, Prayson R. Glioblastoma in the setting of tuberous sclerosis. J Clin Neurosci 2015;22:907–8. 10.1016/j.jocn.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 19. Linzey JR, Marini BL, Pasternak A, et al. Development of the CNS TAP tool for the selection of precision medicine therapies in neuro-oncology. J Neurooncol 2018;137:155–69. 10.1007/s11060-017-2708-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013;381:125–32. 10.1016/S0140-6736(12)61134-9 [DOI] [PubMed] [Google Scholar]
- 21. Franz DN, Belousova E, Sparagana S, et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol 2014;15:1513–20. 10.1016/S1470-2045(14)70489-9 [DOI] [PubMed] [Google Scholar]
- 22. O’Reilly T, McSheehy PM, Kawai R, et al. Comparative pharmacokinetics of RAD001 (everolimus) in normal and tumor-bearing rodents. Cancer Chemother Pharmacol 2010;65:625–39. 10.1007/s00280-009-1068-8 [DOI] [PubMed] [Google Scholar]