Abstract
Background:
Cardiac amyloidosis is a substantially underdiagnosed disease and contemporary estimates of the epidemiology of amyloidosis are lacking. This study aims to determine the incidence and prevalence of cardiac amyloidosis among Medicare beneficiaries from 2000–2012.
Methods and Results:
Medicare beneficiaries were counted in the prevalence cohort in each year they had 1) ≥1 principal or secondary ICD-9 code for amyloidosis and 2) ≥1principal or secondary ICD-9 code for HF or cardiomyopathy within two years after the systemic amyloidosis code. A beneficiary was counted in the incidence cohort only during the first year in which they met criteria. Primary outcomes included the prevalence and incidence of hospitalizations for cardiac amyloidosis. There were 4,746 incident cases of cardiac amyloidosis in 2012 and 15,737 prevalent cases in 2012. There was also a significant increase in the prevalence rate (8 to 17 per 100,000 person-years) and incidence rate (18 to 55 per 100,000 person-years) from 2000–2012, most notable after 2006. Incidence and prevalence increased substantially more among men, the elderly, and in African Americans.
Conclusions:
The incidence and prevalence rates of cardiac amyloidosis are higher than previously thought. The incidence and prevalence rates of cardiac amyloidosis among hospitalized patients have increased since 2000, particularly among specific patient subgroups and after 2006, suggesting improved amyloidosis awareness and higher diagnostic rates with non-invasive imaging. In light of these trends, cardiac amyloidosis should be considered during the initial work up of patients ≥65 years old hospitalized with HF.
Keywords: Epidemiology, cardiomyopathy, heart failure, aging, race and ethnicity, Amyloid, incidence, prevalence
Introduction
Cardiac amyloidosis is a substantially underdiagnosed disease. Improved awareness and advances in imaging over the past two decades have improved the noninvasive diagnosis of cardiac amyloidosis. However, contemporary estimates of the incidence and prevalence of cardiac amyloidosis are lacking. Autopsy data has found wild type transthyretin (ATTRwt) deposits in the myocardium of over 20% of octogenarians with heart failure (HF)1, 2. Current data also suggest that cardiac involvement occurs in as many as 50% of patients with systemic light chain (AL) amyloidosis 3 and that ATTRwt amyloidosis may be responsible for as many as 30% of heart failure with preserved ejection fraction (HFpEF) cases in patients >75 years old.2, 4 Despite these recent trends, the under recognition of cardiac amyloidosis has resulted in significant delays in diagnosis.1
Before targeted treatment was available, the clinical implications of this delay in diagnosis were limited. However, with reliable, non-invasive diagnostic techniques and novel treatments for both light chain reduction in AL amyloidosis5 and novel stabilizing therapies in ATTR amyloidosis,6, 7 timely diagnosis has become paramount. This study aimed to determine the incidence and prevalence of cardiac amyloidosis between 2000 and 2012 among Medicare beneficiaries ≥65 years old and describe the variation in these trends by age, gender, race and geography.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure as access to Medicare data is controlled by the Center for Medicare and Medicaid’s Research Assistance Center (ResDAC) and sharing of the data is not permitted under the terms of our data use agreement. Subjects did not provide informed consent for the study as these data are deidentified nationwide claims data obtained from ResDAC. The project was approved by the Partners Healthcare Institutional Review Board
Study Population
The Medicare Provider and Analysis Review (MedPAR) files from the Centers for Medicare & Medicaid Services (CMS) were used to identify all Medicare beneficiaries aged ≥65 years enrolled in the Medicare Fee-for-Service program for at least 1 month between January 2000 and December 2014. For each year, Medicare beneficiary-years for new enrollment, disenrollment, or death during the study period were calculated. The beneficiary-years data was then linked to the MedPAR inpatient claims to identify beneficiaries who had one or more hospitalizations for cardiac amyloidosis. Cardiac amyloidosis was defined using a combination of International Diagnosis Codes (ICD-9) for systemic amyloidosis and heart failure (Appendix).
Definitions
Person-years enrollment and inpatient data were linked. Then, a prevalence cohort and an incident cohort were created for cardiac amyloidosis. Beneficiaries were counted in the cardiac amyloidosis prevalence cohort in each year that they had 1) ≥1 principal or secondary ICD-9 code for systemic amyloidosis and 2) ≥1 principal or secondary ICD-9 code for heart failure or cardiomyopathy within two years after initial systemic amyloidosis claim (Supplementary Figure 1). Concurrent ICD-9 codes (on the same claim) for systemic amyloidosis and heart failure were included. A beneficiary was counted in the incidence cohort only once -during the year in which they first met criteria for the prevalence cohort (Supplementary Figure 2).
Beneficiary Characteristics
Patient age was assessed in years (64–74, 75–84, ≥85), gender (female, male), race (white, black, other),8, 9 and eligibility for Medicaid coverage (dual-eligibility). In addition, patient geographic region was determined based on the beneficiary zip code of residence (Midwest, Northeast, South and West). Using inpatient claims, the number of beneficiaries with ≥1 HF admission in the past 30-days and 1 year (prior to the index diagnosis of cardiac amyloidosis) were calculated. The frequency of relevant comorbid conditions (acute myocardial infarction (AMI), unstable angina, chronic atherosclerosis, hypertension, atrial fibrillation, stroke, renal failure, chronic obstructive pulmonary disease (COPD), pneumonia, dementia and diabetes) at the start of each study year were determined using the Chronic Condition Warehouse.10 In addition, the frequency of comorbid conditions associated with amyloidosis, i.e. anemia, autonomic neuropathy, carpel tunnel syndrome, orthostatic hypotension, multiple myeloma, nephrotic syndrome and pleural effusions, were determined at the start of each study year using ICD-9 codes from the preceding year.
Outcomes
Our primary outcomes were the prevalence and incidence of hospitalization for cardiac amyloidosis per 100,000 person-years. For each year, the prevalence rate among fee-for-service beneficiaries was calculated by dividing the total number of cardiac amyloidosis-specific hospitalizations per fee-for-service beneficiary in each year by the corresponding number of beneficiary-years of fee-for-service enrollment in that year. This calculation was repeated for the incidence outcome as well.
Statistical Analysis
The changes in beneficiary characteristics, the observed number of prevalent and incident hospitalizations of cardiac amyloidosis, and time to diagnosis from 2000–2012 were assessed. Mantel-Haenszel Chi-squared test was used to evaluate whether these changes were statistically significant. The changes in the incidence of cardiac amyloidosis-related hospitalizations by pre-specified demographic groups based on age, sex, and race subgroups as described previously were examined. To assess geographic variation in the prevalence and incidences rates, the prevalence of cardiac amyloidosis in 2000 and 2012 were mapped with a shading gradient for counties from red to green (lowest prevalence/incidence in red to the highest in green). The same visual/color scale of hospitalizations per 100,000 beneficiary-years was used in 2000 and 2012 so that changes over time could be readily observed. To assess whether geographic variation persisted over time, a Pearson correlation coefficient of the prevalence of cardiac amyloidosis between the two periods, 2000 and 2012, weighted by county-specific population was calculated. This analysis was repeated for the incidence cohort. Analyses were conducted using SAS version 9.4 64-bit Windows version (SAS Institute Inc., Cary, North Carolina). All statistical testing was two-sided and p<0.05 was considered statistically significant.
Results
The prevalence cohort included 121,122 cardiac amyloidosis patients and the incidence cohort included 38,254 newly diagnosed cardiac amyloidosis patients over the 12-year study period. The baseline characteristics of individual patients in the incidence cohort in 2000, 2012, and aggregated from 2000–2012 are displayed in Table 1. By design, all patients were ≥65 years old and the age and gender distributions were similar between the two periods. Almost 15% of patients were black and this percentage increased from 12% in 2000 to 15% in 2012. Approximately 8% of patients hospitalized for cardiac amyloidosis had a HF hospitalization within the previous year. In terms of comorbidities, the rate of hypertension increased from 43% to 63% between 2000 and 2012. The frequency of concurrent renal failure doubled from 8% in 2000 to 16% in 2012. The rates of non-cardiovascular comorbidities, including COPD and dementia changed minimally. The rates of amyloidosis-specific comorbidities were notable for an increase in the prevalence of orthostatic hypotension from 9% in 2000 up to 13% in 2012 and a decline in the prevalence of multiple myeloma from 11% in 2000 to 7% in 2012.
Table 1:
Baseline Characteristics of Individual Patients Newly Diagnosed with Cardiac Amyloidosis (Incidence Cohort), 2000–2012
| 2000 | 2012 | P value (linear trend from 2000 to 2012) | Aggregated (2000–2012) | |
|---|---|---|---|---|
| New Cases of Cardiac Amyloidosis (incidence) | 2101 | 4746 | 38254 | |
| Demographics | ||||
| Age group, (%) | <0.001 | |||
| 65–74 | 853 (40.6) |
1638 (34.5) |
13832 (36.2) |
|
| 75–84 | 927 (44.1) |
1930 (40.7) |
16367 (42.8) |
|
| 85+ | 321 (15.3) |
1178 (24.8) |
8055 (21.1) |
|
| Gender, (%) | 0.177 | |||
| Female | 1079 (51.4) |
2454 (51.7) |
19270 (50.4) |
|
| Race, (%) | <0.001 | |||
| White | 1757 (83.6) |
3763 (79.3) |
31015 (81.1) |
|
| Black | 259 (12.3) |
728 (15.3) |
5451 (14.3) |
|
| Other | 85 (4.0) |
255 (5.4) |
1788 (4.7) |
|
| Gender and Race, (%) | <0.001 | |||
| White Men | 867 (41.3) |
1843 (38.8) |
15687 (41.0) |
|
| White Women | 890 (42.4) |
1920 (40.5) |
15328 (40.1) |
|
| Black Men | 118 (5.6) |
331 (7.0) |
2470 (6.5) |
|
| Black Women | 141 (6.7) |
397 (8.4) |
2981 (7.8) |
|
| Other Men | 37 (1.8) |
118 (2.5) |
827 (2.2) |
|
| Other Women | 48 (2.3) |
137 (2.9) |
961 (2.5) |
|
| Dual-Eligible Enrollment, (%) | 270 (12.9) |
685 (14.4) |
<0.001 | 5186 (13.6) |
| Geography, (%) | <0.001 | |||
| Midwest | 790 (37.6) |
1760 (36.0) |
13912 (36.4) |
|
| Northeast | 475 (22.6) |
1286 (27.1) |
9750 (25.5) |
|
| South | 541 (25.8) |
973 (20.5) |
8391 (21.9) |
|
| West | 295 (14.0) |
781 (16.5) |
6198 (16.2) |
|
| Prior Heart Failure Hospitalizations*, (%) | ||||
| Heart Failure Hospitalization in prior 30-days of the index amyloidosis-related admission (%) | 42 (2.0) |
91 (1.9) |
0.505 | 833 (2.2%) |
| Heart Failure Hospitalization in prior 1-year of the index amyloidosis-related admission (%) | 180 (8.6) |
356 (7.5%) |
0.001 | 3087 (8.1) |
| Past Medical History, f(%) | ||||
| Acute Myocardial Infarction | 26 (1.2) |
126 (2.7) |
0.012 | 883 (2.3) |
| Unstable Angina | 32 (1.5) |
61 (1.3) |
0.328 | 517 (1.4) |
| Cardiac Atherosclerosis | 432 (20.6) |
1055 (22.2) |
0.059 | 8862 (23.2) |
| Hypertension | 894 (42.6) |
2984 (62.9) |
<0.001 | 21288 (55.4) |
| Atrial Fibrillation | 391 (18.6) |
985 (20.8) |
0.025 | 7588 (19.8) |
| Stroke | 142 (6.8) |
393 (8.3) |
0.003 | 2900 (7.6) |
| Renal Failure | 174 (8.3) |
772 (16.3) |
<0.001 | 5314 (13.9) |
| Chronic Obstructive Pulmonary Disease | 338 (16.1) |
539 (11.4) |
<0.001 | 5206 (13.6) |
| Pneumonia | 194 (9.2) |
554 (11.7) |
<0.001 | 4269 (11.2) |
| Dementia | 262 (12.5) |
801 (16.9) |
<0.001 | 6892 (18.0) |
| Diabetes | 289 (13.8) |
933 (19.7) |
<0.001 | 6503 (17.0) |
| Anemia | 532 (25.3) |
1166 (24.6) |
<0.001 | 9337 (24.4) |
| Autonomic Neuropathy | 132 (6.3) |
205 (4.32) |
<0.001 | 1943 (5.1) |
| Carpel Tunnel Syndrome | 16 (0.8) |
16 (0.3) |
0.013 | 137 (0.4) |
| Orthostatic Hypotension | 191 (9.1) |
630 (13.3) |
<0.001 | 4617 (12.07) |
| Multiple Myeloma | 238 (11.3) |
343 (7.2) |
<0.001 | 3553 (9.3) |
| Nephrotic Syndrome | 36 (1.7) |
65 (1.4) |
<0.001 | 574 (1.5) |
| Pleural Effusion | 103 (4.9) |
268 (0.7) |
<0.001 | 2248 (5.9) |
Biceps tendon rupture, macroglossia, periorbital bruising and peripheral neuropathy were excluded due to counts <11
Prior Heart Failure Hospitalization includes any prior hospitalizations within the designated time period with a primary diagnosis of heart failure. No secondary diagnoses are considered.
Temporal incidence and prevalence trends for cardiac amyloidosis between 2000 and 2012 are displayed in Figure 1. The prevalence of cardiac amyloidosis increased from 18.0 (95% CI 117.5–18.5) per 100,000 person-years to 55.2 (95% CI 54.3–56.0) per 100,000 person-years (p<0.01) and the incidence of cardiac amyloidosis increased from 8 (95% CI 7.5–8.2) patients to 16.6 (95% CI 16.2–17.1) patients per 100,000 person-years (p<0.001). Observed in both curves is an inflection point between 2006 and 2007 where the slope of both the prevalence and incidence lines increased. The incidence and prevalence peaked in 2012, when there were 15,737 patients in the prevalence cohort and 4,746 patients in the incident cohort.
Figure 1. Incidence and Prevalence Trends of Hospitalization for Cardiac Amyloidosis, 2000–2012.
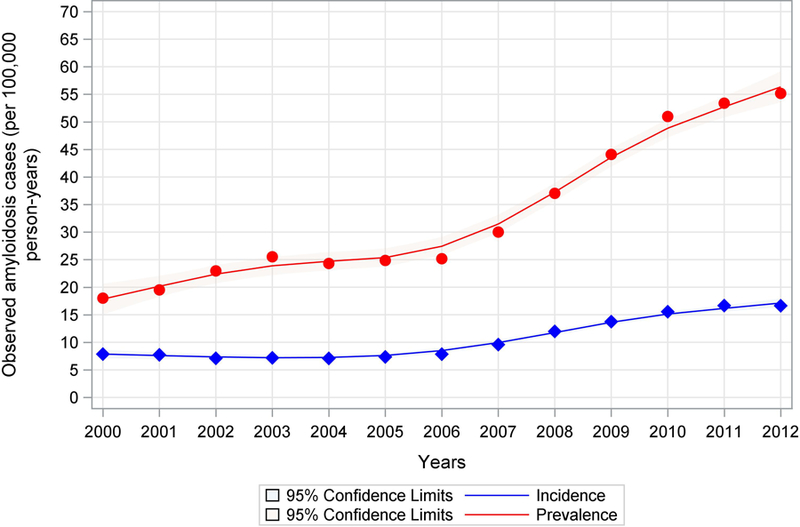
This figure shows the increasing prevalence and incidence rates of hospitalization per 100,000 person years for cardiac amyloidosis between 2000 and 2012. The most notable rate of increase can be observed after 2006–2007. The red line represents the annual prevalence of hospitalization and the blue line represents the annual incidence of hospitalization. The shaded areas represent the 95% confidence intervals.
Age, gender, race and geographic variations in the most contemporary estimates, from 2012, of the incidence and prevalence of cardiac amyloidosis among different groups per 100,000 person years in 2012 is displayed in Table 2. The prevalence of cardiac amyloidosis was higher among men (70 per 100,000 person-years) compared to women (44 per 100,000 person-years). The incidence was similar among men (18 per 100,000 person-years) as compared to women (15 per 100,000 person-years). The highest prevalence was noted among black men (174 per 100,000 person-years) and the highest incidence was also observed in this group (36 per 100,000 person-years). Geographically, the highest prevalence was noted in the Northeast (87 per 100,00 person-years), followed by the Midwest (54 per 100,000 person-years). The highest incidence was also in the Northeast (24 per 100,000 person-years) and the Midwest (16 per 100,000 person-years).
Table 2:
Incidence and Prevalence of Cardiac Amyloidosis per 100,000 person-years among Medicare fee-for-service Beneficiaries aged 65 years or older in 2012
| Incidence (95% Confidence Interval) |
Prevalence (95% Confidence Interval) |
|
|---|---|---|
| N (number of patients in each cohort in 2012) | 4746 | 15737 |
| Overall Patients (per 100,000 person-years) | 16.6 (16.2–17.1) |
55.2 (54.3–56) |
| Gender | ||
| Men | 18.3 (17.5–19) |
69.6 (68.1–71.1) |
| Women | 15.4 (14.7–16) |
43.8 (42.8–44.9) |
| Race & Gender | ||
| White women | 14.2 (13.5–14.8) |
36.2 (35.2–37.2) |
| Black women | 29.5 (26.6–32.4) |
128.9 (122.9–135) |
| White men | 17.2 (16.4–18) |
62.6 (61.1–64.1) |
| Black men | 35.6 (31.8–39.4) |
174.0 (165.5–182.5) |
| Other race women | 12.6 (10.5–14.7) |
33.8 (30.3–37.2) |
| Other race men | 13.1 (10.8–15.5) |
44.5 (40.1–48.8) |
| Geography | ||
| Midwest | 16.2 (15.4–16.9) |
54.4 (53–55.8) |
| Northeast | 24.0 (22.7–25.3) |
87.4 (84.9–89.9) |
| South | 13.5 (12.6–14.3) |
40.5 (39–41.9) |
| West | 14.5 (13.4–15.5) |
44.2 (42.5–46) |
The number (N) in each cohort in 2012 (top row) is displayed in absolute number of patients. The rest of the table is displayed in rates, the number of patients per 100,000 person-years.
Variation in the annual number of cardiac amyloidosis-related hospitalizations by age, sex and race between 2000 and 2012 are displayed in Figure 2. While the number of first hospitalizations for cardiac amyloidosis (incident cases) increased slowly in most age and race subgroups between 2000 and 2012, the number of all hospitalizations (prevalent cases) increased steeply after 2006 and was more pronounced in certain subgroups. Specifically, there were increases in total hospitalization rates for cardiac amyloidosis among black patients of all ages, with marked increases among those >75 years old. There were smaller increases among white and other males >75 years and only minimal increases among white and other women in all age groups.
Figure 2. Variation in the Annual Number of Cardiac Amyloidosis-related Hospitalizations by Age, Sex and Race, 2000–2012.
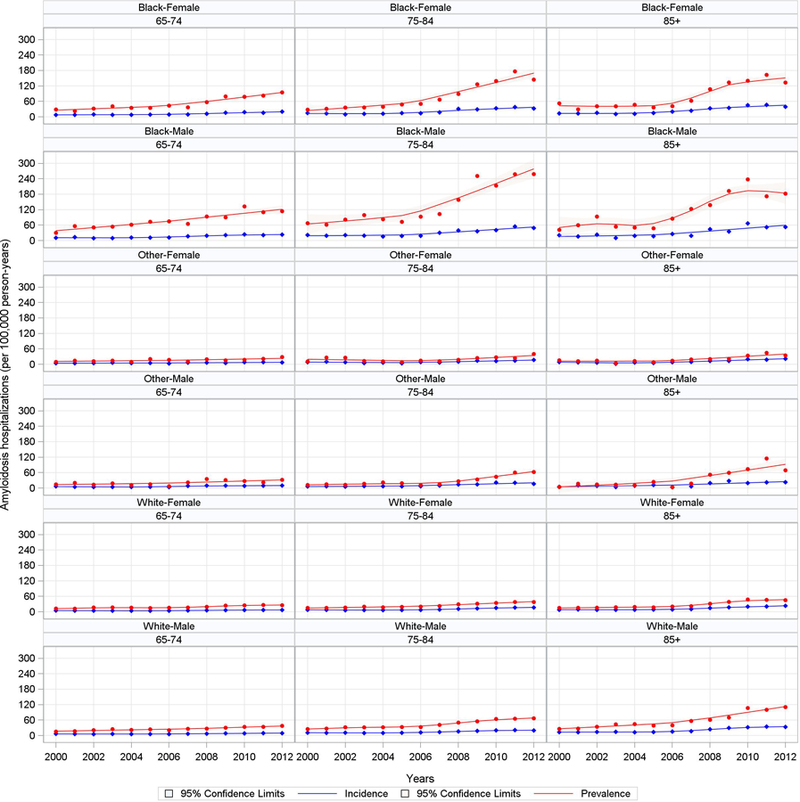
This figure shows the increasing frequency of all hospitalizations (prevalence) and first hospitalizations (incidence) of cardiac amyloidosis among predeteremined age and racial subgroups between 2000 and 2012. The most notable increases are observed among elderly black men and women. The red line shows the annual rate of all hospitalizations (prevalence) and the blue line shows the annual rate of first hospitalizations (incidence) for cardiac amyloidosis. The shaded areas represent the 95% confidence intervals.
Geographic variation in the annual number of cardiac amyloidosis-related hospitalizations are displayed in Figure 3. Similar to the age, sex and race variation figure described above, the number of first hospitalizations for cardiac amyloidosis increased slowly in most regions between 2000 and 2012 but the number of all hospitalizations increased steeply after 2006, again most pronounced in certain geographic regions -the Northeast (16 per 100,000 person-years to 75 per 100,000 person-years) and Midwest (from 15 per 100,000 person-years to 50 per 100,000 person-years).
Figure 3. Variation in the Annual Number of Cardiac Amyloidosis-related Hospitalizations by Geographic Region, 2000–2012.
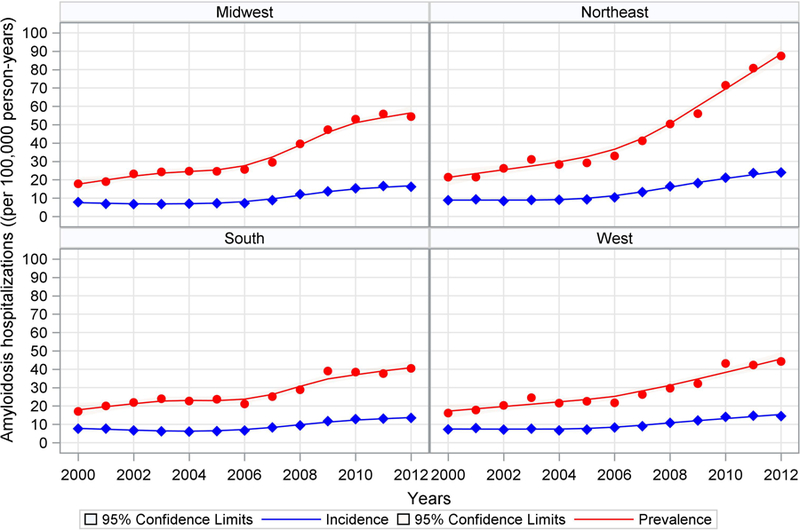
This figure shows the increasing frequency of all hospitalizaations (prevalence) and first hospitalizations (incidence) for cardiac amyloidosis by geographic regions between 2000 and 2012. The frequency of all hospitalizations increases in all geographic regions after 2006–2007. The steepest icnreases can be observed in the Midwest and Northeast. The red line shows the annual rate of all hospitalizations (prevalence) and the blue line shows the annual rate of first hospitalizations (incidence). The shaded areas represent the 95% confidence intervals.
The geographic distribution of hospitalization for cardiac amyloidosis, by beneficiary county of residence, between 2000 and 2012 are displayed in Figures 4a (prevalence) and 4b (incidence). The 2000 prevalence map demonstrates a prevalence rate <15 per 100,000 person-years for most of the country. However, there were three areas of higher hospitalization rates in the Northeast, upper Midwest and West Coast. By 2012, the prevalence rate had increased throughout the country with rates >75 per 100,000 person years in much of the Northeast/Atlantic coast, upper Midwest and Western cost. In 2000, the incidence map demonstrated rates <6 per 100,000 person-years for most of the country, again with a slightly higher rate in the upper Midwest. By 2012, incidence rates had similarly increased across the country with rates approaching 30/100,000 person-years, around Rochester, Minnesota and New York/Boston (30 per 100,000 person-years).
Figure 4. Geographic Distribution of Cardiac Amyloidosis Prevalence, Incidence and Temporal Changes between 2000 and 2012.
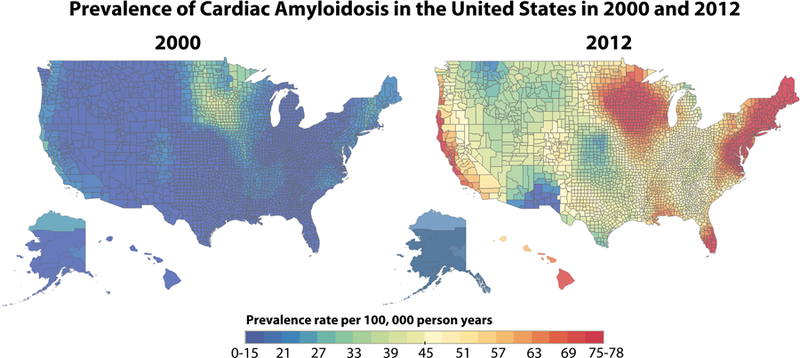
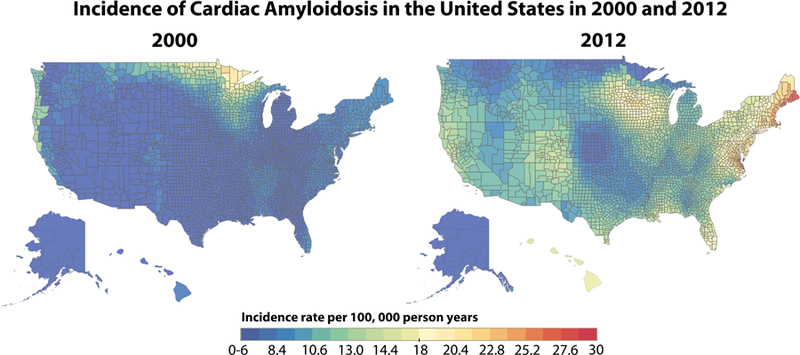
A: Geographic distribution of cardiac amyloidosis prevalence and temporal changes between 2000 and 2012. This figure shows the prevalence of hospitalization for cardiac amyloidosis between 2000 and 2012. The lower limit of prevalence on these maps (blue) is 0–15 cases per 100,000 person-years and the upper limit (red) is 75–78 cases per 100,000 person-years. B. Geographic distribution of cardiac amyloidosis incidence and temporal changes between 2000 and 2012. This figure shows the incidence of hospitalization for cardiac amyloidosis between 2000 and 2012. The lower limit of incidence on these maps (blue) is 0–6 cases per 100,000 person-years and the upper limit (red) is 30 cases per 100,000 person-years.
Discussion
This work builds on prior studies of systemic amyloidosis by establishing the first contemporary, national estimate of the prevalence and incidence of cardiac amyloidosis in the United States. This study measured the incidence and prevalence of hospitalization for all types of cardiac amyloidosis among Medicare beneficiaries ≥65 years old and found that there were at least 15,737 patients living with cardiac amyloidosis including 4,746 patients with new diagnosis of cardiac amyloidosis in 2012. The incidence rate of cardiac amyloidosis in 2012 was 17 per 100,000 person-years, an incidence rate comparable to that of infective endocarditis.11, 12 The prevalence rate of cardiac amyloidosis in 2012 was 55 per 100,000 person-years, a prevalence rate more than double that of pulmonary hypertension.13 Since a majority of the US population ≥65 years are enrolled in the fee-for-service Medicare program, these findings should be applicable to general US population ≥65 years.
Most prior work on epidemiology of amyloidosis has been limited to single-center populations, younger populations and registry populations.2, 14–17 Specifically, prior estimates from Olmstead County in 1992, estimated 3,200 new cases of AL amyloidosis per year in the United States.15 Another more recent study using commercial insurance data (i.e. a younger population aged 18–64 years) of AL amyloidosis, found a prevalence rate of 40.5 per million in 2015 and an incidence rate of 14 cases per million in 2015.16 In contrast, the THAOS (Transthyretin Amyloidosis Outcomes Survey) registry focused only on ATTR amyloidosis, finding that it was a disease of elderly men with a cardiac-predominant phenotype.17 Data from the National Amyloidosis Center in London, which provides care to a large cohort in the United Kingdom, suggests that the diagnosis of ATTRwt has increased exponentially there as well.18
Our study also describes the baseline characteristics of patients with cardiac amyloidosis. We found that among US patients ≥65 years old hospitalized with cardiac amyloidosis, 50% were women, 15% were black and 8% had been hospitalized at least once for HF in the previous year. Notably, blacks were overrepresented (15%) in this cohort of patients hospitalized with HF and amyloidosis compared to the overall US population where blacks comprised 8.7% of the population ≥65 years between 2000–2012 as estimated from the CDC’s WONDER Database.19
Paradoxically, hypertension, rather than hypotension, was present in over half of patients. This is consistent with the inclusion of older patients in this study, who are more likely to have coexistent hypertension and ATTR cardiac amyloidosis. This could also be an artifact of the way Medicare chronic conditions warehouse comorbidities are coded, but also raises the question of how much left ventricular thickening might have been previously misdiagnosed as hypertensive heart disease. Given the high frequency of both hypertensive heart disease and cardiac amyloidosis in African Americans, this observation highlights the importance of disease awareness in the initial work up of left ventricular thickening, especially with concurrent HF. As this study only included subjects ≥65 years, we may have specifically sub-selected for ATTR amyloidosis and the increased incidence and prevalence over time may be partly related to rising awareness and improved detection of ATTR form of amyloidosis. While the overall rate of HF hospitalization has been relatively flat since 2000,20 the rate of hospitalization for cardiac amyloidosis has risen since 2006, most notably among men, the elderly, and African Americans. We believe that the observed increase in incidence and prevalence of cardiac amyloidosis is likely related to increased awareness of amyloidosis and better detection rather than a change in natural history of the disease. It is interesting to note that the cardiac magnetic resonance imaging appearance of cardiac amyloidosis was first described in 2005, at a time when this technique is increasingly used for HF diagnosis. This may be responsible for part of the increase in incidence and prevalence observed after 2006. Moreover, in 2000, there were regional “hot spots” of higher incidence and prevalence clustering around amyloidosis centers of excellence particularly in the Midwest and in the Northeast regions of the country. However, since patients are coded by zip code of residence, this clustering of cardiac amyloidosis incidence and prevalence in certain regions implies better detection of cardiac amyloidosis in these regions, rather than hospitalization of out-of-state referral patients at these amyloidosis centers.
Despite these increases, we believe that cardiac amyloidosis remains underdiagnosed, especially in certain areas. A prior, smaller study from London found that 11.4% of Afro-Caribbean patients presenting with HF were ultimately diagnosed with cardiac amyloidosis, though this number may have been even higher as not all patients were event tested for amyloidosis.21 Moreover, we know that 3–4% of African Americans carry the high risk Val122I ATTR mutation.22 However, in our study, the incidence of cardiac amyloidosis increased less from 2000–2012 in the South than in any other region, despite over 50% of the African American population residing there.23 Taken together, these observations at least suggest that under diagnosis of cardiac amyloidosis may be a larger issue in the Southern United States, compared to other geographic regions.
Limitations
This study utilizes national, claims-based data and the findings should only be interpreted within the limitations of the data. First, we only have access to inpatient claims. This means that outpatient diagnoses of amyloidosis and HF may have been missed. The declining incidence of myeloma in this study from 2000–2012 also suggests that we may not have accurately measured the rates of AL amyloidosis in this study, likely an artifact of inclusion of only subjects aged ≥65 years. However, we do not believe this would affect the overall prevalence or incidence trends and if anything, this renders our incidence and prevalence estimates conservative. Secondly, because no specific ICD-9 code for cardiac amyloidosis exists, our estimates may again be conservative. In creating our claims-based definition of cardiac amyloidosis, we included all ICD-9 codes for HF, rather than just those for HF with preserved ejection fraction. We did this because the ability of ICD-9 codes to accurately distinguish between reduced and preserved ejection fraction is limited 24 and end-stage cardiac amyloidosis patients often develop low ejection fractions as the disease progresses. Ultimately, we believe that our findings are accurate, consistent with prior work and, if anything, conservative.
Conclusion
The incidence and prevalence rates of cardiac amyloidosis among hospitalized patients are increasing, most notably among men, those ≥75 years old and African Americans. These trends may be explained, by a combination of increased awareness and possibly a greater use of cardiac magnetic resonance imaging in clinical practice. To increase early diagnoses, minimize disparities in care, and increase the opportunity to be eligible for novel therapies, cardiac amyloidosis should be considered during the initial work up of HF in patients ≥65 years old.
Supplementary Material
What is new?
This is the first modern assessment of the incidence and prevalence rates of cardiac amyloidosis in the United States using national data.
This work demonstrates that the both the incidence and prevalence of cardiac amyloidosis have increased from 2000–2012, most notably after 2006, and among men, those ≥75 years old and African Americans.
What are the clinical implications?
Given these trends, cardiac amyloidosis should now be considered during the initial work up of a patient ≥65 years old presenting with new heart failure.
Acknowledgments
Sources of Funding
Dr. Gilstrap is supported by NIH/NHLBI K23HL142835 and NIH/NHLBI Heart Failure Research Network, Grant Funding U10HL110337.
Dr. Dorbala is supported by NIH RO1HL130563 and American Heart Association (AHA 16 CSA 2888 0004).
Dr. Falk is supported by NIH R01HL130563.
Footnotes
Disclosures
None of the authors have any relevant disclosures.
References
- 1.Maurer MS, Elliott P, Comenzo R, Semigran M and Rapezzi C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation 2017;135:1357–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR and Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014;2:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M and Falk RH. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM : monthly journal of the Association of Physicians 1998;91:141–57. [DOI] [PubMed] [Google Scholar]
- 4.Dharmarajan K and Maurer MS. Transthyretin cardiac amyloidoses in older North Americans. J Am Geriatr Soc 2012;60:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, Grogan M, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV and Dispenzieri A. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood 2017;129:2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C and Investigators A-AS. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 7.Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, K{Adams r, A. V., Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP, Vita G, Attarian S, Plante-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH 3rd, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA and Suhr OB Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med 2018;379:11–21. [DOI] [PubMed] [Google Scholar]
- 8.Zaslavsky AM, Ayanian JZ and Zaborski LB. The validity of race and ethnicity in enrollment data for Medicare beneficiaries. Health Serv Res 2012;47:1300–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creation of new race-ethnicity codes and socioeconomic status (SES) indicators for Medicare beneficiaries: final report Agency for Healthcare Research and Quality. 2012. Available at: https://pdfs.semanticscholar.org/abd8/d0c761bc4f0fcf9593727e93ef8c04cf1c0e.pdf. Accessed April 24, 2019. [Google Scholar]
- 10.CCW Chronic Conditions: Combined Medicare and Medicaid Data Centers for Medicare and Medicaid Services; 2016. Available at: https://aspe.hhs.gov/system/files/pdf/252376/AppendixA.pdf. Accessed April 24, 2019. [Google Scholar]
- 11.Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA and Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015;65:2070–6. [DOI] [PubMed] [Google Scholar]
- 12.Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH and Egorova NN. Trends in Infective Endocarditis in California and New York State, 1998–2013. JAMA 2017;317:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich S, Chomka E, Hasara L, Hart K, Drizd T, Joo E and Levy PS. The prevalence of pulmonary hypertension in the United States. Adult population estimates obtained from measurements of chest roentgenograms from the NHANES II Survey. Chest 1989;96:236–41. [DOI] [PubMed] [Google Scholar]
- 14.Lee MH, Lee SP, Kim YJ and Sohn DW. Incidence, diagnosis and prognosis of cardiac amyloidosis. Korean circulation journal 2013;43:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O’Fallon WM and Kurland LT. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992;79:1817–22. [PubMed] [Google Scholar]
- 16.Quock TP, Yan T, Chang E, Guthrie S and Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv 2018;2:1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Plante-Bordeneuve V, Coelho T, Mundayat R, Suhr OB, Waddington Cruz M, Rapezzi C and Investigators T. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 2016;68:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banypersad SM, Moon JC, Whelan C, Hawkins PN and Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc 2012;1:e000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS and Buxbaum JN. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997;336:466–73. [DOI] [PubMed] [Google Scholar]
- 20.CDC. Hospitalization for Congestive Heart Failure: United States, 2000–2010. NCHS Data Brief No 108 2012. [PubMed]
- 21.Dungu JN, Papadopoulou SA, Wykes K, Mahmood I, Marshall J, Valencia O, Fontana M, Whelan CJ, Gillmore JD, Hawkins PN and Anderson LJ. Afro-Caribbean Heart Failure in the United Kingdom: Cause, Outcomes, and ATTR V122I Cardiac Amyloidosis. Circ Heart Fail 2016;9:9. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson DR, Alexander AA, Tagoe C and Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid 2015;22:171–4. [DOI] [PubMed] [Google Scholar]
- 23.2010 Census Shows Black Population has Highest Concentration in the South US Census Bureau. 2010. Available at: https://www.census.gov/newsroom/releases/archives/2010_census/cb11-cn185.html. Accessed April 24, 2019. [Google Scholar]
- 24.Bovitz T, Gilbertson DT and Herzog CA. Administrative Data and the Philosopher’s Stone: Turning Heart Failure Claims Data into Quantitative Assessment of Left Ventricular Ejection Fraction. Am J Med 2016;129:223–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


