Abstract
Pancreatic ductal adenocarcinoma (PDAC) is currently the third leading cause of cancer mortality in the United States, with a 5-year survival of ~8%. PDAC is characterized by a dense and hypo-vascularized stroma consisting of proliferating cancer cells, cancer-associated fibroblasts, macrophages and immune cells, as well as excess matrices including collagens, fibronectin, and hyaluronic acid. In addition, PDAC has increased interstitial pressures and a hypoxic/acidic tumor microenvironment (TME) that impedes drug delivery and blocks cancer-directed immune mechanisms. In spite of increasing options in targeted therapy, PDAC has mostly remained treatment recalcitrant. Owing to its critical roles on governing PDAC progression and treatment outcome, TME and its interplay with the cancer cells are increasingly studied. In particular, three-dimensional (3D) hydrogels derived from or inspired by components in the TME are progressively developed. When properly designed, these hydrogels (e.g., Matrigel, collagen gel, hyaluronic acid-based, and semi-synthetic hydrogels) can provide pathophysiologically relevant compositions, conditions, and contexts for supporting PDAC cell fate processes. This review summarizes recent efforts in using 3D hydrogels for fundamental studies on cell-matrix or cell-cell interactions in PDAC.
Keywords: Pancreatic ductal adenocarcinoma, hydrogels, epithelial-mesenchymal transition, 3D culture, tumor microenvironment
Tumor microenvironment in PDAC
Many solid cancers have cancer cells that exhibit self-sufficiency in growth signals, unlimited cell growth, sustained ability to obtain nutrients, apoptosis resistance, insensitivity to growth inhibitory pathways, and the capacity to invade and metastasize [1]. Pancreatic ductal adenocarcinoma (PDAC) has similar characteristics. PDAC is currently the third leading cause of cancer mortality in the United States, with a 5-year survival of ~8% [2]. These slight increases in survival statistics in patients with PDAC are the consequence of improved imaging strategies and advances in chemotherapy but minimal improvement in cancer-directed immune activation strategies [3–5]. Thus, therapies with gemcitabine in combination with nab-paclitaxel or the combination of leucovorin (folinic acid), fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) have led to improvements in overall survival rates [6, 7]. However, in spite of expanding options in targeted therapy [8], PDAC has mostly remained-treatment recalcitrant. Moreover, the incidence of PDAC continues to increase slightly due to the aging of the United States population and the high prevalence of obesity and type 2 diabetes [9]. Moreover, it is anticipated that PDAC will become the second leading cause of cancer death in the United States during the 2020’s [10].
PDAC is generally resistant to chemotherapy or radiotherapy, and only 15% to 20% of patients with PDAC have resectable disease at the time of diagnosis [11]. PDAC’s biological aggressiveness is caused, in part, by the high frequency of major driver mutations that include KRAS, TP53, SMAD4, and CDKN2A, co-existing with numerous low-frequency driver mutations and enhanced cancer cell survival pathways including STAT3, and NFkB, and epigenetic alterations [12–15]. This aberrant genomic landscape leads to multiple dysfunctions that are compounded by the overexpression of tyrosine kinase receptors (TKRs) such as epidermal growth factor (EGF) receptor (EGFR), human EGFR 2 (HER2) and HER3, fibroblast growth factor (FGF) receptors (FGFRs), hepatocyte growth factor (HGF) receptor (MET), the AXL receptor, and insulin-like growth factor 1 (IGF-1) receptor [16]. In addition, serine-threonine kinases, such as the type 1 transforming growth factor beta (TpRI) and bone morphogenetic protein receptors (BMPR) can be overexpressed [17]. Often, the corresponding ligands such as transforming growth factor alpha (TGF-a), fibroblast growth factors (FGFs), insulin-like growth factor 1 (IGF-1), and hepatocyte growth factor (HGF), are also abundant, leading to multiple cross-talk pathways that promote mitogenic signaling and chemoresistance [18].
PDAC is characterized by a dense stroma with limited vascularization. PDAC stroma also contains proliferating cancer associated fibroblasts (CAFs), collagens, fibronectin and hyaluronic acid, leading to increased interstitial pressures, compression of scant vascular beds, impaired drug delivery, and a hypoxic and acidic tumor microenvironment (TME) [19–21]. The TME is also infiltrated with inflammatory cells and macrophages that produce immune suppressive cytokines to suppress cancer directed immune mechanisms [22]. Moreover, CAFs release large quantities of HGF that acts in a paracrine manner on the adjacent pancreatic cancer cells (PCCs) [23]. These cells then produce CXCL12 to attenuate cytotoxic T cell penetration into the tumor mass [22].
Given the above environmental alterations, PCC gain a growth advantage through the utilization of aberrant metabolic pathways, glutamine, alanine and lipids, and acquisition of additional nutrients through macropinocytosis and autophagy [24, 25]. It has therefore been suggested that it is important to reprogram the TME in a manner that decreases the high interstitial pressures, enhances drug delivery into the tumor mass, and interferes with the metabolic advantages created by that above pro-survival processes [26–29]. To achieve these goals, conventional two dimensional (2D) cell culture devices are insufficient, while animal studies may impose additional challenges regarding mechanistic understanding of the complex cell-matrix and cell-cell interactions, as well as the physical alterations that occur in the PDAC TME (Figure 1). As such, extracellular matrix (ECM)-derived and synthetic polymer-based three-dimensional (3D) hydrogels (Table 1) are increasingly developed and used for understanding the influences of microenvironment cues on cancer progression [30–35]. While the use of 3D matrices for PDAC research is in early stage, great strides have been made in recent years (Table 2). The remaining sections highlight recent advances in the use of 3D matrices for studying PDAC cell-matrix and cell-cell interactions.
Figure 1.
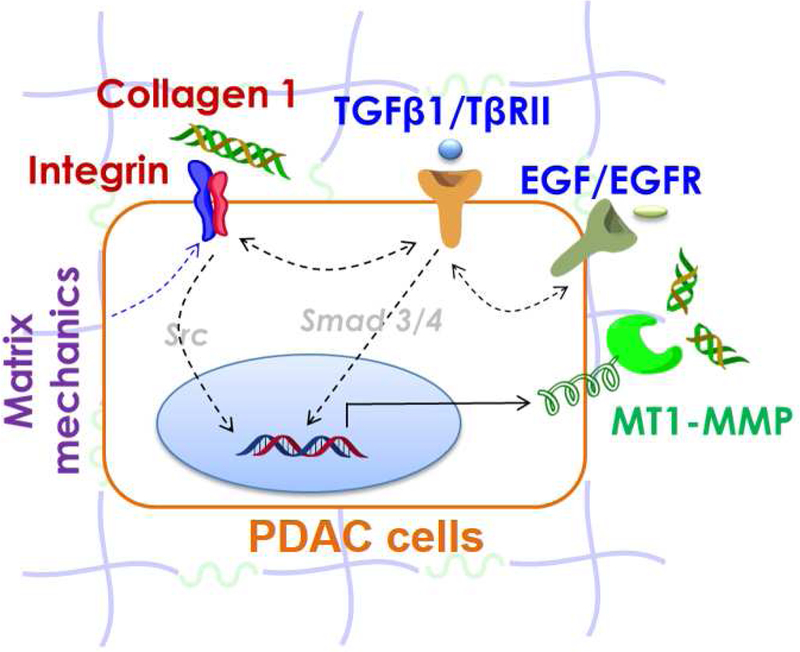
Schematic of PDAC tumor microenvironment. Major matrix factors regulating PDAC cell fate include, but not limited to, matrix mechanics, matrix ligand (e.g., collagen) and cytokine (e.g., TGFpi and EGF) signaling, and matrix metalloproteinase (e.g., MT1-MMP) activation. Src is downstream of many signaling cascades, including integrins and non-canonical TGF-b signaling, whereas Smad3/4 mediated canonical TGF-β signaling.
Table 1.
Advantages and disadvantages of commonly used cell-laden 3D hydrogels
| Materials | Advantages | Disadvantages |
|---|---|---|
| Matrigel | • Derived from basement membrane of animal tissue • Contains bioactive motifs for cell recognition |
• Difficult to tune matrix mechanics • Contains undefined compositions and residual growth factors • Pre-chilled tips/vessels are required as gelation occurs when temperature is above 10°C |
| Collagen gel | • Forms fibrous gel to closely mimic collagen-rich tumor ECM • Contains bioactive motifs for cell recognition |
• Acidic solution is required for dissolving collagen |
| Hyaluronic acid | • Provide HA ligands for receptor recognition • Mimic HA-rich tumor microenvironment |
• Purely HA gel does not provide integrin signaling |
| Purely synthetic polymers | • Well defined and tunable physical properties | • Lack bioactive motifs for cell recognition and degradation |
Table 2.
3D hydrogels for PDAC cell culture
| Materials | Focus | Stiffness | Cell types used | Reference |
|---|---|---|---|---|
| Matrigel | Cell polarity & organization | N/A | Panc-1, MIA PaCa-2, Su.86.86, BxPC-3 | [38] |
| Response to cytokines and inhibitors, and chemotherapeutics | N/A | AsPC-1, BxPC3, COLO- 357, T3M4, PK- 1, PK-2, Rlnk-2 |
[39] | |
| Effect of ruxolitinib on co-culture of PCCs and endothelial cells | N/A | Endothelial cells, Panc-1, IUSCC-PC1 | [41] | |
| Invasion assay | N/A | MIA PaCa-2, Panc-1, Hs766T, HPDE |
[42] | |
| Effect of hypoxia on enhanced aggressive phenotype | N/A | Pa03C, Pa02C, Panc10.05, CAF19, MIA PaCa2, UH1303–02 | [43] | |
| Quiescence/activation of pancreatic stellate cells | N/A | PSC | [44] | |
| Collagen | Gemcitabine resistance | N/A | Panc-1, CD18/HPAF-II | [46] |
| let-7 regulation & TGF-ß1- mediated MT1-MMP expression | N/A | Panc-1 | [47] | |
| MMP expression, EMT | N/A | HPDE, Panc-1, CD18, AsPC-1 | [48] | |
| MMP14 activation & invasion | N/A | Panc-1 | [49] | |
| Cellular contractility & matrix stiffness | 0.05–2 kPa | Panc-1, BxPC- 3, and AsPC-1, HaCat (keratinocyte), MDA-MB-231 (breast cancer) |
[50] | |
| Collagen fibrillar microstructure & EMT | 0.1–1 kPa | Panc-1, BxPC3, MIA PaCa-2 | [51] | |
| Hyaluronic acid | 3D cell carrier for orthotopic implantation of PCCs | N/A | MIA PaCa-2 | [52] |
| Effect of 3D matrix stiffness on PCC growth | 1, 2, 5 kPa | COLO-357 | [53] | |
| Effect of dynamic matrix stiffening on EMT | 1 to 5 kPa | COLO-357, Panc-1 |
[66] | |
| Fibronectin- coated polyacrylamide hydrogels (2D) |
Substrate rigidity on activation & durotaxis of pancreatic stellate cells | 1 to 25 kPa | PSC | [44] |
| PEG-peptide hydrogels |
Effect of integrin ligands & matrix stiffness on cell morphogenesis & EMT | 3 to 6 kPa | Panc-1 | [56] |
| Matrix properties and EGF receptor inhibition on the growth of PANC-1 cells | 2 to 12 kPa | Panc-1 | [55] | |
| Effect of matrix stiffness and collagen 1 on MT1-MMP expression & EMT | 2 kPa | COLO-357 | [54] | |
| Effect of dynamic matrix stiffening on PSC | 1 to 5 kPa | PSC | [65] |
3D culture of PDAC cells with ECM-derived hydrogels
Matrigel®
Matrigel is derived from basement membrane of Engelbreth-Holm-Swarm (EHS) mouse sarcoma and is rich in collagen IV, laminin, heparin sulfate proteoglycans (HSPG), as well as a variety of growth factors. Matrigel solidified/gelled when the temperature is above 10°C. Because of its tumor origin, Matrigel has been used for culturing a variety of cancer cells in 3D [36, 37], including PCCs [38]. For example, Reddy and colleagues showed that pancreatic ductal epithelial cells organized into spheroids with apical-basal polarity in Matrigel, whereas PCCs (e.g., MIA Paca-2, PANC-1) exhibited irregular cell shapes [38]. Using Matrigel overlaid on top of noble agar, Korc and colleagues examined morphological changes and cellular response of PCCs in 3D monocultures (ASPC1, BxPC3, COLO-357, T3M4, PK-1, PK-2, Rlnk-2) to EGF/TGF-β1, pharmacological inhibitors, and chemotherapeutics [39]. The authors concluded that 3D culture and co-incubation with SB431542 and erlotinib enhanced the efficacy of gemcitabine and cisplatin in PCCs and in primary cells derived from genetically-engineered mouse models of PDAC. The same group later developed a 3D co-culture system to show that growth of murine PCCs was enhanced in the presence of murine SVEC4 endothelial cells (ECs) and the enhanced growth was suppressed by ruxolitinib [40]. Interestingly, ruxolitinib failed to inhibit growth of these PCCs or ECs in monoculture, which was attributed to an angiocrine mechanism whereby ECs produce factors that promote PCC proliferation that could be suppressed by targeting JAK1–2 with ruxolitinib [30]. By contrast, when human PCCs were cocultured with human vascular endothelial cells (HUVECs) growth suppression was only obtained through concomitant targeting of TβRI kinase with SB505124 and JAK1 with ruxolitinib [41].
Matrigel-based matrices have also been used to study the influence of physical cues on PDAC cell fate. Rowat and colleagues used a modified scratch wound invasion assay where PCCs were overlaid with Matrigel [42]. PCCs exhibited significantly lower wound confluence and invasion through the Matrigel layer than a non-transformed control cell line. The study also found that cells with higher vimentin levels are more compliant (i.e., softer) but less invasive, which was in contrast to the established phenomenon that cells often express higher levels of vimentin and are more motile/invasive during epithelial-to-mesenchymal transition (EMT). Fisher and colleagues examined the effect of hypoxia on enhanced aggressive phenotype, metastatic potential, and impaired therapeutic efficacy of PCCs [43]. In addition to studying the effect of hypoxia on APE1/Ref-1 redox signaling activity, the authors performed 3D co-culture of PCCs with CAFs in reduced growth factor Matrigel. Results showed that APE1/Ref-1 signaling was dramatically enhanced in ex vivo 3D CAF/PCC co-culture. Dual blockade of APE1/Ref-1 and CA9 (carbonic anhydrase IX) using APX3330 and SLC-0111 induced effective tumor cell killing. del Rio Hernandez and colleagues showed that culturing pancreatic stellate cells (PSCs) on top of Matrigel for 6 days reverted activated PSCs to a quiescent-like phenotype as cells lost their spindle morphology and regain cytoplasmic lipid droplets [44]. The authors also cultured cells on top of fibronectin-coated polyacrylamide hydrogels with different stiffness (1 to 25 kPa). It appeared that increased matrix stiffness alone was sufficient to induce activation of PSCs. Furthermore, matrix with a stiffness gradient caused durotaxis of PSCs. Although these studies were conducted on 2D surface, the combination of Matrigel and synthetic hydrogels provide important insights into PSC activation and quiescence.
Collagen gels
Collagen 1 is one of the most highly secreted ECM proteins in PDAC stroma, or desmoplastic reaction. Depending on the source, the properties of collagen gels vary significantly [45]. For example, bovine collagen exhibits slow gelation and hence can be processed at room temperature. Bovine collagen also forms more regular fibrils that does not resemble collagen structure in vivo. On the other hand, rat tail-derived collagen gels faster and must be placed on ice to prevent pre-mature gelation. Rat tail collagen forms irregular fibril structure that may be more in vivo-like. Regardless of the source, all collagen fibrils are soluble and stable in acidic solutions at low temperatures. At above room temperature (20–25°C), collagen fibrils self-assemble into bundled fibers with diameters ranging from 12 to 120 nm [45]. The fibers further crosslink into 3D microporous matrix through physical association. Notably, the pH value of the gel solution must be adjusted to neutral for cell culture. Collagen regulates many aspects of cell fate processes and has been widely used in 3D culture of cancer cells. For example, Munshi and colleagues studied the effect of 3D PCC cultures in collagen gels on tumor suppression, expression of matrix metalloproteinase (MMP), invasion, and chemo- resistance [46–48]. In particular, PCCs grew in 3D collagen gels repressed tumor-suppressive let-7 family of microRNAs, partly via up-regulation of membrane type-1 MMP (e.g., MT1-MMP, also named MMP-14) expression and ERK1/2 activation [47]. These cells also demonstrated enhanced TGF-β1 signaling in collagen gel, whereas blocking TGF-β1 signaling attenuated collagen-induced signaling (e.g., MT1-MMP expression, ERK1/2 activation, and let-7 repression) [47]. Collagen 1 also induced Snail expression and MT1-MMP-dependent invasion [48]. PCCs were more resistant to gemcitabine treatment when culturing in collagen gels via HMGA2-dependent histone acetyltransferase expression [46]. However, the stiffness of these collagen gels was not reported.
Schneider and co-workers correlated the activity of MMP14 with stiffness of collagen gels. Specifically, MMP14 blocking antibodies were used to reveal its role on activating other soluble MMPs (e.g., MMP-2 and 9) under various matrix stiffness conditions [49]. Furthermore, inhibition of MMP-14 diminished invasion of PANC-1 cells into 3D collagen gels. However, this study did not characterize the exact stiffness of collagen gels formed at different concentrations (1 and 5 mg/mL), nor did it decouple the effect of matrix stiffness and collagen content on MMP expression. To address these issues, the authors used glutaraldehyde and transglutaminase to increase chemical crosslinking and stiffness of collagen gels without changing collagen concentration [50]. As such, ECM density and stiffness was decoupled. It was demonstrated that MMP activity was modulated by substrate stiffness (50–2,000 Pa).
Voytik-Harbin and colleagues examined EMT in PCCs using 3D collagen oligomer gels mixed with different ratios of Matrigel while maintaining constant matrix stiffness [51]. PCCs exhibited more spindle-shaped and single-celled morphology when cultured in soft (100 Pa) collagen oligomer gels, as opposed to round clusters when grew in Matrigel. Furthermore, exposure of PCCs to fibrillar collagen oligomer gels was sufficient to induce EMT. As fibril collagen oligomer density (0.9, 1.5, 2.1 mg/mL) and gel stiffness increased (100, 500, and 1,000 Pa), all PDAC lines (BxPC3 and MIA PaCa-2) growing as tight clusters owing to increased spatial constraints and matrix stiffness. It is worth noting that the stiff hydrogels produced from 2.1mg/mL collagen oligomer had shear modulus (G’) of 1 kPa (equivalent to Young’s modulus (E) of about 3 kPa), which is suboptimal, as recent work has shown that PDAC stroma has a much higher stiffness (i.e., E ~ 12kPa).
Hyaluronic acid (HA) hydrogels
Hyaluronic acid (HA) is a major glycosaminoglycan (GAG) excessively expressed and accumulated in PDAC stroma. HA binds to and activates CD44 and receptor for hyaluronan-mediated motility (RHAMM), leading to cancer cell proliferation, invasion, and drug resistance. Accumulation of HA also causes elevated fluid stress that not only induces abnormal mechanosensing in the cancer cells but also limits penetration of anti-cancer drugs to tumor cells. Chemically crosslinked HA hydrogels have been used to provide stable network for longterm cell study. Scaife and colleagues used thiolated HA, thiolated gelatin, and PEG-diacrylate (PEGDA) to form chemically crosslinked ‘HA-G’ hydrogels via Michael-type addition reaction [52]. MiaPaCa-2 cells were homogeneously encapsulated in the injectable HA-G hydrogels for orthotopic implantation in nude mouse model of PDAC. HA-G gels with PCCs appeared to exhibit more consistent tumor growth and higher rate of metastasis at 8 weeks of tumor growth. While this study showed HA as an effective 3D cell carrier for orthotopic implantation of PCCs, the influence of HA on PDAC cell fate processes was not explored. The use of HA hydrogels as scaffold for 3D culture of PCCs did not receive significant attention until recently. We have developed HA-gelatin hydrogels via visible light based thiol-norbornene photopolymerization [53]. Specifically, thiolated HA (THA) and norbornene-modified gelatin (GelNB) were modularly crosslinked into hydrogels within minutes by means of visible light irradiation. The orthogonal reactivity between thiol and norbornene permits modular control over biochemical and biophysical properties without affecting other critical parameters capable of guiding PDAC cell fate. In particular, THA/GelNB hydrogels were formed at identical chemical compositions but varied matrix stiffness (G’ = 1, 2, and 5 kPa), which was achieved via supplementing PEG-tetra- thiol (PEG4SH). These modular hydrogels were used to examine the effect of stiffness on growth and expression in COLO-357 cells, a PCC line with wild type KRAS. Results showed that gels formed with higher crosslinking density and stiffness (G’ ~ 5 kPa) led to cell cultures with smaller diameters. High stiffness downregulated CTGF mRNA levels, suggesting potential inactivation of the HIPPO pathway that may contribute to tumor progression and metastasis. On the other hand, the expression of sonic hedgehog (SHH) and MMP14 mRNA were significantly upregulated in cells growing in stiffer gels. SHH has been implicated in enhanced drug resistance, whereas upregulation of MMP14 is indicative of higher matrix cleavage and metastatic potential in the encapsulated cells.
3D culture of pancreatic cancer cells with semi-synthetic hydrogels
Semi-synthetic matrices
Hydrogels prepared from synthetic polymers, such as derivatives of poly(ethylene glycol) (PEG), have been used to support the survival of PCCs in 3D [54–56] and to deliver apoptotic/anti-cancer agents to treat PDAC [57–59]. To mimic a cancer cell niche using synthetic hydrogels, it is common to include biomimetic peptides for gel crosslinking and for receptor activation [56, 60]. In one example, multi-arm PEG-based macromers (e.g., PEG-norbornene, PEG-maleimide, PEG-vinylsulfone, etc.) are crosslinked by MMP cleavable peptides flanked with bis-cysteines via step-growth photopolymerizations [60]. The resulting ‘thiol-ene’ networks are more homogeneous and have superior cyto-compatibility when comparing with similar hydrogels formed by random chain-growth polymerization [61].
PEG-peptide hydrogels formed by thiol-norbornene click reaction has been used to evaluate the effect of matrix compositions on PANC-1 cell growth and morphogenesis in 3D. [56] Specifically, PANC-1 cells formed small multicellular clusters in thiol-ene hydrogels within 4 days of in vitro culture. After 10-day, the growth and structures of these clusters were significantly impacted by gel matrix properties, including gel degradability, stiffness, and immobilized peptide ligands. The use of matrix metalloproteinase (MMP) sensitive linker or the immobilization of fibronectin-derived RGDS ligand in the matrix promoted PANC-1 cell growth and encouraged them to adopt ductal cyst-like structures. On the other hand, the encapsulated cells formed smaller and more compact aggregates in non-MMP responsive gels. The incorporation of laminin-derived YIGSR peptide did not enhance cell growth and caused the cells to form compact aggregates. Immobilized YIGSR also enhanced the expression of epithelial cell markers including β-catenin and E-cadherin. Our group used a similar PEG-based thiol-norbornene hydrogel to show that the presence of collagen 1 enhanced cell proliferation and Yes-associated protein (YAP) translocation to nuclei of COLO-357 cells [54]. Furthermore, cytokines and collagen 1 synergistically up-regulated MT1-MMP (i.e., MMP-14) expression and induced cell spreading, which could be attributed to EMT. COLO-357 cells grew in 3D developed chemo-resistance even in the absence of collagen 1 and cytokines, as well as expressed high levels of CD24, SHH, and VEGF. In another example, we studied the influence of matrix properties and EGF receptor inhibition on the growth of PANC-1 cells [55]. Unsurprisingly, cells retained high viability and formed clusters in softer hydrogels (G’ ~ 2 kPa). On the other hand, more cell death and smaller cell clusters were observed whereas cells encapsulated in stiff hydrogels (G’ ~ 12 kPa). Furthermore, the immobilization of an EGFR peptide inhibitor (Asn-Tyr-Gln-Gln-Asn or NYQQN) only caused Akt-dependent cell apoptosis in stiff hydrogels but not in soft hydrogels, highlighting the importance of matrix physical properties on drug sensitivity in PCCs.
Dynamic hydrogels to probe PDAC cell fate
The biochemical compositions and biophysical properties in TME vary greatly depending on the stages of tumor development. The dynamic evolution of stromal tissue stiffness could lead to mechanosensing to both cancer cells and stromal cells. As such, hydrogels capable of recapitulating the dynamic landscape of extracellular microenvironment are of great importance for fundamental understanding of matrix-induced aberrant cell-matrix interactions [62, 63]. Recent work has shown that the stiffness of malignant PDAC tissues ranges from 2–6 kPa in shear modulus (equivalent to ~6–18 kPa in Young’s Modulus), whereas that of the healthy tissue is around 1 kPa [64]. Synthetic approaches commonly used to mimic a stiffening tissue often rely on performing secondary crosslinking within the primary cell-laden hydrogels [62]. For example, we have reported a cytocompatible enzyme-responsive matrix stiffening strategy [65, 66]. The initial gelation and cell encapsulation were achieved by thiol-norbornene crosslinking of 8-arm PEG-norbornene (PEG8NB) and bis-cysteine peptides (i.e., KCYGPQGIWGQYCK or YGKCYGPQGIWGQYCKGY). This simple peptide linker contains sequence sensitive to matrix metalloproteinase (MMP) induced cleavage, as well as additional tyrosine residues for tyrosinase-triggered di-tyrosine crosslinking [65]. Following thiol-norbornene gelation, the tyrosine residues in the primary network served as substrates for exogenously added tyrosinase (TYR). As TYR diffused in hydrogels, it catalyzed dimerization of tyrosines and led to higher gel crosslinking density and stiffness. We demonstrated that enzyme-triggered and on-demand stiffened hydrogels altered morphology of PSCs cultured in 3D. PSCs also expressed higher level of a-smooth muscle actin (αSMA), a signature marker of myofibroblastic activation.
Although the TYR-stiffened PEG-peptide hydrogels have been useful in studying the effect of dynamic matrix stiffening on cancer stromal cell fate, these gels represented minimal tumor-related matrix components. In a separate study, we designed a pathophysiologically relevant dynamic biomimetic hydrogel system where the gels were crosslinked by thiolated HA (THA) and norbornene/hydroxyphenylacetic acid dually-functionalized gelatin (i.e., GelNB-HPA). [66]The initial gel network was formed by orthogonal thiol-norbornene photopolymerization of GelNB, GelNB-HPA, THA, and inert macromer PEG-tetra-thiol (PEG4SH) [53]. With this hybrid dynamic hydrogel system, the effects of matrix biochemical and biophysical cues were easily decoupled for gaining new insights into the effects of matrix compositions on PDAC cell fate processes.
Conclusion and Outlook
Cell-laden hydrogels are increasingly used in cancer cell studies. Overall, animal derived matrices are advantageous owing to their inherent biological motifs for cell attachment and invasion. However, the batch-dependent material compositions and properties, as well as residual growth factors could confound the interpretation of the experimental results (Table 1). While gelation of Matrigel is easily achieved through controlling temperature, pre-cooled pipet tips, microtubes, and cell culture vessels are needed to prevent pre-mature gelation. On the other hand, significant acidic solution is required for preparing collagen gels. Compared with Matrigel and collagen gels, hydrogels crosslinked by derivatives of HA have not been widely used for PDAC cell research. Although HA alone does not provide necessary ligands for integrin signaling, it does activate cell surface receptors such as CD44 and Receptor for hyaluronan-mediated motility (RHAMM). Our work has shown that HA and matrix stiffness synergistically induce EMT in PCCs [66]. Hence, future work should explore the effects of HA and its synergistic signaling with integrin ligands or with other physical cues on PDAC cells fate in 3D.
Conventional hydrogels are often composed of polymers crosslinked by covalent bonds that exhibit purely elastic properties. These elastic hydrogels are excellent artificial tissue mimics for recapitulating aspects of native ECM, including elasticity, permeability, and presentations of bioactive motifs. However, purely elastic hydrogels do not capture the viscoelastic and stress-relaxation properties of native tissues, which may play a significant role in cell fate processes and tissue development. Recent efforts have addressed this through developing advanced hydrogels with reversible crosslinks that can be reformed after breaking up by local cellular processes [67–69]. This new class of reversible/adaptable hydrogels is highly desirable for studies concerning the influence of matrix viscoelastic properties on cell behavior and gene expression. In addition to immobilizing pendant ligand in the presence of cells, one may wish to introduce different integrin ligands at different state of tumor development. In this regard, an addition-fragmentation-chain transfer reaction was developed to allow controlled and reversible exchange of biochemical ligands within an allyl sulfide functionalized PEG hydrogel. [70]Ligand ‘exchange’ could also be achieved via Sortase A mediated peptide ligation or protein labeling [71]. These approaches allows user-defined introduction of immobilized ligands during cell culture, which may be highly useful in understanding the influence of temporal presentation of selective ligands on cancer cell fate. Moreover, bioengineered hydrogels capable of inducing hypoxia could be used to study the synergistic influence of low oxygen tension and other matrix properties on PDAC cell fate [72, 73]. Finally, HA-based hydrogels can be designed/fabricated to enable in vitro evaluation of hydrostatic pressures on drug delivery to PCCs. Collectively, these approaches will not only increase our understanding of the complex PDAC TME, but may lead to the discovery of novel therapeutic options for this deadly cancer.
Highlights.
Tumor microenvironment (TME) governs progression and treatment outcome of pancreatic ductal adenocarcinoma (PDAC).
Three-dimensional (3D) hydrogels derived from or inspired by components in the TME are progressively developed to recapitulate PDAC tumor matrix.
Cell-laden hydrogels (e.g., Matrigel, collagen gel, hyaluronic acid-based, and semi-synthetic hydrogels) can provide pathophysiologically relevant compositions, conditions, and contexts for supporting PDAC cell fate processes.
This review summarizes recent efforts in using 3D hydrogels for fundamental studies on cell-matrix or cell-cell interactions in PDAC.
Acknowledgments
This work was supported, in part, by National Science Foundation (NSF) CAREER Award (#1452390) to C.C.L and National Cancer Institute (NCI) grant (CA-075059) to M.K.
Footnotes
AUTHOR DECLARATION
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from lincc@iupui.edu
Signed by all authors as follows:
Chien-Chi Lin, PhD.
Murray Korc, MD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS- 207) boost vaccines for metastatic pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nature reviews Disease primers. 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- [5].Inman KS, Francis AA, Murray NR. Complex role for the immune system in initiation and progression of pancreatic cancer. World journal of gastroenterology. 2014;20:11160–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. nab- Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. Journal of the National Cancer Institute. 2015;107. [DOI] [PubMed] [Google Scholar]
- [7].Sheahan AV, Biankin AV, Parish CR, Khachigian LM. Targeted therapies in the management of locally advanced and metastatic pancreatic cancer: a systematic review. Oncotarget. 2018;9:21613–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Andersen DK, Korc M, Petersen GM, Eibl G, Li D, Rickels MR, et al. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. 2017;66:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Manji GA, Olive KP, Saenger YM, Oberstein P. Current and Emerging Therapies in Metastatic Pancreatic Cancer. Diabetes. 2017;23:1670–8. [DOI] [PubMed] [Google Scholar]
- [10].Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- [11].Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. The New England journal of medicine. 2014;371:2140–1. [DOI] [PubMed] [Google Scholar]
- [12].Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Humphris JL, Patch AM, Nones K, Bailey PJ, Johns AL, McKay S, et al. Hypermutation In Pancreatic Cancer. Gastroenterology. 2017;152:68–74.e2. [DOI] [PubMed] [Google Scholar]
- [14].Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer research. 2011;71:5020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR. Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer research. 2010;70:2064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Preis M, Korc M. Signaling pathways in pancreatic cancer. Critical reviews in eukaryotic gene expression. 2011;21:115–29. [DOI] [PubMed] [Google Scholar]
- [17].Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. [DOI] [PubMed] [Google Scholar]
- [18].Pothula SP, Xu Z, Goldstein D, Biankin AV, Pirola RC, Wilson JS, et al. Hepatocyte growth factor inhibition: a novel therapeutic approach in pancreatic cancer. British journal of cancer. 2016;114:269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science (New York, NY). 2011;331:1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garg B, Giri B, Modi S, Sethi V, Castro I, Umland O, et al. NFkappaB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Upregulation of CXCL12. Gastroenterology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pothula SP, Xu Z, Goldstein D, Merrett N, Pirola RC, Wilson JS, et al. Targeting the HGF/c- MET pathway: stromal remodelling in pancreatic cancer. Oncotarget. 2017;8:76722–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang A, Herter-Sprie G, Zhang H, Lin EY, Biancur D, Wang X, et al. Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous Mechanisms. Cancer discovery. 2018;8:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer cell. 2014;25:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin D receptor- mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer cell. 2014;25:711–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnology advances. 2014;32:1256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lewis DM, Park KM, Tang V, Xu Y, Pak K, Eisinger-Mathason TS, et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:9292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ozdemir T, Fowler EW, Liu S, Harrington DA, Witt RL, Farach-Carson MC, et al. Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D. ACS biomaterials science & engineering. 2016;2:2217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Park KM, Lewis D, Gerecht S. Bioinspired Hydrogels to Engineer Cancer Microenvironments. Annual review of biomedical engineering. 2017;19:109–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nguyen TV, Sleiman M, Moriarty T, Herrick WG, Peyton SR. Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials. 2014;35:5749–59. [DOI] [PubMed] [Google Scholar]
- [35].Gencoglu MF, Barney LE, Hall CL, Brooks EA, Schwartz AD, Corbett DC, et al. Comparative Study of Multicellular Tumor Spheroid Formation Methods and Implications for Drug Screening. ACS biomaterials science & engineering. 2018;4:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1996;74:833–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nature reviews Cancer. 2005;5:675–88. [DOI] [PubMed] [Google Scholar]
- [38].Gutierrez-Barrera AM, Menter DG, Abbruzzese JL, Reddy SA. Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochemical and biophysical research communications. 2007;358:698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sempere LF, Gunn JR, Korc M. A novel 3-dimensional culture system uncovers growth stimulatory actions by TGFbeta in pancreatic cancer cells. Cancer biology & therapy. 2011;12:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gore J, Craven KE, Wilson JL, Cote GA, Cheng M, Nguyen HV, et al. TCGA data and patient-derived orthotopic xenografts highlight pancreatic cancer-associated angiogenesis. Oncotarget. 2015;6:7504–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Craven KE, Gore J, Wilson JL, Korc M. Angiogenic gene signature in human pancreatic cancer correlates with TGF-beta and inflammatory transcriptomes. Oncotarget. 2016;7:323–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nguyen AV, Nyberg KD, Scott MB, Welsh AM, Nguyen AH, Wu N, et al. Stiffness of pancreatic cancer cells is associated with increased invasive potential. Integrative biology : quantitative biosciences from nano to macro. 2016;8:1232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Logsdon DP, Grimard M, Luo M, Shahda S, Jiang Y, Tong Y, et al. Regulation of HIF1alpha under Hypoxia by APE1/Ref-1 Impacts CA9 Expression: Dual Targeting in Patient-Derived 3D Pancreatic Cancer Models. Molecular cancer therapeutics. 2016;15:2722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lachowski D, Cortes E, Pink D, Chronopoulos A, Karim SA, J PM, et al. Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Scientific reports. 2017;7:2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Antoine EE, Vlachos PP, Rylander MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue engineering Part B, Reviews. 2014;20:683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dangi-Garimella S, Sahai V, Ebine K, Kumar K, Munshi HG. Three-dimensional collagen I promotes gemcitabine resistance in vitro in pancreatic cancer cells through HMGA2-dependent histone acetyltransferase expression. PloS one. 2013;8:e64566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dangi-Garimella S, Strouch MJ, Grippo PJ, Bentrem DJ, Munshi HG. Collagen regulation of let-7 in pancreatic cancer involves TGF-beta1-mediated membrane type 1-matrix metalloproteinase expression. Oncogene. 2011;30:1002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shields MA, Dangi-Garimella S, Krantz SB, Bentrem DJ, Munshi HG. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. The Journal of biological chemistry. 2011;286:10495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Haage A, Nam DH, Ge X, Schneider IC. Matrix metalloproteinase-14 is a mechanically regulated activator of secreted MMPs and invasion. Biochemical and biophysical research communications. 2014;450:213–8. [DOI] [PubMed] [Google Scholar]
- [50].Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:3589–99. [DOI] [PubMed] [Google Scholar]
- [51].Puls TJ, Tan X, Whittington CF, Voytik-Harbin SL. 3D collagen fibrillar microstructure guides pancreatic cancer cell phenotype and serves as a critical design parameter for phenotypic models of EMT. PloS one. 2017;12:e0188870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Scaife CL, Shea JE, Dai Q, Firpo MA, Prestwich GD, Mulvihill SJ. Synthetic extracellular matrix enhances tumor growth and metastasis in an orthotopic mouse model of pancreatic adenocarcinoma. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2008;12:1074–80. [DOI] [PubMed] [Google Scholar]
- [53].Shih H, Greene T, Korc M, Lin CC. Modular and Adaptable Tumor Niche Prepared from Visible Light Initiated Thiol-Norbornene Photopolymerization. Biomacromolecules. 2016;17:3872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ki CS, Lin TY, Korc M, Lin CC. Thiol-ene hydrogels as desmoplasia-mimetic matrices for modeling pancreatic cancer cell growth, invasion, and drug resistance. Biomaterials. 2014;35:9668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ki CS, Shih H, Lin CC. Effect of 3D matrix compositions on the efficacy of EGFR inhibition in pancreatic ductal adenocarcinoma cells. Biomacromolecules. 2013;14:3017–26. [DOI] [PubMed] [Google Scholar]
- [56].Raza A, Ki CS, Lin CC. The influence of matrix properties on growth and morphogenesis of human pancreatic ductal epithelial cells in 3D. Biomaterials. 2013;34:5117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Byeon HJ, Choi SH, Choi JS, Kim I, Shin BS, Lee ES, et al. Four-arm PEG cross-linked hyaluronic acid hydrogels containing PEGylated apoptotic TRAIL protein for treating pancreatic cancer. Acta biomaterialia. 2014;10:142–50. [DOI] [PubMed] [Google Scholar]
- [58].Kim I, Choi JS, Lee S, Byeon HJ, Lee ES, Shin BS, et al. In situ facile-forming PEG crosslinked albumin hydrogels loaded with an apoptotic TRAIL protein. Journal of controlled release : official journal of the Controlled Release Society. 2015;214:30–9. [DOI] [PubMed] [Google Scholar]
- [59].Nogueira DR, Yaylim I, Aamir Q, Kahraman OT, Fayyaz S, Kamran-ul-Hassan Naqvi S, et al. TRAIL mediated signaling in pancreatic cancer. Asian Pacific journal of cancer prevention : APJCP. 2014;15:5977–82. [DOI] [PubMed] [Google Scholar]
- [60].Lin CC. Recent advances in crosslinking chemistry of biomimetic poly(ethylene glycol) hydrogels. RSC advances. 2015;5:39844–398583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lin CC, Raza A, Shih H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials. 2011;32:9685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Brown TE, Anseth KS. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chemical Society reviews. 2017;46:6532–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tam RY, Smith LJ, Shoichet MS. Engineering Cellular Microenvironments with Photo- and Enzymatically Responsive Hydrogels: Toward Biomimetic 3D Cell Culture Models. Accounts of chemical research. 2017;50:703–13. [DOI] [PubMed] [Google Scholar]
- [64].Rubiano A, Delitto D, Han S, Gerber M, Galitz C, Trevino J, et al. Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta biomaterialia. 2018;67:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu HY, Greene T, Lin TY, Dawes CS, Korc M, Lin CC. Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells. Acta biomaterialia. 2017;48:258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Liu HY, Korc M, Lin CC. Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials. 2018;160:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].McKinnon DD, Domaille DW, Brown TE, Kyburz KA, Kiyotake E, Cha JN, et al. Measuring cellular forces using bis-aliphatic hydrazone crosslinked stress-relaxing hydrogels. Soft matter. 2014;10:9230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McKinnon DD, Domaille DW, Cha JN, Anseth KS. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Advanced materials (Deerfield Beach, Fla). 2014;26:865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang H, Heilshorn SC. Adaptable hydrogel networks with reversible linkages for tissue engineering. Advanced materials (Deerfield Beach, Fla). 2015;27:3717–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gandavarapu NR, Azagarsamy MA, Anseth KS. Photo-click living strategy for controlled, reversible exchange of biochemical ligands. Advanced materials (Deerfield Beach, Fla). 2014;26:2521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cambria E, Renggli K, Ahrens CC, Cook CD, Kroll C, Krueger AT, et al. Covalent Modification of Synthetic Hydrogels with Bioactive Proteins via Sortase-Mediated Ligation. Biomacromolecules. 2015; 16:2316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Park KM, Gerecht S. Hypoxia-inducible hydrogels. Nature communications. 2014;5:4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dawes CS, Konig H, Lin CC. Enzyme-immobilized hydrogels to create hypoxia for in vitro cancer cell culture. Journal of biotechnology. 2017;248:25–34. [DOI] [PubMed] [Google Scholar]


