Abstract
Folate metabolism in the brain is critically important and serves a number of vital roles in nucleotide synthesis, single carbon metabolism/methylation, amino acid metabolism, and mitochondrial translation. Genetic defects in almost every enzyme of folate metabolism have been reported to date, and most have neurological sequelae. We report 2 patients presenting with a neurometabolic disorder associated with biallelic variants in the MTHFS gene, encoding 5,10-methenyltetrahydrofolate synthetase. Both patients presented with microcephaly, short stature, severe global developmental delay, progressive spasticity, epilepsy, and cerebral hypomyelination. Baseline CSF 5-methyltetrahydrolate (5-MTHF) levels were in the low-normal range. The first patient was treated with folinic acid, which resulted in worsening cerebral folate deficiency. Treatment in this patient with a combination of oral L-5-methyltetrahydrofolate and intramuscular methylcobalamin was able to increase CSF 5-MTHF levels, was well tolerated over a 4 month period, and resulted in subjective mild improvements in functioning. Measurement of MTHFS enzyme activity in fibroblasts confirmed reduced activity. The direct substrate of the MTHFS reaction, 5-formyl-THF, was elevated 30-fold in patient fibroblasts compared to control, supporting the hypothesis that the pathophysiology of this disorder is a manifestation of toxicity from this metabolite.
Keywords: Folate; Folinic acid; Neurometabolism; Neurodegeneration; 5,10-methenyltetrahydrofolate synthetase
1. Introduction:
Folate metabolism in the brain is critically important, and serves a number of vital roles involving nucleotide synthesis, single carbon metabolism/methylation, amino acid metabolism, and mitochondrial translation. Genetic defects in almost every enzyme of folate metabolism have been reported to date, and most have neurological sequelae. In this paper, we report the phenotype of a 5,10-methenyltetrahydrofolate synthetase deficiency syndrome in 2 patients with biallelic loss-of-function variants in the MTHFS gene.
2. Materials and Methods:
2.1. Measurement of MTHFS enzyme activity in fibroblasts:
The fibroblast samples were procured by The Manton Center for Orphan Disease Research, Gene Discovery Core under informed consent governed by the Institutional Review Board of Boston Children’s Hospital. Control and patient 1 fibroblasts were grown in DMEM based media (Gibco, 11965) supplemented with 10% fetal bovine serum (BenchMark 100–106 or Gibco 26140–079), 2mM L-glutamine (Gibco, 25030), 1 % non-essential amino acids (Gibco, 11140) and 1 mM sodium pyruvate (Gibco, 11360) at a 37°C incubator with humidified atmosphere of 5% CO2. Cells were harvested with cold PBS after they were grown to confluency. The pellet is stored in -80°C for further analyses. The cell lysate was prepared by gently resuspending the cell pellet in 80–150 μL PBS and sonicating for 15 seconds at level 1 (Sonic Dismembrator model 100, Fisher Scientific) three times with one minute intervals. The homogenate was then centrifuged at 14,000 rpm for 10 min at 4°C (5402R, Eppendorf). Protein concentration was quantified by DC kit (Bio-RAD). Cell lysate with adjusted concentration was used in the enzyme reaction. The reaction mixture was prepared by combining one volume of enzyme cocktail with one volume of cell lysate. The enzyme cocktail contains final concentrations of 50 mM MES buffer (pH: 6.0; Sigma M-5287), 10 mM mercaptoethanol, 10 mM magnesium acetate, 1 mM ATP and 0.2 or 0.1 mM folinic acid (Sigma, 47612). The reaction mixture was incubated at 37°C at different time points. The reaction was stopped by combining 1 volume of reaction mixture with 9 volumes of 80μL of ACN/water (8/2, v/v) and 10uL of 0.5M methotrexate (Alfa Aesar, J63075) as internal standard. The mixture was then vortexed for 30 seconds and centrifuged prior to transferring into autosampler vials.
The analyte was measured using an AB Sciex QTrap 5500 mass spectrometer equipped with a Shimadzu HPLC of 2 LC-20AD XR pumps and SIL-20AC XR auto-sampler. The system was operated in positive-ion electrospray ionization mode with multiple reaction monitoring scanning. Acquity UPLC HSS C18 column (Waters, 186004687) was used at room temperature and autosampler tray was kept at 4°C. The parameters for LC-MS/MS are listed in Supplemental Table 1. Analytes were quantified by integrating the LC-MS/MS peak areas with Analyst 4.0 software. A series of standards with final concentrations of 0.01, 0.02, 0.05, 0.1, 0.2, 0.5 and 1.0 μM of 5,10-methenylTHF (Schircks Laboratories, 16.230) and 0.05 μM of methotrexate (internal starndard) were used. Negative control samples included a reaction mixture prepared with PBS instead of cell lysate as well as a blank sample for which reaction mixture with cell lysate was boiled for 3 minutes prior to 37°C incubation.
2.2. Quantification of folate species in fibroblasts:
Fibroblasts were cultured in 10 cm dishes in standard DMEM (Cellgro) supplemented with 20% FBS (Sigma-Aldrich), 1% NEAA, 1% glutamax and 1% sodium pyruvate solution (GIBCO). Fresh media was added to cultures 2 hours before harvesting. Media was removed and cells were lysed immediately without washing using 1.5 mL of ice-cold 1:1 H2O: Acetonitrile lysis buffer freshly made with 25mM sodium ascorbate and 25 mM ammonium acetate (pH 7.0). Samples were incubated on ice for 20 min before scraping into a microcentrifuge tube and heating at 60°C for 5 min. Samples were returned to ice and folates extracted following the procedure of Chen et al (Chen et al. 2018). Upon elution from an SPE column, samples were dried down, resuspended in 50 μL water and ran on a Thermo QExactive Orbitrap mass spectrometer with a Vanquish UHPLC system. 5-formyl-THF standard was obtained from Schrick laboratories and added to certain extracted samples at 50 ng/ mL final. Samples were run in reverse phase separated on a Synergy Hydro-RP column (Phenomenex) and analyzed in negative ion mode (Chen et al. 2018). Data was analyzed using the Maven software suite.
3. Results:
3.1. Case 1
Patient 1 is an 8-year-old male with global developmental delay, microcephaly, short stature, epilepsy, spasticity, and cerebral hypomyelination. He was the product of an uncomplicated pregnancy and term delivery. First concerns arose at 3 months of age when he was noted to have developmental delay, feeding difficulties, an exaggerated startle response, short stature (length approximately 3 standard deviations below mean), and microcephaly (head circumference approximately 4 standard deviations below mean). MRI performed at 3 months of age demonstrated delayed myelination, mild lateral ventriculomegaly and slight prominence of the cerebellar fissures (Figure 1A, E, I). An EEG was performed because of his exaggerated startle and demonstrated multifocal sharp waves and intermittent generalized slowing.
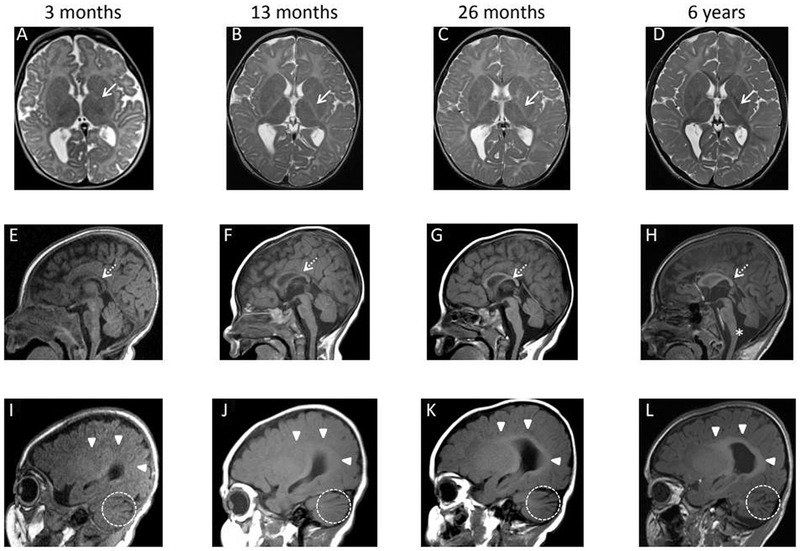
Figure 1.
Axial T2 (A-D), midsagittal T1 (E-H), and right parasagittal T1 (I-L) sequences obtained at 3 months (A, E, I), 13 months (B,F, J), 26 months (C,G, K), and 6 years (D, H, L) of age. The initial MRI at 3 months demonstrates mild lateral ventriculomegaly and delayed myelination for age, best appreciated as absence of posterior limb of the internal capsule myelination despite presence of optic radiation myelination (A). Mild cerebellar fissure widening is also appreciated in I. By 13 months, rudimentary white matter myelination has occurred centrally on T1 weighted imaging in the corpus callosum (F) and periventricular white matter (J). However, myelination remains profoundly delayed, and the corpus callosum has not thickened as expected, now falling below the third percentile for age. By 26 months of age, the myelination within the optic radiations on T2 weighted imaging (C) and the central white matter on T1 weighted imaging (G,K) has become more robust. However, there is no appreciable progression in anatomic extent and the overall myelination remains markedly delayed. On the subsequent MRIs, no further myelination has occurred allowing differences in MRI field strength as shown on the MRI performed at 6 years of age (D, H, L). Hypoplasia of the corpus callosum, widening of the cerebellar fissures, and lateral ventriculomegaly persist without change though the foramen Magendie has grown more patulous over time potentially indicating subtle volume loss (E-H). Posterior limb of the internal capsule (A-D): white arrow (solid stem). Corpus callosum (E-H): white arrow (dashed stem). Periventricular white matter (I-L): white arrowheads. Widened cerebellar fissures (I-L): dashed circle. Widened foramen Magendie (H): asterisk.
Family history was negative for similarly affected individuals. Maternal ancestry is Italian, French, and Portuguese, and paternal ancestry is Italian, Irish, and Scottish. There is no known consanguinity.
At 13 months of age, additional investigations were undertaken. Repeat MRI demonstrated persistently delayed myelination, mild lateral ventriculomegaly, and cerebellar fissure widening with lack of expected growth of the corpus callosum (below the 3rd percentile posteriorly); however, some interval myelination had occurred in the central white matter on T1-weighted imaging (Figure 1 B, F, J). MR spectroscopy was within normal limits for age. Extensive metabolic studies were undertaken. Blood and urine metabolites were normal, including complete blood count, lactic acid, ammonia, free/total carnitine, plasma acylcarnitines, total homocysteine, plasma amino acids, and urine organic acids. Initial cerebrospinal fluid (CSF) studies (and CSF:plasma ratios) were normal, including glucose, lactic acid, amino acids, biogenic amine metabolites, and BH4 metabolites. Five-methyltetrahydrofolate (5-MTHF) was initially at the lower end of the normal range at 53 nmol/L (normal range 40 –150). He also underwent genetic testing that was non-contributory, including chromosomal oligo microarray, Angelman syndrome methylation analysis, GFAP gene sequencing, POLG1 and TYMP gene sequencing, mitochondrial genome analysis, and X-linked intellectual disability NextGen sequencing panel.
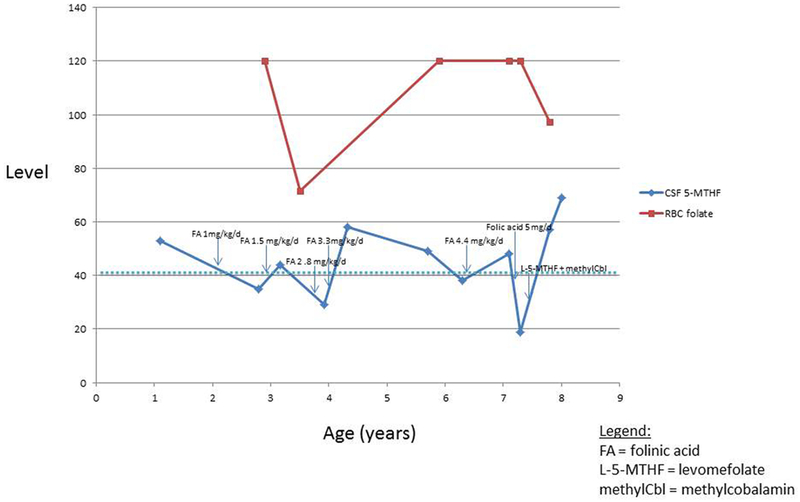
Around 2 years of age, the patient underwent a repeat MRI that re-demonstrated markedly delayed brain myelination with minimal progression since the 13-month-old MRI (Figure 1 C,G, K). The patient was empirically started on 1 mg/kg/d of folinic acid for his previously measured low-normal CSF 5-MTHF. Repeat CSF at 2.5 years on folinic acid supplementation demonstrated a decreased 5-MTHF level of 35 nmol/L (normal range 40–150).
Given the low CSF levels of 5-MTHF, his folinic acid dose was increased to 1.5 mg/kg/d divided twice daily. Between ages 3 to 7 years, the folinic acid dose was gradually increased to 4.4 mg/kg/d. Despite achieving consistently elevated serum folate levels on supplementation, normal CSF 5-MTHF levels were never consistently maintained (see Figure 2). Serum folate receptor antibody titers (IgG and IgM) were negative. FOLR1 gene sequencing was negative. CSF clinical metabolomics analysis was non-diagnostic; in particular, there were no abnormalities of purine or pyrimidine metabolites noted.
Figure 2.
Graph of CSF 5-methyltetrahydrofolate (5-MTHF) and red blood cell (RBC) folate levels over time, and on various treatments. The dotted horizontal line represents the lower limit of the normal range for CSF 5-MTHF levels (40 nmol/L). RBC folate levels are in ng/mL and CSF 5-MTHF levels are in nmol/L.
Clinically, the patient continued to progress slowly in his development without clear regression, although he remained markedly globally delayed. He developed insidiously progressive spastic quadraparesis in late infancy, initially managed with baclofen and ultimately necessitating baclofen pump placement. He required placement of a G-tube at 3 years of age for poor feeding associated with dysphagia. He also developed seizures at age 3 years. Repeat EEG at that time demonstrated generalized slowing, absence of posterior dominant rhythm, and biposterior polyspike-wave discharges. Seizures have remained well controlled on monotherapy with levetiracetam. He has cortical visual impairment. His head circumference is currently below the 1st percentile, with a z-score of -3.8. His height has remained around the 2nd percentile, with weight between the 5th-10th percentiles. He has no additional extra-neurological features. He is non-dysmorphic on examination.
MRI studies since 26 months of age (at 3, 6, and 7 years of age) have demonstrated static, delayed myelination now characterized as hypomyelination (Figure 1 D, H, L). Repeat spectroscopy studies have been normal.
Whole exome sequencing, including proband and parents, demonstrated compound heterozygous variants in the MTHFS gene: paternally inherited c.434G>A (p.R145Q) and maternally inherited c.107T>C (p.L36P). Neither of these variants was observed in approximately 6500 individuals of European and African American ancestry in the NHLBI Exome sequencing Project, indicating that they are not common benign variants in these populations. The p.R145Q variant is reported at a frequency of 0.00003 in the gnomAD database (no homozygotes), while the p.L36P variant is not reported. Both of these variants occur at highly conserved positions, and both are semiconservative amino acid substitutions. In silico analyses (Polyphen, SIFT) predict that both of these variants are probably damaging to protein structure or function. One hundred percent of the coding region was covered at a minimum of 10X for the MTHFS gene. Exome sequencing also detected a maternally inherited pathogenic variant in the AFG3L2 gene, c.2067_2068delCA, that was not felt to fit the patient’s clinical phenotype. There were no additional variants reported. In terms of quality metrics for the exome sequencing, the mean depth of coverage was 107X, with a quality threshold of 97.6%.
Based on his biallelic, suspected loss-of-function variants in the MTHFS gene, the patient’s folinic acid supplementation was stopped. He was then briefly treated with 5 mg/day of folic acid, which resulted in further lowering of CSF 5-MTHF levels (see Figure 2). At 7-½ years of age, he was started on oral levomefolic acid (L-5-methyltetrahydrofolate) at a dose of 3 mg PO daily, and IM methylcobalamin at a dose of 10 mg daily. Within weeks of initiating this treatment, the proband’s parents reported subjectively increased alertness and vocalizations. Repeat CSF 5-MTHF level on this regimen was 57 nmol/L after 2 months of therapy, and 69 after 4 months, which is the highest that his measured 5-MTHF has ever been. Plasma folate after 2 months of therapy was 94 ng/mL. Repeat EEG and MRI (Figure 1) 4 months after starting therapy remained unchanged. To date, the treatment has been well tolerated without adverse events. At present, he is unable to sit or stand without support, he can babble, he has several signs, and has limited comprehension.
3.2. Case 2
The second patient is an 11 year-old male with global developmental delay, microcephaly, short stature, epilepsy, spasticity, and cerebral hypomyelination. The pregnancy was notable for maternal paroxetine use, and ultrasound findings of short humerus and femur lengths. Delivery was induced 9 days post-term, and he was born vaginally without complication, although he was noted to have a short umbilical cord and a nuchal cord.
He was microcephalic at birth, and had feeding difficulties and irritability in the neonatal period. He was also noted to have an exaggerated startle response. His development was globally delayed since birth. Early evaluations in infancy were concerning for diffuse hypertonia. MRI brain at 16 months of age revealed delayed myelination and mild cerebellar atrophy (Figure 3A–C).
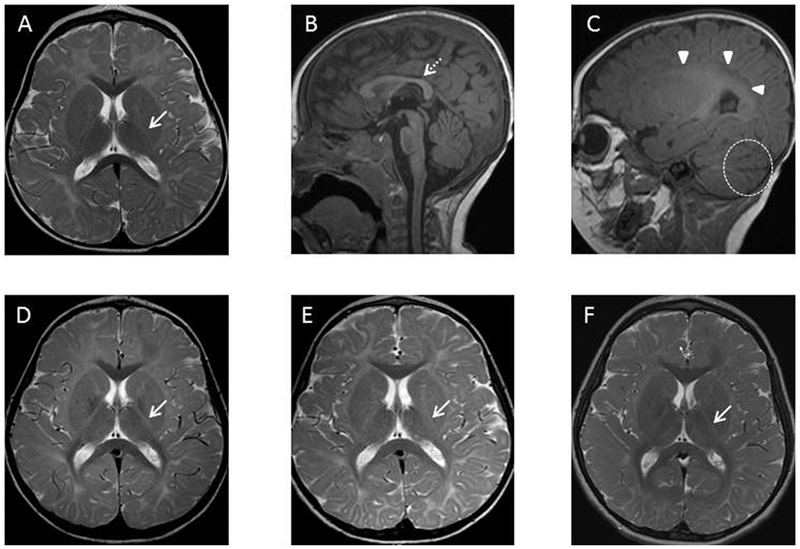
Figure 3.
Axial T2 (A, D, E, F) and sagittal T1 (B,C) images of Patient 2 at 16 months (A-C), 5 years (D), 6 years (E), and 8 years (F) of age. The initial MRI at 16 months (A-C) demonstrates delayed myelination on T1 weighted imaging (absent subcortical white matter myelination) and T2 weighted imaging (absent posterior limb of the internal capsule myelination). Follow-up imaging through 8 years of age (D-F) demonstrates no significant progress in myelination. Posterior limb of the internal capsule (A, D-F) : white arrow (solid stem). Corpus callosum (B): white arrow (dashed stem). Periventricular white matter (C): white arrowheads. Widened cerebellar fissures (C): dashed circle.
The patient learned to roll at roughly 9 months, sat unassisted at 16–18 months, and never crawled. He never developed a pincer grasp. Language acquisition was severely delayed. Repeat imaging at age 2, 6, and 7 years revealed stable hypomyelination and prominence of the cerebellar fissures (Figure 3D–F).
The patient developed epilepsy, starting between 2–3 years of age. His initial seizures were dyscognitive, but evolved to include multiple additional semiologies including atonic seizures, gelastic seizures, and tonic seizures. His seizures have unusual triggers, including changes in temperature, fatigue, valsalva (stooling), and light/dark transitions. Electroencephalograms revealed theta and delta slowing and generalized epileptiform discharges. His seizures have remained refractory to multiple medications (currently on lacosamide and lamotrigine, and previously failed trials of rufinamide, levetiracetam, and clonazepam). His parents have noted subjective improvement in seizure severity with antipyretics. Overall, seizure frequency has been increasing over time.
Also since 2–3 years of age, he has had recurrent episodes of unexplained hyperthermia (104–105 °F) associated with lethargy, flushing, polyuria, and polydipsia. These were occurring every 3–4 weeks, and lasting 3–7 days, but have significantly improved since starting lamotrigine.
Developmentally, he continues to make slow progress without regression. He pulled to stand at about 4 years and walked unassisted shortly thereafter. He was speaking in single words at age 4 years. He currently speaks fluently, but has limited vocabulary and articulation difficulties. He has undergone removal of his salivary glands for severe drooling. He has also received botox injections in his legs for spasticity. His current head circumference is on the 3rd percentile, and height is less than the first percentile (Z=−2). He underwent full skeletal survey x-rays which were normal. He has mild dysmorphisms, including thick eyebrows, full cheeks, epiblepharon, and tapered fingers.
Laboratory evaluations included non-diagnostic serum lactate, folate, CBC, vitamin B12, methylmalonic acid, total homocysteine, plasma amino acids, urine organic acids, plasma acylcarnitine, and urine amino acids. CSF 5-MTHF was 42 nmol/L (normal range 40–150). CSF lactate, protein, glucose, cell count, amino acids, and neurotransmitter metabolites were normal.
An epilepsy gene panel, vanishing white matter disease gene panel, mitochondrial genome sequencing, and gene sequencing of LAMA2, POLR3A, and POLR3B were non-diagnostic. Trio whole exome sequencing revealed compound heterozygous MTHFS variants: Paternally inherited p.Q162X (c.484C>T) and maternally inherited p.R145Q (c.434G>A). The p.R145Q variant was previously described in reference to patient number 1. The p.Q162X variant is predicted to cause loss of normal protein function through protein truncation. The p.Q162X variant was not observed in approximately 6500 individuals of European and African American ancestry in the NHLBI Exome Sequencing Project. This variant is reported at a frequency of 0.00002 in the gnomAD database, and there were no homozygotes. There were no other candidate variants detected. In terms of quality metrics for the exome sequencing, the mean depth of coverage was 70X, with a quality threshold of 94.7%.
3.3. MTHFS enzyme activity in cultured fibroblasts
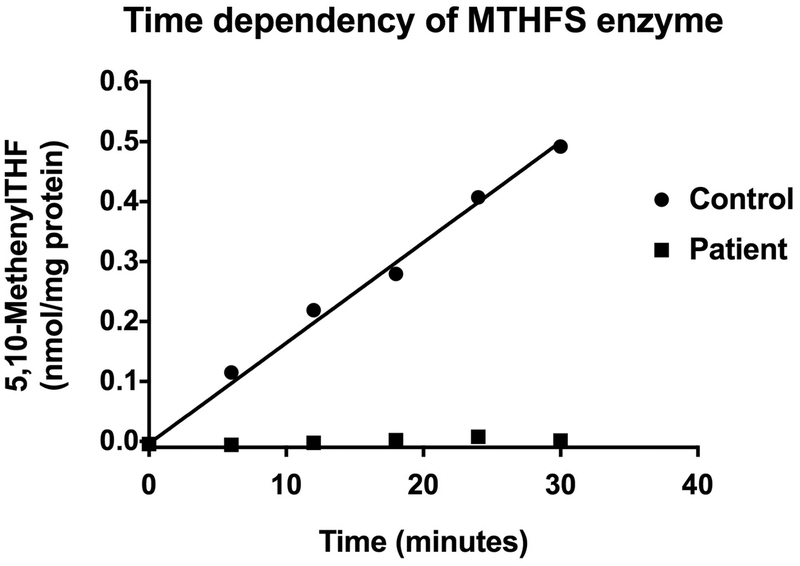
The enzyme activity was measured in control fibroblasts and fibroblasts from patient 1. The enzyme activity from control cells showed linear time dependency when tested up to 30 minutes (n=3). A representative graph of the three independent experiments with 2mg/mL final protein concentration (as cell lysate) is shown in Figure 4. The concentration of 5,10-methenylTHF (product) at each time point from mutant fibroblasts was essentially same as a blank sample (which has the cell lysate but immediately boiled after reaction mixture was prepared), presumably indicating both enzymatic and non-enzymatic production of 5,10-methenylTHF. Under these conditions, no enzyme activity was detected with samples from patient 1.
Figure 4.
Time dependency of MTHFS enzyme activity as shown by the production of 5,10-methenylTHF as measured by LC-MS/MS. The product was measured in one control line of skin fibroblasts and fibroblasts derived from patient 1 at 37°C for 0, 6, 12, 18, 24 and 30 minutes as described in the Material and Methods section. The final protein concentration in the reaction mixture was 2mg/mL and each data point is the average of duplicate measurements. Immediately after reconstitution of the reaction mixture, blank samples for both disease and the control lines were obtained by boiling the time zero samples for 3 minutes. The time zero blank samples were subtracted from each data point. The above figure is representative of one of 3 experiments. The R square value for the linear regression curve for the control samples is 0.9903.
3.4. Folinic acid levels in cultured fibroblasts
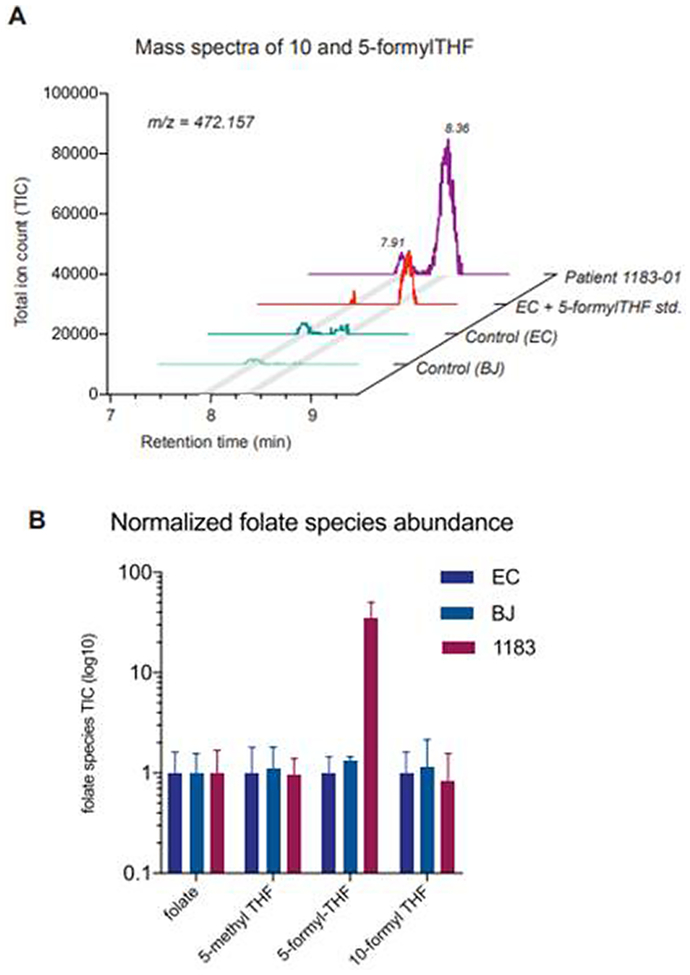
To directly characterize the effects of mutations in MTHFS upon enzymatic function in cells, we next measured the abundance of folate species in patient 1 derived fibroblasts. The direct substrate of the MTHFS reaction, 5-formyl-THF, was elevated some 30-fold in patient fibroblasts compared to control, reaching levels of approximately 0.5 μmol/L cell volume (Figure 5, panel A). Neither 10-formyl-THF nor any other folate species was significantly different between control and mutant cells (Figure 5, panel B). The extreme accumulation of 5-formyl-THF supports the hypothesis that the diverse symptoms are actually manifestations of toxicity of a single metabolite.
Figure 5.
5-formyl-THF accumulates in fibroblasts of the affected patient. (A) Total ion chromatogram (TIC) of m/z = 472.157 corresponding to the molecular weight of 5 and 10-formyl-THF. 2 peaks are present representing 10-formyl-THF (RT= 7.91 min) and 5-formyl-THF (RT= 8.36 min) species. (B) Normalized abundances of folate species in control (EC and BJ) and patient (1183–01) fibroblasts (mean ± stdev, n=3).
4. Discussion:
We present two patients with a very similar phenotype associated with biallelic variants in the MTHFS gene. Both patients have severe global developmental delays without regression, epilepsy, spasticity, microcephaly, and short stature. Both patients also have a history of exaggerated startle response in early infancy. Neuroimaging demonstrates hypomyelination of predominantly subcortical white matter that appears to plateau around 2 years of age, and mild cerebellar atrophy.
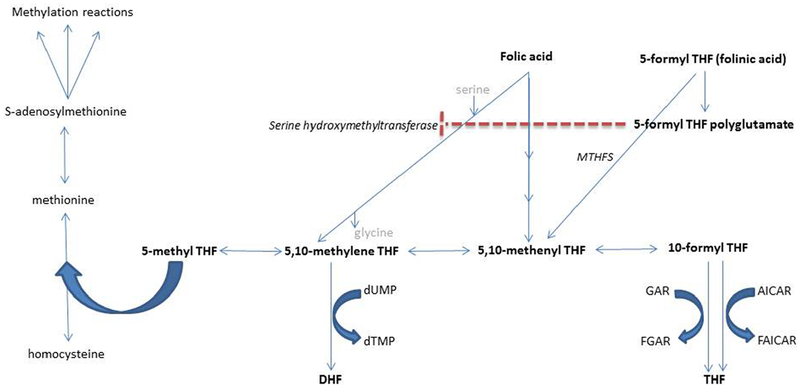
5-formyltetrahydrofolate (folinic acid) is a reduced form of folate that derives from the diet or the metabolism of 5,10-methylenetetrahydrofolate2. 5-formyltetrahydrofolate is not a cofactor for one carbon metabolism like other forms of reduced folate, but rather serves as a storage form of tetrahydrofolate that can be mobilized by the enzyme 5,10-methenyltetrahydrofolate synthetase, encoded by the MTHFS gene.3 This enzyme catalyzes the irreversible ATP and Mg 2+ dependent conversion of 5-formyltetrahydrofolate to 5,10-methenyltetrahydrofolate (see Figure 6). 5,10-methenyltetrahydrofolate can be reversibly converted to other reduced forms of folate, including 5,10-methylenetetrahydrofolate, which is important for single carbon metabolism/methylation and thymidylate metabolism in the cytoplasm and nucleus respectively; and 10-formyltetrahydrofolate, which is important for purine metabolism in the cytoplasm (see Figure 6)4. In fact, the MTHFS enzyme may closely associate with the purinosome3. MTHFS deficient mice demonstrate reduced de novo purine synthesis, and MTHFS null mice are not viable.3 MTHFS is also utilized in the mitochondria, where it may play a role in regulating the intra-mitochondrial folate pool2. Intra-mitochondrial folate is required for the formylation of mitochondrial tRNA-Methionine, which is necessary for translation5.
Figure 6.
Folate metabolism pathway. Folate metabolites are in bold. Enzymes are in italics. The dashed line represents inhibition of the enzyme serine hydroxymethyltransferase by 5-formyl THF polyglutamate. THF = tetrahydrofolate, DHF = dihydrofolate, MTHFS = 5,10-methenyltetrahydrofolate synthetase, FGAR = formylglycinamide ribotide, GAR=glycinamide ribotide, AICAR = aminoimidazole carboxamide ribotide, FAICAR = formylaminoimidazole carboxamide ribotide, dTMP = deoxythmidine monophosphate, dUMP = deoxyuridine monophosphate.
Both of the patients we report had low-normal baseline levels of 5-methyltetrahydrofolate. We believe that this is a result of 5-formyltetrahydrofolate (folinic acid) accumulating in the central nervous system (CNS) as a result of deficient MTHFS function. Surplus folinic acid accumulating in the CNS is likely converted to 5-formyltetrahydrofolate polyglutamate. The latter metabolite has been demonstrated to inhibit serine hydroxymethyltransferase, the enzyme that transfers a methyl group from serine to tetrahydrofolate to produce 5,10-methylene tetrahydrofolate and glycine (i.e. serine + tetrahydrofolate → 5, 10-methylenetetrahydrofolate + glycine)6,7. Inhibition of serine hydroxymethyltransferase could account for the observed reduction in CSF 5-MTHF levels because 5,10 methylenetetrahydrofolate is the direct precursor for 5-MTHF.
MTHFS is the enzyme that converts folinic acid into other reduced forms of folate, thus it is not surprising that supplementation with folinic acid was not able increase CSF levels of 5-MTHF in our first patient. In fact, we suspect that treatment with folinic acid caused a worsening of cerebral folate deficiency by further inhibiting serine hydroxymethyltransferase, a suspicion corroborated by the fact that CSF levels of 5-MTHF prior to folinic acid treatment were higher than following treatment.
The pathophysiology of this disorder is still incompletely understood. It is possible that there is an intracellular folate deficiency despite only borderline-low CSF levels. Our patients’ phenotype is consistent with what has been reported in cerebral folate deficiency syndromes. Abnormal myelination is a well-recognized feature in this group of disorders and is presumed to result from intracellular deficiency of S-adenosylmethionine (SAM), the principle methyl donor involved in the methylation of myelin basic protein and phospholipids, amongst approximately 100 additional reactions6. In an attempt to compensate, a greater amount of choline may be oxidized to betaine (an alternate methyl donor), depleting CNS choline stores and lowering phosphatidylcholine levels, which may also impact myelin composition8. Microcephaly may relate to impaired cell growth and division as a result of the role of folate in purine and thymidylate metabolism, or could also relate to deficient methylation. Another possible disease mechanism is intra-mitochondrial folate deficiency resulting in secondary mitochondrial dysfunction. As previously mentioned, intra-mitochondrial folate is required for the formylation of mitochondrial methionyl-tRNA, which is necessary for intramitochondrial translation. Disorders associated with mitochondrial methionine amino-acyl tRNA synthetase (MARS2 gene) and mitochondrial methionyl-tRNA formyltransferase (MTFMT gene) have been described associated with some overlapping features to our patients.
One case was previously reported of a child with suspected MTHFS deficiency associated with seizures, autism, and cerebral folate deficiency unresponsive to folinic acid, but detailed clinical and molecular information were not provided9. That patient was treated with L-5-methyltetrahydrofolate, methylcobalamin, and pyridoxine, but results were not reported9.
The current treatment strategy for patient one is aimed at supplying an alternative, usable reduced form of folate, namely levomefolate (L-5-methyltetrahydrofolate). The non-reduced form of folate, folic acid, resulted in a worsening of cerebral folate deficiency in our patient, and is generally not recommended in the management of cerebral folate deficiency. Folic acid competes with reduced forms of folate for entry across the blood brain barrier, and the enzymes required to reduce folate are expressed at relatively low levels in the CNS10.
In addition to supplementing with 5-MTHF, we are also utilizing the methylated form of cobalamin, methylcobalamin, in an attempt to produce a sparing effect on the utilization of 5-methyltetrahydrofolate, which would normally be “used up” in the endogenous production of methylcobalamin (see Figure 6). We remain optimistic about some clinical benefit given the early subjective report of improved alertness and vocalizations, but longer-term clinical follow-up, along with repeat EEG and MRI, are required. We did not use pyridoxine or pyridoxal phosphate as was used in the previously described case of MTHFS deficiency, but this remains another treatment consideration because serine hydroxymethyltransferase, the enzyme we believe is inhibited by excess production of 5-formyltetrahydrofolate polyglutamate in this disorder, is a pyridoxine-dependent enzyme and there may be some benefit in treating with its cofactor. Once more long-term data is collected for patient one, we will also consider treating patient 2.
In conclusion, we report a neurometabolic disorder associated with severe global developmental delay, spasticity, epilepsy, microcephaly, short stature, and cerebral hypomyelination caused by biallelic loss-of-function variants in the MTHFS gene. CSF 5-MTHF levels were on the lower end of the normal range, and appear to have been further lowered by supplementation with folinic acid. Thus it appears that a high index of suspicion is required to diagnose this disorder, and caution should be exerted with the non-discriminate use of folinic acid to treat borderline-low CSF 5-MTHF. Treatment with a combination of oral 5-MTHF and IM methylcobalamin was able to increase CSF 5-MTHF levels in our first patient, was well tolerated over a 4-month period, and resulted in subjective mild improvements in function although long-term data are still lacking. This disorder adds to the growing list of diseases associated with folate metabolism, and also CNS hypomyelinating disorders.
Supplementary Material
Acknowledgements:
“We thank the Gene Discovery Core of The Manton Center for Orphan Disease Research for providing resources and support in patient consenting, sample collection, sequencing and sharing of information and samples.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
Appendices. Supplementary data
References:
- 1.Chen L, Ducker GS, Lu W, Teng X, Rabinowitz JD. (2017) An LC-MS chemical derivatization method for the measurement of five different one-carbon states of cellular tetrahydrofolate. Anal. Bioanal. Chem 409 (25): 5955–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertrand R, et al. (1995) mitochondrial methenyltetrahydrofolate synthetase activity. Biochimica et Biophysica Acta. 1266:245–249 [DOI] [PubMed] [Google Scholar]
- 3.Field MS, Anderson DD, Stove PJ (2011) Mthfs is an essential gene in mice and a component of the purinosome. Front. Genet 2: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dayan A, et al. (1995) Cloning and characterization of the human 5,10-methyenyltetrahydrofolate synthetase-encoding cDNA. Gene 165: 307–311. [DOI] [PubMed] [Google Scholar]
- 5.Krupekno NI, et al. (2010) ALDH1L2 is the mitochondrial homolog of 1-formyltetrahydrofolate dehydrogenase. Journal of Biological Chemistry 285(30):23056–23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stover P, Schirch V (1991) 5-Formyltetrahydrofolate polyglutamates are slow tight binding inhibitors of serine hydroxymethyltransferase. J Biol Chem 266(3):1543–1550. [PubMed] [Google Scholar]
- 7.Wu D, et al. (2009) Structural basis for the inhibition of human 5,10-methenyltetrahydrofolate synthetase by N10-substituted folate analogues. Cancer Res 69(18):7294–7301. [DOI] [PubMed] [Google Scholar]
- 8.Steinfeld R, et al. (2009) Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet 85(3):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scaglia F, Blau N. (2013) Disorders of folate metabolism and transport. Berlin, Heidelberg: Springer Berlin Heidelberg, 167–178. [Google Scholar]
- 10.Raemaker VT, Blau N (2004) Cerebral folate deficiency. Dev Med and Child Neurol 46(12):843–851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.