Abstract
A 15-year-old cystic fibrosis patient with a disseminated Mycobacterium abscessus infection was treated with a three-phage cocktail following bilateral lung transplantation. Effective lytic phage derivatives that efficiently kill the infectious M. abscessus strain were developed by genome engineering and forward genetics. Intravenous phage treatment was well tolerated and associated with objective clinical improvement including sternal wound closure, improved liver function, and substantial resolution of infected skin nodules.
Mycobacterial infections impose a substantial global health burden, and drug resistance in Mycobacterium tuberculosis and non-tuberculous mycobacteria (NTM) is widespread1,2. Cystic Fibrosis (CF) patients frequently have NTM infections, including Mycobacterium abscessus3,4, which are antibiotic resistant and difficult to manage clinically3,5. CF is the third most common indication for lung transplantation, but persistent infection – particularly when NTM are present prior to surgery – can result in substantial post-transplant morbidity and mortality4,6,7. Therapeutic bacteriophages are a plausible alternative treatment to antibiotics8, but have not been used for mycobacterial infections in humans9–11; personalized intravenous phage treatments for other bacterial infections have been described (Supplemental Information)12, 13.
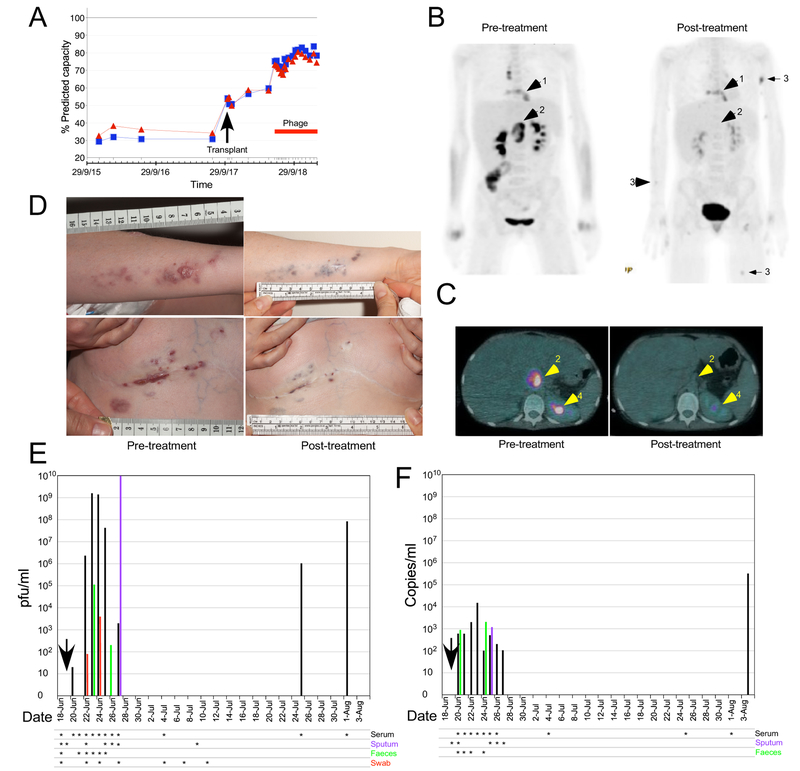
A 15-year-old CF patient (homozygous ΔF508) with co-morbidities of pancreatic insufficiency, insulin-dependent diabetes, CF-related liver disease, Nissen fundoplication and gastrostomy, CF-related osteoporosis and failure to thrive was referred for lung transplant. The patient was chronically infected with Pseudomonas aeruginosa and M. abscessus subspecies massiliense and had been on anti-NTM treatment for 8 years prior to lung transplantation (Extended Data Fig.1). Despite compassionate lumacaftor/ivacaftor treatment for 6 months prior to being placed on the transplant waiting list, FEV1 declined to 0.63 L (29% of predicted normal function for height; Fig. 1A), with hypercapnia and main pulmonary artery enlargement on CT scan. After an uncomplicated bilateral lung transplant, immunosuppressive drugs and multiple intravenous (iv) antibiotics were administered (Extended Data Fig.1). Severe side effects of nausea, anorexia, diarrhea and electrolyte derangement necessitated total parenteral nutrition and discontinuation of iv antibiotics. Within one week of stopping intravenous therapy, redness was noted at the surgical incision. The chest X-ray showed consolidation and M. abscessus grew from sputum (Supplementary Table 1). Clofazimine and bedaquiline were added and previous iv antibiotics were recommenced (Extended Data Fig. 1; Supplemental Information). A PET-CT scan to evaluate ongoing abdominal pain, hepatomegaly, deranged liver function tests and EBV viremia (11 million copies/ml) revealed fluorodeoxyglucose (FDG) activity in a 3.5 cm × 4 cm porta hepatis lesion and a destructive process at the sternum with abnormal adjacent soft tissue (Fig. 1B, C). Mycophenolate mofetil was stopped and 4 doses of iv rituximab were given for presumed post-transplant lymphoproliferative disease, but the node enlarged to 6.8 cm × 4 cm (Fig. 1C). Two skin lesions developed on the forearm, and sternal wound and skin biopsies revealed granulomatous inflammation. The patient was discharged 7 months after transplant with a diagnosis of disseminated mycobacterial infection. Antimicrobial therapy was continued with a palliative care plan in place. Over eight weeks, 20 additional skin nodules appeared on arms, legs, and buttocks, and the surgical wound showed areas of breakdown (Fig. 1D).
Figure 1. Patient status before and after phage treatment.
A. Lung function as percent Predicted Forced Expiratory Volume in One Second (FEV1; blue) and Forced Vital Capacity (FVC; red). Whole body (B) and cross-section (C) PET-CT scans twelve weeks before and six weeks post-phage treatment. Arrows show (1) the sternal area and surrounding soft tissue, (2) abdominal lymph nodes at the porta-hepatis, and (3) skin nodules, respectively. Arrow 4 indicates normal kidney excretion. D. Upper and lower panels show the patient’s left arm and sternal wound, respectively, immediately prior to and six months after phage treatment. E and F. Phage titers by plaque assay (E) or dPCR (F) following phage administration (vertical arrow). Serum (black bars), sputum (purple bars), feces (green bars), and wound swab (red bars) were tested on the dates indicated (asterisks).
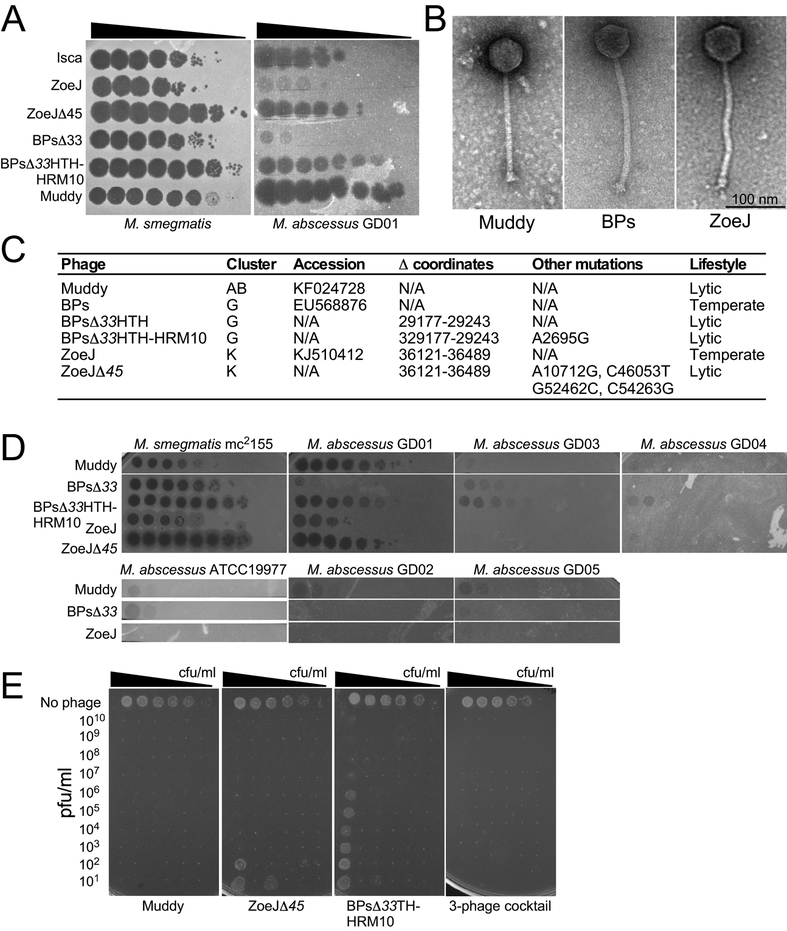
M. abscessus subsp. massiliense with a rough colony morphotype (designated strain GD01; Supplemental Information) isolated 1-month post-transplantation was used to identify potentially therapeutic phages. We exploited a collection of >10,000 phages isolated using Mycobacterium smegmatis by students in the Science Education Alliance Phage Hunters Advancing Genomics and Evolutionary Science (SEA-PHAGES) program, 1,800 of which are genomically defined14. Some of these infect M. tuberculosis15, but other NTM specificities are unknown16. Screening a representative subset identified one (Muddy) that efficiently kills GD01 (Fig. 2A; Supplementary Table 1). A second phage, ZoeJ, infects GD01 with reduced efficiency of plating (EOP), although the plaques are extremely turbid, and difficult to visualize (Fig. 2A). A lytic derivative of ZoeJ was engineered using Bacteriophage Recombineering of Electroporated DNA (BRED)17 to precisely remove its repressor gene (45)18; this efficiently infects and kills GD01 (Fig. 2A). A third phage (BPs) and its lytic derivative (BPsΔ33HTH)19 infect GD01 poorly, but we isolated host range mutants (HRM1 and HRM10) that efficiently infect GD01 and retain M. smegmatis infection (Fig. 2A; Supplemental Information). HRM1 and HRM10 have single base changes in the portal gene 3 (C2083T and A2695G) conferring R66W and N270D amino acid substitutions, respectively.
Figure 2. A three-phage anti-M. abscessus GD01 cocktail.
A. Phage lysates were serially diluted 10-fold and spotted onto M. smegmatis mc2155 and M. abscessus GD01 lawns. These assays were repeated at least ten times with similar results and a representative experiment is shown. B. Electron micrographs of phages Muddy, BPs, and ZoeJ. Representative images are shown. C. Key characteristics of phages. D. Phages were serially diluted 10-fold and spotted onto mycobacteria as indicated. These assays were repeated at least twice with similar results and representative experiments are shown. E. M. abscessus GD01 (~108 cfu/ml) was serially diluted 10-fold in 11 replicates, and either no phage or phage added at indicated concentrations. Cultures were incubated at 37°C for 24 hrs and 3 μl aliquots plated onto solid media and incubated for 7 days. These assays were repeated at least three times with similar results and a representative experiment is shown.
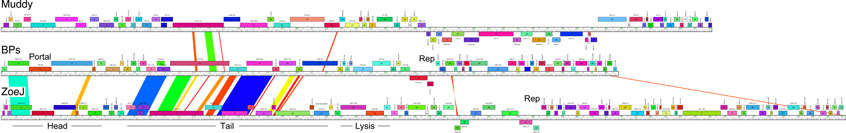
Phages Muddy, ZoeJ, and BPs are morphologically siphoviral (Fig. 2B) and are genomically distinct – grouped in Clusters AB, K and G, respectively (Fig. 2C) – with limited segments of shared sequence similarity (Extended Data Fig. 2). A subsequent search for additional phages included screening of 100 environmental samples and phage Isca was isolated. Isca does not multiply on M. smegmatis, but several close relatives (BabyRay, Heldan, Fred313, Puppy, and TNguyen7) do (Supplementary Table 2). GD01 was also used to enrich large pools of unsequenced phages and we thereby isolated Gabriela and Itos (Supplementary Table 2). Thus, phages for GD01 are uncommon, but can be identified with extensive searches. A search for phages of a second strain (M. abscessus GD02) from a clinically similar patient identified few useful phages (Supplementary Table 3); this patient subsequently died. Muddy, BPs, and ZoeJ do not efficiently kill other M. abscessus clinical isolates, and the three-phage cocktail is not a generalizable treatment (Fig. 2D).
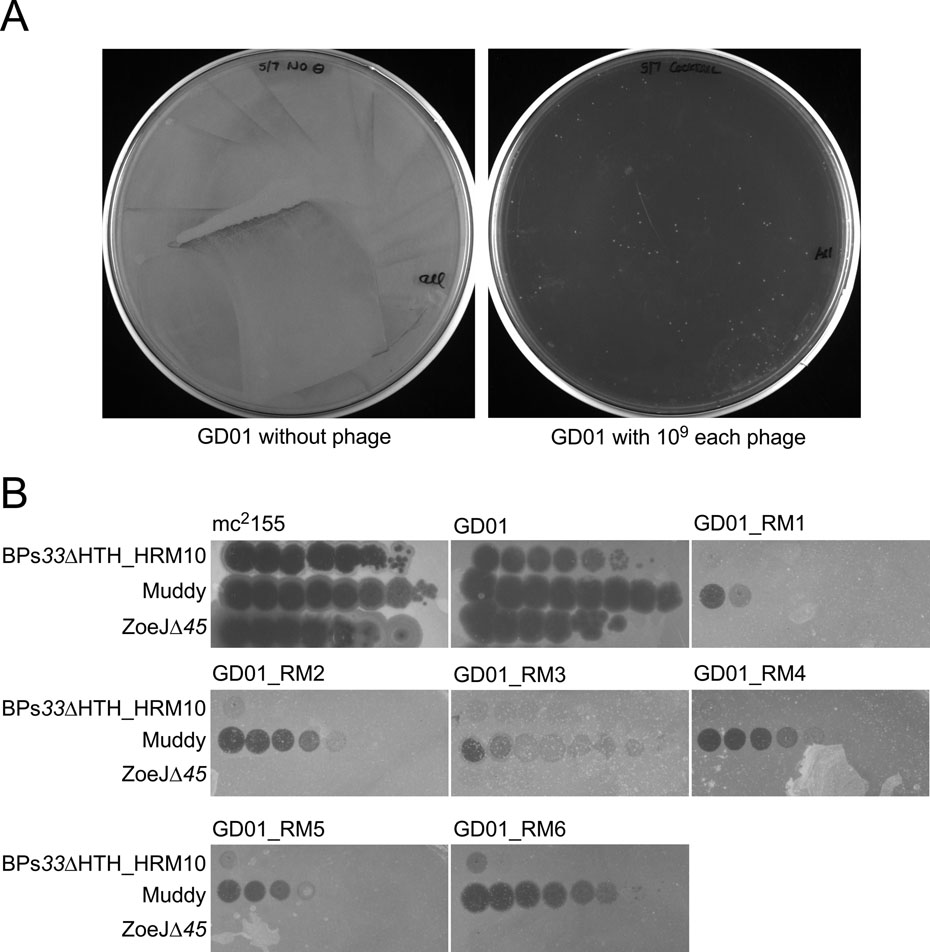
Each of the three phages – Muddy, BPs33ΔHTH-HRM10, and ZoeJΔ45 – infect and kill GD01 over a wide range of cell and phage concentrations, although ZoeJΔ45 and BPs33ΔHTH-HRM10 kill inefficiently at low phage input and higher bacterial numbers (Fig. 2E); however, no bacterial survival in vitro was observed using the three-phage cocktail (Fig. 2E). After challenging a larger GD01 culture with the cocktail some survivors were recovered and shown to be resistant to BPs33ΔHTH-HRM10 and ZoeJΔ45 but at least partially sensitive to Muddy (Extended Data Fig. 3).
Twenty-four hours following a single topical test dose in the sternal wound, iv therapy was initiated with the three-phage cocktail (109 PFU/dose of each phage) every 12-hours for at least 32 weeks (Supplemental Information). During the first two days of treatment the patient felt sweaty and flushed but had no fever or changes on physical exam. Otherwise phage treatment was well tolerated throughout without significant side effects. After nine days, the patient was discharged home and iv 12-hourly administration of the cocktail was continued. After one month of treatment the sternal wound, which had received a topical test dose, had improved more than the other skin lesions and topical daily phage therapy was commenced for both. Over the next six months (to the time of writing) the patient continued to improve clinically with gradual healing of surgical wound and skin lesions (Fig. 2), improvement of lung function (Fig. 1A) and liver function; weight increase was also observed despite stopping overnight supplemental feedings. A repeat CT-PET scan six-weeks into phage treatment showed resolution of FGD activity of the previously enlarged node at the porta-hepatis, although sternal and skin lesion activity remained (Fig. 1B, C).
M. abscessus was not isolated from serum or sputum at any point after initiation of phage treatment, although M. abscessus was cultured from swabs of slowly-resolving skin nodules at 1-, 3-, 4-, and 5-months. Sera showed no evidence of phage-neutralization, although weak antibody responses to phage proteins were seen (Extended Data Fig. 4). Among the weak cytokine responses were IFNγ, IL-6 and IL-10 after 16 days of treatment, TNFα, IL-6 and IL-10 after one month of treatment, and IL-6 after 3- and 4-months of treatment (Supplementary Table 4).
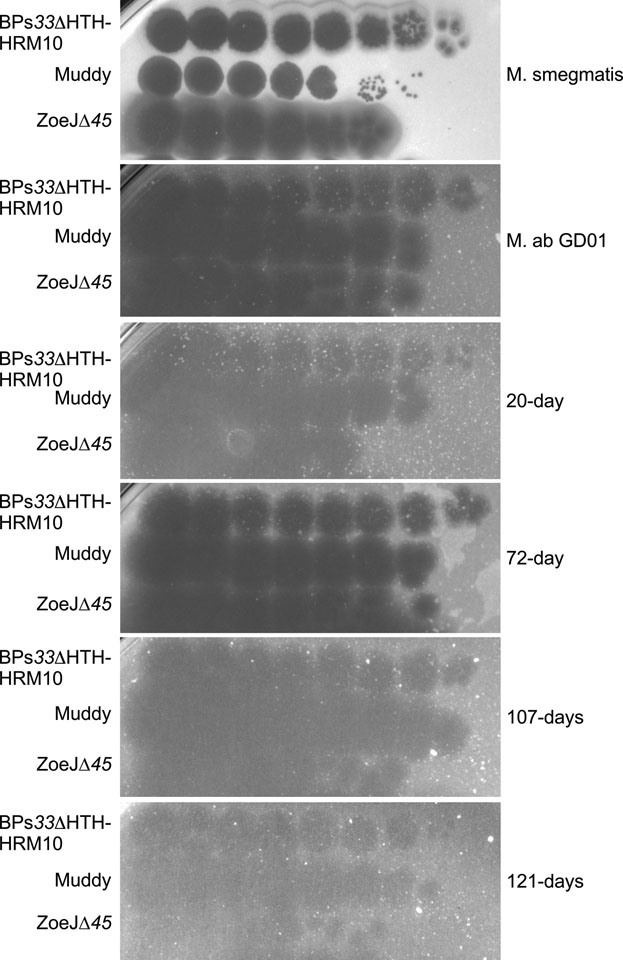
Phages were detected in serum one day after starting treatment, and reached titers in excess of 109 pfu/ml (Fig. 1E); dPCR performed on EDTA-blood samples had a similar temporal profile, although the maximum phage load detected was ~105 copies/ml (Fig. 1F); these observations are consistent with phage replication. Serum phage concentrations fell below detection limits one week after starting treatment although two later samples had mycobacteriolytic activity (Fig. 1E, F). Sputum samples were predominantly saliva and did not contain detectable lytic activity, although a purulent sputum sample collected 9-days after initiation of treatment had a high phage titer (1010 pfu/ml). Lower phage concentrations were detected in feces 4- and 6-days post-treatment, and in wound swabs at 3- and 5-days post-treatment (Fig. 1E, F). M. abscessus isolates were recovered from skin nodule swabs at 20-, 72-, 107-, and 121-days after treatment initiation but remained sensitive to each phage in the cocktail (Extended Data Fig. 5). It is plausible that phage resistance is associated with reduced virulence.
To our knowledge this is the first therapeutic use of phages for a human mycobacterial infection, and the first use of engineered phages. Phage treatment was associated with clinical improvement, although we cannot exclude the possibility that patient gains would have occurred without phage treatment. However, we note that patients with similar clinical conditions typically have high morbidity and mortality, that improvement was not temporally associated with cessation or initiation of other drug administrations, and we show evidence supporting in vivo phage replication. There were no adverse reactions to phage administration. We note there is substantial variation in M. abscessus phage susceptibilities, and phage treatment of similar patients will require expanding our understanding of phage infection of these strains.
Methods
Bacterial strains.
M. smegmatis mc2155 is a laboratory stock strain and was grown as previously described15. M. abscessus ATCC19977 was obtained from the American Type Culture Collection. M. abscessus GD01, GD02, GD03, GD04, and GD05 are patient isolates from London, UK (GD01 and GD02), from Seattle, WA (GD03), Los Angeles, CA (GD04), Winston-Salem, NC (GD05). M. abscessus strains were grown in Middlebrook 7H9 media with OADC and 1 mM CaCl2 for 4–5 days at 37°C, with shaking. These M. abscessus strains grow with a doubling time of approximately six hours, with isolated colonies visible on solid medium in 5–7 days. For plaque assays, M. abscessus cultures were sonicated briefly in a cup-horn sonicator (Q-sonica 500) at 30% amplitude with 15 sec on and 10 sec off until visibly dispersed.
Genome sequencing.
DNA was isolated from a liquid culture of M. abscessus GD01 and sequenced using Illumina (421-fold coverage, 250-bp paired-end reads) and Oxford Nanopore (12-fold coverage, average length 2.6 kb) technologies, assembled with SPAdes23, and evaluated and finished using Consed. The GD01_A and GD01_B sequences are deposited in GenBank with Accession #’s CP035923 and CP03592, respectively. Phage DNAs were isolated and sequenced as described previously24. Sequence features of phages are shown in Fig 2c; other phage information including genome sequences are available at https://phagesdb.org.
Phage susceptibility screening.
Phage susceptibility profiles were screened using a standard plaque assay15, and efficiencies of plating (EOP) were determined by spotting 10-fold serial dilutions on M. smegmatis mc2155, M. abscessus GD01, and other clinical isolates as described previously15. Phages from the collection at the University of Pittsburgh were propagated on M. smegmatis and tested on both strains. For newly isolated phages such as Elmo and Isca that were isolated on M. abscessus, the EOP was determined for phage lysates prepared on both the M. abscessus strain used for isolation and M. smegmatis.
Mycobacteriophage cocktail preparation.
Phages were grown on M. smegmatis mc2155 using solid media and recovered by diffusion into phage buffer (68 mM NaCl, 10 mM Tris HCl pH 7.5, 10 mM MgSO4, 10 mM CaCl2), yielding lysates with titers of >8 × 1010 plaque forming units per ml (PFU/ml). Phage particles were precipitated in 10% PEG8000 and 1 M NaCl, collected by centrifugation, and resuspended in phage buffer. Following clarification by centrifugation, cesium chloride (CsCl) was added to a density of 1.5 g/cm3 (4.1M), subjected to equilibrium density gradient centrifugation for 16 hours, the visible phage band collected (~1.5 mls), centrifuged similarly again, and stored at 4°C; this yielded 1–2 mls of phage with titers of 1012 – 1014 PFU/ml. For cocktail preparation, 1ml of each phage sample was dialysed against 1 liter of phosphate buffered saline (PBS; BupH PBS Thermo Scientific; 0.1 M Na2HPO4, 0.15 M NaCl2, pH 7.2) four times for a minimum of three hours each. Dialysis reduced the cesium concentration to less than 190 parts per billion, as detected by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Phage samples had undetectable levels of endotoxin using an EndoZyme II (Hyglos GmbH) assay. Samples were combined to form a three-phage cocktail, each at 1011 PFU/ml. Phage titers dropped no more than 8-fold over a one-month period when stored in PBS at 10° C, and cocktail batches were prepared monthly.
Phage administration.
The phage cocktail was diluted in PBS to a concentration of 109 pfu/mL. This was further diluted with PBS to 5 mL unit dose syringes, and administered intravenously twice daily. After nine days administration in hospital, the patient continued administration at home through a peripherally inserted central catheter. For topical administration, 109 pfu/7 mL of cocktail was applied to the sternal wound or skin nodules using a gauze pad.
Phage killing of M. abscessus GD01.
Bacterial survival assays were conducted in 96-well trays (Falcon), and were composed of a grid containing 200 μl volumes of 10-fold serial dilutions of bacterial culture (x-axis) and 10-fold serial dilutions of phage lysate (y-axis). Undiluted bacterial cultures contained ~5 × 108 cfu/ml, such that each well contained totals of 108, 107, 106, 105, 104 and 103 cfu. Undiluted phage lysates had titers of 1010 PFU/ml, such that each well contained 2 × 109, 2 × 108, 2 × 107, 2 × 106, 2 × 105, 2 × 104, 2 × 103, 2 × 102, 20, and 2 PFU. Cultures were incubated at 37° C for 24 hrs, and 3 μl of each sample spotted on Middlebrook 7H10 solid media, incubated for seven days, and photographed.
Phage quantitation in patient samples.
Samples were collected for measuring phage counts as follows: For wounds, shortened dry cotton swabs were aseptically placed in 20 ml universal bottles, closed, and weighed. After application, the swab was returned to the bottle, which was re-weighed. After addition of a measured volume of phage buffer and vortexing, the sample was passed through a 0.2 μM filter, serially diluted, and 0.05 ml volumes added to M. smegmatis agar overlays. For sputum, a small sample of sputum was transferred to a pre-weighed bijou bottle which was then re weighed. A measured volume of phage buffer (usually 0.5 ml) was added, vortexed, filtered, and titered as above. For serum, clotted blood samples were centrifuged at 3,000 rpm and the serum serially diluted and counted as above. Phage DNA was quantified by Digital PCR (dPCR) on a QX100 Droplet Digital PCR system (Biorad); real-time PCR (qPCR) was performed using the 7500 Fast real-time PCR system (Thermofisher). The primer and probe sequences used for dPCR and qPCR assays are shown in Table S5.
Patient antibody and cytokine responses.
Patient serum was diluted 1:100 in phosphate buffered saline (PBS), incubated with 108 PFU of each phage separately, and incubated at room temperature for 30 min. Ten-fold serial dilutions were spotted onto top agar overlays of M. smegmatis mc2155 and incubated at 37°C for 1–2 days to determine titer. Cytokines were measured using a Th1/Th2 Cytokine Bead Array (BD Biosciences, Reading, UK) according to manufactures instructions.
Western Blotting.
Phage samples were prepared by adding 75 μl of 20 mM Dithiothreitol (DTT) to 20 μl of phage lysate (1012 PFU/ml) and mixing, followed by addition of 2 μl of 0.5 M EDTA and incubation at 75°C for 5 min. Sample viscosity was reduced by adding 4 μl of 1 M MgSO4 and 2 μl of 1 mg/ml DNAse I (Invitrogen) and incubated further for 30 mins at 37°C. Loading buffer was added and samples were run on an SDS polyacrylamide gel and transferred to a PVDF membrane (BioRad) using a BioRad wet transfer apparatus according to manufacturer instructions. The membrane was blocked with Tris buffered saline (TBS) and 5% milk powder for 1 hr at room temperature and then rinsed with TBS and 0.1% Tween (TBST) for 5 min. The primary antibody (patient serum from 8/29/18) was diluted 1:100 into TBST and incubated with the membrane for 1 hr at room temperature. The membrane was washed with TBST three times for 5 min each. The secondary antibody, Goat anti-human HRP (Novex), was diluted 1:2000 in TBST and added to the membrane and incubated at room temperature for 45 min. The membrane was then washed with TBST three times for 5 min each and then once with TBS for 5 min. The SuperSignal West Dura Extended Duration Substrate kit (Thermo) was used to develop the blot according to manufacturer’s instructions.
Extended Data
Extended Data Fig.1. Timeline of drug administration.
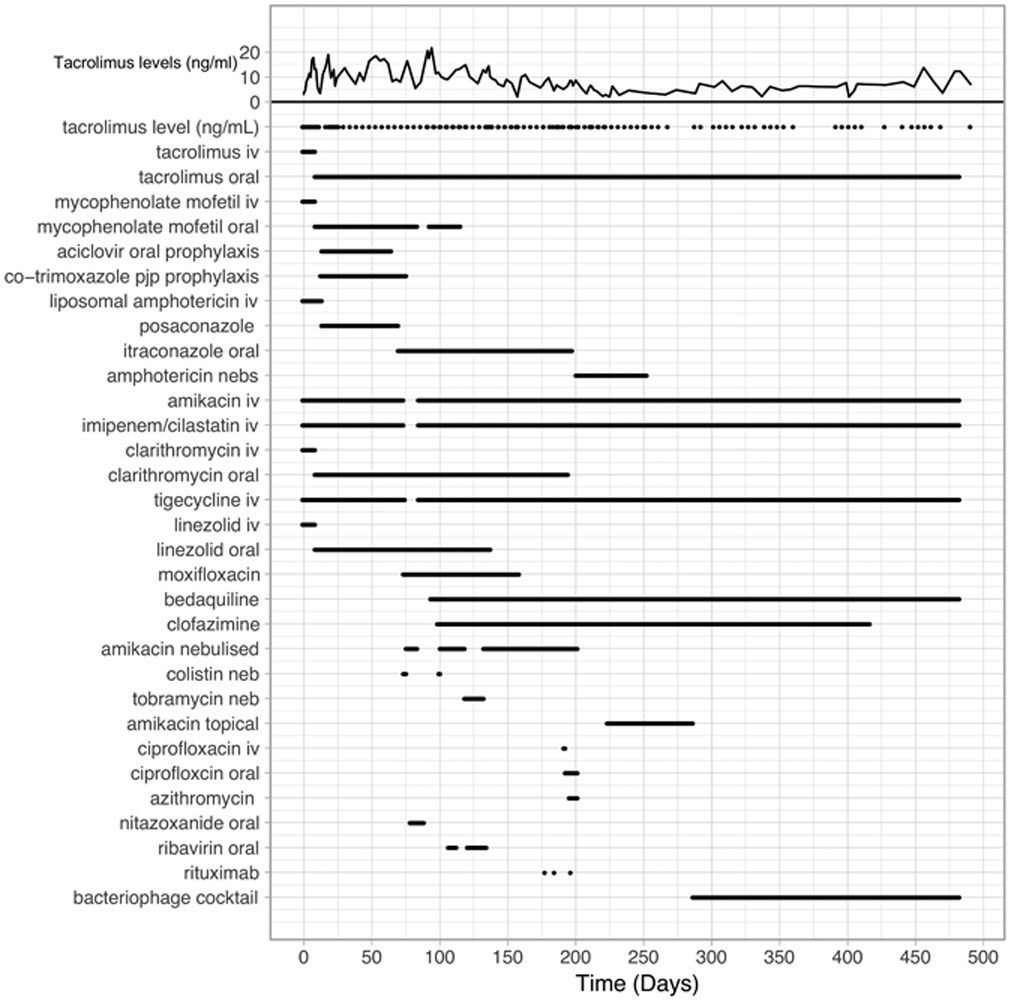
Timeline showing administration of antibiotics, immunosuppressive drugs, and the phage cocktail. Levels of the immunosuppressive drug Tacrolimus are shown at the top, and the administration of drugs is as indicated.
Extended Data Fig.2. Genome maps of phages Muddy, BPs, and ZoeJ.
Genes are shown as colored boxes above or below a genome track, reflecting rightwards and leftwards transcription, respectively. Pairwise nucleotide sequence similarity is indicated by spectrum-colored shading between genomes, with violet representing closest similarity.
Extended Data Fig.3. In vitro selection of phage resistance.
A. Approximately 5 × 108 cells of M. abscessus GD01 in one ml were incubated with a cocktail of 109 pfu each of three phages for one week in liquid culture. Aliquots (100 μl) were plated onto solid media and incubated at 37° C. In the absence of phage, a confluent lawn grew (left), and in the presence of phage (right), approximately 150 small colonies were observed. B. Six individual colonies were picked, grown and retested for phage susceptibilities. Top agar overlays with each strain were plated on solid media and 10-fold serial dilutions of phages (as indicated) were spotted onto each plate.
Extended Data Fig.4. Detection of antisera recognizing phage proteins.
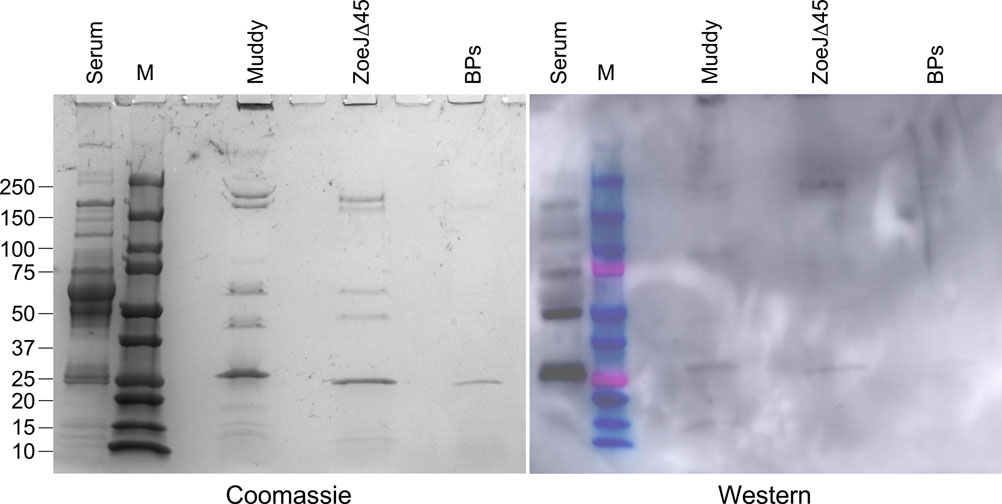
Phage preparations of Muddy, ZoeJD45, and BPs33DHTH-HRM10 (as shown) each containing approximately 2 × 1010 phage particles were separated by SDS-PAGE, together with protein markers (M) and a control sample of 10 μl of a 1:100 dilution of patient serum (serum) collected 72 days after initiation of phage treatment. The gel was stained with Coomassie Blue (left), transferred to a membrane for a Western blot which was probed with the same patient serum and an anti-human Horse Radish Peroxidase conjugated secondary antibody. These assays were repeated three times with similar results; a representative experiment is shown.
Extended Data Fig.5. Phage susceptibilities of GD01 clinical isolates.
M. abscessus were recovered at 20-, 72-, 107, and 121-days after initiation of phage treatment, propagated, and tested for susceptibilities to each of the phages in the cocktail. Each phage was diluted serially 10-fold and spotted onto bacterial lawns. These assays were repeat at least twice with similar results; a representative experiment is shown.
Supplementary Material
Acknowledgements
We thank T. Sampson, L. Holst, and S. Pinches for the discovery of BPs, Muddy, and ZoeJ, respectively, and the students and faculty in the Mycobacterial Genetics Course at the University of KwaZulu Natal and in the SEA-PHAGES program for their contributions in isolating and characterizing the collection of phages used here. Further Information about the phages and who isolated them and from where is available at https://phagesdb.org. We thank the Great Ormond Street Hospital microbiology department and the cell therapy and pharmacy staff for excellent technical assistance, C. Murphy for assistance with strains and sera, J. Standing and B. Margetts for assistance with figures, D. Bain at the University of Pittsburgh for the ICP-MS analysis, and A. Betsko for electron microscopy. We greatly appreciate the advice of J. Hartley on regulatory aspects, and thank C. Hamilton for help with translation. We thank T. Mavrich for comments on the manuscript and are grateful to S. Strathdee for both general advice and comments on the manuscript. This work was funded by National Institutes of Health grant GM116884 to G.F.H. and Howard Hughes Medical Institute grant 54308198 to G.F.H.
Footnotes
Competing Interests
Dr. Schooley serves as an uncompensated member of the AmpliPhi Scientific Advisory Board. Other authors declare no competing interests.
References
- 1.Honda J, Virdi R & Chan ED Front Microbiol 9, 2029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom BR, et al. Tuberculosis in Major Infectious Diseases (eds. Holmes rd, Bertozzi KK, Bloom S, B.R. & Jha P) (Washington (DC), 2017). [Google Scholar]
- 3.Martiniano SL, Davidson RM & Nick JA Pediatr Pulmonol 52, S29–S36 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Furukawa BS & Flume PA Semin Respir Crit Care Med 39, 383–391 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Griffith DE F1000Prime Rep 6, 107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osmani M, Sotello D, Alvarez S, Odell JA & Thomas M Transpl Infect Dis 20, e12835 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Adler FR, et al. Proc Am Thorac Soc 6, 619–633 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakasis A & Panitsa G Int J Antimicrob Agents (2018). [DOI] [PubMed] [Google Scholar]
- 9.Sula L, Sulova J & Stolcpartova M Czech Med 4, 209–214 (1981). [PubMed] [Google Scholar]
- 10.Koz’min-Sokolov BN & Vabilin. Probl Tuberk 4, 75–79 (1975). [PubMed] [Google Scholar]
- 11.Trigo G, et al. PLoS Negl Trop Dis 7, e2183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schooley RT, et al. Antimicrob Agents Chemother 61, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan BK, et al. Evol Med Public Health 2018, 60–66 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatfull GF J Virol 89, 8107–8110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs-Sera D, et al. Virology 434, 187–201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybniker J, Kramme S & Small PL J Med Microbiol 55, 37–42 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Marinelli LJ, et al. PLoS ONE 3, e3957 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dedrick R, et al. Tuberculosis (Edinb) 115, 14–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broussard GW, et al. Mol Cell 49, 237–248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.