Abstract
Clinical trials have demonstrated that 3,4-methylenedioxymethamphetamine (MDMA) paired with psychotherapy is more effective at reducing symptoms of post-traumatic stress disorder (PTSD) than psychotherapy or pharmacotherapy, alone or in combination. The processes through which MDMA acts to enhance psychotherapy are not well understood. Given that fear memories contribute to PTSD symptomology, MDMA could augment psychotherapy by targeting fear memories. The current studies investigated the effects of a single administration of MDMA on extinction and reconsolidation of cued and contextual fear memory in adult, male Long-Evans rats. Rats were exposed to contextual or auditory fear conditioning followed by systemic administration of saline or varying doses of MDMA (between 1 and 10 mg/kg) either 30 min before fear extinction training or immediately after brief fear memory retrieval (i.e. during the reconsolidation phase). MDMA administered prior to fear extinction training failed to enhance fear extinction memory, and in fact impaired drug-free cued fear extinction recall without impacting later fear relapse. MDMA administered during the reconsolidation phase, but not outside of the reconsolidation phase, produced a delayed and persistent reduction in conditioned fear. These findings are consistent with a general memory-disrupting effect of MDMA and suggest that MDMA could augment psychotherapy by modifying fear memories during reconsolidation without necessarily enhancing their extinction.
Keywords: fear conditioning, fear extinction, reconsolidation, renewal, post-traumatic stress disorder, fear memory
1. Introduction
Stress-related disorders such as post-traumatic stress disorder (PTSD) are thought to represent disorders of learning and memory. Indeed, persistent and intrusive traumatic memories contribute to PTSD symptomology. As such, common behavioral strategies to treat PTSD focus on inhibition of fear memories, or establishment of new and stronger competing memories, through exposure- based psychotherapy [1, 2]. Unfortunately, current treatment strategies for PTSD are limited, and demonstrate poor long-term efficacy [3]. Identification of novel strategies for the treatment of PTSD, or means to augment existing approaches, remains of utmost importance.
Recent clinical studies have reported meaningful long-term reduction of PTSD symptoms when psychotherapy is paired with 3,4- methylenedioxymethamphetamine (MDMA, “Ecstasy”)[4–7], and thus MDMA appears to be a promising augmentation strategy for psychotherapy. However, the mechanisms by which MDMA-assisted psychotherapy reduce PTSD symptoms are not fully understood. Because memories of traumatic events are at the core of PTSD, possible mechanisms include strengthening of competing memories formed during psychotherapy (e.g. fear extinction memories), or modification of the original traumatic memory, through the process of reconsolidation.
Fear extinction is learning that previous trauma-associated cues no longer predict threat [8], and is the learning phenomenon underlying exposure therapy. While exposure therapy is often successful at extinguishing fear responses, fear and anxiety symptoms often return even following successful treatment. Relapse phenomena contributing to the poor long-term efficacy of exposure therapy include fear renewal (the return of fear in contexts different from where extinction was learned; [9]), and spontaneous recovery (the return of fear after the passage of time, [8]). Manipulations which reduce the renewal and/or spontaneous recovery of fear could prove beneficial for the long-term remission of PTSD.
When a memory is recalled, it becomes susceptible to modification in a process known as reconsolidation [10]. Repeated recall and reconsolidation is thought to contribute to the persistence of traumatic memories in patients with PTSD, who tend to ruminate over traumatic events. However, just as recalled memories can be strengthened during reconsolidation, they are also susceptible to interference at that time [11]. Persistent symptom remission observed following MDMA-assisted psychotherapy may be attributable to modifications of, or interference with, the reconsolidation of traumatic memories.
In a series of recent studies, Young and colleagues report that, in mice, MDMA administered prior to cued fear extinction enhances fear extinction memory recall, and also reduces fear renewal and spontaneous recovery [12]. These effects of MDMA in mice occurred despite locomotor activating effects of MDMA during fear extinction learning [12], and in the absence of an effect on reconsolidation of cued fear memory.
It is reasonable to infer from prior pre-clinical data that the clinical benefit of MDMA could be occurring through an enhancement of fear extinction during psychotherapy. However, prior work has focused on effects of MDMA on cued fear memories in mice. Whether MDMA modifies the extinction or reconsolidation of contextual fear memories remains unknown. This is an important gap in our knowledge, as memories of contextual features of traumatic events contribute to persistent fear memories in PTSD [13]. Cued and contextual fear memories also involve unique brain circuitry which could be differentially impacted by MDMA. Furthermore, mice and rats have unique strengths as translational models for human neurobehavioral research [14]. Given reported differences in responses to MDMA in mice and rats [15–18], it is important from a mechanistic and translational perspective to determine if MDMA modifies fear extinction and reconsolidation similarly between mice and rats. The goal of the current studies was to determine the impact of MDMA on cued and contextual fear extinction and reconsolidation in rats. We hypothesized that if MDMA demonstrates therapeutic effects in a fear conditioning model in rats as it does in mice, then MDMA would enhance fear extinction and/or impair the reconsolidation of fear memory in rats. This observation could help guide follow-up mechanistic studies in rats, taking advantage of the unique translational benefits they provide.
2. Materials and Methods
2.1. Animals
A total of200 adult male Long Evans rats (Charles River Laboratories International) were obtained between post-natal days 50–55. Animals were pair housed in Nalgene Plexiglas cages (45 cm L × 25.2 cm W × 14.7 cm H) in a humidity-controlled environment at a temperature of 22°C. Rats were kept on a 12- hour light/dark cycle, with lights on between 06:00 – 18:00 h. All rats had ad libitum access to water and food. Rats were acclimated to these housing conditions for 7 days prior to any experimental manipulation. All behavioral procedures were performed during the light cycle between 0800 – 1100. All procedures were approved by the University of Colorado Denver Institutional Animal Care and Use Committee. Precautions were taken to minimize any animal discomfort during all procedures.
2.2. Drugs
The MDMA was synthesized at 100% purity by David E. Nichols, Ph.D., and provided by the Multidisciplinary Association for Psychedelic Studies. The potency of MDMA is tested over time and there is no evidence of decomposition. The MDMA is racemic and in the absence of enantioselective methods being used during synthesis, there is an assumed 1:1 ratio of the R- and S-stereoisomers of MDMA. MDMA in salt form was stored at 23°C, dissolved in 0.9% sterile saline immediately before use, and delivered intraperitoneally (i.p.) at a volume of 1 ml/kg. All rats were habituated to the i.p. injection procedure by handling and belly pinching once daily for 4 days prior to the start of experiments.
Although 3 mg/kg MDMA has been shown to have no significant effect on fear extinction in mice [12], mice require higher doses ofMDMA than rats or humans to reach similar blood levels [19]. In contrast, doses as low as 2 mg/kg in rats have been reported to achieve peak blood concentrations similar to the same dose in humans [20, 21]. Since low doses of 1.5–2.5 mg/kg are administered to humans during MDMA-assisted psychotherapy, we chose to include low doses of 1 and 3 mg/kg in addition to higher doses of 5 and 10 mg/kg, in our initial dose- response experiment. Because our initial experiment did not find any differences between Saline and 1 mg/kg MDMA, and due to the possibility of neurotoxicity produced by 10 mg/kg [22], later experiments use only 3 and 5 mg/kg.
2.3. Behavior
2.3.1. Fear conditioning.
All behavioral protocols closely resembled our previously published protocols [23, 24]. During fear conditioning, rats were placed into rectangular conditioning chambers (Context A; 51 cm W × 25.5 cm D × 30.5 cm H) with a shock grid floor (Coulbourn Instruments, Allentown, PA) housed inside individual sound-attenuating cabinets. Each cabinet was illuminated by individual red lights, while room lights remained off. For auditory conditioning, rats were pre-exposed to Context A for 3 min before exposure to 4, 10-s auditory conditioned stimuli (CS; 80 dB, 2 kH) on a 1-minute inter-trial-interval (ITI). Each of the 4 auditory CS terminated with a foot shock unconditioned stimulus (US; 1-s, 0.8mA). For contextual conditioning, rats were exposed to Context A for 5 min prior to exposure to either 5 (contextual fear extinction experiment) of 3 (contextual fear reconsolidation experiment) foot-shock US (1 s, 0.8 mA, 1 min ITI). Fewer US were used during conditioning in the contextual fear reconsolidation experiment to avoid potential ceiling effects during the proximal memory test. The CS and US were delivered through Coulbourn tone generators and shock scramblers, controlled via a custom interface with Noldus Ethovision- XT software (Leesburg, VA). Rats were transported to and from the conditioning chambers in their home cages. Fear conditioning chambers were cleaned with water between rats. Freezing behavior, an innate fear response defined as the absence of all movement other than that required for respiration, was used as an index of the conditioned fear response. All behavioral tests were video recorded for later analysis of freezing.
2.3.2. Fear extinction training and fear extinction memory tests.
Rats were exposed to fear extinction training 24 hours after conditioning. Saline or MDMA was administered 30 minutes prior to the start of fear extinction training. Auditory fear extinction occurred in a novel context (Context B) that was either a rectangular Plexiglas chamber (38 cm W × 38 cm D × 51 cm H) with a textured floor, or a triangular Plexiglas chamber (38 cm sides x 51 cm H) with a smooth floor. Assignment of chambers to rats in each group was counterbalanced to ensure equal group exposure to both rectangular and triangular chambers. Context B was housed inside the same individual sound-attenuating cabinets as Context A; however, all other cues such as transportation, box lighting, and odor, differed from Context A. In Context B, rats were transported in extinction chambers rather than home cages, cabinets were lit with bright white lights as opposed to red, and a vanilla scent was added. In addition, a fan located near the floor of each cabinet provided ventilation and background noise for Context B. Context B was cleaned with 10% ethanol between trials. Rats were exposed to Context B for 3 min before the auditory CS was delivered 20 times (1-minute ITI) in the absence of foot shock. For contextual fear extinction training, rats were re-exposed to the same Context A in which they were conditioned for 15 minutes. Rats were exposed to auditory or contextual fear extinction memory tests 24 h (proximal) and 7 d (distal) after fear extinction training. Spontaneous recovery of fear occurs after the passage of time following extinction [8]; therefore, freezing during the distal memory test is influenced by spontaneous recovery. During fear extinction memory tests, rats were treated identically to fear extinction training, except no drug was administered. We have observed that both exercise and manipulations of the dopamine (DA) system can enhance fear extinction using identical fear conditioning and extinction methods to those used here [23–25].
Time spent active, defined as pixel displacement above 3%, was calculated by Noldus EthoVision XT during the 3-minute Context B exploration periods prior to the first CS during auditory fear extinction training and auditory fear extinction memory tests.
2.3.3. Fear renewal.
Fear renewal was tested by re-exposing the rats to the extinguished CS in either the same Context B used for extinction or a novel Context C. Rats assigned to Context C were placed into the opposite (rectangular or triangular) extinction chambers from that used during extinction. Context C had red cabinet lighting, raspberry scent, and was washed with 1% acetic acid between rats. A fan located near the top of each cabinet was turned on, and room lights remained off. Rats were exposed to each context for 3 min prior to 20 CS exposures. Differences in freezing observed between the Same and Different contexts are considered fear renewal [26].
2.3.4. Memory reactivation and reconsolidation.
To reactivate cued fear memories in the absence of fear extinction, rats were exposed to a single auditory CS in Context B. We chose to use a single CS to reactivate cued fear memory, as a single auditory CS has been used previously to investigate the effects of MDMA on cued fear reconsolidation in mice [12]. To reactivate contextual fear memories, rats were re-exposed to the conditioning Context B for 3 minutes. A 3-minute retrieval session has been reported to recruit reconsolidation in the absence of extinction [27]. Rats received Saline or MDMA 30 seconds after the single CS or immediately after the 3-minute exposure to Context B. Fear memory tests, identical to fear extinction memory tests, occurred 1 (proximal) or 7 (distal) days later.
2.4. Statistical Analysis
Percent time spent freezing was calculated by averaging freezing data from an individual experimenter blind to treatment condition with immobility times obtained from Noldus EthoVision XT. The inter-rater correlation between the human scorer and EthoVision was calculated at 95%. Because regularly-scheduled ITIs can become a part of the CS, freezing during the 10 second auditory stimulus and the subsequent 1-minute ITI were combined and expressed as total freezing during a trial, as in prior work [23, 24, 28]. Freezing data were combined into blocks of 4 or 5 trials (for auditory fear experiments) or minutes (for contextual fear experiments) and compared with repeated-measures ANOVA with Drug or Drug and Context as factors. Locomotor activity was compared between drug groups using ANOVA. Alpha was set at 0.05. Bonferroni post hoc analyses were performed when appropriate. Statview software was used for all statistical analyses. Power analyses run in G*Power [29] indicated that the statistical power was adequate to detect effect sizes between 0.2 – 0.38 (small effect sizes) during fear extinction memory tests.
3. Results
3.1. Dose-dependent effects of MDMA on auditory fear extinction and extinction retrieval
To investigate dose-dependent effects of MDMA on cued fear extinction and retrieval, rats were exposed to auditory fear conditioning, and then randomly assigned to receive Saline (n = 24), or 1 (n = 8), 3 (n = 10), 5 (n = 10), or 10 (n = 8) mg/kg MDMA, 30 min prior to fear extinction learning (F igure 1A). This large experiment was performed in multiple, smaller replications of various sizes, each including a larger number of Saline-treated rats than any one MDMA-treated group. This provided an anchor of control (Saline) behavior within each replication with which to determine if a drift in freezing levels occurred between replications. No significant differences between Saline-treated rats used in different replications were found, so rats used in each replication were combined to yield the final group sizes reported. One rat given 10 mg/kg MDMA died after fear extinction, resulting in a final 10 mg/kg MDMA group size of n=7.
Freezing during the 3-minute exploration period prior to initial CS exposure during auditory fear conditioning in Context A was negligible, and did not differ between groups (Figure 1B; pre-freezing). All rats acquired fear conditioning (Figure 1B; effect of time: F(3, 162) = 65; p<0.0001) and there was no effect of subsequent group assignment. Freezing in Context B during the 3-minute exploration period prior to the first CS exposure during extinction training differed between groups (F(4, 54) = 3.1; p = 0.02), with the 5 mg/kg and 10 mg/kg MDMA groups freezing less than the Saline group (data not shown). The reduction in freezing produced by 5 and 10 mg/kg MDMA during the 3-minute period prior to the first CS during auditory fear extinction training was paralleled by an increase in locomotor activity (Main effect of drug: (F (4, 54) = 3.1; p = 0.02; Figure 1F). Neither 1 nor 3 mg/kg MDMA increased locomotor activity relative to Saline.
Figure 1.
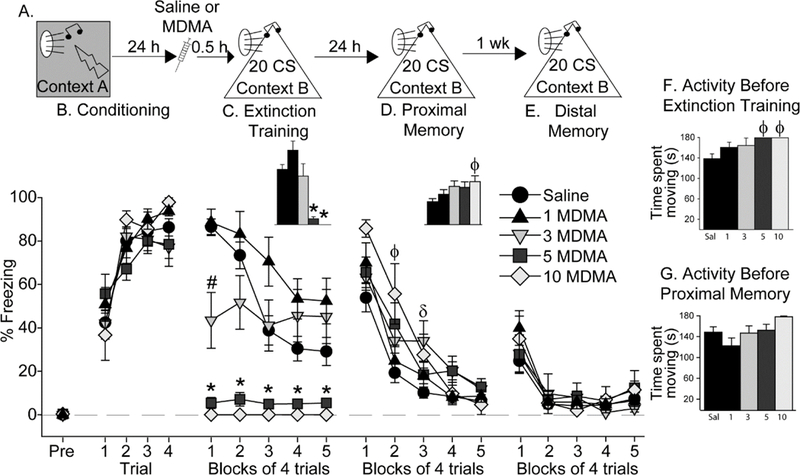
Effect of MDMA on auditory fear extinction. (A) Experimental timeline. Rats received Saline or 1 mg/kg (1 MDMA), 3 mg/kg (3 MDMA), 5 mg/kg (5 MDMA), or 10 mg/kg (10 MDMA) of MDMA 30 minutes prior to auditory fear extinction. (B) Levels of freezing during auditory fear conditioning. (C) Levels of freezing during fear extinction training. Inset shows average freezing over the entire extinction training session. (D) Levels of freezing during proximal memory test. Inset shows average freezing over the entire proximal fear extinction memory test. (E) Levels of freezing during distal memory test. (F) Locomotor activity during the 3-minute exploration period in Context B prior to the first CS during extinction training. (G) Locomotor activity during the 3-minute exploration period in Context B prior to the first conditioned stimulus (CS) during the proximal fear extinction memory test. All data represent means ± SEM. *p<0.05 relative to all other groups except each other; # p<0.05 relative to all other groups; Φ p<0.05 10 mg/kg MDMA versus Saline; δ p<0.05 3 mg/kg MDMA versus Saline.
Repeated measures ANOVA revealed a significant main effect of time (F (4, 216) = 16.3; p < 0.0001), drug (F (4, 54) = 16.2; p < 0.0001), and interaction between time and drug (F (16,216) = 9.4; p < °. 0001) on freezing during the extinction training session (Figure 1C). There was an inverse relationship between dose of MDMA and freezing during extinction, whereby rats given 10 mg/kg MDMA displayed no freezing at all during the entire extinction session. Rats given 5 mg/kg and 3 mg/kg MDMA also displayed less freezing than rats given Saline, whereas 1 mg/kg MDMA did not alter freezing behavior relative to Saline (see Figure 1C for results of post-hoc tests). The dose-responsive reduction in freezing produced by MDMA during fear extinction training can most likely be attributed to the locomotor-activating effects of MDMA observed here (Figure 1F) and in prior work [12, 30–32].
Proximal fear extinction memory, during which rats were again exposed to 20 auditory CS in Context B, was tested drug-free the following day. Neither freezing (data not shown) nor locomotor activity (Figure 1G) prior to the first CS differed between groups. However, prior MDMA dose-dependently increased freezing during the proximal fear extinction memory test (Figure 1D). Repeated measures ANOVA revealed that the main effect of time (F (4, 216) = 105. 4; p < 0.0001), drug (F (4, 54) = 3.6; p = 0.01), and their interaction (F (16, 216) = 2.3; p = 0.004), were all significant. Rats given 10 mg/kg MDMA during prior extinction training froze more than rats given saline during the first and second trial blocks, and rats given 3 mg/kg MDMA froze more than Saline-treated rats during the third trial block. The difference between the Saline and 5 mg/kg MDMA groups during blocks 2 and 4 just missed significance. No other group differences were significant (Figure 1D).
To investigate the persistence of the effects of MDMA, rats were exposed to a distal fear extinction memory test 7 days after the proximal fear extinction memory test in Context B. Freezing and locomotor activity levels prior to the first CS again did not differ between groups (data not shown). All rats acquired within- session fear extinction (main effect of time: (F (4, 216) = 34.9; p < 0.0001), but there was no effect of previous MDMA administration (Figure 1E). In other words, the increase in freezing displayed by the MDMA groups during the proximal memory test was no longer observed during the distal memory test.
3.2. MDMA present during auditory fear extinction training has no impact on fear renewal
Here we investigated the effects of MDMA on fear renewal. Rats were exposed to auditory fear conditioning, and then randomly assigned to receive Saline or 3 mg/kg MDMA (n=16 per group) the next day, 30-minutes prior to fear extinction (Figure 2A). Fear renewal testing occurred 24 h after fear extinction. The 3 mg/kg dose was chosen because this was the lowest dose found to impact freezing without altering locomotor activity in Figure 1. Rats displayed negligible freezing in Context A prior to auditory fear conditioning (Figure 2B, pre-freezing). Rats assigned to subsequent Saline or MDMA groups all acquired auditory fear conditioning (F (3, 90) = 32.3; p < 0.0001), and freezing during conditioning did not differ between groups (Figure 2B). On the following day, rats were randomly assigned to receive either Saline or 3 mg/kg MDMA, 30 minutes prior to fear extinction training in Context B. The Saline group again froze more than the MDMA group during the 3-minute exploration period in Context B before the first CS was delivered (F (1, 30) = 20.5; p < 0.0001; data not shown). During extinction training, we observed a pattern of freezing similar to that observed in Figure 1C. MDMA reduced freezing during the beginning of the extinction session, followed by an increase in freezing later in the session (interaction between time and drug: (F(4, 120) = 7.8; p < 0.0001; Figure 2C).
Figure 2.
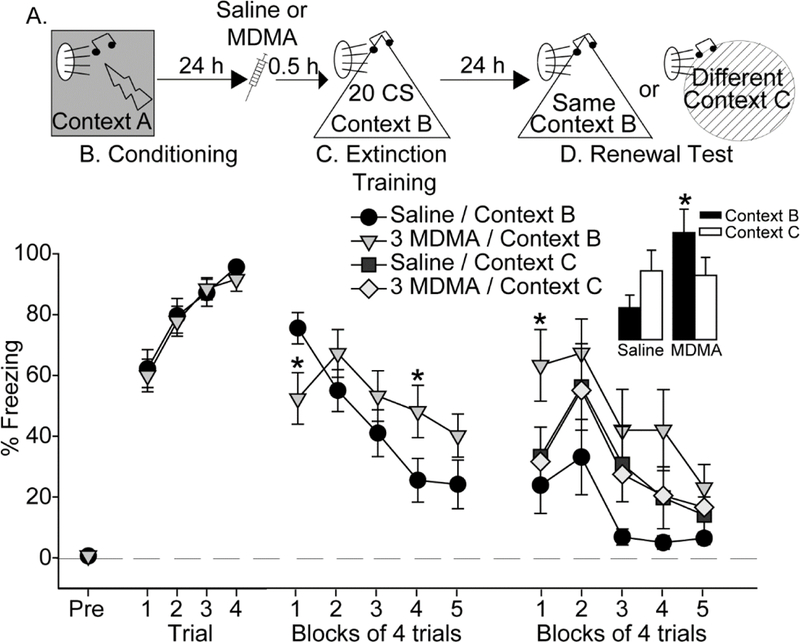
Effect of MDMA on renewal of auditory conditioned fear. (A) Experimental timeline. Rats received Saline or 3 mg/kg MDMA (3 MDMA) 30 minutes prior to auditory fear extinction. (B) Levels of freezing during auditory fear conditioning. (C) Levels of freezing during fear extinction. (D) Levels of freezing during fear renewal. Inset shows average freezing during the entire renewal session. All data represent means ± SEM. *p<0.05 3 mg/kg MDMA / Context B relative to Saline / Context B.
The next day, rats were randomly assigned to one of two groups: Context B or Context C. This resulted in group sizes of n = 8 per group. Rats in the Context B group were reintroduced to Context B, and rats in the Context C group were placed into a novel Context C. Freezing during the 3-minute observation period, prior to the first CS, was negligible regardless of context and did not differ between groups (data not shown). The lack of freezing during this pre-CS period indicates that a negative association with Context B was not formed during extinction training, nor did fear generalize to the novel Context C. All rats were exposed to the auditory CS 20 times in their assigned contexts. The interaction between context and drug just missed significance (F (1, 28) = 4.2; p = 0.05). Rats given Saline tended to freeze more when placed into a different context from where extinction occurred (Context C), relative to Context B, indicating fear renewal (Figures 2D and 2E). Similar to observations in Figure 1D, prior MDMA increased freezing relative to Saline when rats were tested in the same context in which extinction occurred (Context B; p < 0.05), and there was a trend for prior MDMA to increase freezing regardless of context (main effect of drug: F (1, 28) = 3.8; p = 0.06; Figures 2D and 2E). Despite the increase in fear expression displayed by the MDMA group relative to the Saline group in Context B, prior MDMA did not increase fear renewal in Context C (Figure 2D).
3.3. MDMA present during contextual fear extinction has no impact on contextual fear extinction memory retrieval
We next investigated the effects of MDMA on extinction of contextual fear memory. Rats were exposed to contextual fear conditioning in Context A with 5 shocks, to form a strong contextual fear memory. Extinction training occurred 24 h after conditioning, followed the next day by an extinction memory test (Figure 3A). Saline (n = 10), 3 (n = 11), or 5 (n = 11) mg/kg MDMA was administered 30 minutes prior to fear extinction training. None of the rats displayed appreciable freezing prior to the first shock during conditioning (Figure 3B, Pre). Rats acquired contextual fear conditioning (main effect of time: F (4, 18) = 111.1; p < 0.0001; Figure 3B), and there were no differences between subsequent drug groups.
Figure 3.
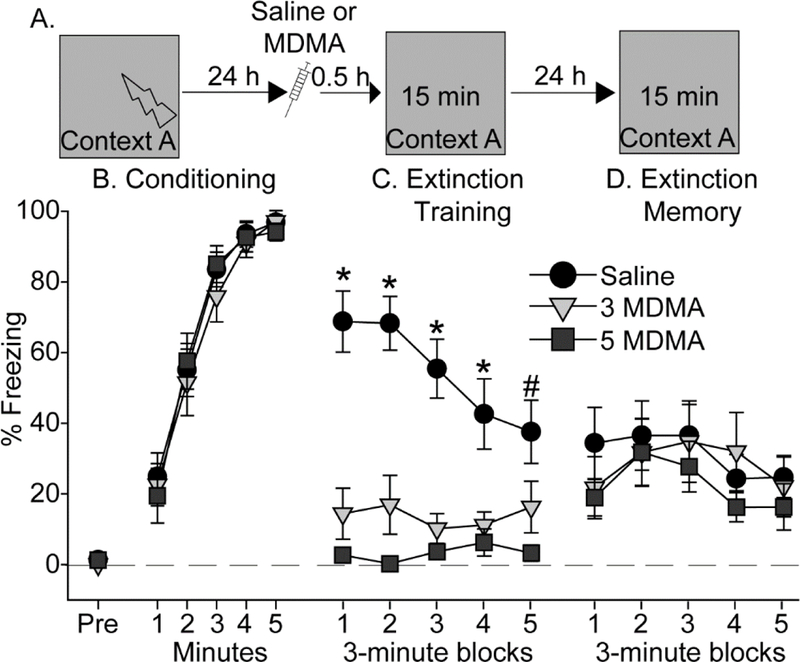
Effect of MDMA on contextual fear extinction. (A) Experimental timeline. Rats received Saline, 3 mg/kg (3 MDMA) or 5 mg/kg (5 MDMA) of MDMA 30 minutes prior to contextual fear extinction. (B) Levels of freezing during contextual fear conditioning. (C) Levels of freezing during contextual fear extinction. (D) Levels of freezing during the contextual fear extinction memory test. All data represent means ± SEM. *p<0.05 relative to all other groups; # p<0.05 Saline relative to 5 mg/kg MDMA.
Twenty-four hours later, rats were randomly assigned to receive either Saline or MDMA, 30-minutes prior to a 15-minute re-exposure to Context A for contextual fear extinction training. Similar to what was observed during auditory fear extinction, MDMA decreased freezing during contextual fear extinction (Figure 3C). The main effect of drug (F (2, 29) = 37.3; p < 0.0001), time (F (4, 116) = 2.4; p < 0.05), and their interaction (F (8, 116) = 3; p < 0.01) were all significant. Both 3 and 5 mg/kg MDMA reduced freezing compared to Saline (p < 0.05). Fear extinction memory was tested drug-free the next day. Rats displayed within-session extinction (F (4, 116) = 2.5; p = 0.04), but there was no effect of prior MDMA (Figure 3D). Freezing during the distal fear extinction memory test was negligible and thus is not shown.
3.4. MDMA administered during fear memory reconsolidation reduces later fear expression
We investigated the effects of MDMA on reconsolidation of both auditory and contextual fear memories. Rats used in the auditory fear reconsolidation study were exposed to auditory fear conditioning in Context A (see Figure 4A for design). All rats acquired auditory fear conditioning (F (3, 86) = 40.1; p < 0.0001; Figure 4B) and there were no differences between groups. Rats were placed into Context B 48 h later and, after a 3-minute exploration period during which rats displayed minimal freezing behavior (Figure 4C; Pre), rats were exposed to a single auditory CS. Relative to the freezing levels during the exploration period, freezing increased during the CS trial (F (1, 29) = 97.4; p < 0.0001) and did not differ between groups, indicating successful retrieval of auditory fear conditioning (Figure 4C). Saline (n = 10), 3 (n = 11) or 5 (n = 11) mg/kg of MDMA was administered 30 seconds after the single CS, during the fear memory reconsolidation period [11], and rats were returned to their home cages.
Figure 4.
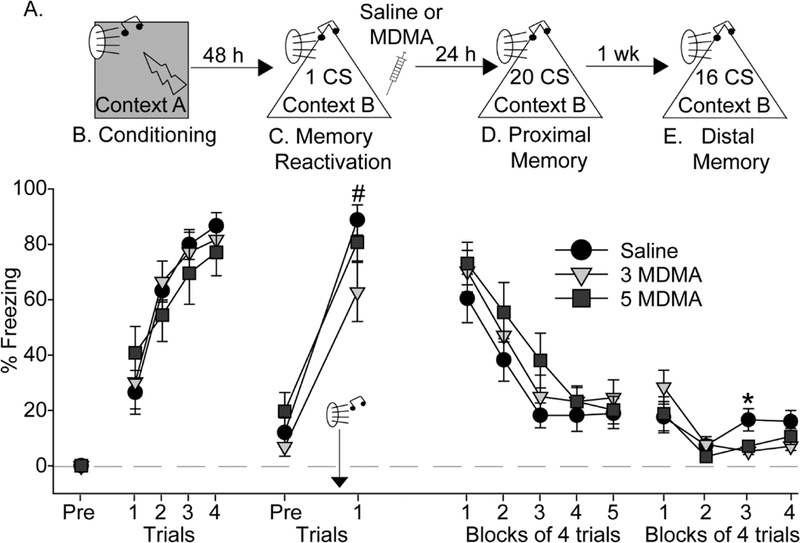
Effect of MDMA on auditory fear memory reconsolidation. (A) Experimental timeline. Rats received Saline, 3 mg/kg (3 MDMA) or 5 mg/kg (5 MDMA) of MDMA 30 seconds after exposure to a single CS. (B) Levels of freezing during auditory fear conditioning. (C) Levels of freezing before (Pre) and after auditory fear memory reactivation with a single conditioned stimulus (CS). (D) Levels of freezing during the proximal auditory conditioned fear memory test. (E) Levels of freezing during the distal auditory conditioned fear memory test. All data represent means ± SEM. #p<0.05 post tone relative to pre tone; *p<0.05 relative to all other groups.
Freezing to the CS was tested 24-hours after memory reactivation in Context B (proximal memory test). Freezing prior to the first CS was minimal and did not differ between groups (data not shown). All rats displayed significant conditioned freezing behavior and within-session extinction (F (4, 116) = 51.8; p < 0.0001), but there was no effect of prior MDMA (Figure 4D). However, during the distal memory test 7 days after fear memory reactivation, ANOVA revealed a significant interaction between time and drug (F (6, 86) = 2.2; p = 0.04). Rats given 3 and 5 mg/kg MDMA during the reconsolidation period displayed less freezing behavior than the Saline group during the third trial block (Figure 4E).
To investigate effects of MDMA given during reconsolidation of contextual fear memory on later conditioned freezing to context, rats were exposed to contextual fear conditioning in Context A (see Figure 5A for design). Rats acquired contextual fear conditioning (F (2, 82) = 9.0; p < 0.001), and freezing during conditioning did not differ between groups (Figure 5B). Freezing during the 3-minute contextual fear memory re-activation period 48 h later increased over time (F (2, 82) = 27.5; p < 0.0001), and also did not differ between groups (Figure 4C), indicating successful retrieval of contextual fear memory in the absence of extinction. Saline (n = 10), 3 (n = 10) or 5 (n = 6) mg/kg of MDMA was administered immediately after the 3-minute re-exposure to Context A and rats were returned to their home cages. To determine if effects of MDMA on later freezing are specific to administration during the reconsolidation phase, 2 additional groups of rats received Saline (n = 10) or 5 mg/kg MDMA (n = 9) 6 h after fear memory reactivation, a time point after the reconsolidation window [11]. Since rats that received saline immediately after fear memory reactivation did not differ from those that received saline 6 hours after reactivation, these rats were combined into one Saline group (n = 20).
Figure 5.
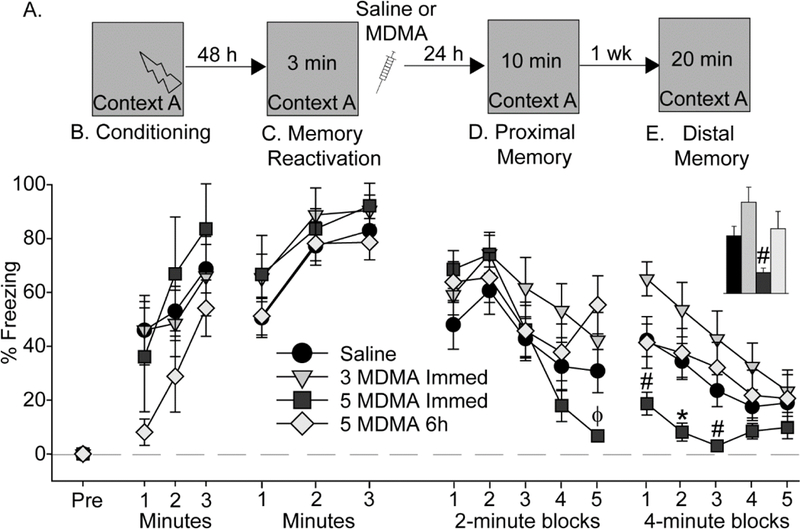
Effect of MDMA on contextual fear memory reconsolidation. (A) Experimental timeline. Rats received Saline, 3 mg/kg (3 MDMA), or 5mg/kg (5 MDMA) MDMA either immediately (Immed) after contextual fear memory reactivation or 6 h later (6h). (B) Levels of freezing during contextual fear conditioning. (C) Levels of freezing during reactivation of contextually conditioned fear memory. (D) Levels of freezing during the proximal fear memory test. (E) Levels of freezing during the distal memory test. Inset shows average freezing over the entire distal contextual fear memory test. All data represent means ± SEM. *p<0.05 relative to all other groups; #p<0.05 relative to 3 MDMA Immed; ϕ p < 0.05 relative to 5 MDMA 6h.
Freezing to Context A was tested 24 h after memory reactivation (proximal memory test). Although the average freezing during re-exposure to Context A did not differ between groups (Figure 5D), repeated measures ANOVA revealed a significant main effect of time (F (4, 164) = 23.4; p < 0.0001) and a significant interaction between time and drug (F (12, 164) = 2.65; p < 0.01). Post-hoc analysis revealed that rats that received 5 mg/kg MDMA immediately after fear memory reactivation froze less than rats in other groups during the final 2-minute block (Figure 5D). Freezing to Context A was assessed again 7 days later (distal memory test). Rats demonstrated within-session fear extinction (main effect of time: (F (4, 164) = 15; p < 0.0001) and freezing levels differed between groups (F (3, 41) = 3.4; p = 0.02). Rats that received 5 mg/kg MDMA immediately after fear memory reactivation froze less than rats given 3 mg/kg MDMA during the first and third 4- minute block and less than all other groups during the second 4-minute block (Figure 5E). Rats given 5 mg/kg MDMA 6 h after memory reactivation displayed freezing levels closely resembling that of the Saline group, indicating that the effect of 5 mg/kg MDMA was dependent on administration during the reconsolidation window. At no time did the 3 mg/kg MDMA group differ from the Saline group.
4. Discussion
Here we report that MDMA fails to facilitate cued or contextual fear extinction memory in rats. Rather, when paired with cued fear extinction training, MDMA interferes with later retrieval of fear extinction. Additionally, MDMA interferes with the reconsolidation of both cued and contextual fear memory. When administered immediately after a brief fear memory retrieval session, MDMA reduced later expression of fear to the conditioned cue or context, even up to a week later. These data are consistent with a memory-impairing effect of MDMA, and implicate interference with fear memory reconsolidation, rather than an enhancement of fear extinction, as a mechanism underlying the long-lasting therapeutic effects of MDMA-assisted psychotherapy.
Contrary to our hypothesis and prior observations in mice [12], both 3 and 10 mg/kg MDMA, given 24 h earlier during auditory fear extinction training, impaired fear extinction retrieval during the proximal cued fear extinction memory test. It is unlikely that conditioned [33] or lingering locomotor-activating effects of MDMA contributed to the observed effects of MDMA on freezing, because there were no group differences in locomotor activity during the first 3-minutes of the fear extinction memory test prior to the first CS, and, rather than decreasing freezing, prior MDMA increased freezing during the proximal memory test. It is also unlikely that prior MDMA increased freeing during the proximal memory test by non-specifically increasing freezing, because MDMA administered noncurrent with extinction or reconsolidation had no effect on later freezing. Furthermore, MDMA had differential effects on freezing depending on the learning process with which it was paired. These observations suggest that the effects of MDMA seem to be dependent on MDMA being present during a learning process, in this case auditory fear extinction.
Given that MDMA needs to be concurrent with auditory fear extinction to increase later freezing to the CS, one explanation for our observations is that a negative association with the context in which MDMA was paired during auditory fear extinction training could have increased fear to the extinction context; thereby obscuring a potential extinction memory enhancement. Indeed, this could explain why prior MDMA increased freezing during the auditory fear extinction memory test, but the not the contextual fear extinction memory test. During contextual fear conditioning, a negative association with the context would have already formed in both Saline and MDMA groups during contextual fear conditioning. If a negative association with the extinction context increased fear during the auditory fear extinction memory tests, then we would expect to observe the MDMA groups freeze more to the extinction context prior to the first CS during cued fear extinction memory tests. This; however, was not observed. Instead, there were no group differences in freezing to the extinction context prior to the first CS during the proximal or distal auditory fear extinction memory tests. Moreover, for MDMA to produce a negative association with the extinction context, MDMA would need to be aversive. In contrast, data indicate that rats find MDMA rewarding [34, 35]. It is therefore unlikely that MDMA produced a negative association with the extinction context with which it was paired.
Another potential explanation for our results is that MDMA present during fear extinction training interfered with the ability of the rats to acquire fear extinction. Indeed, data in humans [36] and rodents [37–40] suggest that MDMA can impair memory acquisition, although effects of a single, low dose administration require additional clarification. One way MDMA could interfere with extinction acquisition is by making it difficult for the rats to attend to the CS (encoding). This possibility could explain why MDMA impaired auditory, but not contextual, fear extinction memory retrieval. Since exposures to the auditory CS during auditory fear extinction are very short compared to the prolonged exposure to conditioned context during contextual fear extinction, it could be easier for MDMA to interfere with the encoding of the brief CS. There is no reason to assume; however, that MDMA would necessarily interfere with the encoding of the CS. Indeed, psychostimulants such as amphetamine given prior to cued or contextual fear extinction training reduce freezing during extinction training similarly to MDMA [41], but have no effect on later auditory fear extinction retention [41, 42]. Even engaging in wheel running activity during cued fear extinction improves extinction memory [28]. Similarly, Young et al. (2015) report that 7.8 mg/kg MDMA increases locomotor activity in mice, yet this dose of MDMA given prior to auditory fear extinction training improved extinction retrieval in the mice. Even if encoding of the CS wasn’t impacted by MDMA, it does remain possible that MDMA interfered with the acquisition of the association between the CS and the lack of the predicted US during extinction. Unfortunately, it is difficult to determine the effects of MDMA on fear extinction acquisition because of the locomotor-activating effects of MDMA observed here and in prior reports [12, 30–32].
A final possibility for the observed increase in freezing behavior to the extinguished CS produced by prior MDMA during auditory fear extinction training is that MDMA interfered with the consolidation of the auditory fear extinction memory. This could have been produced directly by lingering MDMA or indirectly by MDMA altering factors required for later memory consolidation. In mice, the ability of MDMA given 30 min prior to auditory fear extinction to enhance later fear extinction retrieval has been shown to be dependent on an increase in brain- derived neurotrophic factor (BDNF; [12]), a neurotrophin important for memory consolidation [43], including consolidation of cued fear extinction [44]. In contrast, although effects of MDMA on BDNF in rats can depend on dose, administration frequency, timing, and brain region [45, 46], a recent report indicates that a single administration of 10 mg/kg can decrease BDNF [47]. Differences in the effects of MDMA on BDNF and extinction memory consolidation could be due to differential effects of MDMA on monoamine transmission between mice and rats (reviewed in [18]). In general, it is thought that MDMA has a more potent impact on 5-HT in the rat compared to the mouse [18]. This conclusion is supported by more robust effects of MDMA on 5-HT transmission in discrete brain regions and greater 5-HT neurotoxicity in rats [48, 49] than mice [50, 51]. This is important, as 5-HT signaling through the 5-HT2-family receptors can decrease BDNF [52, 53] and impair fear extinction [54–56] in rats. Thus, it is possible that the single-dose administration of MDMA used in the current studies could be impairing the consolidation of cued fear extinction in rats through a 5-HT-dependent reduction in BDNF. While the 5-HT system is thought to more sensitive to MDMA in rats than mice, the DA system seems to be more sensitive to MDMA in mice than rats [18]. In support of a role for DA in the effects of MDMA on fear extinction in mice are the observations that the increase in BDNF produced by MDMA in mice is dependent on DA [57], and manipulations that increase DA transmission or signaling can enhance fear extinction [24, 58–60].
While MDMA increased freezing during the proximal cued fear extinction memory test, MDMA had no impact on contextual fear extinction memory. Cued and contextual fear memories are thought to be supported by unique brain circuits involving the amygdala and hippocampus, respectively, which could be differentially impacted by MDMA. Since MDMA has been reported to impact synaptic plasticity in both amygdala [57, 61, 62] and hippocampus [63, 64], additional research will be required to determine whether effects of MDMA on memory acquisition and consolidation are specific to some types of memories or brain regions.
Despite the proximal fear extinction memory deficit produced by MDMA, fear extinction memories acquired under the influence of MDMA do not appear to be any more susceptible to relapse phenomena, such as spontaneous recovery and renewal, than those acquired after Saline administration. Since freezing to the CS at the distal time point could be influenced by spontaneous recovery of conditioned fear [8], the lack of effects of prior MDMA on freezing during the distal memory tests suggest that prior MDMA does not alter susceptibility to spontaneous recovery. Since the proximal fear extinction memory test was also an extinction training session, it is possible that any potential susceptibility to spontaneous recovery produced by prior MDMA can be eliminated with sufficient extinction. Another group of rats exposed only to the distal fear extinction memory test would be required to test this hypothesis. However, the observation that MDMA did not alter freezing during the fear renewal test in a novel context is also consistent with the idea that MDMA impairs fear extinction without increasing vulnerability to relapse. These data are important from a clinical perspective, because potential strategies to augment psychotherapy that increase fear relapse would be counterproductive to long-term recovery. Thus, the short-lived interference with cued fear extinction memory retrieval produced by MDMA may not impede the long-term therapeutic efficacy of MDMA-assisted psychotherapy.
In addition to exploring the effects of MDMA on fear extinction, we investigated the effects of MDMA on auditory and contextual fear memory reconsolidation. The fear memory reactivation procedures were successful at triggering fear memory recall in the absence of extinction. Exposure to a single CS was sufficient to dramatically increase freezing during auditory fear memory reactivation, and there was little evidence of between-session extinction, as revealed by similar freezing levels between memory reactivation and the first trial block during the proximal fear memory test 24 h later. Similarly, a 3-minute contextual fear retrieval session is insufficient to trigger fear extinction mechanisms, even when a between-session reduction in freezing is observed [27]. These data, along with the observation that MDMA had no impact on freezing when administered outside of the reconsolidation window, suggest that the observed effect of post-retrieval MDMA on later conditioned freezing is due to modification of fear memory reconsolidation.
Interestingly, post-retrieval MDMA had the biggest impact on later freezing to conditioned cues or contexts during the distal memory tests. The delayed effects of MDMA could explain the discrepancy between the current results and the negative results reported previously in mice [12]. Young et al. (2015) administered MDMA prior to cued fear memory reactivation with a single CS, but only assessed auditory conditioned fear memory 24 h after memory reactivation. Had fear memory also been tested at a later time point, a delayed effect of MDMA on freezing might have been observed. The mechanism by which post-retrieval MDMA modifies fear memory reconsolidation to result in a delayed reduction in conditioned freezing requires further study. This observation is clinically relevant; however, considering that an important feature of MDMA-assisted psychotherapy is the long-lasting symptom reduction observed during 2-month, 1-year, and 4-year follow-up sessions, compared to conventional PTSD treatments which, in contrast, have poor long-term efficacy [5, 7].
In summary, we report that MDMA can interfere with both the extinction and reconsolidation of conditioned fear memory in rats. These data are consistent with a general memory-impairing effect of MDMA, as previously reported by others [37, 63, 65]. Inconsistencies between the current results and those in mice could be attributed to differences in the effects of MDMA on monoamine neurotransmission and neurotrophic factor expression between rats and mice, or differences in timing of memory tests between experiments. The current data suggest that MDMA-assisted psychotherapy could provide its long-lasting reduction of PTSD symptoms by interfering with the reconsolidation of traumatic memories that resurface during psychotherapy sessions. This possibility is consistent with clinical studies of MDMA-assisted psychotherapy, which to date utilize non-directive psychotherapy, in which subjects are encouraged to attend to traumatic memories, rather than structured, exposure-based strategies [66]. Thus, MDMA-assisted psychotherapy could trigger the recall and reconsolidation of traumatic memories without necessarily facilitating their extinction. The observation that MDMA does not need to be present during memory reactivation in order to reduce later conditioned fear implies that MDMA-assisted psychotherapy may need not rely on MDMA altering the patient-clinician relationship or the subjective experiences of traumatic memories during recall. Disruption of reconsolidation processes after recall could be a sufficient explanation for the therapeutic effect of MDMA in a clinical setting. Additional research is required to understand the mechanisms by which post-retrieval MDMA produces a delayed and persistent reduction in conditioned fear, though our findings represent an important avenue for future investigation.
Highlights.
MDMA impairs cued fear extinction recall without impacting relapse
Post-retrieval MDMA interferes with reconsolidation of fear memories
The effects of MDMA on reconsolidation are delayed and persistent
Clinical efficacy of MDMA could be attributed to modification of fear memory during reconsolidation
Acknowledgements
This work was funded by a Multidisciplinary Association for Psychedelic Studies (MAPS) research contract, NIH (MH114026), and a University of Colorado Denver faculty development grant awarded to BNG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ursano RJ et al. Practice guideline for the treatment ofpatients with acute stress disorder and posttraumaticstress disorder Am J Psychiatry 2004. 161(11 Suppl): p. 3–31 [PubMed] [Google Scholar]
- 2.Jonas DE et al. in Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder (PTSD) 2013: Rockville (MD). [PubMed] [Google Scholar]
- 3.Kessler RC et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States Int J Methods Psychiatr Res 2012. 21(3): p. 169–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mithoefer MC et al. The safety and efficacy of {+/−}3,4- methylenedioxymethamphetamine-assistedpsychotherapy in subjects with chronic, treatment-resistantposttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol 2011. 25(4): p. 439–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mithoefer MC et al. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study J Psychopharmacol 2013. 27(1): p. 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oehen P et al. A randomized, controlled pilot study of MDMA (+/− 3,4- Methylenedioxymethamphetamine)-assistedpsychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD) J Psychopharmacol 2013. 27(1): p. 40–52 [DOI] [PubMed] [Google Scholar]
- 7.Ot’alora GM GJ Poulter B, Van Derveer JW III, Giron SG, Jerome L, Feduccia AA, Hamilton S, Yazar-Klosinski B, Emerson A, Mithoefer MC, Doblin R, MDMA-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized control trial Biological Psychiatry 2018. Under Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlov IP Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex 1927. Oxford: England: Oxford University Press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouton ME Context and ambiguity in the extinction of emotional learning: implications for exposure therapy Behav Res Ther 1988. 26(2): p. 137–49 [DOI] [PubMed] [Google Scholar]
- 10.Nader K Schafe GE, and Le Doux JE Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval Nature 2000. 406(6797): p. 722–6 [DOI] [PubMed] [Google Scholar]
- 11.Duvarci S and Nader K Characterization of fear memory reconsolidation J Neurosci 2004. 24(42): p. 9269–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young MB et al. 3,4-Methylenedioxymethamphetamine facilitates fear extinction learning Transl Psychiatry 2015. 5: p. e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maren S Phan KL, and Liberzon I The contextual brain: implications for fear conditioning, extinction and psychopathology Nature reviews. Neuroscience 2013. 14(6): p. 417–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellenbroek B and Youn J Rodent models in neuroscience research: is it a rat race? Dis Model Mech 2016. 9(10): p. 1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindlundh-Hogberg AM Schioth HB, and Svenningsson P Repeated intermittent MDMA binges reduce DAT density in mice andSERTdensity in rats in reward regions of the adolescent brain Neurotoxicology 2007. 28(6): p. 1158–69 [DOI] [PubMed] [Google Scholar]
- 16.Trigo JM et al. 3,4-methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice Biol Psychiatry 2007. 62(6): p. 669–79 [DOI] [PubMed] [Google Scholar]
- 17.Oakly AC et al. A genetic deletion of the serotonin transporter greatly enhances the reinforcing properties of MDMA in rats Mol Psychiatry 2014. 19(5): p. 534–5 [DOI] [PubMed] [Google Scholar]
- 18.Easton N and Marsden CA Ecstasy: are animal data consistent between species and can they translate to humans? J Psychopharmacol 2006. 20(2): p. 194–210 [DOI] [PubMed] [Google Scholar]
- 19.Mueller M et al. Studies of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) metabolism and disposition in rats and mice: relationship to neuroprotection and neurotoxicity profile J Pharmacol Exp Ther 2013. 344(2): p. 479–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann MH et al. Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat Drug Metab Dispos 2009. 37(11): p. 2163–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green AR et al. Lost in translation:preclinical studies on 3,4- methylenedioxymethamphetamineprovide information on mechanisms of action, but do not allow accurate prediction of adverse events in humans Br J Pharmacol 2012. 166(5): p. 1523–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soleimani Asl S et al. Attenuation of ecstasy-induced neurotoxicity by N- acetylcysteine Metab Brain Dis 2015. 30(1): p. 171–81 [DOI] [PubMed] [Google Scholar]
- 23.Bouchet CA et al. Acute exercise enhances the consolidation of fear extinction memory and reduces conditionedfear relapse in a sex-dependent manner Learn Mem 2017. 24(8): p. 358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchet CA et al. Activation of Nigrostriatal Dopamine Neurons during Fear Extinction Prevents the Renewal of Fear Neuropsychopharmacology 2018. 43(3): p. 665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanner MK et al. Runningfrom fear: Exercise modulation of fear extinction Neurobiol Learn Mem 2018. 151: p. 28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouton ME et al. Contextual and temporal modulation of extinction: behavioral and biological mechanisms Biol Psychiatry 2006. 60(4): p. 352–60 [DOI] [PubMed] [Google Scholar]
- 27.Cassini LF et al. On the transition from reconsolidation to extinction of contextual fear memories Learn Mem 2017. 24(9): p. 392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mika A et al. Voluntary exercise during extinction of auditory fear conditioning reduces the relapse of fear associated with potentiated activity of striatal direct pathway neurons Neurobiol Learn Mem 2015. 125: p. 224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faul F et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences Behav Res Methods 2007. 39(2): p. 175–91 [DOI] [PubMed] [Google Scholar]
- 30.Callaway CW Wing LL, and Geyer MA Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats J Pharmacol Exp Ther 1990. 254(2): p. 456–64 [PubMed] [Google Scholar]
- 31.Baumann MH Clark RD, and Rothman RB Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain Pharmacol Biochem Behav 2008. 90(2): p. 208–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodsiri R et al. Acute concomitant effects of MDMA binge dosing on extracellular 5-HT, locomotion and body temperature and the long-term effect on novel object discrimination in rats Psychopharmacology (Berl) 2011. 213(2–3): p. 365–76 [DOI] [PubMed] [Google Scholar]
- 33.Gold LH and Koob GF MDMA produces stimulant-like conditioned locomotor activity Psychopharmacology (Berl) 1989. 99(3): p. 352–6 [DOI] [PubMed] [Google Scholar]
- 34.Meyer A et al. Rewarding effects of the optical isomers of 3,4- methylenedioxy-methylamphetamine (‘Ecstasy’) and3,4-methylenedioxy- ethylamphetamine (‘Eve’) measured by conditioned place preference in rats Neurosci Lett 2002. 330(3): p. 280–4 [DOI] [PubMed] [Google Scholar]
- 35.Bilsky EJ et al. MDMA produces a conditioned place preference and elicits ejaculation in male rats: a modulatory role for the endogenous opioids Pharmacol Biochem Behav 1991. 40(2): p. 443–7 [DOI] [PubMed] [Google Scholar]
- 36.Doss MK et al. MDMA Impairs Both the Encoding and Retrieval of Emotional Recollections Neuropsychopharmacology 2018. 43(4): p. 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shariati MB et al. Acute Effects of Ecstasy on Memory Are more Extensive than Chronic Effects Basic Clin Neurosci 2014. 5(3): p. 225–30 [PMC free article] [PubMed] [Google Scholar]
- 38.Kay C Harper DN, and Hunt M The effects of binge MDMA on acquisition and reversal learning in a radial-arm maze task Neurobiol Learn Mem 2011. 95(4): p. 473–83 [DOI] [PubMed] [Google Scholar]
- 39.Harper DN Kay C, and Hunt M Prior MDMA exposure inhibits learning and produces both tolerance and sensitization in the radial-arm maze Pharmacol Biochem Behav 2013. 105: p. 34–40 [DOI] [PubMed] [Google Scholar]
- 40.Trigo JM et al. MDMA modifies active avoidance learning and recall in mice Psychopharmacology (Berl) 2008. 197(3): p. 391–400 [DOI] [PubMed] [Google Scholar]
- 41.Mueller D et al. The effects of yohimbine and amphetamine on fear expression and extinction in rats Psychopharmacology (Berl) 2009. 204(4): p. 599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmack SA Wood SC, and Anagnostaras SG Amphetamine and extinction of cued fear Neurosci Lett 2010. 468(1): p. 18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bekinschtein P Cammarota M, and Medina JH BDNF and memory processing Neuropharmacology 2014. 76 Pt C: p. 677–83 [DOI] [PubMed] [Google Scholar]
- 44.Chhatwal JP et al. Amygdala BDNF signaling is requiredfor consolidation but not encoding of extinction Nat Neurosci 2006. 9(7): p. 870–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemmerle AM et al. (+/−)3,4-methylenedioxymethamphetamine (“ecstasy”) treatment modulates expression of neurotrophins and their receptors in multiple regions of adult rat brain J Comp Neurol 2012. 520(11): p. 2459–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Turrillas R et al. Differential effects of 3,4- methylenedioxymethamphetamine (MDMA, “ecstasy”) on BDNFmRNA expression in rat frontal cortex and hippocampus Neurosci Lett 2006. 402(1–2): p. 126–30 [DOI] [PubMed] [Google Scholar]
- 47.Soleimani Asl S et al. The effect of 3,4- methylenedioxymethamphetamine on expression of neurotrophic factors in hippocampus of male rats Med J Islam Repub Iran 2017. 31: p. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mechan AO et al. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats Br J Pharmacol 2002. 135(1): p. 170–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabol KE and Seiden LS Reserpine attenuatesD-amphetamineand MDMA-induced transmitter release in vivo: a consideration of dose, core temperature and dopamine synthesis Brain Res 1998. 806(1): p. 69–78 [DOI] [PubMed] [Google Scholar]
- 50.Gorska AM. et al. Neurochemical and Neurotoxic Effects of MDMA (Ecstasy) and Caffeine After Chronic Combined Administration in Mice. Neurotox Res. 2017 doi: 10.1007/s12640-017-9831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Shea E et al. Effect of GBR 12909 andfluoxetine on the acute and long term changes induced by MDMA (‘ecstasy’) on the 5-HT and dopamine concentrations in mouse brain Neuropharmacology 2001. 40(1): p. 65–74 [DOI] [PubMed] [Google Scholar]
- 52.Vaidya VA et al. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex J Neurosci 1997. 17(8): p. 2785–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaidya VA Terwilliger RM, and Duman RS Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus Neurosci Lett 1999. 262(1): p. 1–4 [DOI] [PubMed] [Google Scholar]
- 54.Burghardt NS et al. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist Biol Psychiatry 2007. 62(10): p. 1111–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burghardt NS et al. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine Biol Psychiatry 2004. 55(12): p. 1171–8 [DOI] [PubMed] [Google Scholar]
- 56.Greenwood BN et al. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise Psychopharmacology (Berl) 2008. 199(2): p. 209–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mouri A et al. The involvement of brain-derived neurotrophic factor in 3,4-methylenedioxymethamphetamine-induced place preference and behavioral sensitization Behav Brain Res 2017. 329: p. 157–165 [DOI] [PubMed] [Google Scholar]
- 58.Abraham AD Neve KA, and Lattal KM Dopamine and extinction: a convergence of theory with fear and reward circuitry Neurobiology of learning and memory 2014. 108: p. 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abraham AD Neve KA, and Lattal KM Activation of D1/5Dopamine Receptors: A Common Mechanism for Enhancing Extinction of Fear and Reward-Seeking Behaviors Neuropsychopharmacology 2016. 41(8): p. 2072–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo R et al. A dopaminergic switch for fear to safety transitions Nat Commun 2018. 9(1): p. 2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurtman CG et al. Increased anxiety in rats after 3,4- methylenedioxymethamphetamine: association with serotonin depletion Eur J Pharmacol 2002. 446(1–3): p. 89–96 [DOI] [PubMed] [Google Scholar]
- 62.Faria R et al. MDMA in adolescent male rats: decreased serotonin in the amygdala and behavioral effects in the elevated plus-maze test Ann N Y Acad Sci 2006. 1074: p. 643–9 [DOI] [PubMed] [Google Scholar]
- 63.Arias-Cavieres A et al. MDMA (“ecstasy”) impairs learning in the Morris Water Maze and reduces hippocampalLTP in young rats Neurosci Lett 2010. 469(3): p. 375–9 [DOI] [PubMed] [Google Scholar]
- 64.Sajadi A et al. Treadmill exercise alters ecstasy- induced long- term potentiation disruption in the hippocampus of male rats Metab Brain Dis 2017. 32(5): p. 1603–1607 [DOI] [PubMed] [Google Scholar]
- 65.Sprague JE et al. Hippocampal serotonergic damage induced by MDMA (ecstasy): effects on spatial learning Physiol Behav 2003. 79(2): p. 281–7 [DOI] [PubMed] [Google Scholar]
- 66.Amoroso T and Workman M Treatingposttraumatic stress disorder with MDMA-assisted psychotherapy: A preliminary meta-analysis and comparison to prolonged exposure therapy J Psychopharmacol 2016. 30(7): p. 595–600 [DOI] [PubMed] [Google Scholar]


