Abstract
Stress-related disorders are more common in females than males. This difference could arise from differential responses to behavioral interventions that enable stress resistance between sexes. In male rats, regular physical activity prevents the behavioral consequences of uncontrollable stress, such as social avoidance and exaggerated fear conditioning. However, it is unknown if these protective effects are also present in females. Here we demonstrate for the first time in female rats that six weeks of voluntary wheel running buffers against the behavioral sequelae of uncontrollable stress. This observation allows for mechanistic investigations of exercise-induced stress resistance in both sexes.
Women are at a higher risk than men of developing stress-related psychiatric disorders, such as major depression, post-traumatic stress disorder, and anxiety (Kessler et al., 2005). Current pharmacological treatments for stress-related disorders have limited efficacy (Gaynes et al., 2009) and there is a need to identify novel protective and therapeutic strategies that consider sex differences. Physical exercise can be used therapeutically to treat depression and anxiety (Asmundson et al., 2013; Powers et al., 2015; Ravindran and da Silva, 2013), and both males and females who exercise are less likely to develop stress-related psychiatric disorders (Chekroud et al., 2018; Harvey et al., 2018). Exercise participation is therefore a potential means of enabling stress resistance in both sexes.
Animal models have played a critical role in identifying the neural mechanisms underlying exercise-induced stress resistance. Sedentary male rats exposed to uncontrollable stress display numerous behavioral outcomes (reduced social exploration, exaggerated fear, impaired shuttle box escape behavior; (Christianson et al., 2009; Maier and Watkins, 2005)) that do not occur if rats have voluntary access to running wheels for six weeks (Greenwood et al., 2003; Greenwood et al., 2012a). The behavioral consequences of uncontrollable stress are due to hyperactivation and sensitization of serotonin (5-HT) neurons in the dorsal raphe nucleus (DRN) (Maier and Watkins, 2005). In males, 6 weeks of wheel running constrains activation of DRN 5- HT neurons during stress (Clark et al., 2015; Greenwood et al., 2003). Thus, it is thought that constraint over DRN activation is central to exercise-induced stress resistance (Greenwood and Fleshner, 2011; Nicastro and Greenwood, 2016). However, prior work has focused only on males, and it remains unknown whether voluntary exercise protects female rats against the behavioral consequences of uncontrollable stress. The current study sought to determine in females if 6 weeks of voluntary exercise prevents the impact of uncontrollable tail shock on juvenile social exploration (JSE) and shock-elicited freezing; thereby opening the door to investigations of mechanisms underlying exercise-induced stress resistance in both sexes.
Female Sprague Dawley rats (Envigo, Indianapolis, IN, USA) weighing 125–150 g at time of arrival were housed in a temperature- and humidity-controlled room on a 12:12 h light/dark cycle. Rats were housed individually in cages (45 × 25.2 ×14.7 cm, length × width × height) with ad libitum access to food (Lab Chow) and water. Each cage contained a running wheel (1.081 m circumference, Starr Life Sciences, Oakmont, PA) that was rendered immobile for one week prior to experimental manipulation to allow acclimation to housing conditions. Following the acclimation period, rats were randomly assigned to either the voluntary wheel running (“run”; n = 16) or sedentary (“sed”; n = 16) conditions. For 6 weeks, run rats had free access to unlocked running wheels, while wheels in the cages of the sedentary rats remained locked. Six weeks of running was used because it takes between 3 and 6 weeks for wheel running to enable stress resistance in males (Greenwood et al., 2005). Running distances were recorded daily using VitalView Analysis software (Starr Life Sciences) and distance was calculated by multiplying number of revolutions by circumference. Body weight was recorded weekly. All testing occurred within the first 3 h of the light cycle. All experimental procedures were approved by the University of Colorado Denver Animal Care and Use Committee in compliance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Following 6 weeks of sedentary or run conditions, rats in each group were randomly assigned to either be left undisturbed in their home cages (no stress) or exposed to uncontrollable tail shock (stress) as described in prior work (Baratta et al., 2018; Greenwood et al., 2012a). Thus, this experiment used a 2 × 2 experimental design with exercise and stress as the factors and n = 8 per group.
Stressed rats were placed in clear Plexiglas restraint tubes (8 cm × 18 cm, diameter × length) and 2 copper strips were affixed to each tail. 100 trials of 5 sec tail shocks were administered on a 60 sec variable intertrial interval (ITI) by shock scramblers (Coulbourn Instruments, Allentown, PA, USA) controlled by Noldus EthoVision XT (Leesburg, VA) software via a custom interface. To avoid stress-induced analgesia, the intensity of the shock was increased (33 trails at 1.0 mA, 33 trails at 1.3mA, and 34 trails at 1.6mA). Rats were returned to their home cages after stress.
Experimental rats were given a JSE test 24 h after stress as previously described (Baratta et al., 2018; Greenwood et al., 2012a). Experimental rats were transferred to a brightly lit testing room and placed individually into cages identical to home cages except lacking food and water. After 45 min, a juvenile female conspecific (P28 ± 2 d) was added to the cage and exploratory behaviors (sniffing, pinning, and allogrooming) initiated by the adult rat were recorded by observers blind to treatment for 3 min. Total interaction time was calculated by averaging the scores obtained by both observers (inter-rater reliability 95%).
Immediately after the JSE test, rats were transported to a novel fear conditioning chamber (20” × 10” × 12”, length × width × height) with shock grid floors (Coulbourn Instruments) for assessment of shock-elicited freezing, as previously described (Baratta et al., 2018; Greenwood et al., 2012a). Rats were allowed 5 min to explore the chamber and then received 2 foot shocks (1 sec, 0.8 mA) with a 1 min ITI. After the second foot shock, rats remained in the chambers for 20 min, during which time freezing behavior was scored every 10 sec by an observer blind to treatment condition of the animals. Freezing is a fear response in rats and thus is used as an index of fear. Behavior was also recorded by overhead cameras and freezing was calculated by EthoVision XT from the videos. The inter-rater correlation between human scoring and EthoVision XT was calculated at 95 %. Percent time spent freezing was calculated by averaging freeing data obtained from the human scorer and EthoVision XT.
Estrous cycle phase was determined by vaginal lavage immediately following no stress or stress, and again after behavioral testing. A sterile, blunt-tipped eye dropper filled with ~0.5 mL sterile-filtered 0.2% PBS-Brij solution (Brij 35 Solution 30%; Sigma-Aldrich, B4184) was used to gently flush the vagina and collect epithelial cells. The collected fluid was placed onto a microscope slide and cells were examined with a 20X objective lens (Olympus BX53). Characteristic changes in the morphology of cells were used to determine estrous cycle phase as previously described (Bouchet et al., 2017).
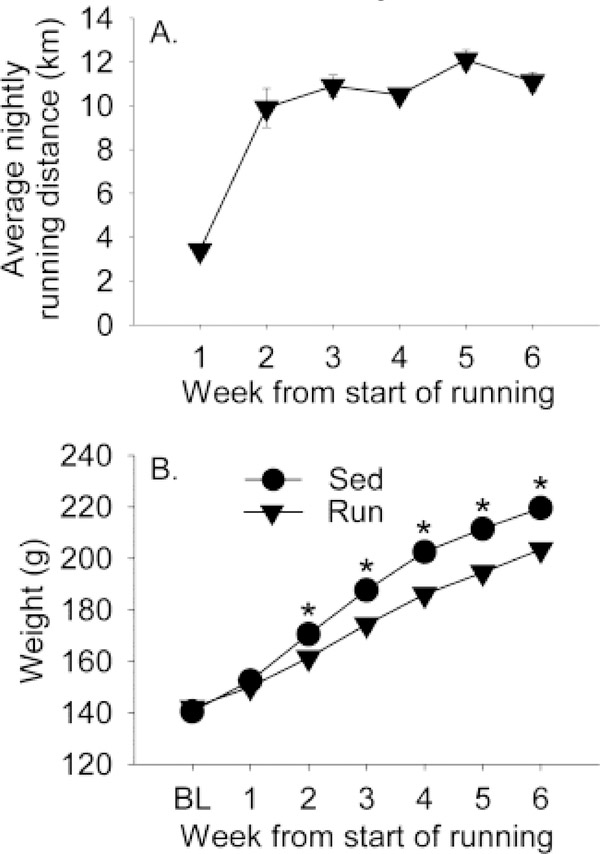
Average nightly running distance and body weight are depicted in Figure 1. Running distance increased nightly (Fig. 1A; F(5,75) = 45.3; p < 0.0001). Repeated measures ANOVA was conducted to determine if no stress and stress groups ran equal amounts prior to stressor exposure. Neither the main effects of stress nor the interaction between stress and time were significant (data not shown). Similar to what is typically observed in males (Greenwood et al., 2003), run rats gained less weight than sedentary rats over the course of the experiment (Fig. 1B). Repeated measures ANOVA revealed significant main effects of time (F(6,180) = 1241.5; p < 0.0001) and exercise (F(1,30) = 27; p < 0.0001) and a time by exercise interaction (F(6,180) = 24.1; p < 0.0001) on body weight.
Figure 1.
Adult female Sprague Dawley rats remained sedentary with access to immobile running wheels (Sed) or were allowed access to voluntary running wheels (Run) for 6 weeks. (A) The mean running distance (kilometers) ran each week by rats with access to voluntary running wheels. (B) The mean weekly body weights (grams) of wheel running rats and sedentary rats. Data represent group means ± SEM (error bars may be obscured by symbols). Repeated measures ANOVA: *p < 0.001 relative to wheel running rats. BL, Baseline.
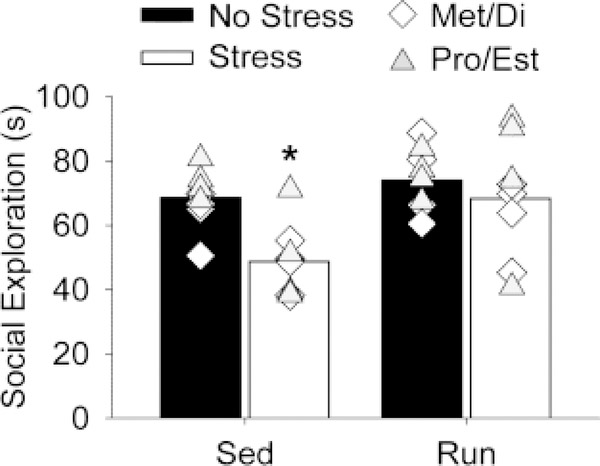
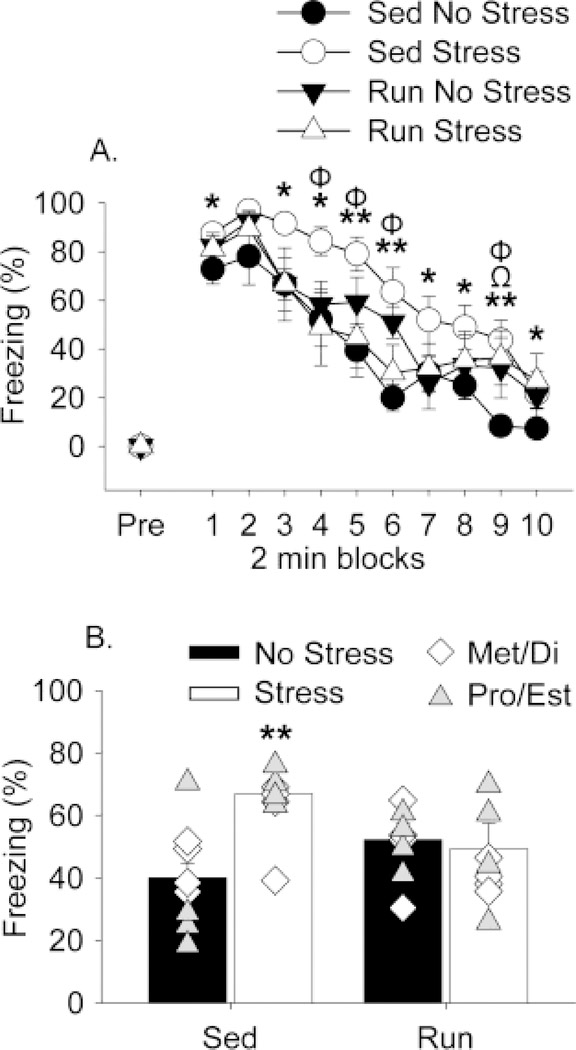
JSE and shock-elicited freezing are shown in Figures 2 and 3, respectively. Similar to what has been reported previously (Baratta et al., 2018), uncontrollable stress reduced JSE in sedentary females (Fig. 2; main effect of stress (F(1,28) = 7.9; p < 0.01)). ANOVA also revealed a significant main effect of exercise (F(1,28) = 7.8; p < 0.01). Post-hoc analyses indicated that prior wheel running prevented the stress-induced reduction in JSE in females (Fig. 2). Similarly, exposure to stress produced exaggerated shock-elicited freezing in sedentary females that was prevented by prior exercise (Fig. 3). Freezing was negligible in all groups prior to the 2 conditioning foot shocks (Fig. 3A, “pre”). All rats demonstrated robust freezing following the 2 foot shocks, and freezing levels decreased over time ((F(9,252) = 75.2; p < 0.0001); Fig. 3A). ANOVA revealed a significant interaction between exercise and stress on shock-elicited freezing (F(1,28) = 9.7; p < 0.01). Post hoc analysis indicated that stress potentiated freezing in sedentary rats, but not in run rats (Fig. 3A and 3B).
Figure 2.
Six weeks of exercise blocked reductions in juvenile social exploration following uncontrollable stress. Sedentary (Sed) and voluntary wheel running (Run) rats remained in their home cages (No stress) or were exposed to 100 uncontrollable tail shocks (Stress). Juvenile social exploration was measured 24 h later. Social exploration was calculated as the total time (seconds) spent performing exploratory behaviors. These behaviors did not differ between rats in metestrus and diestrus (Met/Di) or proestrus and estrus (Pro/Est) phases of the estrous cycle. Symbols represent values of individual rats in either Met/Di (white diamonds, n=4/group) or Pro/Est (gray triangles, n=4/group). Group means Met/Di: Sed No Stress = 63.2, Sed Stress = 47.9, Run No Stress = 79.8, Run Stress = 64.6. Group means Pro/Est: Sed No Stress = 74.1, Sed Stress = 49.7, Run No Stress = 68.1, Run Stress = 74.5. Bars represent group means (regardless of estrous phase) ± SEM. ANOVA: *p < 0.01 relative to all other groups.
Figure 3.
Six weeks of exercise prevented stress-induced exaggerated freezing. Sedentary (Sed) and voluntary wheel running (Run) rats remained in their home cages (No stress) or were exposed to 100 uncontrollable tail shocks (Stress). Shock-elicited freezing behavior was measured 24 h later. (A) Levels of freezing are shown in 2 min blocks. (B) Average levels of freezing over the entire test. Freezing did not differ between rats in metestrus and diestrus (Met/Di) or proestrus and estrus (Pro/Est) phases of the estrous cycle. Symbols represent values of individual rats in either Met/Di (white diamonds, n=4/group) or Pro/Est (gray triangles, n=4/group). Group means Met/Di: Sed No Stress = 44.2, Sed Stress = 59.4, Run No Stress = 57.7, Run Stress = 40.2. Group means Pro/Est: Sed No Stress = 36.0, Sed Stress = 68.4, Run No Stress = 44.1, Run Stress = 50.1. Bars represent group means (regardless of estrous phase) ± SEM. Repeated measures ANOVA: *p < 0.05 Sed No Stress versus Sed Stress; **p < 0.01 Sed No Stress versus Sed Stress; Φp < 0.05 Run Stress versus Sed Stress; Ωp < 0.05 Run No Stress versus Sed Stress. ANOVA: **p < 0.01 Sed Stress relative to Run Stress and Sed No Stress.
ANOVA was used to determine if the effects of exercise were dependent on estrous phase during either stress or behavioral testing. No significant effects of estrous phase were observed. Average JSE and average freezing behavior during metestrus and diestrus (when levels of estrogen are lowest) and proestrus and estrus (when levels of estrogen are highest) were collapsed into two groups corresponding to low (Met/Di) and high (Pro/Est) estrogen and depicted as individual data points in Fig. 2 and Fig. 3B, respectively.
Simple regression analyses were performed between running distances and behavioral outcomes. Consistent with previous studies in males (Greenwood et al., 2003), exercise-induced stress resistance did not vary with running distance. No significant correlations were observed between running distance and JSE or freezing behavior in the run group exposed to stress.
The present results provide evidence that exercise enables stress resistance in females. Consistent with previous studies (Baratta et al., 2018), exposure to uncontrollable tail shock reduces social exploration and potentiates shock-elicited fear in sedentary female rats. Six weeks of voluntary exercise prior to uncontrollable stress blocks the stress-induced social avoidance and exaggerated fear produced by uncontrollable stress. The magnitude of the stress-protective effects of exercise observed in female rats resembles previous results in males (Greenwood et al., 2003; Greenwood et al., 2012a). The current data demonstrate that the stress-protective effects of voluntary exercise are generalizable to both sexes.
It is auspicious for females that exercise is able to provide protection against the behavioral consequences of uncontrollable stress, considering that stress-protective manipulations established in males do not always protect females. Prior exposure to a controllable stressor, for example, protects males against the behavioral consequences of subsequent uncontrollable stress through inhibition of DRN 5-HT activity (Amat et al., 2006). Despite the ability to acquire the wheel-turn escape response, the protective benefits of behavioral control are absent in females. That is, in females, the DRN responds to a controllable stressor as if it was uncontrollable (Baratta et al., 2018). It therefore appears that various stress- protective manipulations do not necessarily act similarly in males and females, even when these manipulations share some common features, such as constraint over DRN 5-HT activity during stress.
The differential sensitivity to stress-protective manipulations observed in females could result from the fact that diverse mechanisms underlie stress resistance produced by controllable stress and exercise, with females only able to benefit from those utilized by exercise. Indeed, even though both controllable stress and voluntary exercise constrain stress-induced activity of 5-HT neurons in the DRN, prior work in male rats reveals multiple means to constrain DRN activation during stress (Christianson and Greenwood, 2014). Controllable stress prevents subsequent uncontrollable stress from activating the DRN through a mechanism involving the prefrontal cortex (PFC) (Amat et al., 2006). The prelimbic (PL) region of the PFC projection to the DRN is recruited by controllable stress and inhibits DRN 5-HT activity (Baratta et al., 2009) presumably by selectively activating local DRN GABA interneurons (Jankowski and Sesack, 2004). Controllable stress alters the PL-DRN pathway in such a way that future uncontrollable stressors, which typically don’t activate this pathway, now do so. Since the behavioral sequelae of uncontrollable stress are dependent on activation of DRN 5-HT neurons, recruitment of the PL-DRN pathway in rats exposed to prior controllable stress provides lasting protection against the typical behavioral outcomes of uncontrollable stress. The PL-DRN pathway exists in females; however, it is not activated during controllable stress (Baratta et al., 2018).
Unlike the protective effects of controllable stress, exercise-induced stress resistance occurs in male rats with lesions of the PFC that include the PL (Greenwood et al., 2013) and is thus independent of the PL-DRN pathway (Christianson and Greenwood, 2014). Females are therefore able to benefit from exercise while remaining insensitive to behavioral control, which enables stress resistance through the PL-DRN pathway. Exercise is thought to protect males by constraining the DRN through either intrinsic changes in the 5-HT system or changes in extrinsic circuits modulating DRN activity (Christianson and Greenwood, 2014; Greenwood and Fleshner, 2011; Nicastro and Greenwood, 2016). Intrinsic changes include an increase in 5-HT1A autoreceptor-mediated inhibition of DRN 5-HT neurons (Greenwood et al., 2003) and a decrease in sensitivity of 5-HT2C receptors in DRN projection sites (Greenwood et al., 2012b), whereas extrinsic changes could involve alterations in dopaminergic (Greenwood, 2018), noradrenergic (Nicastro and Greenwood, 2016) or galaninergic (Sciolino and Holmes, 2012) modulation of DRN activity. We anticipate involvement of parallel mechanisms in females given the similarities in the magnitude of stress protection between males and females, the observation that exercise-induced stress resistance in females doesn’t depend on estrous phase, and the fact that distance run doesn’t influence behavioral outcomes in either sex.
The vast majority of the pre-clinical exercise neuroscience literature focuses on male subjects. Whether there are sex differences in response to exercise is a critical unanswered question. This is particularly true for common stress-related disorders, which are more prevalent in females, and conventional treatments for which have poor long-term efficacy. We have recently observed that an acute bout of voluntary exercise can augment fear extinction in male rats, but fails to provide a similar therapeutic effect in females (Bouchet et al., 2017). Taken in consideration with the current data, it appears that some of the beneficial effects of exercise are sex-dependent, while others are not. It is therefore important to continue to characterize the effects of exercise in both males and females, as an understanding of potential sex differences in the effects of exercise could lead to improved behavioral and pharmacological strategies for the prevention and treatment of stress-related disorders in both sexes.
Acknowledgements
Supported by NIH grants R15 MH114025 (BNG), R01 MH050479 (MVB), R21 MH106817 (MVB), and NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (MVB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, et al. , 2006. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 26, 13264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, et al. , 2013. Let’s get physical: a contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depress Anxiety. 30, 362–73. [DOI] [PubMed] [Google Scholar]
- Baratta MV, et al. , 2009. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, et al. , 2018. Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. Eur J Neurosci. 47, 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet CA, et al. , 2017. Acute exercise enhances the consolidation of fear extinction memory and reduces conditioned fear relapse in a sex-dependent manner. Learn Mem. 24, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud SR, et al. , 2018. Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry. 5, 739–746. [DOI] [PubMed] [Google Scholar]
- Christianson JP, et al. , 2009. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 12, 445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Greenwood BN, 2014. Stress-protective neural circuits: not all roads lead through the prefrontal cortex. Stress. 17, 1–12. [DOI] [PubMed] [Google Scholar]
- Clark PJ, et al. , 2015. Running Reduces Uncontrollable Stress-Evoked Serotonin and Potentiates Stress-Evoked Dopamine Concentrations in the Rat Dorsal Striatum. PLoS One. 10, e0141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, et al. , 2009. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 60, 1439–45. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, et al. , 2003. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 23, 2889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, et al. , 2005. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 1033, 164–78. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M, 2011. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev. 39, 140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, et al. , 2012a. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behavioural brain research. 233, 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, et al. , 2012b. 5-HT(2C) Receptors in the Basolateral Amygdala and Dorsal Striatum Are a Novel Target for the Anxiolytic and Antidepressant Effects of Exercise. PloS one. 7, e46118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, et al. , 2013. Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. The European journal of neuroscience. 37, 469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, 2018. The role of dopamine in overcoming aversion with exercise. Brain Res. [DOI] [PubMed] [Google Scholar]
- Harvey SB, et al. , 2018. Exercise and the Prevention of Depression: Results of the HUNT Cohort Study. Am J Psychiatry. 175, 28–36. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR, 2004. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 468, 518–29. [DOI] [PubMed] [Google Scholar]
- Kessler RC, et al. , 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR, 2005. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 29, 829–41. [DOI] [PubMed] [Google Scholar]
- Nicastro TM, Greenwood BN, 2016. Central monoaminergic systems are a site of convergence of signals conveying the experience of exercise to brain circuits involved in cognition and emotional behavior. Current Zoology. 62, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Asmundson GJ, Smits JA, 2015. Exercise for Mood and Anxiety Disorders: The State-of-the Science. Cogn Behav Ther 44, 237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran AV, da Silva TL, 2013. Complementary and alternative therapies as add-on to pharmacotherapy for mood and anxiety disorders: a systematic review. J Affect Disord. 150, 707–19. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Holmes PV, 2012. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev. 36, 1965–84. [DOI] [PMC free article] [PubMed] [Google Scholar]