Abstract
Extinction-based exposure therapy is the most common behavioral therapy for anxiety and trauma-related disorders, but fear tends to resurface even after successful extinction. Identification of novel strategies to enhance fear extinction and reduce fear relapse is of paramount importance to mental health. Exercise can enhance cognitive function, but it is not yet well understood whether exercise can be an effective augmentation strategy for fear extinction. In the current review, we present the current state of knowledge on the effects of exercise on fear extinction. Effects of exercise duration, explanations for conflicting results, and potential mechanisms, focusing on a hypothesized role for dopamine, are all discussed. We also provide new data suggesting that the timing in which acute exercise occurs relative to fear extinction, is a crucial variable in determining whether exercise can enhance fear extinction. Clinical implications and ideas to guide future research endeavors in this area are provided.
Keywords: Wheel running, fear conditioning, fear renewal, dopamine, voluntary exercise, exposure therapy
Introduction
Fear conditioning is an associative learning phenomenon during which a previously innocuous cue (conditioned stimulus; CS) comes to elicit an emotional fear response due to its association with an aversive event (unconditioned stimulus; US). Because of the involvement of fear conditioning and the pervasiveness of fear memories in anxiety and trauma-related disorders, such as post-traumatic stress disorder (PTSD), therapeutic efforts for the treatment of these disorders focus on the process of fear extinction. Indeed, fear extinction, which is the decrease in fear expression to a CS following repeated presentations of the fear-evoking CS in the absence of the aversive US (Pavlov, 1927), forms the basis of exposure-based psychotherapy. Prior work on identifying means to enhance fear extinction has been quite promising; however, the therapeutic efficacy of these strategies has thus far been inconsistent. This could partly be due to the fact that extinction memories are labile and susceptible to relapse phenomena, including spontaneous recovery of fear (return of fear following the passage of time; (Pavlov, 1927)), fear reinstatement (return of fear following exposure to US; (Rescorla & Heth, 1975)), and fear renewal (return of fear in contexts outside of the extinction context; (Bouton, 1988)). Identification of novel strategies to enhance the effectiveness of existing cognitive and behavioral therapies for these common disorders is therefore of the utmost importance to mental health.
Exercise is an inexpensive and readily available tool for the prevention and treatment of a variety of mental health disorders. Indeed, the ability of exercise to prevent and reverse deleterious effects of adverse events has been well characterized (Greenwood & Fleshner, 2011). In contrast, whether exercise can be used to augment traditional pharmacological or cognitive-behavioral therapies, such as exposure therapy, is not as well understood. Recent human and pre-clinical experiments investigating the effects of exercise on fear extinction have resulted in inconsistent and sometimes conflicting findings, resulting in confusion over the effects of exercise on fear extinction. Here we summarize findings regarding the effects of exercise on fear extinction and offer explanations for conflicting results. Data presented are consistent with the interpretation that although chronic (i.e. several weeks) of exercise does not seem to improve fear extinction, an acute bout of exercise, in the absence of an extensive exercise history, can enhance fear extinction and reduce fear relapse, but only if the acute exercise bout occurs during or immediately following fear extinction acquisition. Potential mechanisms by which acute exercise enhances fear extinction are provided, focusing on a role for dopamine (DA). Clinical implications and ideas for follow-up studies are provided throughout.
Effects of Chronic Exercise on Fear Extinction
Figure 1A summarizes the effects of chronic exercise on fear extinction. A consistent finding across multiple experiments is that a history of repeated, chronic voluntary exercise over a period of several weeks does not facilitate fear extinction. This failure is despite the fact that chronic exercise increases plasticity factors implicated in learning and memory, including brain-derived neurotrophic factor (BDNF; (Neeper et al., 1995)), c-AMP response element binding protein (Chen & Russo-Neustadt, 2009) and mammalian target of rapamycin (Lloyd et al., 2017) in brain regions thought to be critical for fear extinction, such as the hippocampus, amygdala and prefrontal cortex. In fact, if chronic exercise occurs prior to fear conditioning, exercise can delay later extinction. For example, when rats are allowed several weeks of access to running wheels prior to contextual fear conditioning (1 week is without effect; (Greenwood et al., 2009)), exercised rats show enhanced contextual fear memory the next day, and delayed within-session extinction (Van Hoomissen et al., 2004; Burghardt et al., 2006; Greenwood et al., 2009; Falls et al., 2010) that can persist across multiple days of extinction (Greenwood et al., 2009). Rather than chronic exercise somehow impairing extinction mechanisms, this delayed extinction is most likely due to chronic exercise strengthening the initial fear memory, thereby making it more difficult to extinguish. Indeed, it is well established that chronic exercise can enhance memory consolidation, including consolidation of contextual fear conditioning (Falls et al., 2010). Consistent with this interpretation is the observation that chronic voluntary exercise has no impact on fear extinction if extinction is assessed immediately after conditioning (Greenwood et al., 2003; Greenwood et al., 2005a), before exercise-induced plasticity has had a chance to enhance fear memory consolidation. Additionally, chronic exercise has been reported to delay extinction of both conditioned taste aversion (Tsuboi et al., 2015) and cocaine conditioned place preference (Mustroph et al., 2015), but only when exercise also facilitated initial learning. These data suggest that when chronic exercise occurs prior to fear conditioning, it can delay fear extinction by making the initial fear memory more difficult to extinguish. But what if chronic exercise occurs only after initial fear memory acquisition?
Figure 1.
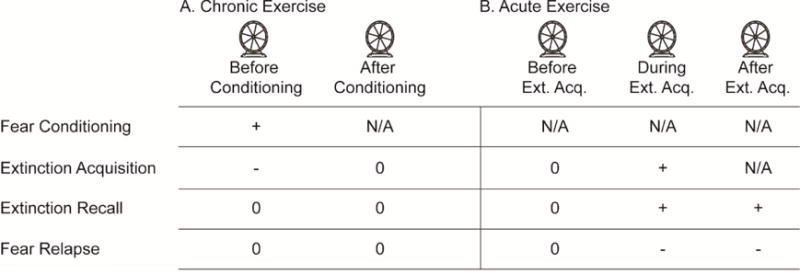
Exercise modulation of fear extinction. A) Chronic exercise prior to fear conditioning delays fear extinction acquisition, likely by strengthening fear memory and making it more difficult to extinguish. Chronic exercise between fear conditioning and extinction has no impact on extinction. B) Acute bouts of exercise prior to fear extinction (Before Ext. Acq.) does not impact extinction acquisition, recall or relapse. Exercise during the acquisition (During Ext. Acq.) or consolidation (After Ext. Acq.) phases of fear extinction learning improves fear extinction memory recall and reduces fear relapse. + enhancement of memory process; − impairment of memory process; 0 no effect on memory process, N/A not applicable.
Experiments limiting chronic exercise only to the period between fear conditioning and extinction can assess the effects of chronic exercise on fear extinction, in the absence of confounding effects of exercise on initial fear learning. We allowed rats either 1 or 6 weeks of access to running wheels after contextual fear conditioning, and observed that neither exercise duration was able to enhance fear extinction or reduce spontaneous recovery (Greenwood et al., 2009). Although these data are consistent with the interpretation that chronic exercise does not impact fear extinction, this is the only experiment of which we are aware that has investigated the effects of chronic exercise specifically after fear conditioning on later fear extinction. Mustroph et al. (2011) report that 30 days of voluntary exercise initiated after the development of cocaine conditioned place preference can facilitate extinction of conditioned place preference in mice. Considering these data, more work should be done to better clarify the effects of post-conditioning chronic exercise on fear extinction. Investigators employing post-conditioning exercise should interpret results with caution, however; as voluntary exercise initiated after contextual fear conditioning has been reported to enhance forgetting of contextual fear memory in both rats (Greenwood et al., 2007) and mice (Akers et al., 2014). Studies investigating effects of post-conditioning exercise on fear extinction should thus be sure to include exercise groups not exposed to fear extinction, to control for exercise-induced forgetting.
Clearly, evidence for beneficial effects of chronic exercise on fear extinction in typical fear conditioning experiments is lacking. Available data do not support the implementation of chronic exercise programs to augment exposure therapy. However, there are reasons other than augmentation of exposure therapy to implement exercise programs for clinical patients, such as improved mood and self-esteem, enhanced cognitive function, and anxiety reduction. Moreover, typical fear conditioning paradigms using only a few shocks during conditioning may not adequately model human anxiety and trauma-related disorders, which typically involve exposure to severely traumatic events. Animal models of PTSD which expose animals to more severe stressors reveal that stressor exposure can impair extinction of fear memories; an observation consistent with human PTSD (Jovanovic et al., 2010). Notably, chronic exercise can prevent the impaired fear extinction that follows severe stressors, such as uncontrollable tail shock, even when exercise occurs prior to conditioning (Greenwood et al., 2003). These data resemble the anxiolytic effects of exercise in tests of conflict anxiety, which appear most robust after the animals are exposed to stressful events (see (Greenwood & Fleshner, 2013) and (Sciolino & Holmes, 2012) for reviews). Therefore, although the animal data indicate a lack of a beneficial effect of chronic exercise on fear extinction under typical experimental conditions, chronic exercise is beneficial during situations which better resemble clinical anxiety and PTSD. Preliminary human data are consistent with this possibility as well. Exercise fails to augment virtual-reality exposure to reduce fear of spiders in a largely non-clinical population (Jacquart et al., 2017), but is able to augment exposure therapy in patients diagnosed with PTSD (Powers et al., 2015). The Powers et al. 2015 study used a small sample size, so more work should be done investigating the therapeutic effects of exercise in clinical populations.
Effects of Acute Exercise on Fear Extinction
The experiments discussed above were designed to test whether stable neuro-plastic changes produced in the brain by chronic exercise can enhance fear extinction. As such, these experiments separated extinction training or memory testing from an exercise bout by at least several hours. If the stable chronic exercise-induced adaptations are not sufficient to enhance fear extinction, then perhaps neurochemical events occurring during an exercise bout are. If this is the case, then in order to augment extinction, an exercise bout might need to be concurrent with fear extinction, or the two might at least need to occur in very close temporal proximity. Fear extinction learning includes critical phases of acquisition and consolidation. During the acquisition phase, the association between the fear-eliciting CS and the lack of the predicted aversive event is initially encoded. Later, during the consolidation phase, molecular processes take place in the neural circuits responsible for forming the long-term memory of fear extinction. Perhaps exercise bouts in close temporal proximity to fear extinction would enable exercise-induced neurochemical events to linger into the acquisition or consolidation phase of fear extinction learning. Moreover, perhaps neither the neurochemical events occurring during acute exercise, nor the potential therapeutic effects of recent exercise bouts, would depend upon an extensive history of exercise. This is an auspicious possibility from a clinical perspective, because while adherence to chronic exercise programs is notoriously low, single exercise bouts would be easy to implement in a clinical setting. If acute exercise bouts can augment fear extinction, then determining in which phase of fear extinction learning these need to occur would provide important mechanistic and clinical insights.
The question of whether an acute exercise bout, in the absence of a history of exercise, occurring in close temporal proximity to fear extinction can augment extinction was first addressed by Siette and colleagues (2014), who reported that 3 h of access to running wheels either immediately prior to or immediately following extinction of contextual fear conditioning improved fear extinction recall the next day. An acute running bout occurring 6 h after extinction was without effect, suggesting that exercise needs to be concurrent with acquisition or consolidation (Siette et al., 2014). Similarly, Mika et al. (2015) reported that rats allowed to run in wheels during auditory fear extinction, but not running by itself, displayed a reduction in fear renewal (indicated by freezing and corticosterone) when re-exposed to the auditory CS in a novel environment 1 week after extinction, compared to rats exposed to extinction in locked wheels. Based on these data, we might conclude that acute exercise bouts can enhance fear extinction regardless of the phase of extinction learning in which exercise occurs: prior to acquisition, during acquisition, or during consolidation. However, follow-up work indicates the relationship between acute exercise and fear extinction may not be so simple (effects of acute exercise, in the absence of a history of chronic exercise, on fear extinction are summarized in Figure 1B).
Two groups followed up on this initial work. Bouchet et al. (2017) investigated the impact of single voluntary exercise bouts occurring after fear extinction on proximal and distal fear extinction memory. Unlike the Siette et al. (2014) study, Bouchet et al. (2017) did not observe a significant improvement in retention of contextual fear extinction during a proximal memory test occurring 24 h following the first extinction session. However, Bouchet et al. (2017) allowed the rats to run again for 2 h after this proximal extinction memory test, which also served as an extinction session. When reassessed for distal retention of extinction memory 10 days later, rats that ran after multiple extinction sessions displayed a significant reduction in freezing that was dependent on the exercise being concurrent with the consolidation of contextual fear extinction, and not due to exercise-induced forgetting. Bouchet et al. (2017) also observed that post-extinction exercise rapidly augmented auditory fear extinction and reduced the later renewal of auditory conditioned fear in a novel context. The more rapid exercise augmentation of auditory, compared to contextual, fear extinction suggests that the extinction of fear memories of discrete cues might be more susceptible to exercise-augmentation than the extinction of contextual fear memories.
A few key differences between the Siette et al. (2014) and Bouchet et al. (2017) studies could explain why additional bouts of post-extinction exercise were required to augment contextual fear extinction in the latter. First are differences in duration or distance run following fear extinction. Rats in the Siette et al. (2014) study were allowed 3 h of wheel access after extinction, whereas rats in the Bouchet et al. (2017) study were only allowed 2 h. Both studies reported significant negative correlations between distance run after fear extinction and freezing during subsequent memory tests, suggesting that potential differences in distance run after extinction could influence the strength of the exercise effect. However, despite the longer duration of wheel access in the Siette et al. (2014) study, it appears that rats in the Siette et al. (2014) study ran less (average distance between 150 – 400 m) than those in the Bouchet et al. (2017) study (average of 707 m). A second, more likely explanation for the conflicting results is that the fear memory in the Bouchet et al. (2017) study was stronger, and therefore more difficult to extinguish. Bouchet et al. (2017) allowed all rats to run in wheels for 2 nights prior to conditioning to reduce neophobia to the wheels and to encourage later running after extinction. As we saw with chronic exercise experiments above, this pre-conditioning exercise could have strengthened the fear memory, making it more difficult to extinguish. Indeed, running distance prior to conditioning was positively correlated with freezing during conditioning (Bouchet et al., 2017a). Adding to this effect could be the fact that Siette et al. (2014) used only a single foot-shock to establish fear conditioning, whereas Bouchet et al. (2017) used 3. Finally, Siette et al. (2014) performed the extinction session during the inactive cycle, whereas Bouchet et al. (2017) performed the extinction sessions during the beginning of the active cycle, because this is when rats naturally choose to run in wheels. However, there is a circadian rhythm of fear extinction learning, whereby rats that learn fear extinction during the active cycle have improved fear extinction retention compared to rats exposed to fear extinction during the inactive cycle (Woodruff et al., 2015). Together, these factors could have made it more difficult to observe an exercise effect in Bouchet et al. (2017) compared to Siette et al. (2014). Nevertheless, the conclusion of both experiments is that brief bouts of voluntary exercise can augment fear extinction when the exercise occurs immediately after the fear extinction session, during the consolidation phase. Most important from a clinical perspective is that the results of Bouchet et al. (2017) also suggest that fear extinction augmented by post-extinction exercise can be resistant to relapse (both spontaneous recovery and renewal).
Jacquart et al. (2017) further investigated the effects of pre-extinction voluntary exercise and observed that acute exercise prior to auditory or contextual fear extinction failed to augment extinction. Conditions of one experiment closely matched those used in the Siette et al. (2014) study. Rats with no history of wheel running were conditioned to fear a context with a single shock during conditioning. Rats were then allowed 3 h of access to a running wheel during the first few hours of the light cycle, followed immediately by a 10-min extinction session in the conditioning context. Extinction memory was assessed 24 h later, with no additional access to wheels. As pointed out by Jacquart et al. (2017), the primary differences in design between their experiment and Siette et al. (2014) were rat strain (Sprague Dawley vs. Wistar) and housing conditions (2 per cage vs. 4 per cage). Jacquart et al. (2017) also failed to find an effect of pre-extinction exercise when rats were habituated to running wheels prior to extinction, when rats were exposed to auditory fear extinction rather than contextual, and when several shocks were used during conditioning. One standout factor in the Jacquart et al. (2017) experiments is the minimal distance run by the rats. The greatest distance run in these experiments appears to be 148 m over a period of 3 h; less than that reported in Siette et al. (2014) and far less than the amount we have observed rats to run over a 2 h period after extinction (Bouchet et al., 2017a). This brings up the possibility that the conflicting reports on the effects of pre-extinction exercise bouts on fear extinction depends on the distance run prior to extinction.
Distance or timing?
Conflicting results on the effects of pre-extinction running on fear extinction memory could be due to differences in distance run by the rats between studies, or could be because exercise prior to fear extinction simply does not elicit as robust an augmentation of fear extinction as exercise after fear extinction, during the memory consolidation phase. Indeed, one could imagine a scenario in which transient neurochemical events elicited during exercise are able to support the consolidation of fear extinction, but these events do not linger long enough after an exercise session to support memory consolidation if exercise only occurs prior to extinction.
We set out to determine if acute exercise prior to fear extinction, like acute exercise after extinction, can improve fear extinction recall and reduce fear renewal. We used methods identical to those used in Bouchet et al. (2017), except that half the rats were placed into locked or mobile wheels for 2 h immediately prior to auditory fear extinction, and the other half immediately after. Adult, male Long Evans rats (n=57; age P50-P55 on arrival) purchased from Charles River (Wilmington, MA) were placed into mobile and locked running wheels on alternating nights for 4 consecutive nights (see Figure 2A for experimental design and timeline). This was done to reduce neophobia, to ensure that locked and mobile wheel environments were equally familiar, and to promote later running behavior. Two days after this wheel habituation period (3 days after the last exposure to a mobile wheel), rats were exposed to auditory fear conditioning, as in our prior work (Bouchet et al., 2017a). All behavior was recorded with overhead cameras, and freezing during both the CS and inter-trial interval was scored automatically by EthoVision XT software (Noldus, Leesburg, VA). All rats acquired auditory fear conditioning equally, as indicated by increased time spent freezing during successive trials (F(3,162)=93.11; p<0.0001; data not shown). Rats were immediately returned to their home cages after fear conditioning and, approximately 36 h later, were exposed to auditory fear extinction, consisting of 20 CS presentations in a novel context in the absence of shock. Either immediately before or immediately after fear extinction, rats were placed into their familiar running wheels, which were either rendered immobile (Locked) or freely mobile (Mobile) for 2 h. Since rats prefer to run during their active (dark) cycle, fear extinction and the 2 h of wheel access occurred at the start of the dark cycle. Freezing levels decreased over trials (F(4, 216)=96.35, p<0.0001; data not shown), indicating successful fear extinction. Freezing during fear extinction did not significantly differ between groups (data not shown), which confirms previous work showing that exercise prior to fear extinction does not impact fear extinction acquisition (Siette et al., 2014; Jacquart et al., 2017). Rats displayed robust running behavior regardless of whether running occurred before or after fear extinction (Figure 2B).
Figure 2.
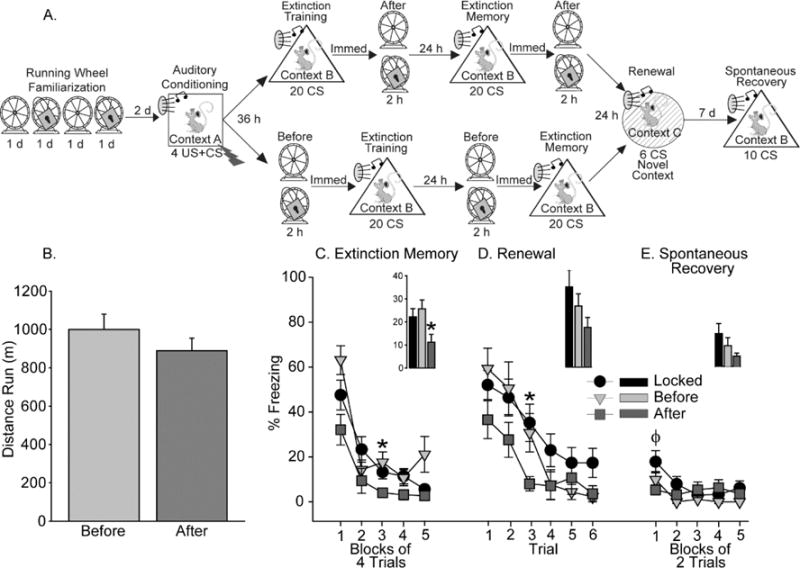
Effects of acute exercise before or after fear extinction on fear extinction memory retention and relapse. A) Experimental timeline. B) Distance run before or after fear extinction. C) Levels of freezing during the 2nd fear extinction training session, which also served as a fear extinction memory test. Exercise afterfear extinction (After) improved fear extinction retention compared to both Locked controls and rats that ran before fear extinction (Before). D) Levels of freezing during re-exposure to the auditory CS in a novel context, indicative of fear renewal. Exercise after, but not before, fear extinction reduced fear renewal. E) Levels of freezing during re-exposure to the auditory CS 7 d after fear renewal. Exercise after, but not before, fear extinction reduced freezing relative to Locked controls during the first trial block. All data represent means ± SEM. * Indicates After group is different from Locked and Before groups (p < 0.05); ɸ Indicates After group is different from Locked controls (p < 0.05).
Exposure to 2 h of locked or mobile wheels either before or after fear extinction was repeated 24 h later, so that all rats received 2 fear extinction training sessions. The second of the fear extinction training sessions also served as a fear extinction memory test. Rats that exercised after the first fear extinction training session displayed significantly less freezing behavior during the fear extinction memory test, compared to both Locked rats and rats allowed to run before fear extinction (Figure 2C; main effect of exercise: F (2, 54) = 4.04; p = 0.02; exercise × trial interaction: F (8, 216) = 2.28; p = 0.02). Similar results were observed upon re-exposure to the CS during both a fear renewal test in a novel context 24 h later (Figure 2D; exercise × trial interaction: F(10, 270) = 1.95;p=0.04) and a spontaneous recovery test in the extinction context 7 d later (Figure 2E). Freezing levels in all groups were very low during the spontaneous recovery test; however, rats that ran after extinction displayed lower levels of freezing than Locked rats during the first trial block (p < 0.05). These data indicate that acute exercise occurring after fear extinction, but not before, can improve extinction memory retention and reduce fear relapse. The failure of a pre-extinction acute exercise bout to enhance fear extinction is consistent with results of Jacquart et al. (2017). However, unlike rats in the Jacquart et al. (2017) experiment that ran minimal distances prior to fear extinction, the current data reveal that acute exercise before fear extinction fails to augment extinction despite robust wheel running prior to fear extinction. Interestingly, these results confirm a recent meta-analysis of the literature, which predicts that the timing of acute exercise relative to fear extinction learning is a critical variable in determining whether acute exercise can successfully augment fear extinction; whereby only post-extinction exercise would augment extinction (Roquet & Monfils, 2018). A final point of consideration is whether acute exercise can augment fear extinction in both sexes, as the work described so far used only male rats. We have observed that acute exercise fails to augment fear extinction in females, regardless of estrous phase and despite vigorous wheel running in females which surpassed that of the males (Bouchet et al., 2017a). Future work is required to determine conditions under which acute exercise can augment fear extinction in females, or whether acute exercise-augmentation of fear extinction is sex-dependent.
Potential Mechanisms
Multiple physiological, endocrine, and neural factors could contribute to the ability of acute exercise to augment fear extinction. Fitzgerald and colleagues (2015) provide an excellent review of various factors that have been reported to enhance fear extinction (Fitzgerald et al., 2014). As acute exercise can impact nearly all of these factors, including monoamine and amino acid neurotransmitters, glucocorticoids, growth factors, and endocannabinoids, any of these factors could be involved in the mechanisms by which exercise could enhance fear extinction. Addressing each of these factors individually is beyond the scope of this short review. Here, we provide insights gleaned from recent experiments that could help guide future mechanistic studies.
We have seen that the stable neural-adaptations that occur with chronic exercise are insufficient to augment fear extinction. Rather, for exercise to strengthen fear extinction, acute bouts of exercise must occur in close temporal proximity to the consolidation of fear extinction memory. These data imply that factors involved in exercise-augmentation of fear extinction must be sensitive to acute exercise, whereas their sensitivity to chronic exercise is irrelevant. Thus, exercise adaptations such as neurogenesis, altered gene expression within central serotonergic systems (Greenwood et al., 2005b), as well as other changes requiring several days or weeks to develop, are unlikely to be involved in exercise-augmentation of extinction. Instead, rapid changes in hormonal, neurochemical and/or neuromodulator signaling are more likely involved. Glucocorticoids, for example, are elevated during acute bouts of voluntary exercise and can enhance fear extinction (Bentz et al., 2010). Central norepinephrine (NE) and dopamine (DA) circuits are also recruited during exercise, and their signaling has been reported to enhance fear extinction (Abraham et al., 2014; Fitzgerald et al., 2014). Further supporting involvement of these monoamines, NE and DA can rapidly increase intracellular signaling molecules and growth factors thought to be important for supporting fear extinction, such as extracellular-regulated kinase and BDNF. Powers and colleagues (2015) provide an excellent review of the potential role of BDNF in exercise-augmentation of fear extinction.
Acute exercise occurring during or immediately following fear extinction is unique in that it not only enhances fear extinction memory, it also reduces fear relapse. This point is critical, because factors that can enhance fear extinction memory are not necessarily successful at reducing later relapse. The disconnect between extinction and relapse could partly explain why strategies that successfully enhance exposure therapy in the clinic often have poor long-term efficacy. For example, administration of the NMDA agonist D-cycloserine can enhance extinction, but D-cycloserine does not seem to prevent renewal (Woods & Bouton, 2006). Similarly, increasing NE transmission with the α2A adrenergic inhibitory-autoreceptor antagonist yohimbine can facilitate fear extinction, yet yohimbine’s effect on extinction is context dependent, and fear extinction facilitated by yohimbine remains susceptible to renewal (Morris & Bouton, 2007). When searching for mechanistic factors by which exercise augments fear extinction, these examples illustrate the need to consider the effects of potential factors on relapse phenomena, rather than extinction memory alone.
Even after narrowing potential mechanisms by which exercise enhances fear extinction down to those sensitive to acute exercise and able to prevent fear relapse, a laundry list of potential mechanisms remains. In fact, multiple factors could contribute to the mechanisms by which acute exercise enhances fear extinction. If exercise augments fear extinction through multiple mechanisms, then it is unlikely that blocking any one factor or mechanism would significantly reduce the exercise effect. Rather than attempting to identify which of the potential mechanisms is most involved in the exercise effect, research efforts in this area might be better spent using exercise as a tool to identify novel therapeutic targets. In other words, can activation of one of the potential exercise mechanisms be sufficient to enhance fear extinction in the absence of exercise? Such efforts could lead to novel therapeutic strategies for long-term treatment of anxiety and trauma-related disorders. Heeding this advice, we have investigated whether activation of a circuit traditionally viewed in the context of motor behavior, the nigrostriatal DA circuit, is sufficient to mimic the effects of acute exercise on fear extinction and relapse.
Proposed Role for Dopamine
DA stands out among the factors sensitive to acute exercise that could contribute to the ability of exercise to augment fear extinction and reduce relapse. DA circuits are likely recruited during acute exercise bouts to promote locomotor activity or goal-directed processes such as learning to run or choosing to run, and could contribute to the reinforcing effects of exercise (Knab & Lightfoot, 2010). Several observations are consistent with the possibility that DA neurotransmission recruited during exercise could enhance fear extinction. High frequency (phasic) activity of DA neurons resulting in large concentrations of extracellular DA is thought to contribute to new learning. Phasic DA release (Badrinarayan et al., 2012) and cfos mRNA in D1 receptor-expressing neurons (Bouchet et al., 2017b), which are preferentially activated by phasic DA, are both increased in the striatum during fear extinction. These observations suggest that phasic DA signaling could be involved in the acquisition or consolidation of fear extinction memory. Furthermore, augmenting DA signaling can enhance fear extinction and reduce relapse. For example, systemic administration of DA reuptake inhibitors (Abraham et al., 2012), the DA precursor L-DOPA (Haaker et al., 2013), and D1 receptor agonists (Abraham et al., 2016), during either the acquisition or consolidation phases of fear extinction learning, have all been reported to enhance recall of fear extinction and reduce various forms of relapse. The effects of DA on fear extinction; however, seem to be dependent on the specific DA receptor or brain region targeted. Indeed, systemic D2 receptor agonists can impair fear extinction (Ponnusamy et al., 2005), potentially through actions in the hippocampus (Fiorenza et al., 2012) or infralimbic region (IL) of the medial prefrontal cortex (Hitora-Imamura et al., 2015).
The question of where in the brain DA acts to enhance fear extinction remains an active area of investigation. The nigrostriatal DA pathway, which consists of DA neurons originating in the substantia nigra (SN) and terminating in the dorsal striatum (DS), plays a critical role in movement, and thus represents a potential circuit through which acute exercise could augment fear extinction. The nigrostriatal pathway may seem like a strange circuit to associate with fear extinction; however, data suggest that exercise augments fear extinction through a circuit independent from typical fear extinction circuitry. Consistent with the established role of the IL in supporting fear extinction memory, levels of activity markers are often higher in the IL of rats that display improved fear extinction memory recall compared to their more fearful counterparts (Knapska & Maren, 2009). Mika et al. (2015), however, observed that levels of cfos mRNA in the IL of rats allowed to run during fear extinction and expressing low levels of fear during a relapse test were no higher than levels observed in the IL of rats extinguished in locked wheels. Acute exercise may thus augment fear extinction through unique neural circuits independent of IL-mediated inhibition of fear. Consistent with a role for the nigrostriatal circuit in exercise-augmented fear extinction, we observed potentiated expression of cfos mRNA in D1-expressing neurons in the DS during recall of relapse-resistant fear extinction in rats that previously ran during fear extinction, compared to rats previously extinguished in locked wheels (Mika et al., 2015). These data suggest that D1-expressing neurons in the DS could be a site of plasticity involved in exercise-augmented fear extinction.
One way in which nigrostriatal DA and D1 receptor signaling in the DS could facilitate fear extinction is by contributing to the assignment of a positive emotional value to the CS. Emotional value has been suggested to contribute to fear extinction; whereby a negative emotional value is associated with the CS during fear conditioning, and a positive emotional value, stemming from the relief that comes from the lack of the predicted US, is associated with the CS during extinction (Tronson et al., 2012). As nigrostriatal DA and DS D1-expressing neurons are increasingly associated with reward and reinforcement (Kravitz & Kreitzer, 2012; Lenz & Lobo, 2013), nigrostriatal DA could support this emotional component to fear extinction.
If phasic DA and D1 receptor signaling in the DS contribute to the ability of exercise to augment fear extinction, then D1-expressing neurons sensitive to phasic DA must be either directly or indirectly connected to circuits able to inhibit fear. In the rodent, the primary output nucleus of the DS is the entopeduncular nucleus (EPN; analogous to the human internal globus pallidus). Inhibitory neurons in the EPN projecting to the motor thalamus are inhibited by GABAergic, D1-expressing neurons of the DS; thereby disinhibiting motor activity. This is the primary circuit through which the “direct pathway” of the DS promotes movement. However, the thalamus is not the only target of the EPN. A population of glutamatergic neurons in the EPN projects to, and activates, the lateral habenula (Shabel et al., 2012), a stress-responsive region known for its involvement in aversion. The lateral habenula can influence the activity of downstream monoaminergic nuclei, including dorsal raphe serotonergic (5-HT) neurons implicated in anxiety and delayed fear extinction (Dolzani et al., 2016). In fact, lesions of the lateral habenula have been recently reported to facilitate fear extinction (Song et al., 2017). These data suggest that by inhibiting the EPN-to-lateral habenula circuit, phasic activity of nigrostriatal DA neurons and subsequent D1 receptor signaling in the DS could communicate with the fear circuit and even facilitate fear extinction (Figure 3).
Figure 3.
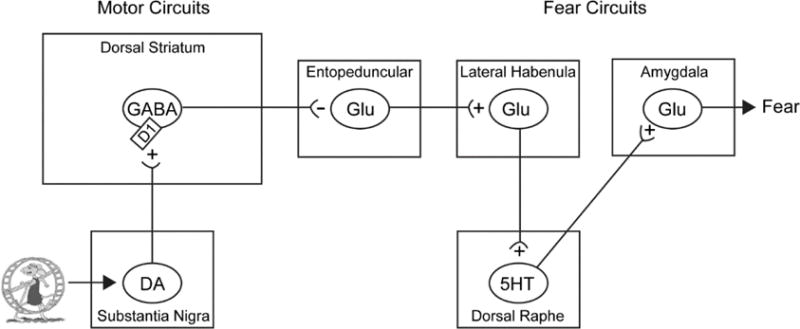
Proposed circuit through which nigrostriatal dopamine DA neurons recruited during acute exercise could communicate with fear circuits. Exercise-induced activation of D1 receptor (D1)-expressing, GABAergic neurons in the dorsal striatum could inhibit fear by inhibiting glutamatergic (Glu) neurons in the entopeduncular nucleus that activate the lateral habenula. Potential exercise-induced plasticity could occur within the nigrostriatal dopamine pathway or within D1 receptor-expressing neurons in the dorsal striatum. DA, dopamine; 5-HT, serotonin; GABA, gamma-Aminobutyric acid; + excitatory pathway; − inhibitory pathway.
If activation of SN DA neurons and subsequent D1 signaling in the DS is an important contributor to the mechanisms by which acute exercise facilitates fear extinction, then activating this circuitry in the absence of exercise might be sufficient to enhance fear extinction and reduce relapse. If so, then the nigrostriatal DA circuit would represent a potential novel target for the treatment of anxiety and trauma-related disorders. Emerging technologies such as designer receptors exclusively activated by designer drugs (DREADDs) allow investigators unprecedented control over discrete neural populations during ongoing behavior. Viral-mediated expression of Gq-coupled DREADDs in midbrain DA neurons elicits an increase in phasic activation of DA neurons in response to systemic administration of the synthetic ligand clozapine-N-oxide (CNO). Recent data indicate effects of CNO on their target DREADDs are mediated by back-metabolism of CNO to clozapine, which can have off-target effects (Gomez et al., 2017). Inclusion of CNO control groups is therefore critical to interpretation of experiments using DREADDs. Using appropriate CNO controls consisting of rats with off-site viral injections receiving CNO, we observed that selective activation of SN DA neurons during fear extinction facilitates later recall of fear extinction memory and reduces fear renewal in a manner similar to exercise (Bouchet et al., 2017b). These data indicate that phasic activation of SN DA neurons is sufficient to mimic the effects of acute exercise on fear extinction. The nigrostriatal DA circuit and D1-expressing neurons in the DS could be an important circuit integrating motor activity during exercise with emotional consequences of exercise, including augmented fear extinction (Figure 3).
Conclusions
Although the stable neuro-plastic changes induced in the brain by exercise are not sufficient to enhance fear extinction, an acute bout of exercise temporally proximal to the consolidation phase of fear extinction learning can enhance the later recall of fear extinction memory and reduce fear relapse. Critical from a clinical perspective is the fact that an acute exercise bout can enhance fear extinction even in the absence of an extensive exercise history. These promising pre-clinical data should provide the impetus for further clinical investigations into the ability of acute exercise to augment exposure therapy. Exercise might have maximal effects if exercise bouts follow, rather than precede, sessions of exposure therapy, and if subjects are those most likely to have impaired fear extinction, such as patients diagnosed with clinical anxiety or trauma-related disorders. Clinical studies should include subjects of both sexes to determine if sex differences in the effects of exercise on fear extinction observed in rodents exist in humans. Neural circuits through which exercise augments fear extinction could involve multiple factors and circuits not before considered in fear extinction research, such as phasic activation of nigrostriatal DA neurons and subsequent D1 receptor signaling in the DS. Future research efforts should utilize acute exercise as a tool for identifying novel therapeutic factors.
Highlights.
Chronic exercise adaptations are insufficient to enhance fear extinction
Acute exercise can enhance fear extinction and reduce relapse
Acute exercise can enhance fear extinction even in previously sedentary rats
The timing of acute exercise relative to fear extinction is a critical variable
Nigrostriatal dopamine could contribute to exercise-augmentation of fear extinction
Acknowledgments
Funding: This work was supported by the National Institutes of Health (grant number R15 MH114026).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abraham AD, Cunningham CL, Lattal KM. Methylphenidate enhances extinction of contextual fear. Learn Mem. 2012;19:67–72. doi: 10.1101/lm.024752.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiology of learning and memory. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AD, Neve KA, Lattal KM. Activation of D1/5 Dopamine Receptors: A Common Mechanism for Enhancing Extinction of Fear and Reward-Seeking Behaviors. Neuropsychopharmacology. 2016;41:2072–2081. doi: 10.1038/npp.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz D, Michael T, de Quervain DJ, Wilhelm FH. Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. Journal of anxiety disorders. 2010;24:223–230. doi: 10.1016/j.janxdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Bouchet CA, Lloyd BA, Loetz EC, Farmer CE, Ostrovskyy M, Haddad N, Foright RM, Greenwood BN. Acute exercise enhances the consolidation of fear extinction memory and reduces conditioned fear relapse in a sex-dependent manner. Learn Mem. 2017a;24:358–368. doi: 10.1101/lm.045195.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet CA, Miner MA, Loetz EC, Rosberg AJ, Hake HS, Farmer CE, Ostrovskyy M, Gray N, Greenwood BN. Activation of Nigrostriatal Dopamine Neurons during Fear Extinction Prevents the Renewal of Fear. Neuropsychopharmacology. 2017b doi: 10.1038/npp.2017.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and ambiguity in the extinction of emotional learning: implications for exposure therapy. Behav Res Ther. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacol Biochem Behav. 2006;84:306–312. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009;19:962–972. doi: 10.1002/hipo.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzani SD, Baratta MV, Amat J, Agster KL, Saddoris MP, Watkins LR, Maier SF. Activation of a Habenulo-Raphe Circuit Is Critical for the Behavioral and Neurochemical Consequences of Uncontrollable Stress in the Male Rat. eNeuro. 2016;3 doi: 10.1523/ENEURO.0229-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behavioural brain research. 2010;207:321–331. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232:210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain research bulletin. 2014;105:46–60. doi: 10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exercise and sport sciences reviews. 2011;39:140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Mechanisms Underlying the Relationship Between Physical Activity and Anxiety: Animal data. Routledge; New York, NY: 2013. [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005a;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT(1A), 5-HT(1B), and alpha(1b)-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005b;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: Wheel running reverses stress-induced interference with shuttle box escape. Behav Neurosci. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Gaburro S, Sah A, Gartmann N, Lonsdorf TB, Meier K, Singewald N, Pape HC, Morellini F, Kalisch R. Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2428–2436. doi: 10.1073/pnas.1303061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitora-Imamura N, Miura Y, Teshirogi C, Ikegaya Y, Matsuki N, Nomura H. Prefrontal dopamine regulates fear reinstatement through the downregulation of extinction circuits. Elife. 2015;4 doi: 10.7554/eLife.08274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquart J, Roquet RF, Papini S, Powers MB, Rosenfield D, Smits JAJ, Monfils MH. Effects of acute exercise on fear extinction in rats and exposure therapy in humans: Null findings from five experiments. Journal of anxiety disorders. 2017;50:76–86. doi: 10.1016/j.janxdis.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM, Lightfoot JT. Does the difference between physically active and couch potato lie in the dopamine system? International journal of biological sciences. 2010;6:133–150. doi: 10.7150/ijbs.6.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology. 2012;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Lloyd BA, Hake HS, Ishiwata T, Farmer CE, Loetz EC, Fleshner M, Bland ST, Greenwood BN. Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav Brain Res. 2017;323:56–67. doi: 10.1016/j.bbr.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika A, Bouchet CA, Bunker P, Hellwinkel JE, Spence KG, Day HE, Campeau S, Fleshner M, Greenwood BN. Voluntary exercise during extinction of auditory fear conditioning reduces the relapse of fear associated with potentiated activity of striatal direct pathway neurons. Neurobiol Learn Mem. 2015;125:224–235. doi: 10.1016/j.nlm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Merritt JR, Holloway AL, Pinardo H, Miller DS, Kilby CN, Bucko P, Wyer A, Rhodes JS. Increased adult hippocampal neurogenesis is not necessary for wheel running to abolish conditioned place preference for cocaine in mice. Eur J Neurosci. 2015;41:216–226. doi: 10.1111/ejn.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. conditioned refles: An investigation of the physiological activity of the cerebral cortex. Oxford University Press; Oxford, England: 1927. [Google Scholar]
- Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Medina JL, Burns S, Kauffman BY, Monfils M, Asmundson GJ, Diamond A, McIntyre C, Smits JA. Exercise Augmentation of Exposure Therapy for PTSD: Rationale and Pilot Efficacy Data. Cogn Behav Ther. 2015;44:314–327. doi: 10.1080/16506073.2015.1012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;1:88–96. [PubMed] [Google Scholar]
- Roquet RF, Monfils MH. Does exercise augment operant and Pavlovian extinction: A meta-analysis. J Psychiatr Res. 2018;96:73–93. doi: 10.1016/j.jpsychires.2017.09.018. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev. 2012;36:1965–1984. doi: 10.1016/j.neubiorev.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siette J, Reichelt AC, Westbrook RF. A bout of voluntary running enhances context conditioned fear, its extinction, and its reconsolidation. Learn Mem. 2014;21:73–81. doi: 10.1101/lm.032557.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Jo YS, Lee YK, Choi JS. Lesions of the lateral habenula facilitate active avoidance learning and threat extinction. Behav Brain Res. 2017;318:12–17. doi: 10.1016/j.bbr.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends in neurosciences. 2012;35:145–155. doi: 10.1016/j.tins.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi H, Hirai Y, Maezawa H, Notani K, Inoue N, Funahashi M. Effects of treadmill exercise on the LiCl-induced conditioned taste aversion in rats. Physiol Behav. 2015;138:1–5. doi: 10.1016/j.physbeh.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behav Neurosci. 2004;118:1378–1390. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- Woodruff ER, Greenwood BN, Chun LE, Fardi S, Hinds LR, Spencer RL. Adrenal-dependent diurnal modulation of conditioned fear extinction learning. Behav Brain Res. 2015;286:249–255. doi: 10.1016/j.bbr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behavioral neuroscience. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]


