Abstract
Inflammatory bowel disease (IBD) is a chronic, idiopathic inflammatory set of conditions that can affect the entire gastrointestinal (GI) tract and is associated with an increased risk of colorectal cancer. To date there is no curative therapy for IBD; therefore life-long medication can be necessary for IBD management if surgery is to be avoided. Drug delivery systems specific to the colon have improved IBD treatment and several such systems are available to patients. However, current delivery systems for IBD do not target drugs to the site of inflammation, which leads to frequent dosing and potentially severe side effects that can adversely impact patients’ adherence to medication. There is a need for novel drug delivery systems that can target drugs to the site of inflammation, prolong local drug availability, improve therapeutic efficacy, and reduce drug side effects. Nanoparticulate (NP) systems are attractive in designing targeted drug delivery systems for the treatment of IBD because of their unique physicochemical properties and capability of targeting the site of disease. This review analyzes the microenvironment at the site of inflammation in IBD, highlighting the pathophysiological features as possible cues for targeted delivery; discusses different strategies and mechanisms of NP targeting IBD, including size-, charge-, ligand-receptor, degradation- and microbiome-mediated approaches; and summarizes recent progress on using NPs towards improved therapies for IBD. Finally, challenges and future directions in this field are presented to advance the development of targeted drug delivery for IBD treatment.
Keywords: Nanoparticulate systems, Targeted drug delivery, Inflammatory bowel disease, Inflammation
I. Introduction
Inflammatory bowel disease (IBD) is a chronic, idiopathic inflammatory set of conditions that can affect the entire gastrointestinal (GI) tract and is associated with an increased risk of colorectal cancer [1]. IBD consists of two major clinically defined forms, Crohn’s disease (CD) and ulcerative colitis (UC) [2]. CD generally involves the ileum and the colon, but it can affect any region of the GI tract discontinuously, and the inflammation is often transmural; in contrast, UC is confined to the large intestine, extending proximally from the rectum to potentially involve the entire colon in an uninterrupted pattern with extensive superficial mucosal ulceration [2]. Both CD and UC are associated with high morbidity and decreased quality of life [3]. The incidence of IBD is increasing worldwide with a substantial burden on public healthcare [4, 5]. Currently, IBD affects roughly 1.4 million individuals in the U.S., with an estimated annual direct cost exceeding $6 billion [6].
To date, the etiology of IBD is not completely understood. Multiple factors including genetic predisposition, immunoregulatory dysfunction, environmental triggers, and microbial exposure contribute to the development of the disease [7, 8]. Dysbiosis (alterations in the development or compositions of the microbiota) and pathogenic infections have also been reported to play a role [7]. Conventional medication for IBD comprises anti-inflammatory drugs (e.g., 5-aminosalicyclic acid, corticosteroids) and immunosuppressive agents (e.g., azathioprine, 6-mercaptopurine). The introduction of anti-tumor necrosis factor (TNF)-a monoclonal antibodies as biologic therapies significantly increased the treatment options for IBD [9, 10]. However, there is no cure for IBD, and available therapies fail to control symptoms in a large number of patients. The loss of response to biologics can lead to colectomy or small bowel resections in IBD patients [11, 12].
IBD predominantly affects the colon, and consequently colon-targeted drug delivery systems have received significant attention for IBD treatment. Traditional drug delivery approaches for IBD treatment have applied pH-, time-, microflora-, and pressure-triggered systems to control drug release in the colon [13]. Examples include methacrylate derivatives applied as coatings of formulations (pH-dependent), an erodible hydrogel plug in a capsule (time-dependent), azo-conjugated pro-drugs [14] or ethylcelluose/amylose modified systems (microflora-triggered) [15, 16], and osmotic agents with a semi-permeable membrane inside a capsule (pressure-controlled) [17]. These approaches have been extensively studied and the Food and Drug Administration (FDA) has approved several of them for clinical use (Table 1).
Table 1.
| Drugs | Brand name | Formulation | Mechanism for drug release |
|---|---|---|---|
| Aminosalicyl ate (5-ASA) | Dipentum | 5-ASA dimer linked by azo bond | Enzymatic reduction# |
| Azulfidine | 5-ASA linked to sulfapyridine by azo bond | Enzymatic reduction# | |
| Salazopyrin | 5-ASA linked to sulfapyridine by azo bond | Enzymatic reduction# | |
| Colazal | 5-ASA linked to 4-aminobenzoyl-β-alanine by azo bond | Enzymatic reduction# | |
| Colazide | 5-ASA linked to 4-aminobenzoyl-β-alanine by azo bond | Enzymatic reduction# | |
| Asacol | A Eudragit S formulation coated 5-ASA tablet | pH-responsive | |
| Asacol HD | A Eudragit S formulation coated 5-ASA tablet | pH-responsive | |
| Claversal | 5-ASA coated with Eudragit-L | pH-responsive | |
| Salofalk | 5-ASA coated with Eudragit-L | pH-responsive | |
| Calitoflak | Eudragit-L 100 coated 5-ASA tablets | pH-responsive | |
| Salofalk Granu-Stix | 5-ASA coated with Eudragit-L100, polyacrylate-dispersion, povidone K (Eudragit-NE 40D, Nonoxynol 100), simethicone | pH-responsive | |
| Apriso | 5-ASA coated with Eudragit-L100, polyacrylate-dispersion, povidone K (Eudragit-NE40D, Nonoxynol 100), simethicone | pH-responsive | |
| Pentasa | Microgranules coated in ethylcellulose containing 5-ASA | Time-dependent | |
| Lialda | Multi-Matrix System using a Eudragit S coating a matrix core containing hydrophobic/ hydrophilic excipients | pH-responsive + time-delayed | |
| Mezavant | 5-ASA coated with Multi Matrix system with lipophilic and hydrophilic matrices | pH-responsive + time-delayed | |
| CODES | Polysaccharide coating coupled with a pH-sensitive polymer coating | Degradation by bacteria + pH-responsive | |
| Budesonide | Uceris | Multi-Matrix System using a Eudragit S coating a matrix core containing hydrophobic/hydrophilic excipients | pH-responsive + time-delayed |
| Entocort EC | Eudragit L coated beads with ethyl cellulose matrix | pH-responsive + time-dependent | |
| Beclomethasone | Clipper | Eudragit L 100–55 coated tablet | pH-responsive |
Note:
All administered orally.
The azo bond is cleaved by azoreductases produced by colonic microflora [14].
Recently, novel drug delivery systems for IBD treatment capable of specifically targeting the site of inflammation, instead of the entire colon, have attracted substantial attention [18, 19]. Since IBD is a chronic, incurable condition, the therapeutic goal is to induce and maintain long-term remission. Inflammation-targeting drug delivery in IBD potentially offers a safe and efficacious approach for disease treatment and management. First, it provides high local drug concentration at the site of disease for prolonged pharmacological activities and maximized drug efficacy. Studies with IBD patients have shown that higher drug concentrations in affected tissues are associated with lower histological degrees of inflammation and improved disease control [20, 21]. Second, targeted delivery may prevent or reduce drug degradation and loss of efficacy prior to reaching the site of action. Biological therapies such as small interfering RNA (siRNA) and proteins generally display a short half-life in the circulation and undergo rapid degradation in vivo, thereby requiring targeted delivery to the site of action as well as nucleic acid modification to render the anti-sense therapies resistant to nucleases. Third, targeted drug delivery in IBD has the potential to reduce dosing frequency and minimize systemic side effects. By guiding drugs to the site of action, targeted drug delivery can reduce non-specific distribution of drugs throughout the body; consequently, decreases in dosing frequency may be achieved for the required amount of drug. Since drugs are directly released at the inflamed tissue, systemic drug exposure and the associated side effects can be minimized. Frequent dosing, complex tapering regimens, and fear of side effects have been reported to negatively impact patients’ adherence to medications in IBD treatment [22]. With the potential to reduce dosing frequency and drug side effects, targeted drug delivery could contribute to improving medication adherence.
Nanoparticulate (NP) drug delivery systems are of particular interest among the inflammation-targeting approaches in IBD, due to their small size and versatile surface chemistry [23]. The intestinal tissue permeability is increased in the inflamed colon - both at the level of the endothelium and the epithelium, where the tight junctions (TJs) between cells are enlarged due to the loss of cellular integrity upon the activation of proinflammatory cytokines [24]. Taking advantage of the “leaky” intestine, NPs passively accumulate at the sites of inflammation due to the increased intestinal permeability [25], which is likely accompanied by enhanced NP uptake by the infiltrating immune cells [26]. Due to their small size, NPs can also penetrate deeply into the target tissue, which could be beneficial for the treatment of CD that is characterized by transmural inflammation [27]. Additionally, NPs provide the potential of modifying drug properties, including solubility, stability, and immunogenicity. Furthermore, surface tailoring of NPs facilitates targeted and controlled drug release to maximize drug concentration for a prolonged period of time at the site of inflammation while minimizing systemic side effects [28].
A large number of reviews have been conducted on drug delivery systems for IBD treatment, including prodrugs, micro-/nanoparticles, hydrogels, and biomimetic approaches. [17, 29–32]. In this review, however, we focus on NP systems targeting the site of inflammation for therapeutic applications in IBD. We analyze the general considerations for targeted drug delivery in IBD, including the GI physiology, the choice of delivery route, and the pathophysiological features at the site of inflammation as instrumental cues to anchor delivery systems. We then discuss the targeting mechanisms of different strategies and provide recent progress on using NPs towards improved therapies for IBD. Furthermore, we briefly review NP-loaded hybrid systems for oral administration in IBD treatment. We conclude with a summary of the challenges in the field and potential future directions for research and development.
II. General Considerations for Targeted Drug Delivery in IBD
2.1. The Physiology of the Gastrointestinal Tract
The major difficulty in designing drug delivery systems targeting IBD lies in the dynamic and wide-ranging environmental extremes encountered in the GI tract. The pH gradients, variable transit times, diverse digestive enzymes, and bacterial loads present significant challenges to the stability of drug delivery systems, especially when orally administered [33]. Moreover, GI physiology is affected by the chronic inflammation in IBD, and can be altered remarkably.
Under normal conditions, pH varies along the GI tract from the acidic stomach at 1.0–2.5, rapidly increasing to 5.0–7.0 in the duodenum, and gradually increasing to 7.5±0.5 in the terminal ileum, which then undergoes a slight decrease to 6.4±0.6 past the ileocaecal valve and slowly rises along the colon to the rectum (7.0±0.7) [34] (Table 2). The unique pH ranges in the macro-anatomic compartments of the GI tract have motivated colon-specific delivery using multiple pH-responsive systems such as an Eudragit S coating (methacrylic acid-methyl methacrylate copolymer) in Asacol® HD [35], Lialda® and Uceris® [36, 37], or a methacrylic acid-ethyl acrylate copolymer coating in Mongersen [38]. These polymer coatings respond to pH change and become soluble under a pH greater than 7. However, luminal pH is affected by many factors, including mucosal bicarbonate and lactate production, bacterial fermentation of carbohydrates, mucosal absorption of short chain fatty acids, and the intestinal transit; all of which can be disrupted in IBD and potentially contribute to abnormal pH in the inflamed colon [39]. Studies have shown that the colonic pH in IBD patients can be significantly more acidic (pH 2.3–5.5) [39–41]. Therefore, colon-targeting systems may have lower efficacy when relying solely on pH-responsive drug release. With the use of wireless motility/pH capsules measuring the colonic pH in situ [42], the luminal pH could be better profiled to guide the design of future delivery systems.
Table 2.
| Location | Healthy | Active UC | Active CD |
|---|---|---|---|
| Esophagus | ~ 7.0 | n/a | n/a |
| Stomach | 1–2.5 | n/a | n/a |
| Proximal small intestine | 5.9–6.8 | 6.1–7.3 | 6.3–6.5 |
| Distal small intestine | 7.3–7.7 | 6.8–8.3 | 7.3–7.5 |
| Proximal colon | 5.7–6.8 | 2.3–7.2 | 6.2–7.2 |
| Distal colon | 6.1–7.2 | 4.8–7.3 | 5.3–6.8 |
Note: n/a indicates “Not Available”.
Time-dependent drug release systems could overcome the limitations of pH-triggered delivery systems, though one challenge time-dependent systems face is the inter-individual GI transit variability. The transit time in the GI tract varies in healthy individuals, as well as those suffering from IBD, with multiple factors affecting transit, including the intestinal fluid and motility, the composition of the microbiome, and the length of the intestine (if surgery has been conducted to remove a section of the GI tract) [33]. Delayed transit times have been reported in both UC and CD patients, except when patients experience dysbiotic conditions which can be associated with faster transit times [43]. As diarrhea is common in IBD, the colonic transit time typically decreases, likely due to the increased luminal fluid content. The microbiome in IBD is affected by showing reduced diversity typically characterized by depletion of commensal bacteria such as Firmicutes and Bacteroidetes but with an expansion of Proteobacteria [44], which may influence the transit time [45]. Furthermore, many CD patients undergo ileocaecal resection [46], which may reduce the small intestinal transit time due to the shorter time spent at the ileocaecal junction. Overall, the inter-individual variability of transit time may prove too broad for time-dependent systems that are designed entirely based on the transit time for triggered release.
The changing enzyme repertoire encountered along the GI tract needs to be considered to ensure the stability of novel delivery systems, which includes proteases such as pepsin in the stomach, trypsin and chymotrypsin in the small intestine, multiple lipases, and carbohydrate-degrading enzymes. In addition, bacteria-derived enzymes in the colon must be considered, as they consist of a wide range of reductive and hydrolytic enzymes such as β-glucosidase, β-xylosidase, β-arabinosidase, β-galactosidase, nitroreductase, azoreductase, deaminase and urea hydroxylase, which provide further breakdown of polysaccharides and dietary fibers undigested in the small intestine [47]. The azoreductase activity of colonic bacteria has been extensively studied for colontargeting systems especially in prodrug development [14]. For example, sulfasalazine is the earliest prodrug that provides targeted release of active 5-ASA in the colon since the 1960s [48]. Recent studies on bacterial reduction of disulfide and nitro bonds suggest their potential as azo counterparts [49]. Importantly, the pathological changes in the microflora in IBD affect the composition and diversity of bacterial species, and hence the secretion of these enzymes, which should be taken into consideration for the design of novel drug delivery systems.
2.2. The Choice of Delivery Route
2.2.1. Oral administration
Both patients and physicians prefer orally delivered formulations. The challenges for oral drug delivery in IBD include the acidic pH (~1.0–2.5) in the stomach, the abundant enzymes encountered in saliva, stomach, small intestine, and colon, the variable GI transit time, the bacterial load in the colon, and the complex microenvironment at the site of inflammation. The physiological characteristics of the respective sections in the GI tract have been previously well defined and as such have been exploited for traditional oral drug delivery systems based on pH-, time-, microflora-, and pressure-mediated mechanisms for colonic-targeting in IBD treatment. After oral administration, these formulations release active drugs into the colonic lumen, where they are absorbed through the GI mucosa. Recently, the trend for such delivery systems has been towards the combination of more than one colon-targeting mechanism, such as CODES® (for 5-aminosalicylic acid) and TARGIT® (for budesonide) using both pH-responsiveness and microflora-mediated degradation [17].
To develop oral drug delivery systems specifically targeting and releasing drugs at the inflamed colonic mucosa instead of the colonic lumen, particular attention should be paid to the pathophysiological changes in the GI tract in IBD, including the increased intestinal tissue permeability, the accumulated positively-charged proteins, the overexpressed inflammatory molecules/receptors, and the shifted bacteria species (as well as their secretions). In addition, the drug activity needs to be factored in for targeted drug release. For example, siRNA drugs require a system for high cellular uptake and endosomal escape, while DNA drugs require nuclear localization [50]. Furthermore, premature drug release caused by acidic erosion or enzymatic degradation before reaching the site of inflammation needs to be prevented, which requires the judicious use of enteric coatings or degradation-resistant delivery systems.
2.2.2. Rectal administration
The fact that UC is confined to the colon and CD generally involves the ileum and colon [2] makes the colon the area most affected by IBD. Rectal administration of drugs, including the use of enemas, suppositories or foams, offers topical delivery and can provide local high concentrations of drugs for the treatment of distal colonic inflammation. Rectal administration of drugs is recommended as first-line treatment for patients with mild to moderate distal colitis, and is considered effective and feasible for both the induction and maintenance of remissions [51]. Compared to oral delivery and intravenous injection/infusion, rectal administration of drugs has been reported to induce better response rates, deliver higher concentrations of active drugs to the affected tissue, and lead to lower systemic drug exposure (potentially with reduced side effects). In the UC population, 50–60% of patients have proctosigmoiditis, 20–30% have left-sided colitis up to the splenic flexure, and 20% have pancolitis even though the disease is often most severe in the rectum [52]. However, rectal therapies are generally unpopular and remain underused, despite the recognized advantages rectal administration can provide [52].
The GI distribution of enemas and foams has been studied by y-scintigraphy, which has shown that enemas can reach up to or more proximally than the splenic flexure [53, 54], while foams spread more continuously in the rectum and sigmoid but not as far as enemas [55]. While enemas reduce the risk for systemic drug side effects, poor drug retention and limited medication adherence remain challenging. Topical therapies may have problems with leakage, retention, and bloating [52]. Serious complications such as rectal perforation are rare. Adherence to topical therapies is significantly lower than orally administered therapies, given the same dose regimen (32% vs. 60%) [56].
2.2.3. Injectable administration
Injectable administration of drugs, including subcutaneous (SC) injection and intravenous (IV) infusion, is less desirable compared with oral administration, as injections are associated with pain, require specialized personnel for their administration in the case of IVs or patient education for SC injections. Currently, anti-TNF-α antibodies, antibodies against integrins, and sometimes corticosteroids are administered through IV or SC injections [57]. IV administration provides high systemic bioavailability and reduces first-pass metabolism of drugs. However, the high systemic bioavailability inevitably increases the possibility of severe drug side effects. For example, IV administration of anti-TNF-α antibodies may suppress the immune system non-specifically. With the aid of delivery systems, drugs could circulate in the bloodstream and leave the circulation when the delivery systems specifically anchor to sites of disease and release their cargo locally. IV administration of drugs is particularly useful for IBD treatment when the disease manifests at extraintestinal locations, commonly including the joints, the skin, and the eyes [58]. However, if the disease is limited to the GI tract, oral and/or rectal drug delivery would be preferred, which would be guided by the anatomical location of the inflammation and the disease severity.
2.3. The Microenvironment at the Site of Inflammation
Traditional colon-targeting systems for IBD treatment, exploiting pH-, time-, pressure-, and microflora-triggered drug release, are associated with inconsistent efficacy in patients [59], which can be attributed to the diverse pathophysiological alterations in IBD and inter-patient variability in the GI tract. To develop drug delivery systems specifically targeting inflammation in IBD, it is important to consider the pathophysiological features at the site of inflammation, and harness them for the design of targeted drug delivery and release systems.
2.3.1. Mucus and Epithelium
Mucus is the first line of defense limiting the access of potential pathogens, toxins, hydrochloric acid, and digestive enzymes to the GI epithelium [60]. Studies have shown that the mucus layer is discontinuous and less well defined in the small intestine, with the tips of the villi not always covered. In contrast, there are two mucus layers in the stomach and colon: an inner “firm” layer of membrane-anchored mucin and an outer “loose” layer of secreted mucin [61]. The inner, adherent mucus adjacent to the epithelial cells contains high concentrations of antimicrobial molecules (e.g., defensins and secretory IgA) and provides a bacteria-free environment; however, the outer layer harbors bacterial populations and, partially owing to the degradation by bacterial hydrolytic enzymes, it has been partly solubilized, contributing to the mucus turnover [62]. The glycoarray presented by mucin allows selective attachment of strain-specific bacterial adhesins [63, 64]. The mucus barrier plays a crucial role in preventing enteric microflora and pathogens from colonizing the epithelial surface. The intestinal epithelium is covered by mucus, mainly composed of highly glycosylated MUC2 mucin [62]. In MUC2-deficient mice, spontaneous development of colitis was observed [65]. In active UC, a thinner mucus layer or regions devoid of mucus has also been observed [66, 67]. The reduction in mucus thickness in IBD has been linked to the depletion of goblet cells in affected colorectal mucosa, which reduces mucin production. Evaluation of biopsies from UC patients has indicated that the thickness of mucus and its spread decreases with increasing severity of inflammation [68]. The glycans on mucin are important for selection and maintenance of the intestinal microbiota, and the altered glycosylation and depletion of sulfation in UC increases mucin degradation by the microflora, in which shorter glycans lead to faster mucin degradation by bacteria and erosion of the mucus barrier [69]. A degraded mucus layer exposes the GI mucosa to higher levels of bacteria, leading to a vicious cycle [70, 71]. Conversely, an increased mucin secretion has been observed in CD in association with the nucleotide-binding oligomerization domain 2 (NOD2) [67, 72].
The intestinal epithelium is lined by a single layer of columnar intestinal epithelial cells (IECs) comprising several specialized cell types with distinct functions [73]. Goblet cells secrete the gel-forming mucins; Paneth cells secrete antibacterial peptides close to the IEC surface; and M cells sample the luminal antigens and present them to the underlying mucosal immune system [74, 75]. IECs also actively transport secretory IgA produced by plasma B cells in the lamina propria across the epithelial barrier into the intestinal lumen, to regulate commensal bacterial populations and maintain immune homeostasis [76, 77]. The TJs between IECs help to seal the paracellular space between IECs, and defects in TJs lead to increased epithelial permeability, which has been observed in IBD [78]. It has been suggested that the overexpressed cytokines such as TNF-α and IFNγ in the inflamed tissue can affect the regulation of the TJs [79]. To date, it is still not clear whether the loss of barrier function is a cause or a consequence of the intestinal inflammation in IBD. In health, IECs constitutively express toll-like receptor 3 (TLR3) and TLR5 basolaterally, while TLR2 and TLR4 are undetectable. In contrast, IECs significantly down-regulate the surface expression of TLR3 in CD, while strongly up-regulating the expression of TLR4 in both CD and UC [80]. In addition, up-regulation of NOD2 in IECs has also been observed in IBD, which can augment itself further in a feedback loop when the NF-κB cascade is activated [81, 82]. IECs from IBD patients have also been reported to express alternative co-stimulatory molecules that could transform them into antigen-presenting cells to activate T cells [83]; whereas others have suggested that IECs might also directly activate T cells in IBD through the expression of lectins and other carbohydrates on their surfaces [84, 85].
2.3.2. Immune cells
Separated by a single cell-layered epithelium from the lumen, the intestinal immune system - including the innate and the adaptive immune systems - functions to prevent the invasion of pathogens while remaining tolerant of food and commensal microorganisms [86]. The innate immune system comprises a diverse array of cell types including monocytes, macrophages, dendritic cells (DCs), and granulocytes such as neutrophils. Innate lymphoid cells are the most recently identified constituent of the innate immune system: they play an important role in the initiation of inflammation at barrier surfaces, contribute to the transition from innate to adaptive immunity, and promote chronic inflammation [87, 88]. In addition to M cells that sample luminal antigens, transepithelial DCs also sense and scan the lumen for invaders using their extended pseudopods across the epithelial lining. In active IBD, there is a pronounced infiltration of innate immune cells including neutrophils, macrophages, DCs, natural killer cells, and adaptive immune cells, including B cells and T cells, into the lamina propria [2]. Specifically, the infiltration of neutrophils and macrophages is considered a hallmark of the disease pathophysiology of IBD [74]. Neutrophils are recruited to the basal side of IECs in response to CXC-chemokine ligand 8 (CXCL8), which is produced by inflamed IECs [89]. Along the gradient of CXCL8, neutrophils translocate across the epithelial lining, reaching into the gut lumen, and exert their antibacterial function through increased expression of antibacterial proteins. Similarly, the inflamed microenvironmental factors drastically alter macrophage surface receptor expression and cytokine secretion: the infiltrating macrophages show elevated expression of surface marker CD14 and produce pro-inflammatory cytokines such as interleukin-23 (IL-23), TNF-α, and IL-6 in IBD; in contrast, under healthy conditions, macrophages lack CD14 expression and secrete anti-inflammatory molecules such as IL-10 [86]. (Please note that CD, when followed by a number, refers to “cluster of differentiation”, not to Crohn’s disease.)
Antigen-presenting cells, including DCs, present relevant antigens to T cells for effective adaptive immune response [90]. During the activation of the adaptive immune system, T cells differentiate into different subsets, including effector T cells that promote the inflammatory response and regulatory T cells (Tregs) that suppress inflammation. In IBD, DCs have an increased frequency of activation compared with their circulating, immature, and potentially tolerogenic counterparts in health. These latter DCs express CD103 and maintain intestinal homeostasis [91], while the former (activated, inflammatory) DCs up-regulate the expression of TLR4 and E-cadherin and produce IL-6 and IL-23 to promote T cell-mediated colitis [92]. Activated DCs lack regulatory capacity, repeatedly activate certain memory T cells, and fail to delete the overreactive T cell populations [93]. Consequently, the effector T cells become predominant over Tregs, and contribute to the chronic inflammatory state. The persistently activated T cells have also been postulated to disrupt the TJs between IECs, thereby increasing intestinal tissue permeability [94, 95]. In contrast to T cells, the subject of many studies, B cells are poorly understood, even though the gut is also massively infiltrated with B cells and plasma cells (effector B cells) in IBD. Significantly increased proliferation of immature plasma cells has been observed in the ulcer bases and inflamed mucosa in UC patients, and was considered to be an important source for the abnormal humoral immune responses, including autoantibody production in IBD [96]. A recent study showed that IBD-related inflammation is marked by mucosal accumulation of cytotoxic, granzyme B (GrB)-expressing CD19+ B cells and IgA+ plasma cells [97]. GrB is capable of inducing cell death in IECs, which possibly contributes to the IBD-associated epithelial damage [97]. Additionally, B cells were found to express elevated levels of IL-8 and TLR2 in IBD patients, which were correlated with disease activity [98].
2.3.3. Extracellular matrix
The extracellular matrix (ECM) is being increasingly recognized as a pivotal source of modulation of the immune response [99]. The ECM consists of numerous macromolecules such as collagens, elastins, proteoglycans, and non-collagenous glycoproteins that are not only essential structural components, but also exhibit important functional roles in the control of key cellular events such as adhesion, migration, proliferation, differentiation, and survival [100]. The ECM in the inflamed tissue is remodeled in a complicated manner, affected by the infiltrating immune cells and the activated tissue-resident cells. These cells release high concentrations of inflammatory regulators such as reactive oxygen species/reactive nitrogen species (ROS/RNS), proteases such as matrix metalloproteinases (MMPs), and cytokines such as IFNγ, TNF-α, and TGF-β [99]. The activation of inflammatory cells results in the production of large amounts of superoxide and nitric oxide from NAD(P)H oxidase (NOX2) and inducible nitric oxide synthase (iNOS), respectively. ROS/RNS are generated from the NOX enzymes and iNOS. Myeloperoxidase (MPO), which is primarily derived from infiltrating neutrophils and involved in the generation of hypochlorous acid, has also been implicated in the generation of ROS [101]. Additionally, esterases are up-regulated at the site of inflammation and their concentrations correlate with the degree of inflammation [102, 103].
The released inflammatory regulators not only have a crucial role in controlling intestinal inflammation [104], but also are capable of modulating a wide range of ECM molecules. The proteases, especially MMPs, selectively cleave ECM molecules into bioactive peptides that may activate immune cells or alter immune cell activities. For example, MMP8 (or MMP9) can cleave type I collagen and generate fragments that mimic CXCL8 to promote neutrophil recruitment [105, 106]. The fragments of ECM constituents, such as tenascin C isoform, fibronectin, and hyaluronan, can activate TLRs, especially TLR4 and TLR2, on resident immune cells and stimulate the secretion of pro-inflammatory cytokines and chemokines in the ECM [107, 108]. These activities, in turn, lead to the remodeled ECM affecting the intestinal immune system, and contribute to chronicity.
Inflammation of colonic mucosa is also accompanied by an in situ secretion and accumulation of positively charged proteins, such as transferrin [109], bactericidal/permeability-increasing proteins [110, 111], and antimicrobial peptides [112, 113]. Transferrin is an iron-carrying, circulation-borne protein; however, a large amount of transferrin has been observed in the inflamed colon [109]. This may be due to intestinal bleeding from the inflamed tissue, which stimulates a hepatic de novo generation of transferrin. Additionally, the increased mucosal permeability may allow transferrin to diffuse across the epithelium into the mucus layer. Owing to the lumen acidification in colitis, transferrin is positively charged in the inflamed colon. Other proteins contribute to the positive charges at the inflamed mucosal surface, including eosinophil cationic proteins secreted by eosinophils in CD lesions [114], bactericidal/permeability-increasing proteins secreted by neutrophils in UC lesions [111], and short bactericidal proteins such as β-defensins accumulated at the inflamed epithelium [112, 113]. Another factor that may contribute to the collective positive charge is the altered glycosylation of mucin in the inflamed colon, which lacks sulfation, thereby decreasing the net negative charges on mucin [69].
2.3.4. Microbiota
The human intestine is home to an estimated 1014 commensal bacteria, which is 10 times greater than the combined number of somatic and stem cells in the human body [115, 116]. The collective genome of intestinal microbes is estimated to contain at least 100 times more genes than the human genome [117]. Factors that affect community membership of microbiota include diet, inflammation, and host genotypes [118]. Microbiota typically includes bacteria, fungi, and viruses; here we focus on the bacterial component. It is increasingly being recognized that the microbiota is able to modulate the expression of host genes that participate in diverse and fundamental physiological functions [119]. For example, specific flagellin epitopes expressed by a minor constituent of the normal microbiota Clostridium spp. can induce effector rather than regulatory responses, posing a pathogenic risk [120], while probiotics and some bacterial products, such as polysaccharide A produced by Bacteroides fragilis [121], are able to control inflammation by increasing IL-10 production and the induction of Tregs [122–124].
Many studies have observed significant alterations in the microbiota of IBD patients compared with non-IBD controls, including the composition, diversity, and location [118]. There are four phyla dominating adult human intestinal habitats: Bacteroidetes (~ 67%), Firmicutes (~28%), Proteobacteria (2%) and Actinobacteria (1%). The rest show considerable inter-individual variations. Intestinal inflammation is typically associated with significant decreases in the frequencies of Bacteriodetes and Firmicutes, especially in Clostridium leptum and Clostridium coccoides groups [125, 126], which combat bacterial dysbiosis. In contrast, the frequencies of Proteobacteria and Actinobacteria are increased in IBD [118], especially Enterobacteriaceae, adherent invasive Escherichia coli, and Clostridium spp. [120, 127, 128]. In general, an overall decrease in microbial diversity and stability of the microbiota has been observed in IBD patients [129]. On average, IBD patients harbor 25% fewer microbial genes than non-IBD individuals, in accordance with the finding that the former have lower bacterial diversity than the latter [117]. CD and UC primarily occur in the colon (and distal ileum for CD), where the highest intestinal bacterial concentrations are found. The concentration of mucosally associated bacteria identified in inflammatory lesions in the intestine appears to increase with the severity of IBD [130].
Perturbation of the microbiota in inflammation can further contribute to the inflammatory state through disturbances of the microbiota metabolome. Enteric bacteria metabolize indigestible dietary components to provide useful products to the host. For example, Clostridia and Bacteroides preferentially produce butyrate, acetate, and other short chain fatty acids (SCFAs) that are energy substrates for colonic epithelial cells. These SCFAs have been implicated in the regulation of adaptive immune responses by stimulating the proliferation of Foxp3+IL-10-producing colonic Tregs, which help to protect the colon against colitis [131]. The decrease in Clostridia and Bacteroides manifests in reduced SCFA concentrations in fecal extracts of IBD patients [132]. In addition, the overgrowth of sulfate-reducing bacteria increases hydrogen sulfide production, which blocks butyrate utilization by colonocytes and potentially leads to epithelial nutrient deficiency [133]. These compositional and functional changes of the microbiota in IBD patients suggest that the intestinal microbiota likely plays an important role in the etiology and pathogenesis of IBD.
III. Nanoparticulate Drug Delivery Systems Targeting IBD
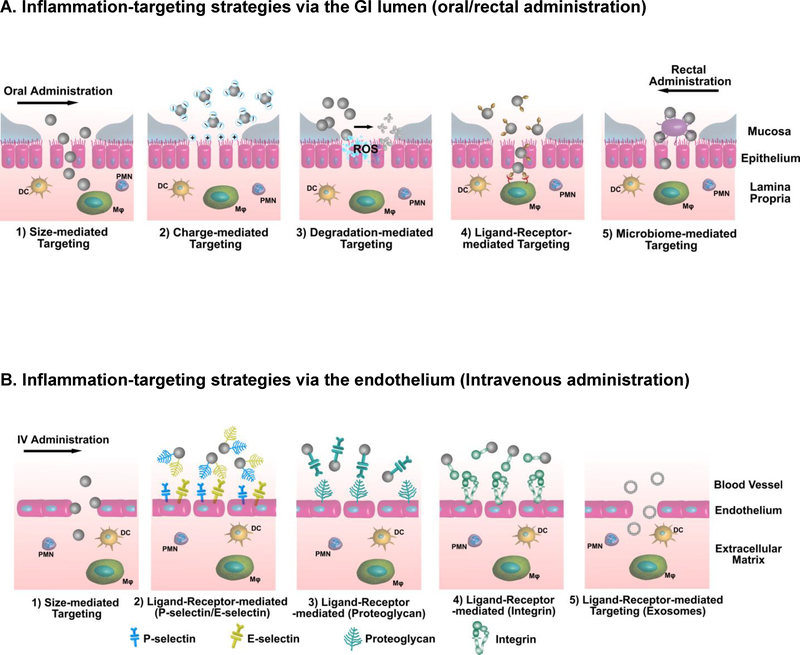
Targeted drug delivery in IBD has the potential to augment local drug concentrations in inflamed tissue, improve drug efficacy, decrease dosing frequency, and reduce drug side effects. NP systems targeting inflammation in IBD have been attracting increasing attention. In general, there are two main approaches to administer NPs targeting the site of inflammation in IBD - from the epithelium via oral or rectal administration and from the endothelium via IV administration (Fig. 1). The presence of NPs in the human body tends to initiate immunological responses upon detection by the host typically by the mononuclear phagocytic system (MPS). The size, shape, and surface chemistry of NPs contribute to the interaction of NPs with the MPS, which, in turn, strongly affects the fate of NPs after administration, including NP internalization, biodistribution, and clearance from the host. NP internalization by phagocytes is commonly considered as a “problem”, since NPs generally undergo rapid clearance after being internalized, which not only limits the targeting efficiency of NPs, but also potentially induces toxicological effects in the clearance organs such as spleen, kidney, and liver [134]. Therefore, NP systems have been intentionally designed to be “bioinvisible” to the host for a “stealth effect” (e.g., PEGylation [135]) to avoid the MPS recognition, prolong NP circulation time, and improve NP targeting efficacy. This utilization of the “stealth effect” still holds true for the IV administration of NPs targeting inflammation in IBD, since these NPs are expected to accumulate at the site of inflammation after circulating in the bloodstream (where avoiding MPS recognition is necessary). However, for oral or rectal administration of NPs targeting inflammation in IBD, NPs could be designed to specifically harness this host recognition system in order to maximize the NP uptake by the infiltrating immune cells, thereby augmenting the NPs’ targeting efficiency. Below we discuss different mechanisms employed by current NP systems to achieve inflammation-targeting drug delivery in IBD (Table 3 summarizes drugs loaded in the NP systems discussed).
Figure 1.
Delivery strategies and targeting motifs for inflammation-targeting NPs in IBD. NPs employ different targeting mechanisms for efficient delivery to the site of action to maximize local drug concentration, improve drug efficacy, and minimize systemic drug side effects. (A) Through oral or rectal administration, NPs target the inflamed colon from the epithelium by mechanisms mediated by size, charge, degradation, ligand-receptor, and microbiome. (DC: dendritic cells; MΦ: macrophages; PMN: polymorphonuclear leukocytes; ROS: reactive oxygen species;) (B) Through intravenous administration, NPs target the inflamed colon from the endothelium via mechanisms mediated by size and various ligand-receptor interactions including the selective homing specificity of exosomes.
Table 3.
Drugs delivered by NPs to the site of inflammation in IBD.
| Drugs | NP System | Surface Charge | Mechanism | Route | model | Ref. |
|---|---|---|---|---|---|---|
| Small-molecule drugs | ||||||
| 5-amino salicyclic acid | Covalently bound to PCL | 200 nm (emulsion) −1.2 ± 1.5 mV; 350 nm (nanoprecipitation) −2.5 ± 2.5 mV | Size | Oral | TNBS mice | [210] |
| Budesonide | Nanostructured lipid carrier (NLCs) | 204.1 ± 8.7 nm −42.2 ± 7.2 mV | Size | Oral | DSS mice | [139] |
| Superoxide dismutase/ 4-amino tempol/ catalase | HSPC:HSPG: cholesterol = 4:2:3 | 400 nm −66 ± 5 mV | Charge | Rectal | DNBS rat | [143] |
| Methotrexate | Grapefruit-derived nanovesicles (GDNs) | 210.8 ± 48.62 nm −49.2 to −1.52 mV | Ligand-receptor | Oral | DSS mice | [162] |
| Rolipram | PLGA NPs | 332 nm 474 nm | Size | Oral | TNBS rat | [137] |
| Doxorubicin | DSPC:Chol:BPCNe | 100 ± 10 nm Not reported | Ligand-receptor | I.V. | B-cellB-cell lymphoma in mice | [158] |
| Protein drugs | ||||||
| Leukemia inhibitory factor | Anti-CD4 modified PLGA NP | 165 −170 nm Not reported | Ligand-receptor | Infusion | Heart allograft transplant ation in | [157] |
| TGF-β1 | Exosomes from TGF-β1 gene-modified dendritic cells | 50 – 100 nm Not reported | Ligand-receptor | IV. | DSS mice | [161] |
| Ovalbumin (model protein) | Mannose modified PEG-PCL | 212 ± 8 nm −7 ± 2 mV | Ligand-receptor | N.A. (Ex vivo) | DSS mice | [140] |
| siRNA and Antisense oligonucleotide | ||||||
| Antisense oligonucleotide against TNF-α | Cationic konjac glucomannan (cKGM) | ~200 nm +16.7 mV | Ligand-receptor | Oral | DSS mice | [189] |
| miRNA-155 inhibitor | B cell-derived exosomes | 30 – 100 nm Not reported | Ligand-receptor | DSS mice | [211] | |
| TNF-α siRNA | TPP-PPM | 211 – 275 nm Not reported | Ligand-receptor | Ex vivo | DSS mice | [153] |
| CD98 siRNA | CD98siRNA/ CD98 antibody modified urocanic acid/PEI NP | 211 – 275 nm Not reported 147 – 261 nm +7.9 to +17.3 mV |
Ligand-receptor | Oral | DSS mice CD4+CD4 5RBhi T cell transfer colitis | [152] |
| Cyclin D1 siRNA | Liposome-based, β7 integrin-targeted NP | ~ 150 nm −24 to −18 mV | Ligand-receptor | I.V. | DDS mice | [150] |
| Antisense oligonucleotide against TNF-α | Galactosylated low molecular weight chitosan | Not reported | Ligand-receptor | Rectal | TNBS mice CD4+CD4 5RBhi T cell transfer colitis mice | [154] |
| Map4k4 siRNA | Galactosylated trimethyl chitosan- cysteine/TPP NPs | 140 –160 nm +20 to +42 mV | Ligand-receptor | Oral | DSS mice | [155] |
| TNFα siRNA | PLA-PEG NP bearing Fab’ portion of F4/80 antibody | 376 ± 19 nm ~+ 2.56 mV | Ligand-receptor | Oral | DSS mice | [156] |
| Map4k4 siRNA | β1,3-D-glucan MP | 2–4 μm Not reported | Ligand-receptor | Oral | LPS-induced lethality | [212] |
| TNF-α siRNA | Thioketal NPs | 600.1 ± 281 nm 5.84 ± 0.8 mV | Degradation | Oral | DSS mice | [165] |
| Probiotic | ||||||
| Lyophilized probiotic bacteria (L. casei ATCC 39392) | Chitosan-coated PLGA NPs | 226.3 ± 0.97 nm 25.65 ± 7.02 mV | Degradation | Oral | TNBS rat | [172] |
3.1. Size-mediated targeting
As vascular permeability and epithelial permeability are increased in IBD [25], size-mediated targeting is the most commonly used mechanism for NPs targeting the inflamed colon. Lamprecht et al. reported a size-dependent accumulation of NPs in the inflamed intestine by comparing fluorescent polystyrene particles at sizes of 10 μm, 1 μm, and 100 nm (in diameter, likewise below) [136]. The particles were orally gavaged to healthy and trinitrobenzenesulfonic acid (TNBS)-induced colitis rats, and the 100 nm particles demonstrated the highest attachment to inflamed colons compared to healthy colons among the particles tested. In a subsequent study, Lamprecht et al. demonstrated a prolonged anti-inflammatory effect and reduced systemic absorption of rolipram-loaded poly(lactic-co-glycolic acid) (PLGA) NPs (470 nm and 330 nm, respectively), in comparison with rolipram solution in a TNBS-induced colitis model in rats [137]. Later, in vivo fluorescent imaging showed the localization of 200 nm budesonide-loaded PLGA NPs at the inflamed colon in oxazolone-induced colitis in mice [138]. Nanostructured lipid carriers (NLCs) were used to investigate the efficacy of budesonide in dextran sulfate sodium (DSS)-induced colitis in mice [139]. The budesonide-loaded NLCs decreased neutrophil infiltration, reduced the levels of IL-1β and TNF-α in the colon, and maintained the mucosal architecture of the colon in DSS-induced colitic mice, compared with DSS-induced colitic mice treated with budesonide solution or phosphate buffered saline (PBS).
However, size-mediated targeting results in passive accumulation of NPs at the site of inflammation, which is less likely to be a dominating factor when NPs interact with tissue and/or cells in the inflamed colon; other factors such as internalization of NPs by the significant number of local immune cells may also importantly affect the outcome. Coco et al. compared the targeting of NPs prepared from different strategies to the inflamed mouse colon, including PLGA NPs (~180 nm), peptidic ligand grafted PLGA NPs (~210 nm), mannosylated PLGA NPs (~210 nm), trimethylchitosan NPs (~340 nm), and Eudragit® S100 NPs (~620 nm) [140]. The mannosylated PLGA NPs that could interact specifically with the mannose receptors on macrophages showed the highest accumulation (2–3 fold higher than other formulations) in the inflamed colon. Similarly, another study compared the internalization of denatured albumin NPs with native albumin NPs (both of ~100 nm) by adherent neutrophils in the inflamed endothelium; the results suggested that the denatured albumin NPs were internalized specifically via surface Fcy receptors by the adherent neutrophils, while there was no internalization of native albumin NPs of the same size [141]. These denatured albumin NPs loaded with drugs blocking β2 integrin signaling were able to specifically detach the adherent neutrophils and inactivate them, thereby inhibiting inflammation. Compared with microparticles, NPs may penetrate deeper into the inflamed tissue rather than remaining at the mucosal surface. One recent study has compared the targeting effect of fluorescently labeled PLGA particles of 250 nm and 3 μm, respectively, to rectal mucosa in human IBD patients and healthy controls [27]. A significantly higher accumulation of 3 μm particles in the ulcerous lesions was found than with the 250 nm NPs, which were only observed in traces at the mucosal surface. Further analysis of biopsies taken from these patients and controls indicated that the majority of 250 nm NPs penetrated the tissue and translocated to the “serosal” compartment. Although it was attributable to the translocation of NPs to a deeper tissue compartment in human biopsy studies, the discrepancy of NP accumulation in rodent colitis models and human patients suffering from IBD reveals significant species difference in size-mediated targeting and demands further studies to bridge the gap between data obtained from animal models and human patients, including the use of large animal models. Furthermore, evaluations of therapeutic efficacy are needed for drugs delivered by these particles of different sizes in animal models of IBD.
3.2. Charge-mediated targeting
Inflammation of the colonic mucosa is accompanied by depletion of the mucus layer and in situ accumulation of positively charged proteins, which results in the buildup of positive charges at the damaged epithelium and provides a molecular target and anchor for drug carriers with negative surface charges. The earlier observation of negatively charged drug carriers preferentially binding to the inflamed colon was reported by Jubeh et al., in which they used colon explants isolated from rats with DNBS-induced colitis to study the differential adhesion of neutral, cationic, and anionic liposomes to inflamed colons compared with healthy colons [142]. The results showed that anionic liposomes preferentially adhered to the inflamed colonic mucosa (2-fold higher than neutral or cationic liposomes), while cationic liposomes preferentially adhered to the healthy colonic mucosa (3 times higher than neutral or anionic liposomes). The authors then tested the treatment of DNBS-induced colitis in rats using antioxidants delivered by anionic liposomes [143]. They showed that antioxidants delivered by anionic liposomes were more effective than free antioxidants in the treatment of colitis, which was attributed to the preferential adhesion of negatively charged liposomes to the inflamed colon, and consequently, a longer residence time and better uptake of antioxidants by the inflamed epithelium.
Under certain conditions, the optimization of surface charges of particles could precisely target a particular cell population and effectively treat the disease, without the need for functionalization of particles using molecular ligands. Getts et al. demonstrated that negatively charged microparticles, not positively charged or neutral ones, were specifically engulfed by inflammatory monocytes leading to monocyte apoptosis, thereby markedly improving disease symptoms in multiple inflammatory disorders in animal models, including DSS-induced colitis [144]. Recently, we have developed a hydrogel microfiber system prepared from ascorbyl palmitate, a “generally recognized as safe” (GRAS) material, for local drug delivery in IBD [145]. The preferential adhesion of negatively charged hydrogel microfibers to inflamed mucosa was tested in two murine colitis models, T-bet−/−Rag2−/− ulcerative colitis and DSS-induced colitis, as well as in tissue biopsies from UC patients. Administered via enema, dexamethasone-loaded hydrogel microfibers were therapeutically more efficacious and resulted in less systemic drug exposure than free dexamethasone solution.
3.3. Ligand-receptor mediated targeting
During the inflammatory process, certain ligands or receptors are overexpressed on cell surfaces, which offer molecular targets for anchoring drug carriers through specific interactions. One example of the ligand-receptor interaction is inspired by the leukocyte-endothelial biochemistry that mediates selective recruitment of leukocytes to the site of inflammation [146]. Upon inflammation, endothelial cells are activated by inflammatory cytokines to up-regulate the expression of adhesion molecules such as P-selectin, E-selectin, vascular cell adhesion molecule (VCAM)-1, and intercellular adhesion molecule (ICAM)-1 on their luminal surfaces. While on the surfaces of leukocytes, ligands that participate in rolling and mediate firm leukocyte adhesion are expressed at elevated levels: carbohydrate glycoconjugates such as P-selectin glycoprotein ligand-1 (PSGL-1), which mainly binds to P-selectin to initiate leukocyte binding to vascular endothelium, and also binds to L-selectin to mediate neutrophils rolling on adherent neutrophils [147]; and integrins, such as α4β7, α4β1, αLβ2, and αMβ2 [146], which bind avidly to the adhesion molecules overexpressed on inflamed endothelial cells. To mimic the leukocyte-endothelial biochemistry and design targeted systems to the site of inflammation, Sakhalkar et al. synthesized PEGylated PLA particles conjugated with recombinant PSGL-1, which demonstrated significant rolling adhesion to the inflamed endothelium in vivo [148]. Similarly, another study utilized a carbohydrate Sialyl LewisX (sLex), which binds to both P- and E-selectins, attaching to the surface of PLGA spheres to mimic leukocyte adhesion on selectins [149]. However, neither of the particles discussed above were loaded with drugs for therapeutic evaluation in IBD. In contrast, Peer et al. developed liposome-based, β7 integrin-targeted NPs (β7 I-tsNPs) entrapped with siRNAs to target specific subsets of leukocytes and treat DSS-induced murine colitis through silencing Cyclin D1 (CyD1), a cell cycle-regulating molecule aberrantly up-regulated in both epithelial and immune cells in IBD [150]. The β7 I-tsNP-delivered CyD1-siRNA led to a drastic reduction in intestinal tissue damage, a potent suppression of leukocyte infiltration into the colon, and a reversal in body weight loss and hematocrit decrease.
Besides adhesion molecules expressed on the endothelial cells, other ligands/receptors are up-regulated on colonic epithelial cells and/or immune cells in IBD, and have been applied for NP targeting to the inflamed intestine through ligand-receptor interactions. Expression of glycoprotein CD98 is increased in colonic epithelial cells and infiltrating immune cells in the inflamed state. To target such cells, CD98 Fab’-bearing PLA NPs [151], and single-chain CD98 antibody conjugated chitosan/polyethyleneimine (PEI) NPs [152] were synthesized, respectively, for treatment of colitis in mouse models. Targeting macrophages through the overexpressed surface receptors/ligands has also been intensively studied in NP-based therapies in IBD, for instance, the mannose receptors have been targeted by mannosylated poly(amido amine)-based NPs [153]; the macrophage galactose/N-acetyl galactosamine-specific lectins (MGL) by galactosylated chitosan NPs [154, 155]; and the F4/80 membrane proteins by the Fab’ portion of F4/80 antibodies-grafted-PLA-PEG NPs [156]. Furthermore, T cells and B cells have been targeted through the antibody/carbohydrate-surface protein interaction, although current studies focus more on transplantation and cancer applications. Anti-CD4 mAB-coated PLGA NPs were utilized to deliver a leukemia inhibitory factor to CD4+ T cells, which promoted the development of CD4+CD25+Foxp3+ Tregs in a murine transplantation model [157]. BPCNeuAc-pegylated liposomal NPs were synthesized as a carbohydrate recognition-based approach to target CD22 on B cells for the treatment of B-cell lymphoma in mice [158, 159].
The third approach employing the ligand-receptor interaction is utilizing biologically derived exosomes, which are naturally occurring nanovesicles (30–100 nm) consisting of a lipid bilayer membrane and play a major role in intercellular communication. In addition to passive targeting to the site of inflammation through the enhanced permeability effect (EPR) effect, exosomes bear specific surface proteins originating from their parent cells, and have a highly selective homing specificity for active targeting [160]. Cai et al. found that exosomes derived from TGF-β1 gene-modified DCs could specifically interact with T cell subsets, induce the CD4+Foxp3+ Tregs, and decrease the proportion of Th17 cells at inflammatory sites, thereby inhibiting the development of DSS-induced colitis in mice [161]. Nanovesicles derived from plants, such as grapefruit, were harnessed for macrophage targeting, in which these vesicles facilitated their internalization by macrophages via both macropinocytosis and clathrin-dependent pathways due to the enrichment of phosphatidylethanolamine and phosphatidylcholine on the nanovesicles’ surfaces [162].
3.4. Degradation-mediated targeting
Specific molecules or enzymes present in the ECM or microbiota in the inflamed colon can digest the delivery systems locally, leading to degradation-mediated targeting to the site of inflammation. Substantial evidence suggests that an overproduction of ROS is associated with chronic intestinal inflammation, and that the elevated mucosal ROS concentrations are confined to the site of inflammation and correlated with the disease progression [163, 164]. Wilson et al. developed thioketal NPs that are degraded in response to ROS at the site of inflammation to release TNF-α siRNA locally for the treatment of DSS-induced colitis in mice [165]. Polyesters bearing boronic ester-triggering groups have also been utilized to synthesize NPs that were degraded upon exposure to hydrogen peroxide for drug delivery specifically to inflamed tissue [166]. Proteolytic enzymes such as MMPs are known to be up-regulated in the inflamed ECM. It is postulated that MMP3 and MMP12 in the inflamed mucosa in IBD patients may degrade the anti-TNF-α antibodies and contribute to non-responsiveness to such treatment [167]. MMP-sensitive delivery systems have been synthesized [168 and could be used to protect biologics and target inflammation in IBD. Furthermore, a growing number of examples indicate that specific ECM components such as hyaluronan could have a dramatic effect on disease phenotypes in IBD [169], which warrants studies on NP systems targeting such ECM components for IBD treatment.
A wide range of reductive enzymes and hydrolytic enzymes are secreted by bacteria in the colon, creating enzyme-based fermentation systems. For example, high levels of polysaccharidases of microbial origin present in the human colon [170, 171] have motivated extensive studies of polysaccharide-based delivery systems, including the utilization of pectin, guar gum, chitosan, and amylose [47]. Chitosan-coated PLGA NPs were utilized to deliver probiotic extracts to the inflamed colon for the treatment of TNBS-induced colitis in rats [172]. However, the composition and concentration of microbiota are significantly altered in IBD, requiring the identification of dominant species and associated enzymes for the development of such targeted delivery systems in IBD. Recently, the small subunit ribosomal RNA (16s rRNA)-based terminal restriction fragment length polymorphism (T-RFLP) techniques have been applied to provide a “snapshot” of the complex bacterial population of the gut in real time for comparative analysis [173]. In light of such technology advances, the colitis-associated bacteria can be identified, and enzymes secreted by these bacteria can be harnessed to specifically degrade NP systems thereby enabling drug release by the inflammatory dysbiotic state, which may provide novel approaches for IBD treatment.
3.5. Microbiota-mediated targeting
In IBD, the host-bacterial interaction tends to be detrimental, leading to pathological inflammation, rather than beneficial, helping to maintain controlled physiological inflammation. Prebiotics support the growth of probiotics and other beneficial microbes, and probiotics may introduce desirable microbes [174]. The interaction between gut microflora and host may be manipulated to change from detrimental back to beneficial to restore intestinal homeostasis. Genetically engineered probiotic bacteria have been developed to colonize a specific niche in the colon where they grow and subsequently secrete biologically active proteins to produce a desired pharmacological response. Lactococcus lactis has been used to deliver an anti-inflammatory cytokine IL-10 [175], immunomodulatory Yersinia LcrV protein [176], and cytoprotective molecule trefoil factors [177 to treat colitis in vivo in mice. Besides Lactococcus lactis, a diverse spectrum of engineered bacteria strains, such as Streptococcus gordonii [178], Lactobacillus casei BL23 [179], and Lactococcusplantarum [180], have been used to deliver various molecules to treat colitis in animal models. However, safety concerns were raised regarding the amount of proteins produced and released in vivo, which motivated studies to add controllers as a safety measure to the delivery systems. For example, engineered Bacteroides ovatus was used to deliver TGF-β under the control of a plant polysaccharide xylan by adding the xylanase promoter to the xylan operon of the Bacteroides ovatus to treat DSS-induced colitis in mice [181]. Alternatively, a stress-inducible controlled expression system was used in Lactococus lactis to regulate the IL-10 production and delivery for treatment of DNBS-induced murine colitis [182].
Novel strategies have also harnessed proteins on bacteria surfaces as docking stations for NPs to deliver drugs. The simultaneous use of NPs and bacteria to deliver drugs in vivo has been demonstrated by plasmid DNA-loaded streptavidin-coated polystyrene NPs attaching to biotinylated bacteria surfaces termed “microbots” [183]. In such systems, the Listeria monocytogenes bacteria are about 1 μm in length, and their surfaces were equipped with NPs ranging in size of 40 – 200 nm. Mice injected with these “microbots” successfully expressed the loaded genes in vivo, as demonstrated by luminescence in different organs.
IV. Hybrid Drug Delivery Systems Targeting IBD
Oral drug delivery is the preferred route of administration. However, limitations including premature drug release in the upper GI tract or lack of targeting to the site of disease can prevent efficient use of oral drug delivery systems. To protect the loaded drugs and allow for selective targeting, hybrid drug delivery systems have been developed to load drugs in the interior of NPs, and then embed the drug-loaded NPs in a protective external compartment such as a microsphere (NP-in-MP) or a hydrogel (NP-in-hydrogel). This external compartment typically consists of a material that dissolves or degrades specifically in the colon, where the embedded NPs are liberated to target the site of inflammation and release drugs locally (Table 4).
Table 4.
Drugs delivered by hybrid systems to the site of inflammation in IBD.
| Drugs | NP System | External Compartment | Targeting Mechanisms | Colitis model | Ref. |
|---|---|---|---|---|---|
| Plasmid DNA encoding IL- 10 | Gelation NP | PCL MP | PCL slow degradation and gelatin NP size- mediated targeting | DSS mice | [185] |
| TNF-α siRNA | Gelation NP | PCL MP | PCL slow degradation and gelatin NP size- mediated targeting | DSS mice | [186] |
| Tacrolimus | PLGA NP | Eudragit P- 4135F MP | pH-sensitive microspheres and PLGA NP size-mediated targeting | TNBS rat | [184] |
| TNF-α siRNA | TNF-α siRNA/ PEI NP | polylactide (PLA) NPs covered with polyvinyl alcohol (PVA) | PVA shell and PLA matrix structure prevent quick degradation of NPs and PEI/siRNA NP size- mediated targeting | LPS-treated mice | [188] |
| Antisense oligonucleoti de against TNF-α | Cationic konjac glucomannan (cKGM)/anti- TNF-α nucleotides NP | cKGM/phytag el MPs | Polysaccharide microparticles degraded by bacterial enzymes in the colon and mannose ligand-receptor mediated targeting | DSS mice | [189] |
| Lys-Pro-Val (KPV) | PLA NP | Alginate and chitosan hydrogel | Polysaccharide hydrogel degraded by bacterial enzymes in the colon and PLA NP size-mediated targeting | DSS mice | [190] |
| Prohibitin 1 | PLA NP | Alginate and chitosan hydrogel | Polysaccharide hydrogel degraded by bacterial enzymes in the colon and PLA NP size-mediated targeting | DSS mice | [191] |
| CD98 siRNA | CD98 siRNA/ PEI NP | Alginate and chitosan hydrogel | Polysaccharide hydrogel degraded by bacterial enzymes in the colon and PEI/siRNA NP size- mediated targeting | DSS mice | [192] |
| CD98 siRNA | CD98siRNA/ CD98 antibody modified urocanic acid/PEI NP | Alginate and chitosan hydrogel | Polysaccharide hydrogel degraded by bacterial enzymes in the colon and CD98 antibody ligand- receptor mediated targeting | CD4+CD45RB hlgh T cell transfer colitis and DSS mice | [152] |
The MPs in NP-in-MP systems are commonly formulated using pH-sensitive polymers, slowly degradable polymers, or polysaccharides to cope with the acidic environment of the stomach. One study reported that tacrolimus-loaded PLGA NPs were entrapped in pH-sensitive MPs, prepared from Eudragit P-4135F, to treat TNBS-induced colitis in rats [184]. Rats with colitis were orally administered drug-loaded NP-in-MP, drug-loaded NP only, drug-loaded MP only, or free drug solution. Significantly lower colon/body ratio and MPO activity were observed in the drug-loaded NP-in-MP group, but not the remaining experimental colitis groups. Alternatively, slowly degradable polymers such as PCL have been used to prepare PCL MPs loaded with gelatin NPs for delivery of a spectrum of therapeutics, including IL-10-expressing plasmid DNA [185], TNF-α siRNA [186], and dual delivery of TNF-α siRNA and CyD1 [187]. For nucleic acid delivery, siRNA or plasmid DNA have been typically complexed with cationic polymers or polysaccharides to form nanocomplexes (i.e., NPs), which were then loaded into MPs to target colonic inflammation. For example, TNF-α siRNA/PEI nanocomplexes in PVA MPs were used to treat colonic inflammation in LPS-treated mice [188], and anti-TNF-α nucleotides/cationic konjac glucomannan (cKGM) nanocomplexes in cKGM/phytagel MPs were employed to target mannose receptors on macrophages in DSS-induced colitis in mice [189].
The hydrogels in NP-in-hydrogel systems are often formulated using the electrostatic interactions of calcium or sulfate ions to crosslink alginate and chitosan that are degraded by colonic enzymes. An anti-inflammatory peptide Lys-Pro-Val (KPV) was encapsulated in PLA NPs, which were then embedded in an alginate/chitosan hydrogel to treat DSS-induced murine colitis [190]. Mice given DSS followed by KPV in NP-in-hydrogel were protected against inflammation, compared with mice given DSS only. The same system was also tested for delivering a biologically active protein, Prohibitin 1 (PHB), to epithelial cells in DSS colitis in mice, in which mice treated with PHB-loaded NPs-in-hydrogel exhibited an increased level of PHB in the colonic epithelial cells and reduced severity of DSS-induced colitis [191]. Besides peptide/protein delivery, the alginate/chitosan hydrogel was also used to deliver NPs loaded with CD98 siRNA [152, 192] through the addition of PEI for complexation. The alginate/chitosan polysaccharide hydrogel seemed to provide protection for the biologics-loaded NPs to achieve site-specific action for reduced levels of inflammation in animal models of IBD.
V. Conclusions and Future Directions
IBD is a global disease with steadily increasing incidence in the Western world, and gradual emergence and expansion in the newly industrialized countries in Asia, South Africa, and the Middle East [4]. The primary intestinal sites of pathology provide a strong case for drug delivery targeting the inflamed colon in the treatment of IBD. Engineering materials at the nanoscale enables the combination of several beneficial features into one multicomponent, multifunctional NP system. Recent years have witnessed an exponential increase in the number of studies employing NPs for the treatment of IBD in animal experiments, although none of them have yet entered clinical trials. Developing robust NP systems that are able to specifically target the site of inflammation and release drug locally to treat IBD can address this unmet medical need. The size, shape, and surface chemistry of NPs each play a role in determining the performance and specific targeting of NPs towards the site of inflammation and, in an integrated manner, these factors affect the overall targeting effect. NP systems hold great promise in improving drug therapeutic efficacy, reducing side effects, and promoting patients’ adherence to medication, which could provide novel therapeutics that may revolutionize the management of IBD and open a new era for IBD treatment.
Advances in the understanding of IBD pathophysiology, immunology, and gut microbiome will facilitate the development of novel drug delivery systems targeting IBD, given the complexity at the site of inflammation. First, the identification of relevant ligands at the site of inflammation through understanding IBD pathophysiology will help in developing effective mechanistically-based targeting approaches. Previous studies on mucoadhesive NPs have primarily investigated the interaction of NPs with the mucus layer under healthy conditions. Future investigation of the interaction between NPs and mucin from the GI mucosa with evidence of active inflammation stands to increase our understanding and potentially provide novel drug delivery approaches. Recently, it was reported that smaller glycans are at higher frequencies in UC patients than in healthy individuals due to decreased sulfation and reduced complexity of glycans on MUC2 mucin in IBD [70]. Glycan ligands have been documented for targeting glycan-binding proteins expressed specifically on activated immune cells or inflamed endothelial cells. Therefore, strategies targeting altered mucin glycans present at the site of inflammation would be of great interest in IBD treatment. Secondly, employing NPs to selectively regulate immune response would potentially promote intestinal homeostasis in IBD treatment. The autologous Tregs infusion therapy has been explored in the treatment of IBD patients, but it was hindered by problems with homing to inflamed tissue and apoptosis of the infused Tregs [193]. NPs loaded with drugs that modulate the expression of “homing molecules”, such as CCR9, α4β7, or GPR15, would be useful to target the infused Tregs to inflamed colon; and drugs that block the proinflammatory cytokines can improve the survival of Tregs after infusion [194, 195]. Such NPs infused together with Tregs may enhance the success of this therapy. Also, NP systems that are able to stimulate the generation of tolerogenic DCs [196] or Tregs [197] in vivo would be beneficial in shifting the immunity towards homeostasis. Furthermore, T cell genome engineering with CRISPR/Cas9 [198] holds great promise in cell-based therapies for many immune-related diseases including IBD, and the combination of such tools with NPs could provide completely new genetic regulatory approaches to reprogram the aberrant cell signaling in IBD. Thirdly, a better understanding of the composition, diversity, and function of gut microbiota is becoming increasingly relevant to the management and treatment of IBD, and has the potential to inform inflammation-targeting strategies. Characterization of the difference between healthy and diseased conditions in microbiota is ongoing through large-scale projects such as the European Metagenomics of the Human Intestinal Tract (metaHIT) and the U.S. Human Microbiome project (HMP) [199]. The identification of antigenic components of the microbiota in the inflamed intestine, specifically the dominant bacterial species residing at the site of inflammation associated with the pathogenesis of IBD and disease development, could lead to future microbiome-mediated NP delivery systems targeting the pathogenic bacteria or responding to secretions from such bacteria, thereby increasing the treatment efficacy in IBD.
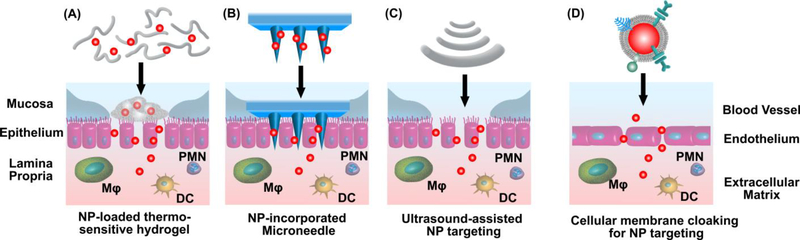
The versatility of NP systems enables the incorporation of multiple drugs and different targeting modalities, as well as biomimetic components to enhance the inflammation-targeting effect and treatment efficacy, which appears to be an avenue worth pursuing. The delivery of multiple drugs using NPs can integrate anti-inflammatory actions across multiple pathways to treat IBD. Infliximab together with azathioprine has been investigated in clinical trials for combination therapies, and was shown to be superior to either drug alone in both UC and CD [200, 201]. The loading of such drug combinations in NPs could reduce the non-specific immunosuppression of these drugs and further improve drug efficacy. NPs can also be aided with different targeting modalities to additionally augment the targeting effect (Fig. 2), such as thermo-sensitive hydrogels [202], ultrasound-assisted delivery [203], and microneedle-based systems [204, 205]. In addition to synthetic NPs, biomimetic NPs have been developed employing components derived from human immune cells such as neutrophils [206], platelets [207], and macrophages [208], which selectively target the inflamed vasculatures in animal models, mimicking the endogenous inflammation-targeting mechanisms. Such biomimetic NPs combine the unique functionalities of cellular components and the capability of nano-engineering to manipulate materials at the nanometer scale for effective delivery of therapeutic agents. This approach could be applied for targeted delivery in IBD.
Figure 2.
NP incorporation with multiple modalities to target inflammation in IBD. (A) Thermo-sensitive hydrogels, (B) Microneedle-based delivery systems, (C) Ultrasound-assisted drug delivery, and (D) Cellular components derived from human immune cells mimicking endogenous inflammation-targeting mechanisms. These systems in combination with NPs have the potential to further augment the targeting effect of NPs to the site of inflammation, thereby improving efficacy in IBD treatment.
Targeting is the first step; the second step is how to advance to clinical trials. Further elucidation of the in vivo fate of NPs in animal models may facilitate the translational aspect of NPs towards clinical trials. Very little is known about the fate of NPs after they reach the site of inflammation, and therefore the mode of local drug release is unclear. Whether NPs being degraded by enzymes secreted under the inflammatory condition resulting in intercellular drug release, or NPs being internalized by phagocytes leading to intracellular drug release is not clear. Consequently, the possible correlations between these two events, with respect to size, shape and surface chemistry of NPs, have not been investigated thoroughly in animal studies. The potential effects of NPs and their individual or degraded components on the immune system and general toxicity remain largely unknown. In vivo studies aiming to examine these processes are challenging, but the findings will provide invaluable information for a comprehensive, mechanistic understanding of the complex and differential subcellular effects of NPs in the inflamed microenvironment, which will greatly aid the design of targeted NP delivery systems and improve the clinical reliability and translation of such systems. Acting at the site of disease, inflammation-targeting NP systems could, in the near future, revolutionize the regimen for the treatment of IBD.
Research Highlights.
IBD pathophysiologic characteristics present unique challenges and opportunities in drug delivery
Inflammation in the GI tract provides instrumental cues for drug targeting
Nanoparticulate systems can employ a broad range of mechanisms to target IBD inflammation
Advances in disease understanding and rational design could result in optimal targeting of IBD
Acknowledgements
The authors thank Dr. Aaron C. Anselmo and Dr. Vikash P. Chauhan for their critical reading of the manuscript. This work was supported in part by a Research Fellowship Award from the Crohn’s & Colitis Foundation of America (to S.Z.), NIH Grant EB-000244, and the Max Planck Research Award, Award Ltr Dtd. 2/11/08, Alexander von Humboldt-Stiftung Foundation (to R.L.) and the Division of Gastroenterology, Brigham and Women’s Hospital (to G.T.).
Author’s Biographical Sketch
Sufeng Zhang, Ph.D.

Dr. Sufeng Zhang is a postdoctoral fellow in the laboratory of Institute Professor Robert Langer at the Massachusetts Institute of Technology. Dr. Zhang’s postdoctoral work focuses on targeted drug delivery for treatment of gastrointestinal disorders, particularly inflammatory bowel disease. She received her Ph.D. in Chemical Engineering from the Department of Chemical and Materials Engineering at the University of Alberta in Edmonton, Canada. Dr. Zhang’s research is focused on understanding the biological changes associated with disease types and disease states, and applying these pathophysiological features as cues in systems for drug delivery and tissue regeneration. Dr. Zhang has received several awards, including postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Crohn’s and Colitis Foundation of America (CCFA).
Robert Langer, Sc.D.

Robert Langer is the David H. Koch Institute Professor at MIT (there are 13 Institute Professors at MIT; being an Institute Professor is the highest honor that can be awarded to a faculty member). He has written nearly 1,380 articles and has over 1,130 issued and pending patents worldwide. His many awards include the US National Medal of Science, the US National Medal of Technology and Innovation, the Charles Stark Draper Prize (considered the engineering Nobel Prize), Albany Medical Center Prize (largest US medical prize), the Wolf Prize for Chemistry, the 2014 Kyoto Prize and the Lemelson-MIT prize, for being “one of history’s most prolific inventors in medicine.” Langer is one of the very few individuals ever elected to the National Academy of Medicine, the National Academy of Engineering, and the National Academy of Sciences.
Giovanni Traverso, M.B., B.Chir., Ph.D.

Dr. Traverso is an Assistant Professor of Medicine and Associate Physician in the Division of Gastroenterology, Brigham and Women’s Hospital (BWH), Harvard Medical School. Dr. Traverso is a research affiliate of the Massachusetts Institute of Technology. His work straddles the fields of molecular biology and biomedical engineering and is focused on developing the next generation of sensing and drug delivery systems to enable efficient monitoring of patients as well as delivery of therapeutics through the gastrointestinal tract. Dr. Traverso’s work has been published in the New England Journal of Medicine, The Lancet, Nature, Nature Biotechnology, Nature Materials, Science Translational Medicine and Cancer Research. He has been the recipient of the Grand Prize of the Collegiate Inventors Competition, a Research Fellowship from Trinity College, and was named one of the most promising innovators under 35 by the MIT Tech Review’s TR 35.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kaser A, Zeissig S, Blumberg RS, Annu Rev Immunol, 28 (2010) 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abraham C, Cho JH, N Engl J Med, 361 (2009) 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moradkhani A, Beckman LJ, Tabibian JH, J Crohns Colitis, 7 (2013) 467–473. [DOI] [PubMed] [Google Scholar]
- [4].Kaplan GG, Nat Rev Gastroenterol Hepatol, 12 (2015) 720–727. [DOI] [PubMed] [Google Scholar]
- [5].Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. , Gastroenterology, 142 (2012) 46–54 e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- [6].Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA, et al. , Gastroenterology, 135 (2008) 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chassaing B, Darfeuille-Michaud A, Gastroenterology, 140 (2011) 1720–1728. [DOI] [PubMed] [Google Scholar]
- [8].Cho JH, Nat Rev Immunol, 8 (2008) 458–466. [DOI] [PubMed] [Google Scholar]
- [9].Dretzke J, Edlin R, Round J, Connock M, Hulme C, Czeczot J, et al. , Health Technol Assess, 15 (2011) 1–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Billiet T, Rutgeerts P, Ferrante M, Van Assche G, Vermeire S, Expert Opin Biol Ther, 14 (2014) 75–101. [DOI] [PubMed] [Google Scholar]
- [11].Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP, Gut, 54 (2005) 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marzano AV, Borghi A, Meroni PL, Crosti C, Cugno M, Autoimmunity, 47 (2014) 146–153. [DOI] [PubMed] [Google Scholar]
- [13].Jain SK, Jain A, Expert Opin Drug Deliv, 5 (2008) 483–498. [DOI] [PubMed] [Google Scholar]
- [14].Roldo M, Barbu E, Brown JF, Laight DW, Smart JD, Tsibouklis J, Expert Opin Drug Deliv, 4 (2007) 547–560. [DOI] [PubMed] [Google Scholar]
- [15].Siew LF, Man SM, Newton JM, Basit AW, Int J Pharm, 273 (2004) 129–134. [DOI] [PubMed] [Google Scholar]
- [16].Sinha VR, Kumria R, Eur J Pharm Sci, 18 (2003) 3–18. [DOI] [PubMed] [Google Scholar]
- [17].Patel MM, Expert Opin Drug Deliv, 8 (2011) 1247–1258. [DOI] [PubMed] [Google Scholar]
- [18].Lamprecht A, Nat Rev Gastroenterol Hepatol, 12 (2015) 195–204. [DOI] [PubMed] [Google Scholar]
- [19].Moulari B, Beduneau A, Pellequer Y, Lamprecht A, Curr Drug Deliv, 10 (2013) 9–17. [DOI] [PubMed] [Google Scholar]
- [20].Naganuma M, Iwao Y, Ogata H, Inoue N, Funakoshi S, Yamamoto S, et al. , Inflamm Bowel Dis, 7 (2001) 221–225. [DOI] [PubMed] [Google Scholar]
- [21].Frieri G, Giacomelli R, Pimpo M, Palumbo G, Passacantando A, Pantaleoni G, et al. , Gut, 47 (2000) 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Osterberg L, Blaschke T, N Engl J Med, 353 (2005) 487–497. [DOI] [PubMed] [Google Scholar]
- [23].Langer R, Weissleder R, JAMA, 313 (2015) 135–136. [DOI] [PubMed] [Google Scholar]
- [24].Barua S, Mitragotri S, Nano Today, 9 (2014) 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tolstanova G, Deng X, French SW, Lungo W, Paunovic B, Khomenko T, et al. , Lab Invest, 92 (2012) 9–21. [DOI] [PubMed] [Google Scholar]
- [26].Lamprecht A, Nat Rev Gastroenterol Hepatol, 7 (2010) 311–312. [DOI] [PubMed] [Google Scholar]
- [27].Schmidt C, Lautenschlaeger C, Collnot EM, Schumann M, Bojarski C, Schulzke JD, et al. , J Control Release, 165 (2013) 139–145. [DOI] [PubMed] [Google Scholar]
- [28].Torchilin VP, Nat Rev Drug Discov, 13 (2014) 813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Talaei F, Atyabi F, Azhdarzadeh M, Dinarvand R, Saadatzadeh A, Eur J Pharm Sci, 49 (2013) 712–722. [DOI] [PubMed] [Google Scholar]
- [30].Lautenschlager C, Schmidt C, Fischer D, Stallmach A, Adv Drug Deliv Rev, 71 (2014) 58–76. [DOI] [PubMed] [Google Scholar]
- [31].Wolk O, Epstein S, Ioffe-Dahan V, Ben-Shabat S, Dahan A, Expert Opin Drug Deliv, 10 (2013) 1275–1286. [DOI] [PubMed] [Google Scholar]
- [32].Hua S, Marks E, Schneider JJ, Keely S, Nanomedicine, 11 (2015) 1117–1132. [DOI] [PubMed] [Google Scholar]
- [33].McConnell EL, Fadda HM, Basit AW, Int J Pharm, 364 (2008) 213–226. [DOI] [PubMed] [Google Scholar]
- [34].Hatton GB, Yadav V, Basit AW, Merchant HA, J Pharm Sci, 104 (2015) 2747–2776. [DOI] [PubMed] [Google Scholar]
- [35].Schroeder KW, Tremaine WJ, Ilstrup DM, N Engl J Med, 317 (1987) 1625–1629. [DOI] [PubMed] [Google Scholar]
- [36].Lichtenstein GR, Travis S, Danese S, D’Haens G, Moro L, Jones R, et al. , J Crohns Colitis, 9 (2015) 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kamm MA, Sandborn WJ, Gassull M, Schreiber S, Jackowski L, Butler T, et al. , Gastroenterology, 132 (2007) 66–75; quiz 432–433. [DOI] [PubMed] [Google Scholar]
- [38].Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, et al. , N Engl J Med, 372 (2015) 1104–1113. [DOI] [PubMed] [Google Scholar]
- [39].Nugent SG, Kumar D, Rampton DS, Evans DF, Gut, 48 (2001) 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sasaki Y, Hada R, Nakajima H, Fukuda S, Munakata A, Am J Gastroenterol, 92 (1997) 114–118. [PubMed] [Google Scholar]
- [41].Fallingborg J, Christensen LA, Jacobsen BA, Rasmussen SN, Dig Dis Sci, 38 (1993) 1989–1993. [DOI] [PubMed] [Google Scholar]
- [42].Saad RJ, Hasler WL, Gastroenterol Hepatol (N Y), 7 (2011) 795–804. [PMC free article] [PubMed] [Google Scholar]
- [43].Rana SV, Sharma S, Malik A, Kaur J, Prasad KK, Sinha SK, et al. , Dig Dis Sci, 58 (2013) 2594–2598. [DOI] [PubMed] [Google Scholar]
- [44].Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR, Proc Natl Acad Sci U S A, 104 (2007) 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, et al. , Gastroenterology, 144 (2013) 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lewis RT, Maron DJ, Gastroenterol Hepatol (N Y), 6 (2010) 587–596. [PMC free article] [PubMed] [Google Scholar]
- [47].Shah N, Shah T, Amin A, Expert Opin Drug Deliv, 8 (2011) 779–796. [DOI] [PubMed] [Google Scholar]
- [48].Baron JH, Connell AM, Lennard-Jones JE, Jones FA, Lancet, 1 (1962) 1094–1096. [DOI] [PubMed] [Google Scholar]
- [49].Saphier S, Haft A, Margel S, J Med Chem, 55 (2012) 10781–10785. [DOI] [PubMed] [Google Scholar]
- [50].Xiao B, Merlin D, Expert Opin Drug Deliv, 9 (2012) 1393–1407. [DOI] [PubMed] [Google Scholar]
- [51].Klotz U, Schwab M, Adv Drug Deliv Rev, 57 (2005) 267–279. [DOI] [PubMed] [Google Scholar]
- [52].Frei P, Biedermann L, Manser CN, Wilk M, Manz M, Vavricka SR, et al. , Digestion, 86 Suppl 1 (2012) 36–44. [DOI] [PubMed] [Google Scholar]
- [53].Brunner M, Vogelsang H, Greinwald R, Kletter K, Kvaternik H, Schrolnberger C, et al. , Aliment Pharmacol Ther, 22 (2005) 463–470. [DOI] [PubMed] [Google Scholar]
- [54].Nyman-Pantelidis M, Nilsson A, Wagner ZG, Borga O, Aliment Pharmacol Ther, 8 (1994) 617–622. [DOI] [PubMed] [Google Scholar]
- [55].Campieri M, Corbelli C, Gionchetti P, Brignola C, Belluzzi A, Di Febo G, et al. , Dig Dis Sci, 37 (1992) 1890–1897. [DOI] [PubMed] [Google Scholar]
- [56].D’Inca R, Bertomoro P, Mazzocco K, Vettorato MG, Rumiati R, Sturniolo GC, Aliment Pharmacol Ther, 27 (2008) 166–172. [DOI] [PubMed] [Google Scholar]
- [57].Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. , Gut, 60 (2011) 571–607. [DOI] [PubMed] [Google Scholar]
- [58].Ott C, Scholmerich J, Nat Rev Gastroenterol Hepatol, 10 (2013) 585–595. [DOI] [PubMed] [Google Scholar]
- [59].Talley NJ, Abreu MT, Achkar JP, Bernstein CN, Dubinsky MC, Hanauer SB, et al. , Am J Gastroenterol, 106 Suppl 1 (2011) S2–25; quiz S26. [DOI] [PubMed] [Google Scholar]
- [60].Johansson ME, Inflamm Bowel Dis, 20 (2014) 2124–2131. [DOI] [PubMed] [Google Scholar]
- [61].Johansson ME, Larsson JM, Hansson GC, Proc Natl Acad Sci U S A, 108 Suppl 1 (2011) 4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Corfield AP, Biochim Biophys Acta, 1850 (2015) 236–252. [DOI] [PubMed] [Google Scholar]
- [63].Juge N, Trends Microbiol, 20 (2012) 30–39. [DOI] [PubMed] [Google Scholar]
- [64].Springer SA, Gagneux P, J Biol Chem, 288 (2013) 6904–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. , Gastroenterology, 131 (2006) 117–129. [DOI] [PubMed] [Google Scholar]
- [66].Strugala V, Dettmar PW, Pearson JP, Int J Clin Pract, 62 (2008) 762–769. [DOI] [PubMed] [Google Scholar]
- [67].Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, et al. , Gut, 35 (1994) 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, et al. , Gut, 56 (2007) 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M, Gut, 47 (2000) 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Larsson JM, Karlsson H, Crespo JG, Johansson ME, Eklund L, Sjovall H, et al. , Inflamm Bowel Dis, 17 (2011) 2299–2307. [DOI] [PubMed] [Google Scholar]
- [71].Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, et al. , J Clin Invest, 121 (2011) 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB, Gastroenterol Res Pract, 2013 (2013)431231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Artis D, Nat Rev Immunol, 8 (2008) 411–420. [DOI] [PubMed] [Google Scholar]
- [74].Maloy KJ, Powrie F, Nature, 474 (2011) 298–306. [DOI] [PubMed] [Google Scholar]
- [75].Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A, Mucosal Immunol, 6 (2013) 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, et al. , Proc Natl Acad Sci U S A, 101 (2004) 1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Johansen FE, Kaetzel CS, Mucosal Immunol, 4 (2011) 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Henderson P, van Limbergen JE, Schwarze J, Wilson DC, Inflamm Bowel Dis, 17 (2011) 382–395. [DOI] [PubMed] [Google Scholar]
- [79].Turner JR, Nat Rev Immunol, 9 (2009) 799–809. [DOI] [PubMed] [Google Scholar]
- [80].Cario E, Podolsky DK, Infect Immun, 68 (2000) 7010–7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Berrebi D, Maudinas R, Hugot JP, Chamaillard M, Chareyre F, De Lagausie P, et al. , Gut, 52 (2003) 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, et al. , Gastroenterology, 124 (2003) 1001–1009. [DOI] [PubMed] [Google Scholar]
- [83].Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, et al. , Gastroenterology, 126 (2004) 1347–1357. [DOI] [PubMed] [Google Scholar]
- [84].Hokama A, Mizoguchi E, Sugimoto K, Shimomura Y, Tanaka Y, Yoshida M, et al. , Immunity, 20 (2004) 681–693. [DOI] [PubMed] [Google Scholar]
- [85].Srikrishna G, Turovskaya O, Shaikh R, Newlin R, Foell D, Murch S, et al. , J Immunol, 175 (2005) 5412–5422. [DOI] [PubMed] [Google Scholar]
- [86].Cader MZ, Kaser A, Gut, 62 (2013) 1653–1664. [DOI] [PubMed] [Google Scholar]
- [87].Artis D, Spits H, Nature, 517 (2015) 293–301. [DOI] [PubMed] [Google Scholar]
- [88].McKenzie AN, Spits H, Eberl G, Immunity, 41 (2014) 366–374. [DOI] [PubMed] [Google Scholar]
- [89].Grbic DM, Degagne E, Larrivee JF, Bilodeau MS, Vinette V, Arguin G, et al. , Inflamm Bowel Dis, 18 (2012) 1456–1469. [DOI] [PubMed] [Google Scholar]
- [90].Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. , Nat Immunol, 2 (2001) 361–367. [DOI] [PubMed] [Google Scholar]
- [91].Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, et al. , J Exp Med, 202 (2005) 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Siddiqui KR, Laffont S, Powrie F, Immunity, 32 (2010) 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Baumgart DC, Carding SR, Lancet, 369 (2007) 1627–1640. [DOI] [PubMed] [Google Scholar]
- [94].Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. , Gastroenterology, 129 (2005) 550–564. [DOI] [PubMed] [Google Scholar]
- [95].Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, et al. , J Clin Invest, 110 (2002) 1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Jinno Y, Ohtani H, Nakamura S, Oki M, Maeda K, Fukushima K, et al. , Virchows Arch, 448 (2006) 412–421. [DOI] [PubMed] [Google Scholar]
- [97].Cupi ML, Sarra M, Marafini I, Monteleone I, Franze E, Ortenzi A, et al. , J Immunol, 192 (2014) 6083–6091. [DOI] [PubMed] [Google Scholar]
- [98].Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, et al. , J Leukoc Biol, 86 (2009) 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sorokin L, Nat Rev Immunol, 10 (2010) 712–723. [DOI] [PubMed] [Google Scholar]
- [100].Daley WP, Peters SB, Larsen M, J Cell Sci, 121 (2008) 255–264. [DOI] [PubMed] [Google Scholar]
- [101].Klebanoff SJ, J Leukoc Biol, 77 (2005) 598–625. [DOI] [PubMed] [Google Scholar]
- [102].Mahida YR, Inflamm Bowel Dis, 6 (2000) 21–33. [DOI] [PubMed] [Google Scholar]
- [103].Wiener E, Levanon D, Science, 159 (1968) 217. [DOI] [PubMed] [Google Scholar]
- [104].Neurath MF, Nat Rev Immunol, 14 (2014) 329–342. [DOI] [PubMed] [Google Scholar]
- [105].Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, et al. , J Immunol, 180 (2008) 5662–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, et al. , Nat Med, 12 (2006) 317–323. [DOI] [PubMed] [Google Scholar]
- [107].McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, et al. , J Clin Invest, 98 (1996) 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. , J Exp Med, 195 (2002) 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tirosh B, Khatib N, Barenholz Y, Nissan A, Rubinstein A, Mol Pharm, 6 (2009) 1083–1091. [DOI] [PubMed] [Google Scholar]
- [110].Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, et al. , Proc Natl Acad Sci U S A, 99 (2002) 3902–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Monajemi H, Meenan J, Lamping R, Obradov DO, Radema SA, Trown PW, et al. , Gastroenterology, 110 (1996) 733–739. [DOI] [PubMed] [Google Scholar]
- [112].Wehkamp J, Fellermann K, Herrlinger KR, Baxmann S, Schmidt K, Schwind B, et al. , Eur J Gastroenterol Hepatol, 14 (2002) 745–752. [DOI] [PubMed] [Google Scholar]
- [113].Ramasundara M, Leach ST, Lemberg DA, Day AS, J Gastroenterol Hepatol, 24 (2009) 202–208. [DOI] [PubMed] [Google Scholar]
- [114].Winterkamp S, Raithel M, Hahn EG, J Clin Gastroenterol, 30 (2000) 170–175. [DOI] [PubMed] [Google Scholar]
- [115].Round JL, Mazmanian SK, Nat Rev Immunol, 9 (2009) 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ley RE, Peterson DA, Gordon JI, Cell, 124 (2006) 837–848. [DOI] [PubMed] [Google Scholar]
- [117].Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. , Nature, 464 (2010) 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Honda K, Littman DR, Annu Rev Immunol, 30 (2012) 759–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI, Science, 291 (2001)881–884. [DOI] [PubMed] [Google Scholar]
- [120].Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, et al. , Gastroenterology, 128 (2005) 2020–2028. [DOI] [PubMed] [Google Scholar]
- [121].Mazmanian SK, Round JL, Kasper DL, Nature, 453 (2008) 620–625. [DOI] [PubMed] [Google Scholar]
- [122].O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, et al. , PLoS Pathog, 4 (2008) e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M, J Immunol, 174 (2005) 3237–3246. [DOI] [PubMed] [Google Scholar]
- [124].Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. , Nature, 500 (2013) 232–236. [DOI] [PubMed] [Google Scholar]
- [125].Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, et al. , Inflamm Bowel Dis, 12 (2006) 106–111. [DOI] [PubMed] [Google Scholar]
- [126].Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. , Gut, 55 (2006) 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, et al. , Gastroenterology, 127 (2004) 412–421. [DOI] [PubMed] [Google Scholar]
- [128].Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, et al. , Cell Host Microbe, 8 (2010) 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hansen J, Gulati A, Sartor RB, Curr Opin Gastroenterol, 26 (2010) 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, et al. , Gastroenterology, 122 (2002) 44–54. [DOI] [PubMed] [Google Scholar]
- [131].Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. , Science, 341 (2013) 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Marchesi JR, Holmes E, Khan F, Kochhar S, Scanlan P, Shanahan F, et al. , J Proteome Res, 6 (2007) 546–551. [DOI] [PubMed] [Google Scholar]
- [133].Roediger WE, Duncan A, Kapaniris O, Millard S, Gastroenterology, 104 (1993) 802–809. [DOI] [PubMed] [Google Scholar]
- [134].Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H, Nano Today, 10 (2015) 487–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Owens DE 3rd, Peppas NA, Int J Pharm, 307 (2006) 93–102. [DOI] [PubMed] [Google Scholar]
- [136].Lamprecht A, Schafer U, Lehr CM, Pharm Res, 18 (2001) 788–793. [DOI] [PubMed] [Google Scholar]
- [137].Lamprecht A, Ubrich N, Yamamoto H, Schafer U, Takeuchi H, Maincent P, et al. , J Pharmacol Exp Ther, 299 (2001) 775–781. [PubMed] [Google Scholar]
- [138].Ali H, Weigmann B, Collnot EM, Khan SA, Windbergs M, Lehr CM, Pharm Res, (2015) 788–793. [DOI] [PubMed] [Google Scholar]
- [139].Beloqui A, Coco R, Alhouayek M, Solinis MA, Rodriguez-Gascon A, Muccioli GG, et al. , Int J Pharm, 454 (2013) 775–783. [DOI] [PubMed] [Google Scholar]
- [140].Coco R, Plapied L, Pourcelle V, Jerome C, Brayden DJ, Schneider YJ, et al. , Int J Pharm, 440 (2013) 3–12. [DOI] [PubMed] [Google Scholar]
- [141].Wang Z, Li J, Cho J, Malik AB, Nat Nanotechnol, 9 (2014) 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Jubeh TT, Barenholz Y, Rubinstein A, Pharm Res, 21 (2004) 447–453. [DOI] [PubMed] [Google Scholar]
- [143].Jubeh TT, Nadler-Milbauer M, Barenholz Y, Rubinstein A, J Drug Target, 14 (2006) 155–163. [DOI] [PubMed] [Google Scholar]
- [144].Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vreden C, et al. , Sci Transl Med, 6 (2014) 219ra217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Zhang S, Ermann J, Succi MD, Zhou A, Hamilton MJ, Cao B, et al. , Sci Transl Med, 7 (2015) 300ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kim E, Schueller O, Sweetnam PM, Lab Chip, 12 (2012) 2255–2264. [DOI] [PubMed] [Google Scholar]
- [147].Spertini O, Cordey AS, Monai N, Giuffre L, Schapira M, J Cell Biol, 135 (1996) 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, et al. , Proc Natl Acad Sci U S A, 100 (2003) 15895–15900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Eniola AO, Rodgers SD, Hammer DA, Biomaterials, 23 (2002) 2167–2177. [DOI] [PubMed] [Google Scholar]
- [150].Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M, Science, 319 (2008) 627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Xiao B, Yang Y, Viennois E, Zhang Y, Ayyadurai S, Baker M, et al. , J Mater Chem B Mater Biol Med, 2 (2014) 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Xiao B, Laroui H, Viennois E, Ayyadurai S, Charania MA, Zhang Y, et al. , Gastroenterology, 146 (2014) 1289–1300 e1281–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Xiao B, Laroui H, Ayyadurai S, Viennois E, Charania MA, Zhang Y, et al. , Biomaterials, 34 (2013) 7471–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Zuo L, Huang Z, Dong L, Xu L, Zhu Y, Zeng K, et al. , Gut, 59 (2010) 470–479. [DOI] [PubMed] [Google Scholar]
- [155].Zhang J, Tang C, Yin C, Biomaterials, 34 (2013) 3667–3677. [DOI] [PubMed] [Google Scholar]
- [156].Laroui H, Viennois E, Xiao B, Canup BS, Geem D, Denning TL, et al. , J Control Release, 186 (2014) 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Park J, Gao W, Whiston R, Strom TB, Metcalfe S, Fahmy TM, Mol Pharm, 8 (2011) 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC, Blood, 115 (2010) 4778–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, von Drygalski A, et al. , J Clin Invest, 123 (2013) 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tran TH, Mattheolabakis G, Aldawsari H, Amiji M, Clin Immunol, 160 (2015) 46–58. [DOI] [PubMed] [Google Scholar]
- [161].Cai Z, Zhang W, Yang F, Yu L, Yu Z, Pan J, et al. , Cell Res, 22 (2012) 607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q, et al. , Mol Ther, 22 (2014) 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zhu H, Li YR, Exp Biol Med (Maywood), 237 (2012) 474–480. [DOI] [PubMed] [Google Scholar]
- [164].Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. , Dig Dis Sci, 41 (1996) 2078–2086. [DOI] [PubMed] [Google Scholar]
- [165].Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N, Nat Mater, 9 (2010) 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].de Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, et al. , J Am Chem Soc, 134 (2012) 15758–15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Biancheri P, Brezski RJ, Di Sabatino A, Greenplate AR, Soring KL, Corazza GR, et al. , Gastroenterology, 149 (2015) 1564–1574 e1563. [DOI] [PubMed] [Google Scholar]
- [168].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. , Proc Natl Acad Sci U S A, 100 (2003) 5413–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R, Pharmacol Rev, 61 (2009) 198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sinha VR, Kumria R, Drug Dev Ind Pharm, 30 (2004) 143–150. [DOI] [PubMed] [Google Scholar]
- [171].Karrout Y, Dubuquoy L, Piveteau C, Siepmann F, Moussa E, Wils D, et al. , J Control Release, 197 (2015) 121–130. [DOI] [PubMed] [Google Scholar]
- [172].Saadatzadeh A, Atyabi F, Fazeli MR, Dinarvand R, Jamalifar H, Abdolghaffari AH, et al. , Fundam Clin Pharmacol, 26 (2012) 589–598. [DOI] [PubMed] [Google Scholar]
- [173].Samanta AK, Torok VA, Percy NJ, Abimosleh SM, Howarth GS, J Microbiol, 50 (2012) 218–225. [DOI] [PubMed] [Google Scholar]
- [174].Preidis GA, Versalovic J, Gastroenterology, 136 (2009) 2015–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, et al. , Science, 289 (2000)1352–1355. [DOI] [PubMed] [Google Scholar]
- [176].Foligne B, Dessein R, Marceau M, Poiret S, Chamaillard M, Pot B, et al. , Gastroenterology, 133 (2007) 862–874. [DOI] [PubMed] [Google Scholar]
- [177].Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, et al. , Gastroenterology, 127 (2004) 502–513. [DOI] [PubMed] [Google Scholar]
- [178].Ricci S, Macchia G, Ruggiero P, Maggi T, Bossu P, Xu L, et al. , BMC Biotechnol, 3 (2003)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Watterlot L, Rochat T, Sokol H, Cherbuy C, Bouloufa I, Lefevre F, et al. , Int J Food Microbiol, 144 (2010) 35–41. [DOI] [PubMed] [Google Scholar]
- [180].Han W, Mercenier A, Ait-Belgnaoui A, Pavan S, Lamine F, van S II, et al. , Inflamm Bowel Dis, 12 (2006) 1044–1052. [DOI] [PubMed] [Google Scholar]
- [181].Hamady ZZ, Scott N, Farrar MD, Wadhwa M, Dilger P, Whitehead TR, et al. , Inflamm Bowel Dis, 17 (2011) 1925–1935. [DOI] [PubMed] [Google Scholar]
- [182].Benbouziane B, Ribelles P, Aubry C, Martin R, Kharrat P, Riazi A, et al. , J Biotechnol, 168 (2013) 120–129. [DOI] [PubMed] [Google Scholar]
- [183].Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K, Robinson JP, et al. , Nat Nanotechnol, 2 (2007) 441–449. [DOI] [PubMed] [Google Scholar]
- [184].Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y, J Control Release, 104 (2005) 337–346. [DOI] [PubMed] [Google Scholar]
- [185].Bhavsar MD, Amiji MM, Gene Ther, 15 (2008) 1200–1209. [DOI] [PubMed] [Google Scholar]
- [186].Kriegel C, Amiji M, J Control Release, 150 (2011) 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Kriegel C, Attarwala H, Amiji M, Adv Drug Deliv Rev, 65 (2013) 891–901. [DOI] [PubMed] [Google Scholar]
- [188].Laroui H, Theiss AL, Yan Y, Dalmasso G, Nguyen HT, Sitaraman SV, et al. , Biomaterials, 32 (2011) 1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Huang Z, Gan J, Jia L, Guo G, Wang C, Zang Y, et al. , Biomaterials, 48 (2015) 26–36. [DOI] [PubMed] [Google Scholar]
- [190].Laroui H, Dalmasso G, Nguyen HT, Yan Y, Sitaraman SV, Merlin D, Gastroenterology, 138 (2010) 843–853 e841–842. [DOI] [PubMed] [Google Scholar]
- [191].Theiss AL, Laroui H, Obertone TS, Chowdhury I, Thompson WE, Merlin D, et al. , Inflamm Bowel Dis, 17 (2011) 1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Laroui H, Geem D, Xiao B, Viennois E, Rakhya P, Denning T, et al. , Mol Ther, 22 (2014) 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Geem D, Harusato A, Flannigan K, Denning TL, Inflamm Bowel Dis, 21 (2015) 1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY, Nat Immunol, 6 (2005) 11421151. [DOI] [PubMed] [Google Scholar]
- [195].Kinoshita M, Kayama H, Kusu T, Yamaguchi T, Kunisawa J, Kiyono H, et al. , J Immunol, 189 (2012) 2869–2878. [DOI] [PubMed] [Google Scholar]
- [196].Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, et al. , Proc Natl Acad Sci U S A, 112 (2015) E156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Johannes Herkel AC, Freund Barbara, Heeren Jorg, Nielsen Peter, Bruns Oliver, Lohse Ansgar, Lüth Stefan, Weller Horst, Salmen Sunhild, in: E.P. APPLICATION (Ed.) 2013. [Google Scholar]
- [198].Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, et al. , Proc Natl Acad Sci U S A, 112 (2015) 10437–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R, Nature, 489 (2012) 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Panaccione R, Ghosh S, Middleton S, Marquez JR, Scott BB, Flint L, et al. , Gastroenterology, 146 (2014) 392–400 e393. [DOI] [PubMed] [Google Scholar]
- [201].Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. , N Engl J Med, 362 (2010) 1383–1395. [DOI] [PubMed] [Google Scholar]
- [202].Sinha SR, Nguyen LP, Inayathullah M, Malkovskiy A, Habte F, Rajadas J, et al. , Gastroenterology, 149 (2015) 52–55 e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Schoellhammer CM, Schroeder A, Maa R, Lauwers GY, Swiston A, Zervas M, et al. , Sci Transl Med, 7 (2015) 310ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Traverso G, Schoellhammer CM, Schroeder A, Maa R, Lauwers GY, Polat BE, et al. , J Pharm Sci, 104 (2015) 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Zaric M, Lyubomska O, Touzelet O, Poux C, Al-Zahrani S, Fay F, et al. , ACS Nano, 7 (2013) 2042–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN, J Immunol, 186 (2011) 5543–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, et al. , Nature, 526 (2015) 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, et al. , Nat Mater, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ, Lancet, 380 (2012) 1606–1619. [DOI] [PubMed] [Google Scholar]
- [210].Pertuit D, Moulari B, Betz T, Nadaradjane A, Neumann D, Ismaili L, et al. , J Control Release, 123 (2007) 211–218. [DOI] [PubMed] [Google Scholar]
- [211].Momen-Heravi F, Bala S, Bukong T, Szabo G, Nanomedicine, 10 (2014) 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, et al. , Nature, 458 (2009) 1180–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]