Abstract
Staphylococcus aureus infections represent a major public health threat, but previous attempts at developing a universal vaccine have been unsuccessful. We attempted to identify a vaccine that would be protective against both skin/soft tissue and bloodstream infections. We first tested a panel of staphylococcal antigens that are conserved across strains, combined with aluminum hydroxide as an adjuvant, for their ability to induce protective immunity in both skin and bacteremia infection models. Antigens were identified that reduced dermonecrosis during skin infection, and other non-overlapping antigens were identified that showed trends to protection in the bacteremia model. However, individual antigens were not identified that mediated substantial protection in both the skin and bacteremia infection models. We therefore tested a variety of combinations of proteins to seek a single combination that could mediate protection in both models. After iterative testing, a vaccine consisting of 3 antigens, ABC transporter protein (SACOL2451), ABC2 transporter protein (SACOL0695), and α-hemolysin (SACOL1173), was identified as the most effective combination. This combination vaccine provided protection in a skin infection model. However, these antigens were only partially protective in the bacteremia infection model. Even by testing multiple different adjuvants, optimized efficacy in the skin infection model did not translate into efficacy in the bacteremia model. Thus protective vaccines against skin/soft tissue infections may not enable effective protection against bloodstream infections.
Introduction
Staphylococcus aureus is the most common cause of skin infections and the second most common cause of bacteremia [1–4]. The spread of community-associated methicillin-resistant S. aureus (MRSA) has made treatment of such infections challenging [5–8]. Given their high incidence and drug-resistance, a vaccine to prevent such S. aureus infections would have an enormous impact on US and global health.
Recent vaccine attempts have not yielded promising clinical results. Tefibazumab was developed as passive immunotherapy that targeted clumping factor A (ClfA) but showed poor efficacy in combination with antibiotics [9,10]. A phase II clinical trial involving V710 (Merck), a vaccine developed against iron-regulated surface determinant protein B (IsdB) was terminated early due to increased mortality among vaccinated patients [11]. StaphVax, a vaccine directed against the capsular polysaccharides CP5 and CP8, advanced to a phase III clinical trial but was also unsuccessful [12].
Many of these attempts have largely targeted single antigens rather than combinations of antigens (multivalent vaccine), which may be too simplistic with regards to the complex host-pathogen interactions that develop during infection. There is evidence that combinatorial strategies elicit greater protection than targeting of single antigens. A vaccine composed of IsdB, iron-regulated surface determinant protein A (IsdA), serine aspartate containing protein D (SdrD), serine aspartate containing protein E (SdrE) protected mice from lethal infection better than any of the single components [13]. However, underscoring the complexity of staphylococcal vaccination, clinical development of the quadrivalent vaccine (Pfizer) was terminated after a phase IIb trial demonstrated it did not reduce invasive infections following spinal surgery [14].
We hypothesized that active vaccines targeting S. aureus may require a combination of multiple antigens [15–17]. In order to support basic vaccinology research, Merck agreed to supply numerous recombinant proteins which they have internally demonstrated to have varying measures of protective efficacy against S. aureus infection in mice. We selected this panel of antigens, in combination with several novel candidates identified by the authors. We hypothesized that such a multivalent vaccine might protect the host against the two predominant infectious syndromes caused by S. aureus: bloodstream and skin. We also hypothesized that antigenic targets and host defense elements required for protection would differ during bloodstream and skin infections.
Results
Single antigen immunization
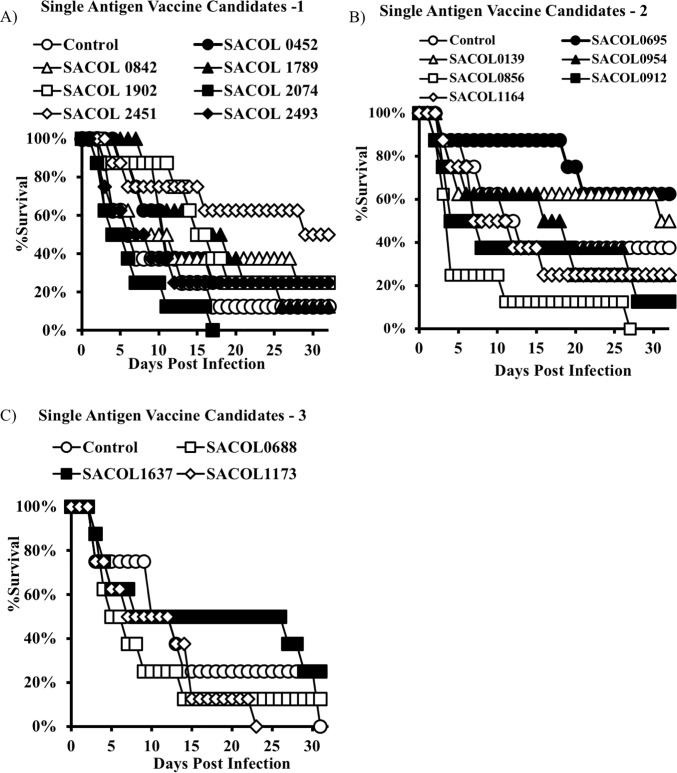
We tested each antigen alone to determine if the antigen was sufficient to provide protection in the bacteremia or skin infection models. Because antibody titers have not been found to be accurate surrogate markers of efficacy in prior studies in mice or humans,[15,16,18] we chose not to rely on antibody titers to select candidates to move forward. Rather, we used clinical endpoints to select candidate proteins. Specifically, we used survival time for the bacteremia model, which is a lethal model. The skin model does not produce a lethal infection and vaccine efficacy is defined as the development of smaller skin lesions (area).
We found minimal overlap in the antigens that were found to be protective in each respective model (Figs 1 and 2). For the bacteremia model, SACOL0695 and SACOL2451 provided the best trend to protection, although statistical significance was not achieved. However, for the skin model SACOL0856, SACOL1164, SACOL1173, and SACOL1789 provided significant protection (Fig 2).
Fig 1. Single antigen immunization, bacteremia model.
BALB/c mice (n = 8) were immunized with individual antigens (20 micrograms) and then challenged with a S. aureus IV infection. Mice were monitored daily and euthanized when moribund. Immunization with SACOL2451 and SACOL0695 resulted in the highest percentage of surviving mice.
Fig 2. Single antigen immunization, skin model.
BALB/c mice (n = 8) were immunized with individual antigens (20 micrograms) and then challenged with a S. aureus in a skin infection model. Mice were monitored daily and the wound size was recorded. The single antigens that provided the best protection in the skin model were SACOL1173 and SACOL1164.
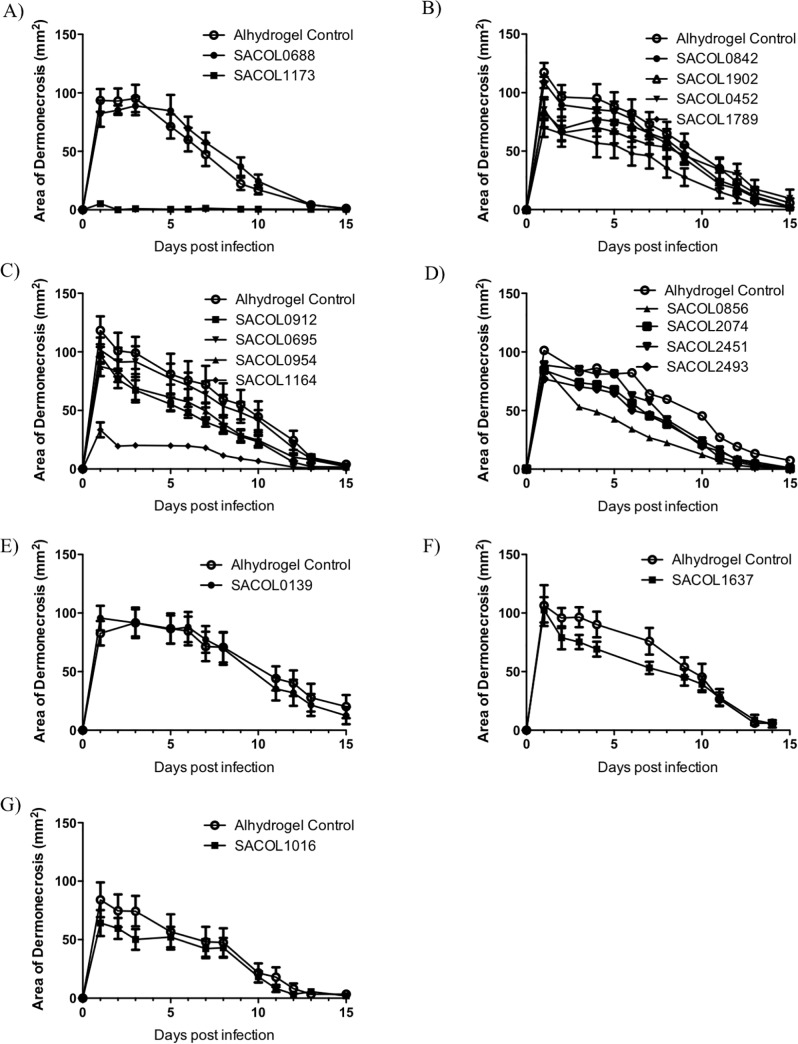
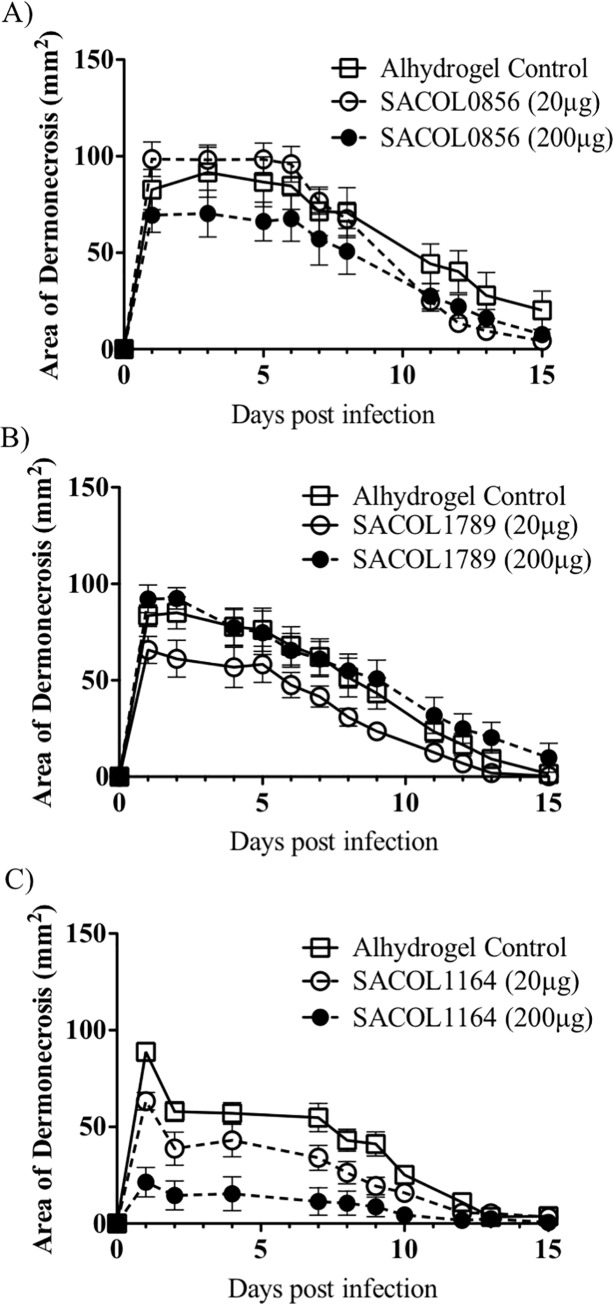
To determine the potential for improved efficacy with dose optimization, we repeated the immunization strategy but tested both the original 20 μg and a higher 200 μg dose (except for SACOL1173, which was fully protective in the skin model at the 20 μg dose). Protection was again seen in the skin model (Fig 3), but once again not in the bacteremia model irrespective of dose (Fig 4).
Fig 3. Dose optimization.
A-C) Skin infection model. BALB/c mice (n = 8) were immunized with individual antigens that were found to be protective during the initial screen and then challenged with a S. aureus in a skin infection model. Mice were monitored daily and the wound size was recorded. Increasing the antigen dose 10-fold did not result in a statistically significant benefit.
Fig 4. Dose optimization.
A, B) Bacteremia model. BALB/c mice (n = 8) were immunized with individual antigens (20 or 200 micrograms) and then challenged with a S. aureus IV infection. Mice were monitored daily and euthanized when moribund. No benefit was observed in follow up studies in the bacteremia model. Increasing the antigen dose 10-fold did not result in a statistically significant benefit.
Combination vaccine
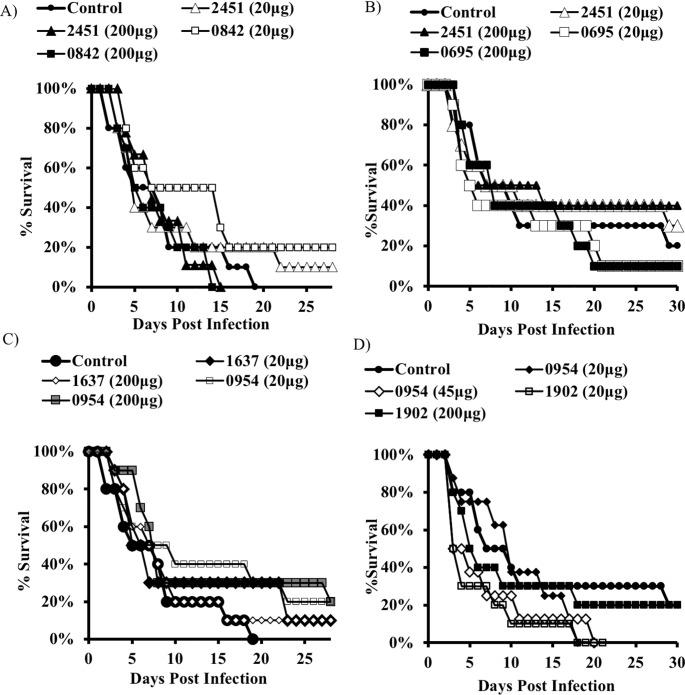
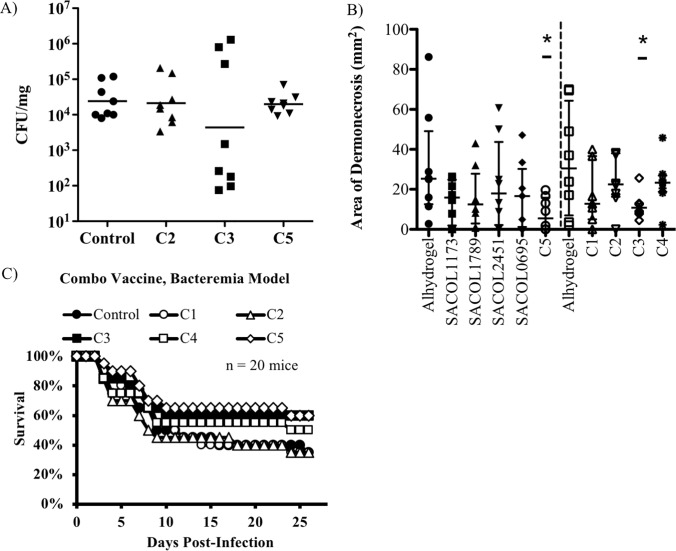
Thus a single antigen vaccine strategy was not feasible for protecting against both skin infection and bacteremia. Therefore, we systematically developed vaccine combinations by combining individual antigens (Table 1) that provided the best protection in each respective model. As before, we evaluated the ability of the vaccine to delay the time to moribund condition in the bacteremia model and to reduce CFU and/or lesion size in the skin model. Reduced lesion size in the skin model was observed for combinations “C3” and “C5”. However, only combination “C3” resulted in a reduction in CFUs as well. Immunization with combinations “C1” and “C2” resulted in the best numerical survival in the bacteremia model, however no group achieved statistically significant improvements in survival (Fig 5).
Table 1. Summary of combination immunizations.
| Combo Group | Description |
|---|---|
| C1 | SACOL1173, SACOL1789, SACOL2451, and SACOL0695 |
| C2 | SACOL0695, SACOL1789, and SACOL2451 |
| C3 | SACOL0695, SACOL1173, and SACOL2451 |
| C4 | SACOL1173, SACOL1789, and SACOL0695 |
| C5 | SACOL1173, SACOL1789, and SACOL2451 |
Fig 5. Combination vaccines.
Combinations of antigens that offered protection in the bacteremia model (SACOL2451 and SACOL0695) were combined with antigens that were found to be protective in the skin model (SACOL1173 and SACOL1789). A, B) Skin infection model. BALB/c mice (n = 8) were immunized and then challenged with a S. aureus in a skin infection model. Combination groups “C3” and “C5” were able to reduce the area of dermonecrosis relative to the Alhydrogel control (Wilcoxon rank-sum, p < 0.05) but the reduction in CFU burden was not statistically significant (n = 8 mice per group). C) Bacteremia model. BALB/c mice (n = 20) were immunized with antigen combinations and then challenged with a S. aureus IV infection. Mice were monitored daily and euthanized when moribund. Combination groups "C3", "C4", and "C5" resulted in a greater number of mice surviving but this was not statistically significant (n = 20 mice per group). “C1” = SACOL1173, SACOL1789, SACOL2451, and SACOL0695; “C2” = SACOL0695, SACOL1789, and SACOL2451; “C3” = SACOL0695, SACOL1173, and SACOL2451; “C4” = SACOL1173, SACOL1789, and SACOL0695. “C5” = SACOL1173, SACOL1789, and SACOL2451. * P < 0.05, relative to the alhydrogel control (Wilcoxon rank-sum).
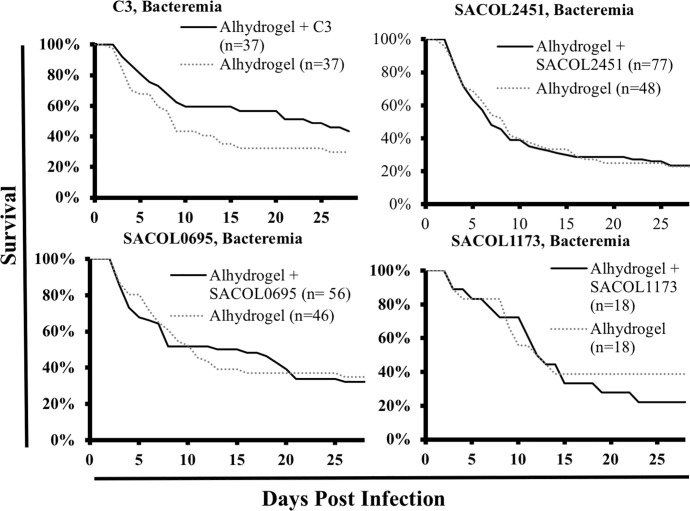
As group “C3” was the most promising in the skin model, we decided to repeat the bacteremia model using the components of group "C3" alone and in combination. Once again, we found no significant benefit of vaccination in the bacteremia model (Fig 6).
Fig 6. Group "C3" efficacy, bacteremia model.
BALB/c mice were immunized and then challenged with a S. aureus IV infection. Mice were monitored daily and euthanized when moribund. We had previously observed mixed results when immunizing mice with the different regimens and therefore we carried out 4 independent experiments (combined n = 37 mice). Immunization and infections were standardized across experiments. No significant difference was observed between immunized and control mice. "C3" is composed of an equal mixture of SACOL1173, SACOL2451, and SACOL0695 antigens.
Alternative adjuvants
Vaccines require the use of an adjuvant to help direct the proper host immune system to yield a protective response. All previous experiments had been carried out using Alhydrogel (aluminum hydroxide) as an adjuvant and we wanted to expand the adjuvants tested to include MPLA (which activates TLR4), Imiquimod (which activates TLR7), and/or whole glucan particles (WGP, which activates Dectin-1). Alhydrogel was also included to enable depot formations. We tested variable combinations of adjuvants with the fixed antigen combination of group "C3". We elected to test only group "C3" because it had resulted in protection in the skin model and a trend to protection in the bacteremia model. Of note, “C3” did not include SACOL1164, which performed well in individual skin infection experiments. We chose “C3” to focus more on the bacteremia model, for which protection was much more challenging to identify, particularly since SACOL1173 was completely protective in the skin model, and was included in “C3”.
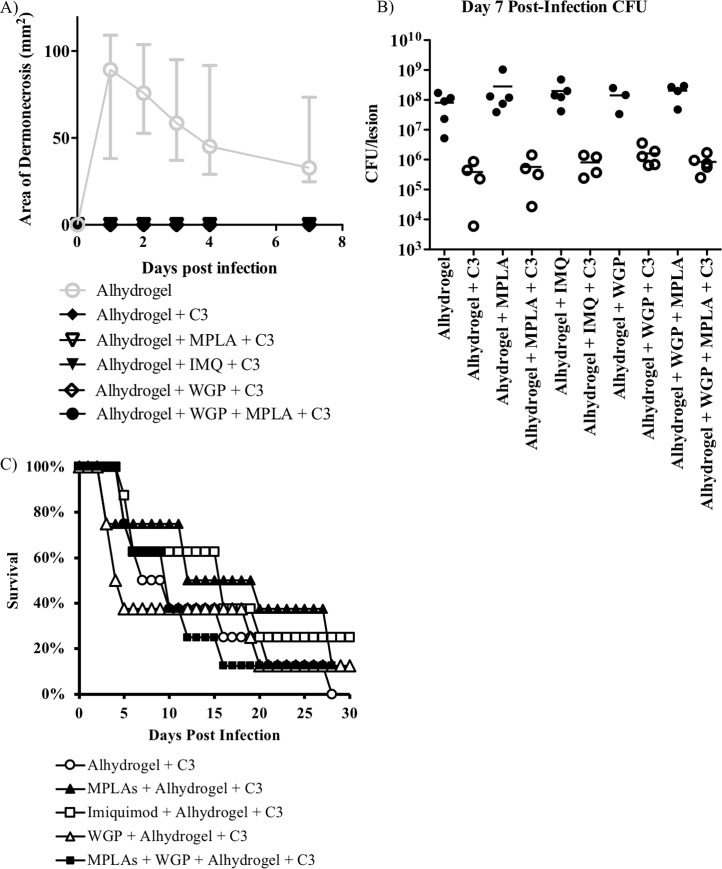
We again observed that mice immunized with the combination group "C3" antigens resulted in protection in the skin model (Fig 7). Vaccine efficacy was observed for each of the various adjuvant combinations tested. However, no statistically significant difference was observed in the bacteremia model when group "C3" antigens were added to any of the adjuvant formulations (Fig 7).
Fig 7. Adjuvant optimization.
In attempt to improve the activity of "C3", we tried to substitute the adjuvant used. Skin infection model. BALB/c mice (n = 8) were immunized with “C3” and then challenged with a S. aureus in a skin infection model. Mice were monitored daily and the wound size was recorded. For the bacteremia model, BALB/c mice were immunized and then challenged with a S. aureus IV infection. Mice were monitored daily and euthanized when moribund. (A) A significant benefit (P <0.05, nonparametric log-rank test) was observed for both the prevention of dermonecrosis. (B) A significant reduction in CFU burden relative to the adjuvant only control (P < 0.05, Wilcoxon rank-sum) was also observed. All vaccine formulations that contained "C3" performed similarly. This is in contrast to the limited observed benefit in the bacteremia model (C). "C3" = SACOL0695, SACOL1173, and SACOL2451. n = 8 mice per group.
Discussion
There is an urgent need for preventative vaccines against S. aureus infections of all kinds, given their frequency, virulence, and antibiotic resistance. We have previously hypothesized that a mixture of multiple antigens would be necessary to protect against both skin and bloodstream infections [15–17]. We set about performing a systematic test of candidate staphylococcal proteins to test for vaccine efficacy, in both skin and lethal bloodstream models of infection.
We identified a number of staphylococcal protein antigens that display protective efficacy in either a bacteremia or skin model of infection. Interestingly, individual antigens that demonstrated efficacy in one disease model had little overlap with the other disease model. This led us to pursue a combination vaccination strategy. The combination was considerably more effective in the skin model, but we were not able to achieve substantial protection in the bloodstream infection model irrespective of antigen combinations or adjuvants tested.
One possible explanation for the lack of protection in both models is the use of different strains of S. aureus (both of which are USA300) for each model. However, for a vaccine to be useful, it must protect against most clinical isolates encountered, and as such, failure to protect against different isolates still makes translational development problematic. Furthermore, the proteins used are identical across the strains tested, so antigenic variation across the strains is not the explanation for lack of efficacy across both models.
Another possible explanation is that the cellular physiology of the bacteria differ at different disease sites. Other studies have also found that vaccine effectiveness will vary between disease models [19]. For example, the composition of bacterial surface antigens can differ in different host anatomical locations. It is also not necessarily true that the most abundant antigen is the most immunogenic and we therefore chose not to preselect our candidate antigens based solely on the relative abundance of the antigen present in each disease model. We took an unbiased approach and tested individual antigens for efficacy in each disease model.
The majority of work presented here utilized Alhydrogel as the adjuvant. Aluminum is an FDA approved adjuvant and is currently included the hepatitis A, hepatitis B, DTaP, Hib, and HPV vaccine formulations [20–22]. Aluminum adjuvants establish a depot formation for the sustained release of antigen after immunization, and also stimulates antigen presenting cells by activating the NALP3 inflammasome. MPL is a detoxified version of LPS and functions by activating immune cells through TLR4. Glucan can signal through multiple receptors, however the WGP product that we used functions by signaling only through Dectin-1 to stimulate macrophages and dendritic cells. Imiquimod is a TLR7 agonist and polarizes a Th1 response. The 4 adjuvants that we tested in total are not an exhaustive list and the possibility exists that the protection provided by the group "C3" antigens in the bacteremia model could be further improved if paired with a different adjuvant [21]. However, there is not an obvious way to select the proper adjuvant because the optimal immune response to protect against S. aureus infections is still unknown [17,19].
In summary, this combinatorial approach to vaccine testing underscores the ongoing challenges of vaccine development targeting S. aureus. Antigens that are protective at one anatomical site of infection may well not be effective at protecting against infection at other sites. We have no established surrogate marker to predict in vivo efficacy. Furthermore, the immunological mechanisms of protection may differ at different sites of infection. Further research is needed to identify antigens that are broadly protective in vivo, and adjuvants that will enhance protection at various sites of infection.
Methods
All animal experiments were approved by the Institutional Committee on the Use and Care of Animals at the USC Keck School of Medicine and the University of Chicago, following the National Institutes of Health guidelines for animal housing and care. Mice were housed in standard cages at a maximum density of five per cage, and given food and water ad libitum. Mice were euthanized by CO2 followed by cervical dislocation when they reached moribund condition, defined as inability to ambulate on tactile stimulation.
Immunization protocol
Female BALB/c mice (9–15 weeks) were purchased from Taconic. Immunizations done using a prime-boost strategy. After the initial “prime” immunization, the “boost” immunization was administered 21 days later. Mice were then challenged S. aureus 14 days after the boost immunization.
Concentrations of purified antigens were determined by BCA assay, and endotoxin levels were assessed using the Pierce LAL Chromogenic Endotoxin Quantitation Kit (ThermoFisher 88282). Antigens were mixed with 0.1% Alhydrogel (Brenntag, Westbury, NY) in PBS, and injected subcutaneously in a volume of 200 μL. Mice were primed, then boosted with the same formulation.
We additionally tested adjuvant components including Imiquimod (Invivogen vac-imq), WGP Dispersible (Invivogen tlrl-wgp), and MPLA Synthetic VacciGrade (Invivogen vac-mpls). MPLAs, Imiquimod, and WGP were administered at 10, 50, and 100 μg per dose respectively.
Antigens
To identify antigens against which immune responses are generated during infection, we inoculated mice with a sublethal dose of S. aureus LAC (USA300). We bled the mice prior to immunization and then again at 14 days post-immunization to harvest immune serum. We used lysostaphin cell surface preparations[23,24] of the S. aureus to run 2D gel Western blots (size vs. isoelectric focusing) as we have previously described.[18] The immune serum identified several novel antigens that were not recognized by pre-immune sera. Cell wall protein antigens were identified by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass-Spectrometry (MALDI-TOF) analysis. None had previously been assessed in vaccine studies. These antigens were: SACOL1637, SACOL0695, and SACOL0954 (Table 2). The cDNAs encoding these antigens were cloned into the expression vector pQE-1 (Qiagen) and expressed by Isopropyl β-D-1-thiogalactopyranoside (IPTG) induction, followed by His-tag protein purification. Endotoxin was removed from protein preparations using a Detoxigel endotoxin removal kit (Pierce) or ToxinEraser kit (GenScript). Final endotoxin levels were less than 5 EU per immunization dose.
Table 2. Summary of the antigens tested.
| Gene | Symbol | Description | Source |
|---|---|---|---|
| SACOL0139 | Cap5E | Capsular polysaccharides 5E subunit | Jean Lee |
| SACOL0452 | - | Alkyl hydroperoxide reductase | Merck |
| SACOL0688 | - | Mg/Zn transport system protein | Merck |
| SACOL0695 | ABC2 | ABC transporter protein 2 | This study |
| SACOL0842 | Eno | Enolase | Merck |
| SACOL0856 | ClfA | Clumping factor A | Merck |
| SACOL0912 | - | Hypothetical protein | Merck |
| SACOL0954 | EfTu | Elongation Factor | This study |
| SACOL1016 | FABI | Enoyl-(acyl-carrier-protein) reductase | This study |
| SACOL1164 | Ecb | Extracellular complement binding protein | Merck |
| SACOL1173 | Hla | α-hemolysin | Merck |
| SACOL1637 | Hsp70 | Heat shock protein 70 | Merck |
| SACOL1789 | Mce | Mammalian cell entry protein | Merck |
| SACOL1902 | - | Hypothetical protein | Merck |
| SACOL2074 | - | D-alanyl-alanine synthetase | Merck |
| SACOL2451 | - | ABC Transporter | Merck |
| SACOL2493 | - | Hypothetical protein | Merck |
| SACOL2493 | - | Hypothetical protein | Merck |
In addition, Merck provided a panel of recombinant protein antigens (Table 2) that had previously displayed protective efficacy in their internal testing, and that also were immunogenic in macaques as determined by a Th17 Enzyme-Linked ImmunoSpot (ELISPOT) assay.
Bacteremia infection model
We re-struck fresh cultures of the USA300 strain S. aureus LAC (Los Angeles County Jail) from frozen stock monthly to ensure no loss of virulence. For the bacteremia infection model. Overnight cultures of LAC were subcultured 1:100 in fresh tryptic soy broth on the day of challenge, and harvested 3 hours later at OD600 of 0.5. Bacterial cells were pelleted by centrifugation and washed three times, resuspended in sterile PBS at a density of 7E7 CFU/200 μL, and injected intravenously into the tail vein of pre-warmed mice. Inocula were confirmed by plating serial dilutions on tryptic soy agar and counting colonies. Mice were followed for >28 days for signs of morbidity; mice that were moribund were euthanized according to IACUC guidelines.
Skin infection model
Skin infections were done as previously described.[25] For skin infections we used the USA300 clinical isolate SF8300 (provided by Henry Chambers, University of California, San Francisco).[26] Overnight cultures of SF8300 were subcultured in fresh tryptic soy broth at 1:100 on the day of challenge, and harvested 2–3 hours later at an approximate OD600 of 1.8. Bacterial cells were pelleted by centrifugation, washed, and resuspended in sterile PBS at a density of 1.5 x 107 CFU/50μL. Inocula were confirmed by plating serial dilutions on tryptic soy agar and counting colonies.
Mice were sedated, and their flanks were shaved and disinfected. Mice were injected subcutaneously with 50 μl of the S. aureus suspension at a concentration of 1.5 × 107 CFU/50 μl. Skin lesions were photographed each day for 15 days using a 100-mm2 square as a standard. The size of the lesion was measured using Adobe Photoshop software. To determine the bacterial burden, skin lesions were excised on day 7 post-infection. Serial dilutions of the tissue homogenates were plated on mannitol salt agar to quantify the bacterial load. For the lethal sepsis model, mice were challenged by tail vein injection of a 250-μl suspension of S. aureus of 2.5 × 108 CFU/mL and monitored for survival up to 28 days. At 24 hours post-infection, blood collected from tail vein is diluted and plated on mannitol salt agar to quantify bacterial burden.
Subcutaneous inoculation of mice on the flank with a USA300 CA-MRSA strain (SF8300) results in the formation of localized dermonecrotic lesions in 100% of mice. These lesions initially increase in size, before eventual spontaneous clearance by naïve animals. Control mice developed lesions that reached a maximal size within 1 day of challenge and then steadily decreased in size over the following two weeks. Mice that showed signs of immunity either developed lesions that were initially much smaller than those on control mice, had lesions that decreased in size more rapidly, or both.
Statistical analysis
Survival was compared by the nonparametric log-rank test. Surface staining and bacterial killing were compared with the Wilcoxon rank-sum test for unpaired comparisons. Group comparisons were done using the Wilcoxon rank-sum test. Differences were considered significant if the P value was < .05. All statistics were run using KyPlot software.
Supporting information
(ZIP)
Acknowledgments
This article was prepared while Susan Boyle-Vavra (aka Susan Daum) was employed at the University of Chicago. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Data Availability
All relevant data underlying the study are available within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Institute of Allergy and Infectious Diseases, R01 AI103342 to RD. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298: 1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 2.Fluit AC, Jones ME, Schmitz FJ, Acar J, Gupta R, Verhoef J. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin Infect Dis. 2000;30: 454–460. 10.1086/313710 [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39: 309–317. 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 4.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368: 874–885. 10.1016/S0140-6736(06)68853-3 [DOI] [PubMed] [Google Scholar]
- 5.Kumar N, David MZ, Boyle-Vavra S, Sieth J, Daum RS. High Staphylococcus aureus colonization prevalence among patients with skin and soft tissue infections and controls in an urban emergency department. J Clin Microbiol. 2015;53: 810–815. 10.1128/JCM.03221-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daum RS. Skin and Soft-Tissue Infections Caused by Methicillin-Resistant Staphylococcus aureus. N Engl J Med. Massachusetts Medical Society; 2007;357: 380–390. 10.1056/NEJMcp070747 [DOI] [PubMed] [Google Scholar]
- 7.Alegre M-L, Chen L, David MZ, Bartman C, Boyle-Vavra S, Kumar N, et al. Impact of Staphylococcus aureus USA300 Colonization and Skin Infections on Systemic Immune Responses in Humans. J Immunol. 2016;197: 1118–1126. 10.4049/jimmunol.1600549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray GT, Suaya JA, Baxter R. Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2013;76: 24–30. 10.1016/j.diagmicrobio.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 9.Patti JM. A humanized monoclonal antibody targeting Staphylococcus aureus. Vaccine. 2004;22 Suppl 1: S39–43. [DOI] [PubMed] [Google Scholar]
- 10.Weems JJ, Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, et al. Phase II, Randomized, Double-Blind, Multicenter Study Comparing the Safety and Pharmacokinetics of Tefibazumab to Placebo for Treatment of Staphylococcus aureus Bacteremia. Antimicrob Agents Chemother. 2006;50: 2751–2755. 10.1128/AAC.00096-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeely TB, Shah NA, Fridman A, Joshi A, Hartzel JS, Keshari RS, et al. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum Vaccin Immunother. 2014;10: 3513–3516. 10.4161/hv.34407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giersing BK, Dastgheyb SS, Modjarrad K, Moorthy V. Status of vaccine research and development of vaccines for Staphylococcus aureus. Vaccine. 2016;34: 2962–2966. 10.1016/j.vaccine.2016.03.110 [DOI] [PubMed] [Google Scholar]
- 13.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103: 16942–16947. 10.1073/pnas.0606863103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagonowsky E. Pfizer cans $500M staph vaccine hopeful after phase 2b trial failure [Internet]. [cited 19 Feb 2019]. Available: https://www.fiercepharma.com/vaccines/pfizer-s-s-aureus-vaccine-faces-uncertain-future-after-phase-2b-trial-failure
- 15.Spellberg B, Daum R. A new view on development of a Staphylococcus aureus vaccine: insights from mice and men. Hum Vaccin. 2010;6: 857–859. 10.4161/hv.6.10.12469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54: 560–567. 10.1093/cid/cir828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol. 2012;34: 335–348. 10.1007/s00281-011-0293-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, et al. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS One. 2012;7: e29446 10.1371/journal.pone.0029446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagnoli F, Fontana MR, Soldaini E, Mishra RPN, Fiaschi L, Cartocci E, et al. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A. 2015;112: 3680–3685. 10.1073/pnas.1424924112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baylor NW, Egan W, Richman P. Aluminum salts in vaccines—US perspective. Vaccine. 2002;20: S18–S23. [DOI] [PubMed] [Google Scholar]
- 21.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11: 865–872. 10.1038/nri3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. Vaccine Excipient & Media Summary [Internet]. 6 Jan 2017 [cited 1 Aug 2017]. Available: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- 23.Spellberg B, Ibrahim AS, Yeaman MR, Lin L, Fu Y, Avanesian V, et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun. 2008;76: 4574–4580. 10.1128/IAI.00700-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung AL, Bayer AS, Peters J, Ward JI. Analysis by gel electrophoresis, Western blot, and peptide mapping of protein A heterogeneity in Staphylococcus aureus strains. Infect Immun. 1987;55: 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194: 1761–1770. 10.1086/509506 [DOI] [PubMed] [Google Scholar]
- 26.Montgomery CP, Boyle-Vavra S, Daum RS. The arginine catabolic mobile element is not associated with enhanced virulence in experimental invasive disease caused by the community-associated methicillin-resistant Staphylococcus aureus USA300 genetic background. Infect Immun. 2009;77: 2650–2656. 10.1128/IAI.00256-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data underlying the study are available within the paper and its Supporting Information files.