Abstract
Background
The majority of studies that link antibiotic usage and resistance focus on simple associations between the resistance against a specific antibiotic and the use of that specific antibiotic. However, the relationship between antibiotic use and resistance is more complex. Here we evaluate selection and co-selection by assessing which antibiotics, including those mainly prescribed for respiratory tract infections, are associated with increased resistance to various antibiotics among Escherichia coli isolated from urinary samples.
Methods
Monthly primary care prescribing data were obtained from National Health Service (NHS) Digital. Positive E. coli records from urine samples in English primary care (n = 888,207) between April 2014 and January 2016 were obtained from the Second Generation Surveillance System. Elastic net regularization was used to evaluate associations between prescribing of different antibiotic groups and resistance against amoxicillin, cephalexin, ciprofloxacin, co-amoxiclav and nitrofurantoin at the clinical commissioning group (CCG) level. England is divided into 209 CCGs, with each NHS practice prolonging to one CCG.
Results
Amoxicillin prescribing (measured in DDD/ 1000 inhabitants / day) was positively associated with amoxicillin (RR 1.03, 95% CI 1.01–1.04) and ciprofloxacin (RR 1.09, 95% CI 1.04–1.17) resistance. In contrast, nitrofurantoin prescribing was associated with lower levels of resistance to amoxicillin (RR 0.92, 95% CI 0.84–0.97). CCGs with higher levels of trimethoprim prescribing also had higher levels of ciprofloxacin resistance (RR 1.34, 95% CI 1.10–1.59).
Conclusion
Amoxicillin, which is mainly (and often unnecessarily) prescribed for respiratory tract infections is associated with increased resistance against various antibiotics among E. coli causing urinary tract infections. Our findings suggest that when predicting the potential impact of interventions on antibiotic resistances it is important to account for use of other antibiotics, including those typically used for other indications.
Introduction
In England, approximately three-quarters of antibiotics are dispensed in primary care [1]. A substantial proportion of these antibiotics are unnecessary, being used for viral or self-limiting respiratory tract infections [2,3]. When antibiotics are used for a viral infection an effect on the pathogen causing the infection, both in terms of outcome of the infection as well as resistance against antibiotics, is not expected. However, because antibiotics typically used for respiratory tract infections, such as amoxicillin, have a systemic effect, they can select for antibiotic resistances among bacteria that are carried by the host at the moment of treatment, i.e. bacteria forming the microflora or microbiota [4]. If those bacteria are pathogenic or act as a reservoir of resistance elements this may lead to an increased incidence of symptomatic infections caused by bacteria that are resistant to clinically important antibiotics [5,6]. Moreover, antibiotic prescriptions are often longer than necessary, which could further increase antibiotic resistance levels without clinical benefit [7].
However, the relationship between antibiotic use and antibiotic resistance is more complex. A particular antibiotic may not only select for resistance against that same antibiotic i.e. ‘selection’, but also for resistance against other antibiotics i.e. ‘co-selection’. There may be cross-resistance between antibiotics, such as observed for ampicillin and amoxicillin [8]. Resistance genes may be linked on the same mobile genetic element, such as observed for amoxicillin and trimethoprim resistance genes [8,9]. Therefore treatment with one antibiotic may select for resistance against another antibiotic via cross-resistance and co-selection [8,9]. Treatment with one antibiotic may also simply kill competing bacteria, thereby providing bacteria resistant to another antibiotic more space and nutrients, such as anti-anaerobic antibiotics that promote the overgrowth of vancomycin-resistant enterococci [10,11]. Moreover, mutations or acquired genes conferring resistance to one antibiotic can not only increase but also decrease resistance to another antibiotic [12]. Such collateral sensitivity, where resistance against one antibiotic confers sensitivity against another has been mainly explored for spontaneous resistance mutations [12,13].
The vast majority of studies that link antibiotic usage and resistance at the population level focus on simple associations between the resistance against a specific antibiotic and the use of that specific antibiotic or antibiotic group, or alternatively group all antibiotics together [14]. There is a lack of studies that simultaneously take into account use of different antibiotics and potential co-selection.
We therefore evaluated associations between prescribing levels of antibiotic groups in primary care across geographical areas in England and resistance against amoxicillin, cephalexin, ciprofloxacin, co-amoxiclav and nitrofurantoin, among Escherichia coli isolated from urinary samples across these same areas in England, thereby taking into account prescribing of other antibiotics groups. Because we only had data on antibiotic prescribing in primary care, we focused on E. coli sampled from the urinary tract by general practitioners. We used elastic net regularization [15,16], because this method–which combines the advantages of both least absolute shrinkage and selection operator (lasso) [17] and ridge regression [18]–works particularly well in situations with high collinearity and relative large number of variables compared to the amount of observations [15,16]. This is particularly relevant, because there are many different antibiotic groups and there are likely strong correlations between prescribing patterns of antibiotics leading to sparsity and multicollinearity problems with standard regression techniques [19].
The vast majority urinary tract infections are caused by E. coli infections and uropathogenic E. coli strains are often part of the human intestinal microbiota. Given the systemic nature of systemic antibiotics, this research may shed light on the question whether and to what extent antibiotics typically being used to treat (viral) respiratory tract infections, such as amoxicillin [1], may result in resistance problems against not only the same antibiotic, but also other antibiotics among bacteria for which the antibiotic courses were not initially intended.
The work presented in this paper provides evidence about which antibiotics are associated with higher and lower levels of antibiotic resistance against common antibiotics among Escherichia coli bacteria sampled from the urinary tract by comparing antibiotic prescribing and resistance in different geographical areas in England. We evaluated variation in prescribing by geographic area in lieu of linked data on antibiotic prescribing and antibiotic resistance at an individual patient level. However, unmeasured confounding may actually be larger at the individual patient level. While patient characteristics that are also prognostic factors of having a resistant infection are influencing decisions to prescribe an antibiotic to an individual patient [14,20], variation in prescribing between general practices or areas is to a large extent due to the general tendency to prescribe antibiotics [21,22]. In addition, with geographical data it is more likely that the outcome of one unit is unaffected by the particular assignment of exposure to other units than when analysing individual patient level data, thereby increasing the chances of meeting the Stable Unit Treatment Value Assumption (SUTVA).
Our models show that amoxicillin, the most commonly used antibiotic in England and mainly used for respiratory tract infections, is associated with increased resistance against several other antibiotics among bacteria causing urinary tract infections. The methods used in this study, that overcome several of the limitations of previous studies, can be used to explore the complex relationships between antibiotic use and antibiotic resistance in other settings.
Methods
Data
All data were collected as part of routine surveillance and were anonymized. Ethics Committee approval was therefore not required. Antibiotic prescribing data were obtained from NHS Digital, who collate for all general practices in England the total number of items that are prescribed and dispensed (http://digital.nhs.uk/). Antibiotic groups were created based on the first five characters of the Anatomical Therapeutic Chemical (ATC) classification system (Fig 1). Antibiotic prescribing was expressed in daily defined doses (DDDs) per 1000 persons per day for each calendar month at the clinical commissioning group (CCG) level. Antibiotics were expressed in DDDs as this at least partly captures the dose and duration of treatment, while this would not be the case when expressing use in terms of items. This is important, because dose and duration has been shown to be an important driver of antibiotic resistance [23–25]. Moreover, using DDDs would facilitate incorporating of hospital prescribing when this data becomes available, as antibiotics used in the hospital are typically expressed in terms of DDDs. CCGs were set up by the Health and Social Care Act 2012 to organize the delivery of NHS services in England. From April 2018, general practices in England belong to one of 209 CCGs.
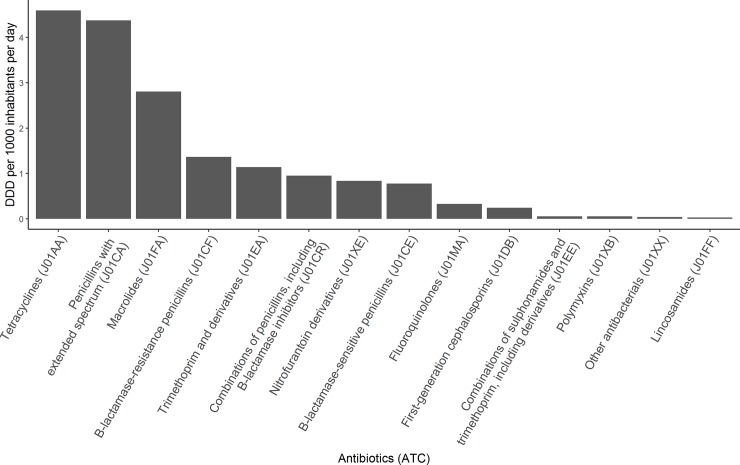
Fig 1. The average DDD per 1000 inhabitants per day for the 14 most common antibiotic groups during the study period.
Antibiotic groups–e.g. Tetracyclines (J01AA) were created based on the first 5 characters of the Anatomical Therapeutic Chemical classification system.
Reports of E. coli isolated from urine samples from general practice patients between April 2014 and January 2016 in England were extracted from PHE’s Second Generation Surveillance System (SGSS) (https://fingertips.phe.org.uk/profile/amr-local-indicators) (Data in S3 File). Therefore, urine samples taken in the hospital were excluded from this study. SGSS is a national voluntary laboratory surveillance system which captures antimicrobial susceptibility data of all microorganisms tested. The database contains laboratory reports supplied electronically by approximately 98% of NHS hospital microbiology laboratories in England. Repeat specimen reports received from the same patient with matching causative agents were excluded if the specimen dates were within 30 days [8]. A 30 day cut-off is often used to distinguish between same and new urinary tract infection episodes. Both samples categorized as intermediate (I) and resistant (R) were treated as being resistant. The following antibiotic susceptibility test results for E. coli urine samples were analyzed: amoxicillin, cephalexin, ciprofloxacin, co-amoxiclav and nitrofurantoin. At least 75% of reported E. coli urine isolates extracted from SGSS were tested for resistance against these antibiotics; levels of susceptibility testing for other antibiotics were not reported frequently enough for a useful analysis. For each calendar month the number of samples tested for resistance against each antibiotic and the number of samples confirmed as resistant against each antibiotic were measured at the CCG level. Measurements were only included when at least 10 samples and at least 75% of samples were tested for resistance against the antibiotic of interest in the CCG.
Analyses
Elastic net regularization was used to evaluate the association between the different antibiotic groups and the five resistances of interest [8,15,16]. Elastic net regularization combines the advantages of least absolute shrinkage and selection operator (lasso) [17] and ridge regression [18]. Elastic net regularization is especially useful when encountering situations with high collinearity, such as strong correlations in antibiotic usage, and a relatively large number of variables (antibiotic groups) compared to the amount of observations [16,18,19]. More conventional regression techniques would likely result in multi-collinearity and sparsity bias issues [18,19].
We fitted a separate Poisson model with elastic net regularization for each resistance. The number of E. coli isolates from urinary samples reported to be resistant each month was included as the dependent variable. The natural logarithm of the number of samples being tested was included as an offset to account for the fact that there is variation in the number of samples tested between CCGs, thereby effectively modelling resistance as a proportion.
Potential explanatory variables were all antibiotics groups (e.g. ATC codes J01AA and J01CA) prescribed in the month before the monthly measured resistance prevalence (expressed in DDDs per 1000 persons per day), month of the year, calendar year and the test rate. The test rate was defined as the number of E. coli urinary samples tested for the resistance of interest per 1000 persons-months. The test rate was included because we have previously observed a relatively strong negative relationship between the test rate and the proportion of samples that are resistant [8].Antibiotics and the test rate were standardized by mean-centering and dividing by two standard deviations. To keep the antibiotic groups on the same scale, all antibiotic groups were mean-centered and divided by two standard deviations of penicillins with extended spectrum (ATC code J01CA) instead of using the standard deviations of individual antibiotic groups. To keep all variables, including binary (dummy) variables, on the same scale all variables were divided by two standard deviations [26]. After performing the elastic net regularization variables were back transformed to the original ‘DDD per 1000 persons per day’ scale.
All elastic net analyses were performed using the ‘glmnet’ package in R version 3.4.3 [16]. To reduce the false discovery rate often observed with standard application of regularization methods, we estimated the optimal shrinkage parameter λ using the Akaike information criterion (AIC) [8,27]. Confidence intervals (CIs) were obtained by taking 1000 clustered bootstrap samples, resampling at the highest level (CCG) with replacement.
Secondary analysis
In a secondary analysis we varied the lag time between antibiotic prescribing and the resistances of interest. First, instead of using antibiotic prescribing in the month before the resistance measurement as a potential covariate, we evaluated the association between antibiotics used in 1–3 months before the resistance measurements. Further, we assessed the association between antibiotics used in the year (1–12 months) before the resistance measurements.
In addition, we performed a sensitivity analysis, restricting to months with at least 20 measurements to assess the potential influence of potential random error and systematic error due to low sampling rates.
Results
The antibiotic groups that were used most intensively with ≥1 daily defined doses (DDD) per 1000 inhabitants per day, were tetracyclines, penicillins with extended spectrum (mainly amoxicillin) [1], macrolides, Beta-lactamase-resistant penicillins (mainly Flucloxacillin) [1], and trimethoprim (Fig 1).
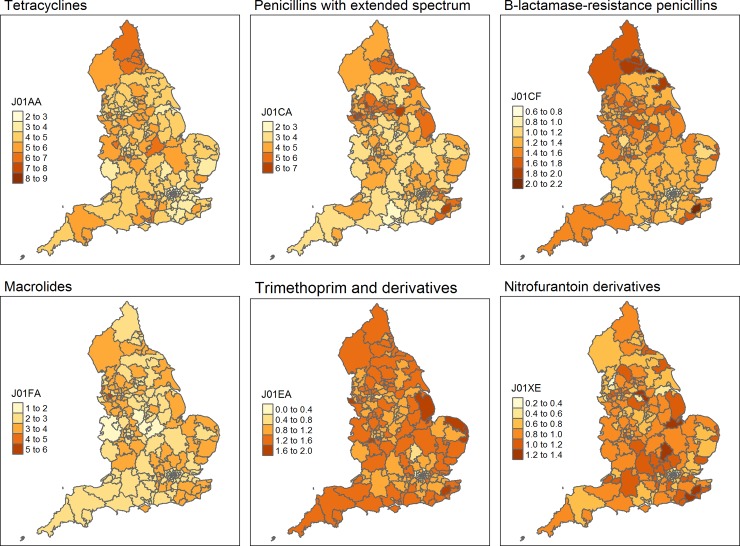
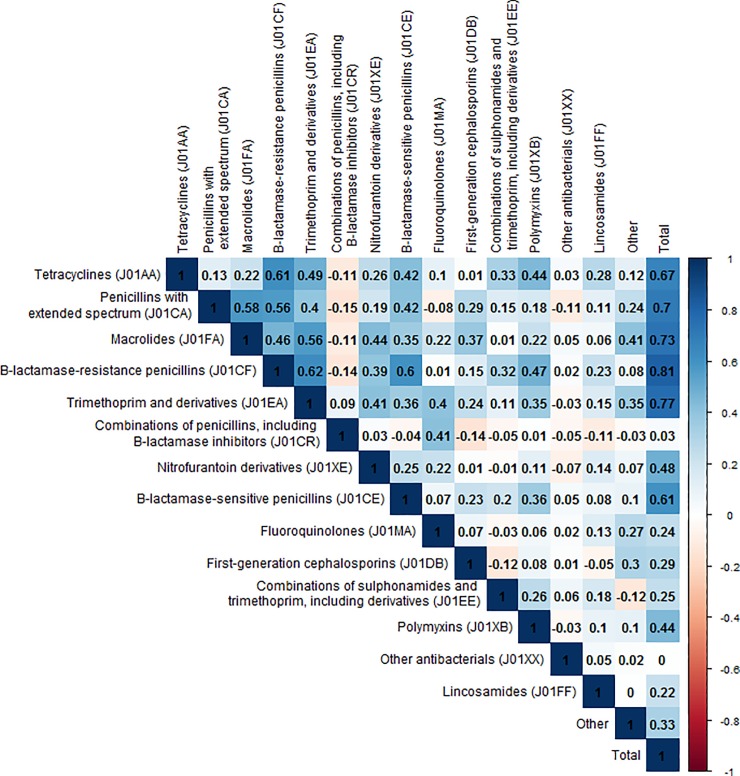
While we evaluated the association between resistances of interest and all antibiotic groups, Fig 2 shows the variation in prescribing between the different clinical commissioning groups (CCGs) for the 4 antibiotic groups that are prescribed the most. In addition, these maps show the variation in nitrofurantoin and trimethoprim, which are the antibiotics typically used to treat urinary tract infections. There was substantial variation in antibiotic prescribing between the different CCGs (Fig 2), some CCGs had high antibiotic prescribing levels for all antibiotics, especially in the North of England. There was generally more variation in antibiotic prescribing between CCGs than variation over time within CCGs. However, for some antibiotics there were clear peaks or seasonality in the amount of dispensed antibiotics, in line with peaks in the incidence of respiratory tract infections (winter) and skin infections (summer) (Figure A and B in S1 File). Univariate correlations between prescribing of the different antibiotic groups are shown in Fig 3. In general there were positive correlations between prescribing of different antibiotic groups. However, prescribing of combinations of penicillins (mainly co-amoxiclav) was negatively correlated with several other commonly prescribed antibiotics.
Fig 2. Maps of the average number of DDD per 1000 inhabitants per day for the 209 clinical commissioning groups during the study period.
Not that different scales are used for the different antibiotics. J01AA = tetracyclines; J01CA = penicillins with extended spectrum (mainly amoxicillin); J01CF = Beta-lactamase-resistant penicillins (mainly flucloxacillin); J01FA = macrolides; J01EA = trimethoprim; J01XE = nitrofurantoin.
Fig 3. Univariate correlations between prescribing of the different antibiotic groups.
Antibiotic groups–e.g. Tetracyclines (J01AA) were created based on the first 5 characters of the Anatomical Therapeutic Chemical classification system.
Between April 2014 and January 2016, nearly all (99%, n = 888,207) E. coli urinary samples from general practice patients sent in for laboratory testing were tested for resistance against nitrofurantoin. The percentages of samples tested for resistance against the other included antibiotics varied between 78% for amoxicillin and 90% for co-amoxiclav.
There was substantial variation in the percentage of E. coli urinary isolates that were resistant to the antibiotics tested (Table 1).
Table 1. Variation in antibiotic resistance among E. coli urinary samples, measured on a monthly basis at the clinical commissioning group level.
| Percentage of E. coli samples resistant to antibiotic, median (25th– 75th percentile) | |
|---|---|
| Amoxicillin | 53% (49% - 58%) |
| Nitrofurantoin | 2% (1% - 4%) |
| Cephalexin | 8% (6% - 11%) |
| Ciprofloxacin | 11% (8% - 16%) |
| Co-amoxiclav | 11% (7% - 23%) |
There was less variation in the percentage of isolates that were resistant to the antibiotics test over time (Figures C-G in S1 File). The variation in testing rate, which may influence apparent antibiotic resistance proportions, and variation in the measured antibiotic resistance proportions are shown in Fig 4. As is apparent from the maps, part of the variation in the apparent proportion of samples that are resistant to antibiotics can be explained by the test rate. When few tests are determined, most of the samples are resistant. However, there are also regions with a relatively high test rate and still relatively high resistance, such as in the North-East, indicating that the resistance prevalence may indeed be relatively high.
Fig 4. Proportion of samples resistant to antibiotics and antibiotic resistance test rates.
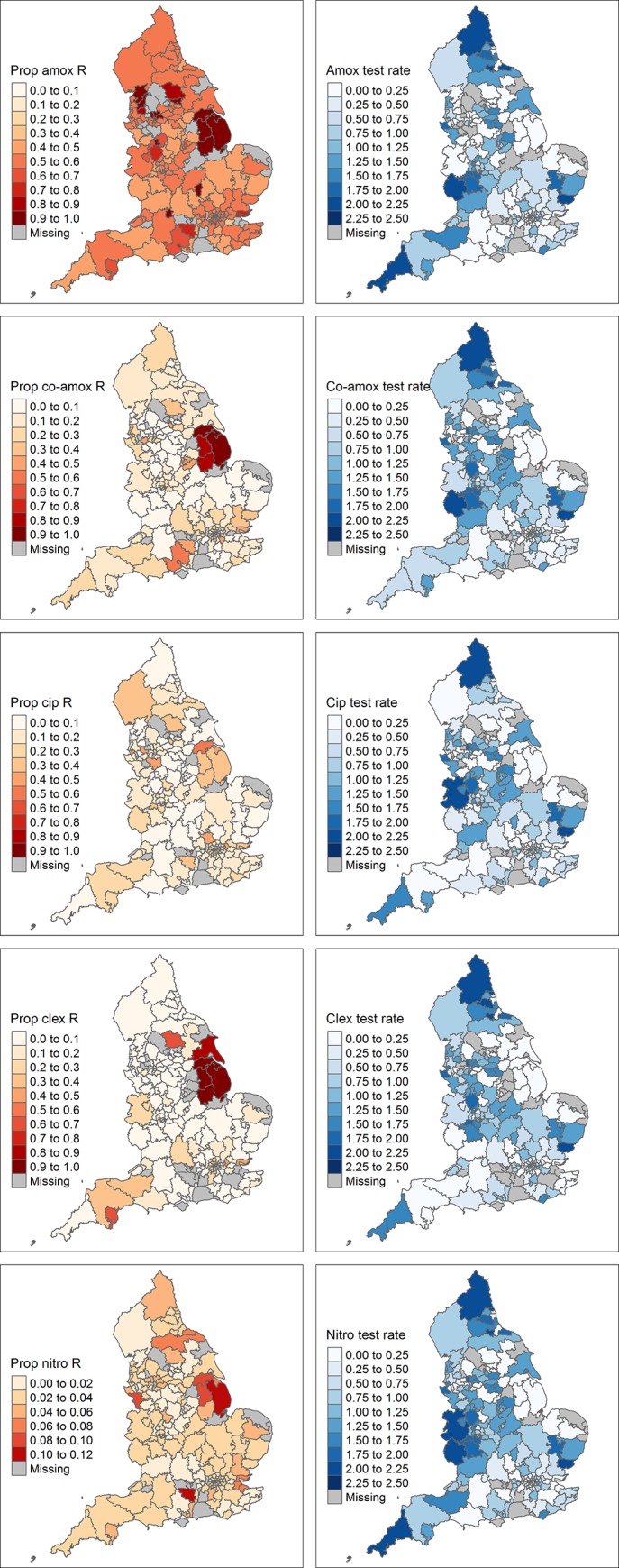
The left column shows the proportion of E. coli urinary samples that are resistant to amoxicillin, co-amoxiclav, ciprofloxacin, cephalexin and nitrofurantoin, respectively. The right column shows the number of samples tested for resistance against these antibiotics per 1000 person-months.
Results from the elastic net regularization models showed amoxicillin resistance was positively associated with prescribing of penicillins with extended spectrum (mainly amoxicillin in England) [1] in the month (RR 1.03, 95%CI 1.01 to 1.04), quarter (RR 1.03, 95%CI 1.01 to 1.04) and year (RR 1.04, 95%CI 1.01 to 1.06) (Table 2; the full results including the coefficients for the test rate are provided in Table A in S2 File) before the specimen date.
Table 2. Associations between amoxicillin resistance among E. coli urinary samples and antibiotic prescribing (DDD per 1000 persons per day).
| Antibiotic prescribed | Amoxicillin resistance, antibiotic prescribing 1 month before. RR (2.5th–97.5th percentile of bootstrap) | Amoxicillin resistance, antibiotic prescribing 1–3 month before. RR (2.5th–97.5th percentile of bootstrap) | Amoxicillin resistance, antibiotic prescribing 1 year before. RR (2.5th–97.5th percentile of bootstrap) |
|---|---|---|---|
| Tetracyclines (J01AA) | 1.00 (0.98–1.01) | 1.00 (0.98–1.02) | 1.00 (0.98–1.04) |
| Penicillins with extended spectrum (J01CA) | 1.03 (1.01–1.04)a | 1.03 (1.01–1.04) a | 1.04 (1.01–1.06) a |
| Beta-lactamase-sensitive penicillins (J01CE) | 1.02 (0.97–1.12) | 1.00 (1.00–1.19) | - |
| Beta-lactamase-resistant penicillins (J01CF) | 1.03 (0.98–1.12) | 1.03 (0.96–1.13) | 1.04 (0.95–1.17) |
| Combinations of penicillins, including β-lactamase inhibitors (J01CR) | 1.02 (0.95–1.08) | - | - |
| First-generation cephalosporins (J01DB) | 1.01 (0.91–1.07) | - | - |
| Second-generation cephalosporins (J01DC) | 1.00 (0.85–1.02) | - | - |
| Trimethoprim and derivatives (J01EA) | 1.01 (0.98–1.08) | 1.01 (1.00–1.12) | 1.00 (1.00–1.17) |
| Macrolides (J01FA) | 0.99 (0.97–1.02) | 1.00 (0.97–1.03) | 1.00 (0.97–1.04) |
| Lincosamides (J01FF) | 0.98 (0.71–1.00) | - | - |
| Fluoroquinolones (J01MA) | 0.93 (0.78–0.99) a | 0.87 (0.69–1.00) | 0.88 (0.61–1.00) |
| Polymyxins (J01XB) | 1.01 (0.93–1.35) | - | - |
| Nitrofuran derivatives (J01XE) | 0.92 (0.84–0.97) a | 0.91 (0.82–0.96) a | 0.91 (0.83–0.98) a |
| Other antibacterials (J01XX) | 0.98 (0.87–1.13) | - | - |
aAssociations for which 2.5th and 97.5th percentile of the clustered bootstrap are both indicating an increased or decreased risk. Results are adjusted for differences in the test rate, time and use of other antibiotics (full model results shown in Table A in S2 File).
A similar direct association was seen in that CCGs that used more nitrofurantoin had a higher percentage of E. coli samples that tested resistant to nitrofurantoin (RR 1.52, 95%CI 1.00 to 2.24) (Table 3). The data did not confirm such a relationship between first-generation cephalosporin use (mainly cephalexin in England) [1] and cephalexin resistance, between fluoroquinolone use (mainly ciprofloxacin in England) [1] and ciprofloxacin resistance, or between combinations of penicillins, including β-lactamase inhibitors (mainly co-amoxiclav in England) [1] and co-amoxiclav resistance (Tables 4–6).
Table 3. Associations between nitrofurantoin resistance among E. coli urinary samples and antibiotic prescribing (DDD per 1000 persons per day).
| Antibiotic prescribed | Nitrofurantoin resistance, antibiotic prescribing 1 month before. RR (2.5th–97.5th percentile of bootstrap) | Nitrofurantoin resistance, antibiotic prescribing 1–3 month before. RR (2.5th–97.5th percentile of bootstrap) | Nitrofurantoin resistance, antibiotic prescribing 1 year before. RR (2.5th–97.5th percentile of bootstrap) |
|---|---|---|---|
| Tetracyclines (J01AA) | 1.01 (0.89–1.14) | 1.02 (0.91–1.19) | 1.01 (0.83–1.23) |
| Penicillins with extended spectrum (J01CA) | 1.02 (0.96–1.15) | 1.03 (0.96–1.21) | 1.05 (0.91–1.28) |
| Beta-lactamase-sensitive penicillins (J01CE) | 1.50 (0.73–2.02) | 1.46 (0.61–2.09) | 1.47 (0.47–2.31) |
| Beta-lactamase-resistant penicillins (J01CF) | 1.01 (0.75–1.72) | - | - |
| Combinations of penicillins, including β-lactamase inhibitors (J01CR) | - | - | 1.01 (0.21–3.04) |
| First-generation cephalosporins (J01DB) | 1.76 (0.56–3.18) | 1.78 (0.41–3.27) | 1.85 (0.27–7.39) |
| Trimethoprim and derivatives (J01EA) | 1.52 (1.15–2.08)a | 1.50 (1.10–2.24) a | 1.55 (1.00–2.56) |
| Combinations of sulfonamides and trimethoprim, including derivatives (J01EE) | - | - | 1.02 (0.09–56.66) |
| Macrolides (J01FA) | 0.93 (0.80–1.06) | 0.92 (0.78–1.09) | 0.90 (0.72–1.06) |
| Other aminoglycosides (J01GB) | 45.87 (1.00–2.12x103) | 59.16 (1.00–2.34*104) | 21.05 (1.00–2.77*104) |
| Polymyxins (J01XB) | - | - | 1.02 (0.10–12.39) |
| Nitrofuran derivatives (J01XE) | 1.52 (1.00–2.24) a | 1.60 (1.05–2.58) a | 1.68 (1.01–3.04) a |
| Other antibacterials (J01XX) | 0.84 (0.47–1.89) | 0.85 (0.48–2.14) | 0.76 (0.40–2.59) |
a Associations for which 2.5th and 97.5th percentile of the clustered bootstrap are both indicating an increased or decreased risk. Results are adjusted for differences in the test rate, time and use of other antibiotics (full model results shown in Table B in S2 File).
Table 4. Associations between cephalexin resistance among E. coli urinary samples and antibiotic prescribing (DDD per 1000 persons per day).
| Antibiotic prescribed | Cephalexin resistance, antibiotic prescribing 1 month before. RR (2.5th–97.5th percentile of bootstrap) | Cephalexin resistance, antibiotic prescribing 1–3 month before. RR (2.5th–97.5th percentile of bootstrap) | Cephalexin resistance, antibiotic prescribing 1 year before. RR (2.5th–97.5th percentile of bootstrap) |
|---|---|---|---|
| Tetracyclines (J01AA) | 0.97 (0.89–1.02) | 0.97 (0.86–1.02) | 0.96 (0.85–1.05) |
| Penicillins with extended spectrum (J01CA) | 0.99 (0.94–1.06) | 0.98 (0.90–1.06) | 0.96 (0.87–1.08) |
| Beta-lactamase-sensitive penicillins (J01CE) | 0.98 (0.63–1.00) | 0.97 (0.56–1.00) | 0.95 (0.42–1.00) |
| Beta-lactamase-resistant penicillins (J01CF) | 0.98 (0.70–1.35) | 0.98 (0.73–1.45) | 1.00 (0.69–1.65) |
| Combinations of penicillins, including β-lactamase inhibitors (J01CR) | 0.98 (0.69–1.10) | 0.97 (0.67–1.13) | 0.95 (0.59–1.15) |
| First-generation cephalosporins (J01DB) | 1.01 (0.94–1.41) | 1.01 (0.95–1.50) | 1.04 (0.92–1.78) |
| Trimethoprim and derivatives (J01EA) | 1.03 (0.98–1.75) | 1.04 (0.98–1.87) | 1.08 (0.97–2.04) |
| Macrolides (J01FA) | 0.95 (0.75–1.00) | 0.94 (0.73–1.00) | 0.92 (0.70–1.00)a |
| Fluoroquinolones (J01MA) | 1.00 (0.81–1.30) | 1.00 (0.77–1.34) | 1.01 (0.63–1.44) |
| Nitrofuran derivatives (J01XE) | 1.01 (0.94–1.44) | 1.01 (0.94–1.51) | 1.04 (0.93–1.72) |
| Other antibacterials (J01XX) | - | 1.00 (0.91–1.52) | - |
a Associations for which 2.5th and 97.5th percentile of the clustered bootstrap are both indicating an increased or decreased risk. Results are adjusted for differences in the test rate, time and use of other antibiotics (full model results shown in Table C in S2 File).
Table 6. Associations between co-amoxiclav resistance among E. coli urinary samples and antibiotic prescribing (DDD per 1000 persons per day).
| Antibiotic prescribed | Co-amoxiclav resistance, antibiotic prescribing 1 month before. RR (2.5th–97.5th percentile of bootstrap) | Co-amoxiclav resistance, antibiotic prescribing 1–3 month before. RR (2.5th–97.5th percentile of bootstrap) | Co-amoxiclav resistance, antibiotic prescribing 1 year before. RR (2.5th–97.5th percentile of bootstrap) |
|---|---|---|---|
| Tetracyclines (J01AA) | 1.03 (0.89–1.31) | 1.04 (0.87–1.33) | 1.04 (0.87–1.35) |
| Penicillins with extended spectrum (J01CA) | 0.99 (0.74–1.00) | 0.98 (0.70–1.00) | 0.97 (0.68–1.02) |
| Beta-lactamase-sensitive penicillins (J01CE) | - | - | 1.00 (1.00–8.86) |
| Combinations of penicillins, including β-lactamase inhibitors (J01CR) | - | - | 1.00 (0.51–2.62) |
| Trimethoprim and derivatives (J01EA) | - | - | 1.00 (0.54–2.30) |
a Associations for which 2.5th and 97.5th percentile of the clustered bootstrap are both indicating an increased or decreased risk. Results are adjusted for differences in the test rate, time and use of other antibiotics (full model results shown in table E in S2 File).
However, it should be noted that a substantial proportion of these specific antibiotics are used in the hospital settings, for which no data was available [28]. Besides the obvious associations between prescribing of a particular antibiotic and resistance to that same antibiotic, we also observed associations between prescribing of a particular antibiotic and resistance against an antibiotic from another group. Amoxicillin use was not only associated with higher levels of amoxicillin resistance, but also with increased ciprofloxacin resistance (RR 1.09 95%CI 1.04 to 1.17) (Table 5) and increased trimethoprim resistance (as we have previously shown [8]). CCGs with high prescribing of trimethoprim also had higher levels of nitrofurantoin resistance (RR 1.52 95%CI 1.15 to 2.08) and ciprofloxacin resistance (RR 1.34 95%CI 1.10 to 1.59) (Tables 3 and 5).
Table 5. Associations between ciprofloxacin resistance among E. coli urinary samples and antibiotic prescribing (DDD per 1000 persons per day).
| Antibiotic prescribed | Ciprofloxacin resistance, antibiotic prescribing 1 month before. RR (2.5th–97.5th percentile of bootstrap) | Ciprofloxacin resistance, antibiotic prescribing 1–3 month before. RR (2.5th–97.5th percentile of bootstrap) | Ciprofloxacin resistance, antibiotic prescribing 1 year before. RR (2.5th–97.5th percentile of bootstrap) |
|---|---|---|---|
| Tetracyclines (J01AA) | 0.92 (0.88–0.98)a | 0.92 (0.87–0.98) a | 0.93 (0.85–1.01) |
| Penicillins with extended spectrum (J01CA) | 1.09 (1.04–1.17) a | 1.10 (1.03–1.19) a | 1.13 (1.02–1.25) |
| Beta-lactamase-resistant penicillins (J01CF) | - | - | 0.96 (0.65–1.24) |
| Trimethoprim and derivatives (J01EA) | 1.34 (1.10–1.59) a | 1.35 (1.13–1.72) a | 1.35 (1.11–1.78) a |
| Macrolides (J01FA) | 0.85 (0.76–0.94) a | 0.84 (0.75–0.94) a | 0.84 (0.73–0.95) a |
| Fluoroquinolones (J01MA) | 1.24 (1.00–2.81) | 1.29 (1.00–3.55) | 1.38 (1.00–4.40) |
| Nitrofurantoin | - | - | 1.00 (0.65–1.14) |
a Associations for which 2.5th and 97.5th percentile of the clustered bootstrap are both indicating an increased or decreased risk. Results are adjusted for differences in the test rate, time and use of other antibiotics (full model results shown in Table D in S2 File).
There were also some antibiotics that had negative associations with antibiotic resistances. Nitrofurantoin use was associated with decreased amoxicillin resistance (RR 0.92 95%CI 0.84 to 0.97) (Table 2). Previously, we observed a similar negative association between nitrofurantoin use and trimethoprim resistance levels [8]. Tetracycline and macrolide use was associated with decreased ciprofloxacin resistance (Table 5), while fluoroquinolone use was associated with lower amoxicillin resistance levels (Table 2).
Results were very similar when restricting the analyses to months with at least 20 measurements (Tables F-J in S2 File), suggesting that random error or remaining systematic error due to low testing probability were not large after adjusting for the test rate.
Discussion
We found evidence of both selection and co-selection, as well as geographical patterns in antibiotic use and resistance. Amoxicillin use, an antibiotic that is mainly used for respiratory tract infections (~83%) and rarely for urinary tract infections (~2%) [1], is associated with increased resistance against amoxicillin and ciprofloxacin among urinary tract infections caused by E. coli. Areas that used more trimethoprim had higher levels of ciprofloxacin and nitrofurantoin resistance among E. coli urinary samples. These positive associations between prescribing of a particular antibiotic and resistance against another antibiotic suggest that co-selection may play a role.
We found that use of amoxicillin and trimethoprim were associated with resistance against ciprofloxacin, which suggests that co-selection may be occurring. Isolates from the common E. coli urinary pathogenic clonal group ST131 are often non-susceptible to both fluoroquinolones and trimethoprim-sulfamethoxazole and/or β-lactam antibiotics [29,30], which may explain why use of trimethoprim and amoxicillin would select for ciprofloxacin resistance as amoxicillin use likely also selects for bacteria with trimethoprim resistance genes [8]. This link is further supported by the ECO·SENS study that found that resistance to any agent was correlated with increased resistance to all other agents tested, except for fosfomycin [9].
Nitrofurantoin had a negative association with amoxicillin resistance. This is in line with our previous finding that areas with relatively high nitrofurantoin use have lower trimethoprim resistance levels.7 Nitrofurantoin resistance genes are, in contrast to trimethoprim and amoxicillin resistance genes, not frequently found on mobile genetic elements with multiple resistances or correlated with multiple resistances in other ways [8,11,31]. Therefore, nitrofurantoin use may select for E. coli that are susceptible to amoxicillin and trimethoprim. Collateral sensitivity (and collateral resistance) has previously been observed for resistance against several antibiotics among E. coli isolates in an experimental setting [13]. Selecting for resistance against ampicillin was associated with increased sensitivity to nitrofurantoin compared to wild type E. coli strains [13], suggesting that collateral sensitivity may partly explain the observed negative association between amoxicillin and trimethoprim.
Given the high fitness cost of nitrofurantoin resistance [31], the positive association between trimethoprim use and nitrofurantoin resistance is not likely due to co-selection, but may be due to the possibility that CCGs with high trimethoprim usage have more patients on long-term treatment or prophylaxis with trimethoprim and nitrofurantoin.
The negative association between prescribing of tetracyclines or macrolides and ciprofloxacin resistance is harder to explain. Macrolides are typically active against Gram-positive bacteria, although they can be effective against Gram-negative bacteria when used in combination with antibiotics that do have outer-membrane disruptive activity [32]. Given the lack of selective pressure, E coli are unlikely to frequently harbor resistance mechanisms against antibiotics like macrolides, which could make such a synergistic combination therapy particularly effective against Gram-negative bacteria resistant to multiple antibiotics including ciprofloxacin [32]. However, this is unlikely the cause of the negative association between macrolide use and ciprofloxacin resistance, as such a combination therapy is not frequently being used or necessary in England. Collateral sensitivity has been observed for resistance against azithromycin (a macrolide) and sensitivity to nalidixic acid (a quinolone) among a pathogenic E. coli strain [13]. This may partly explain why macrolides use had a negative association with ciprofloxacin resistance. However, further studies are needed to evaluate whether these associations are causal.
Amoxicillin is the most frequently used antibiotic for respiratory conditions which are responsible for the largest share in inappropriate antibiotic prescribing in primary care [1–3]. Based on the current and a previous study, amoxicillin prescribing appears to be associated with increased resistance to amoxicillin, ciprofloxacin and trimethoprim [8]. Together these findings suggest that there is a substantial potential to reduce selective pressure via (co-)selection through reduction in the amount of unnecessary treatment with amoxicillin.
In many countries nitrofurantoin has been adopted as the first-line treatment for uncomplicated urinary tract infections [33,34]. Compared to other European countries, such as the Netherlands, the proportion of urinary tract infections treated with nitrofurantoin is much lower in England, though between June 2017 and June 2018 the ratio of trimethoprim prescribing over trimethoprim plus nitrofurantoin prescribing substantially decreased from 0.53 to 0.38, subsequent to the national quality premium [1,34].
Recent recommendations are to prescribe nitrofurantoin as the first choice treatment for uncomplicated urinary tract infections [8]. We found nitrofurantoin prescribing to be associated with lower levels of resistance to amoxicillin and it has a negative association with trimethoprim resistance [8]. Conversely, we previously showed trimethoprim prescribing to be associated with increased resistance to trimethoprim [8], and, here, associated with high ciprofloxacin resistance.
Our findings therefore suggest that a shift towards more nitrofurantoin instead of trimethoprim for uncomplicated urinary tract infections could potentially reduce antibiotic resistance among E. coli.
Patterns of co-resistance and co-selection likely differ between various parts of the world as there is substantial variation in selection pressure by antibiotics and infection prevention and control between countries [35,36]. However, we would even caution against direct comparison of results from another recent study from a region in England [37], as that study did not take into account prescribing of other antibiotics or the potential differences in the propensity to send in samples from patients. Our results show that areas with low testing rates have artificial high resistance proportions. This finding emphasizes the importance of accounting for differences in testing practices when comparing resistance prevalences between different countries or more granular areas. Ideally sentinel surveillance systems with systematic and standardized testing would be set up to facilitate less biased between-area comparisons and local pre-test resistance probabilities, thereby potentially improving future association studies and clinical practice.
The most obvious limitation of this work is that the associations we found are not necessarily causal. As with any observational study we could not take into account confounding by unmeasured factors, such as antibiotic use in hospitals and other potential selective pressures. Although we only included samples taken from patients presenting in primary care, the unavailability of hospital prescribing data may have especially affected the analyses focusing on co-amoxiclav and cephalexin as less than half of co-amoxiclav prescribing and approximately half of cephalosporin prescribing occurs in the general practice setting [28]. In addition roughly 40% of fluoroquinolones are prescribed in the hospital setting in England [28]. Such misclassification of exposure/confounders makes the estimated impact of antibiotics that are commonly used in the hospital less reliable. This may partly explain why amoxicillin use–only 13% of penicillins are used in the hospital [28]–is associated with ciprofloxacin resistance, while fluoroquinolone use was not associated with increased amoxicillin prescribing.
Besides the influence of unmeasured confounding, we cannot exclude the possibility that prescribing differs as a consequence of resistance rather than the other way around, i.e. reverse causation. We tried to reduce such reverse causation by looking at antibiotic prescribing happening before the resistance measurement. Nonetheless, reverse causation may reduce the strength of a positive association or even reverse the association. In addition, some of our results may be partly due to other types of model misspecification.
If we were only interested in the influence of one particular antibiotic, a regularization approach that penalizes all coefficients except the antibiotic of interest might provide better estimates [38]. However, because different antibiotics may influence resistance levels in various ways, we decide to penalise all coefficients in the same way, potentially leading to an underestimation of the effect of antibiotics that increase the prevalence of resistance. Therefore, our estimates should be regarded as conservative.
Given the limitations described above and the relatively small effect sizes observed, one should be cautious in making policy recommendations based on our observations alone. Nevertheless, our results add to the increasing body of evidence that suggest that co-selection may play a relevant role in driving antibiotic resistance rates.
Conclusions
Amoxicillin prescribing is associated with increased resistance to amoxicillin, ciprofloxacin and trimethoprim. Amoxicillin is the most frequently used antibiotic for respiratory conditions, which are responsible for the largest share in inappropriate antibiotic prescribing in primary care. These findings suggest that there is a potential to reduce selective pressure via (co-)selection with unnecessary use of amoxicillin for viral and self-limiting respiratory tract infection.
Nitrofurantoin prescribing is associated with lower levels of resistance to amoxicillin and trimethoprim, while trimethoprim prescribing is associated with increased levels of amoxicillin, ciprofloxacin and trimethoprim resistance. This suggests that replacing trimethoprim prescribing with nitrofurantoin prescribing where possible for uncomplicated urinary tract infections may also be associated with a reduction in trimethoprim, amoxicillin and ciprofloxacin resistance among E. coli. The methodology used in this study, that can cope with correlated antibiotic use, can be used in other settings to further explore the complex relationships between antibiotic use and levels of antibiotic resistance.
Supporting information
Figs A-G. Variation in antibiotic prescribing and proportion of urinary samples with E. coli resistant to antibiotics.
(DOCX)
Tables A-J. Associations between antibiotic resistances among E. coli urinary samples and antibiotic prescribing.
(DOCX)
(CSV)
Data Availability
The antibiotic prescribing data is available at the general practice level via https://digital.nhs.uk/data-and-information/publications/statistical/practice-level-prescribing-data. These data provide a granular breakdown of antibiotics at the GP level. The following link provides information about which general practice belongs to which Clinical Commissioning Group (CCG): https://digital.nhs.uk/services/organisation-data-service/data-downloads/gp-and-gp-practice-related-data (GP Practices linked to CCG/LHG). The antibiotic resistance data is added as a data supplement.
Funding Statement
SH and JV are supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at the University of Oxford in partnership with Public Health England (PHE) (HPRU-2012-10041) (https://www.nihr.ac.uk/about-us/how-we-are-managed/our-structure/research/health-protection-research-units.htm). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, Department of Health, or PHE.
References
- 1.Dolk FCK, Pouwels KB, Smith DRM, Robotham J V, Smieszek T. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother. 2018; 73 Suppl 2: ii2–ii10. 10.1093/jac/dkx504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smieszek T, Pouwels KB, Dolk FCK, Smith DRM, Hopkins S, Sharland M, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother. 2018; 73 Suppl 2: ii36–ii43. 10.1093/jac/dkx500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouwels KB, Dolk FCK, Smith DRM, Robotham J V, Smieszek T. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother. 2018; 73 Suppl 2: 19–26. 10.1093/jac/dkx502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan Å, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001; 1: 101–114. 10.1016/S1473-3099(01)00066-4 [DOI] [PubMed] [Google Scholar]
- 5.Bailey JK, Pinyon JL, Anantham S, Hall RM. Commensal Escherichia coli of healthy humans: a reservoir for antibiotic-resistance determinants. J Med Microbiol. 2010; 59: 1331–1339. 10.1099/jmm.0.022475-0 [DOI] [PubMed] [Google Scholar]
- 6.Dale AP, Woodford N. Extra-intestinal pathogenic Escherichia coli (ExPEC): Disease, carriage and clones. J Infect. 2015; 71: 615–626. 10.1016/j.jinf.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 7.Pouwels KB, Hopkins S, Llewelyn MJ, Walker AS, McNulty CAM, Robotham JV. Duration of antibiotic treatment for common infections in English primary care: cross-sectional analysis and comparison with guidelines. 2019; 10.1136/bmj.l440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pouwels KB, Freeman R, Muller-Pebody B, Rooney G, Henderson KL, Robotham JV, et al. Association between use of different antibiotics and trimethoprim resistance: going beyond the obvious crude association. J Antimicrob Chemother. 2018; 73: 1700–1707. 10.1093/jac/dky031 [DOI] [PubMed] [Google Scholar]
- 9.Kahlmeter G, Menday P. Cross-resistance and associated resistance in 2478 Escherichia coli isolates from the Pan-European ECO.SENS Project surveying the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections. J Antimicrob Chemother. 2003; 52: 128–131. 10.1093/jac/dkg280 [DOI] [PubMed] [Google Scholar]
- 10.Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of Antibiotic Therapy on the Density of Vancomycin-Resistant Enterococci in the Stool of Colonized Patients. N Engl J Med. 2000. 343: 1925–1932. 10.1056/NEJM200012283432604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipsitch M, Samore MH. Antimicrobial Use and Antimicrobial Resistance: A Population Perspective. Emerg Infect Dis. 2002; 8: 347–354. 10.3201/eid0804.010312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baym M, Stone LK, Kishony R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science. 2016; 351: aad3292 10.1126/science.aad3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imamovic L, Sommer MOA. Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development. Science Transl Med. 2013; 5: 204ra132. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee A, Modarai M, Naylor NR, Boyd SE, Atun R, Barlow J, et al. Quantifying drivers of antibiotic resistance in humans: a systematic review. Lancet Infect Dis. 2018; 18: e368–e378. 10.1016/S1473-3099(18)30296-2 [DOI] [PubMed] [Google Scholar]
- 15.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B (Statistical Methodol). 2005; 67: 301–320. [Google Scholar]
- 16.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010; 33: 1–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Tibshirani R. Regression Shrinkage and Selection via the Lasso. J R Stat Soc Ser B. 1996; 58: 267–288. [Google Scholar]
- 18.Hoerl AE, Kennard RW. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics. 1970; 12: 55. [Google Scholar]
- 19.Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ. 2016; 352: i1981 10.1136/bmj.i1981 [DOI] [PubMed] [Google Scholar]
- 20.Shallcross L, Beckley N, Rait G, Hayward A, Petersen I. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J Antimicrob Chemother 2017;72:1818–1824. 10.1093/jac/dkx048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hope EC, Crump RE, Hollingsworth TD, Smieszek T, Robotham JV, Pouwels KB. Identifying English practices that are high antibiotic prescribers accounting for comorbidities and other legitimate medical reasons for variation. EClinicalMedicine 2018;6:36–41. 10.1016/j.eclinm.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pouwels KB, Dolk FCK, Smith DRM, Smieszek T, Robotham JV. Explaining variation in antibiotic prescribing between general practices in the UK. J Antimicrob Chemother 2018;73 Suppl 2:ii27-ii35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts JA, Kruger P, Paterson DL, Lipman J. Antibiotic resistance—What’s dosing got to do with it? Crit Care Med. 2008; 36: 2433–2440. 10.1097/CCM.0b013e318180fe62 [DOI] [PubMed] [Google Scholar]
- 24.Day T, Read AF. Does High-Dose Antimicrobial Chemotherapy Prevent the Evolution of Resistance? PLOS Comput Biol. 2016; 12: e1004689 10.1371/journal.pcbi.1004689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of Exposure to Antipseudomonal β-Lactam Antibiotics in the Critically Ill and Development of New Resistance. Pharmacother J Hum Pharmacol Drug Ther. 2018; 10.1002/phar.2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med. 2008; 27: 2865–2873. 10.1002/sim.3107 [DOI] [PubMed] [Google Scholar]
- 27.Ternès N, Rotolo F, Michiels S. Empirical extensions of the lasso penalty to reduce the false discovery rate in high-dimensional Cox regression models. Stat Med. 2016; 35: 2561–2573. 10.1002/sim.6927 [DOI] [PubMed] [Google Scholar]
- 28.Public Health England. English surveillance programme for antimicrobial utilisation8and resistance (ESPAUR) report—GOV.UK. https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report.
- 29.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis. 2010; 51: 286–294. 10.1086/653932 [DOI] [PubMed] [Google Scholar]
- 30.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, et al. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. MBio. 2013; 4: e00377–13. 10.1128/mBio.00377-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandegren L, Lindqvist A, Kahlmeter G, Andersson DI. Nitrofurantoin resistance mechanism and fitness cost in Escherichia coli. J Antimicrob Chemother. 2008; 62: 495–503. 10.1093/jac/dkn222 [DOI] [PubMed] [Google Scholar]
- 32.MacNair CR, Stokes JM, Carfrae LA, Fiebig-Comyn AA, Coombes BK, Mulvey MR, et al. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat Commun. 2018; 9: 458 10.1038/s41467-018-02875-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother. 2015; 70: 2456–2464. 10.1093/jac/dkv147 [DOI] [PubMed] [Google Scholar]
- 34.Pouwels KB, Visser ST, Bos HJ, Hak E. Angiotensin-converting enzyme inhibitor treatment and the development of urinary tract infections: A prescription sequence symmetry analysis. Drug Saf. 2013; 36: 1079–1086. 10.1007/s40264-013-0085-z [DOI] [PubMed] [Google Scholar]
- 35.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000. to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014; 14: 742–750. [DOI] [PubMed] [Google Scholar]
- 36.Moreland LD, Gore FM, Andre N, Cairncross S, Ensink JHJ. Monitoring the inputs required to extend and sustain hygiene promotion: findings from the GLAAS 2013/2014 survey. Trop Med Int Heal 2016; 21: 1029–1039. [DOI] [PubMed] [Google Scholar]
- 37.Ironmonger D, Edeghere O, Verlander NQ, Gossain S, Hopkins S, Hilton B, et al. Effect of general practice characteristics and antibiotic prescribing on Escherichia coli antibiotic non-susceptibility in the West Midlands region of England: a 4 year ecological study. J Antimicrob Chemother. 2018; 73: 787–794. 10.1093/jac/dkx465 [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, Nian H, Zhu Y, Talbot HK, Griffin MR, Harrell FE Jr. Too many covariates and too few cases?—a comparative study. Stat Med. 2016; 35: 4546–4558. 10.1002/sim.7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs A-G. Variation in antibiotic prescribing and proportion of urinary samples with E. coli resistant to antibiotics.
(DOCX)
Tables A-J. Associations between antibiotic resistances among E. coli urinary samples and antibiotic prescribing.
(DOCX)
(CSV)
Data Availability Statement
The antibiotic prescribing data is available at the general practice level via https://digital.nhs.uk/data-and-information/publications/statistical/practice-level-prescribing-data. These data provide a granular breakdown of antibiotics at the GP level. The following link provides information about which general practice belongs to which Clinical Commissioning Group (CCG): https://digital.nhs.uk/services/organisation-data-service/data-downloads/gp-and-gp-practice-related-data (GP Practices linked to CCG/LHG). The antibiotic resistance data is added as a data supplement.