Abstract
Riemerella anatipestifer is a gram-negative bacterium that mainly infects ducks, turkeys and other birds. In a previous study, we established a markerless mutation system based on the pheS mutant as a counterselectable marker. However, the toxic effect of p-Cl-Phe on the R. anatipestifer strain expressing the pheS mutant was weak on blood agar plates. In this study, we successfully obtained streptomycin-resistant derivative of R. anatipestifer ATCC11845 using 100 μg/mL streptomycin as a selection pressure. Then, we demonstrate that rpsL can be used as a counterselectable marker in the R. anatipestifer ATCC11845 rpsL mutant strain, namely, R. anatipestifer ATCCs. A suicide vector carrying wild-type rpsL, namely, pORS, was constructed and used for markerless deletion of the gene RA0C_1534, which encodes a putative sigma-70 family RNA polymerase sigma factor. Using rpsL as a counterselectable marker, markerless mutagenesis of RA0C_1534 was also performed based on natural transformation. R. anatipestifer ATCCsΔRA0C_1534 was more sensitive to H2O2-generated oxidative stress than R. anatipestifer ATCCs. Moreover, transcription of RA0C_1534 was upregulated under 10 mM H2O2 treatment and upon mutation of fur. These results suggest that RA0C_1534 is involved in oxidative stress response in R. anatipestifer. The markerless gene mutation method developed in this study provides new tools for investigation of the physiology and pathogenic mechanisms of this bacterium.
Introduction
Riemerella anatipestifer (referred to herein as R. anatipestifer or RA) is a gram-negative bacterium belonging to the family Flavobacteriaceae, phylum Bacteroidetes, and genus Riemerella [1]. To date, more than 21 serotypes have been reported, and there is no cross-protection among them, and there may exist different epidemic serotypes in the same duck farm [2–4]. Thus, the effects of vaccination have been unsatisfactory. In addition, R. anatipestifer is naturally resistant to various antibiotics [5–9]. The application of antibiotics to prevent and treat the disease leads to severe contamination and poses a threat to human health. To prevent this disease completely, an understanding of the pathogenic mechanisms is required.
Currently, OmpA [10]; TonB, which is involved in heme transportation [11–13]; the TonB-dependent receptors B739_1208 and B739_1343 [14, 15]; the wza-like gene, which is involved in capsule biosynthesis [16]; and AS87_04050, M949_1556 and M949_RS01915, which are involved in lipopolysaccharide biosynthesis [17–19] have been identified as potential virulence factors. In these studies, the functions of these genes were identified via gene deletion using an antibiotic cassette for replacement. However, this method could lead to a “polar effect”, and the development of a markerless mutation method for R. anatipestifer is required.
In Escherichia coli, the rpsL gene encodes the S12 ribosomal protein of the 30S subunit. Mutations in rpsL confer antibiotic resistance to E. coli and other bacteria, including Streptococcus pneumoniae [20], Thermus thermophilus [21], Staphylococcus aureus [22] and so on. However, the streptomycin-resistant rpsL mutant strain becomes sensitive to streptomycin when wild-type rpsL is expressed in trans, indicating that the antibiotic susceptibility phenotype is dominant [21]. Furthermore, growth of merodiploid rpsL strains in the presence of streptomycin can be used to select for loss of wild-type rpsL, demonstrating the utility of this gene as a counterselectable marker [23].
Although the pheS mutant has been used for counterselection, allowing markerless deletion in R. anatipestifer ATCC11845, the toxic effect of p-Cl-Phe on R. anatipestifer ATCC11845 grown on a blood agar plate was weak when the pheS mutant was expressed in trans [24]. In this study, we established a method for markerless gene deletion in the genome of R. anatipestifer ATCC11845 using rpsL as a counterselectable marker. RA0C_1534 encodes a putative sigma factor, and as a proof of concept, the homolog of this gene in R. anatipestifer CH-1 was shown to be upregulated under iron-limited conditions [25]. RA0C_1534 was disrupted using the knockout strategy established by this study and its function was investigated.
Materials and methods
Bacterial strains, primers and growth conditions
The bacterial strains used in this study are listed in Table 1. The primers used in this study are listed in Table 2. For culture conditions, the E. coli strains were grown on LB agar and in LB broth. R. anatipestifer strains were routinely grown on LB agar supplemented with 5% sheep blood and in GC broth (GCB) [26]. Antibiotics were used at the following concentrations when needed: kanamycin (Kan) at 50 μg/mL and ampicillin (Amp) at 100 μg/mL for E. coli; cefoxitin (Cfx) at 1 μg/mL, streptomycin (Str) at 100 μg/mL and erythromycin (Erm) at 1 μg/mL for R. anatipestifer ATCC11845. The concentration of Erm used in this study was determined according to the minimum inhibitory concentration of erythromycin on R. anatipestifer ATCC11845 (0.5 μg/mL).
Table 1. Strains and plasmids used in this study.
| Strains | Genotype | Source or reference |
| XL1-blue | F-supE44 hdsR17 recA1 endA1 gyrA46 thi relA1 lac- F’ proAB-
lacIq lacZΔM15 Tn10, TetR |
Laboratory collection |
| S17-1 | hsdR17 recA1 RP4-2-tet::Mu-1 kan::Tn7; SmR | [27] |
| Riemerella anatipestifer strains | Phenotype or genotype | Source or reference |
| R. anatipestifer ATCC11845 | RA ATCC11845 | [28] |
| R. anatipestifer ATCCs | RA ATCC11845, rpsL mutant, StrR | This study |
| R. anatipestifer ATCCs pLMF03::rpsL | RA ATCC11845, rpsL mutant, pLMF03::rpsL, StrS, CfxR | This study |
| R. anatipestifer ATCCsΔRA0C_1534 | RA ATCC11845, rpsL mutant, RA0C_1534 mutant, StrR | This study |
| R. anatipestifer ATCCsΔRA0C_1534 pLMF03::RA0C_1534 | RA ATCC11845, rpsL mutant, RA0C_1534 mutant, pLMF03::RA0C_1534, StrR, CfxR | This study |
| R. anatipestifer ATCC11845 RA0C_1912* (Δfur) | RA ATCC11845, fur mutant | [24] |
| R. anatipestifer ATCC11845Δfur pLMF03::fur | RA ATCC11845, fur mutant, pLMF03::fur, CfxR | This study |
| Plasmids | Genotype | Source or reference |
| pMM47.A | ermF promoter, oriColE1, ori pCC7, AmpR, CfxR | [29] |
| pMM47.B | Suicide vector, ermF promoter, oriColE1, AmpR, CfxR | This study |
| pMM47.C | Suicide vector, oriT, ermF promoter, oriColE1, AmpR, CfxR | This study |
| pLMF03 | Shuttle plasmid, AmpR, CfxR | [12] |
| pLMF03::rpsL | pLMF03 carrying wild-type rpsL from R. anatipestifer ATCC11845, AmpR, CfxR, StrS | This study |
| pEX18GM | oriT+, sacB+, gene replacement vector with MCS from pUC18, GenR | [30] |
| pORS | Suicide vector, oriT, ermF promoter, rpsL from R. anatipestifer ATCC11845, oriColE1, AmpR, CfxR | This study |
| pORS::RA0C_1534 up-down | pORS carrying R. anatipestifer ATCC11845 RA0C_1534 upstream and downstream fusion fragment, AmpR, CfxR | This study |
| pBAD24 | pBR322 araC, arabinose-inducible promoter, AmpR | Laboratory collection |
| pBAD24::ermR-rpsL | pBAD24 carrying ermR from R. anatipestifer CH-1, rpsL from R. anatipestifer ATCC11845, AmpR, ErmR, StrS | This study |
| pBAD24::RA0C_1534 up-ermR-rpsL-RA0C_1534 down | pBAD24::ermR-rpsL carrying RA0C_1534 upstream and downstream regions from R. anatipestifer ATCC11845, AmpR, ErmR, StrS | This study |
| pLMF03::RA0C_1534 | pLMF03 carrying RA0C_1534 from R. anatipestifer ATCC11845, AmpR, CfxR | This study |
| pLMF03::fur | pLMF03 carrying fur from R. anatipestifer ATCC11845, AmpR, CfxR | This study |
AmpR, ampicillin resistance; CfxR, cefoxitin resistance; StrS, streptomycin sensitive; StrR, streptomycin resistance; ErmR, erythromycin resistance.
Table 2. Primers used in this study.
| Primer | Organism | Sequence (5'−3') |
|---|---|---|
| rpsLP1 | RA ATCC11845 | GGGACGAAAGTTGTTCTATGGAAG |
| rpsLP2 | RA ATCC11845 | CTGAATGCGATAGACTTCTTACCG |
| rpsL expP1 | RA ATCC11845 | ACGCGTCGACGTCGGCCATAGCGGATCAAAAATAATTTGGTTTTCG |
| rpsL expP2 | RA ATCC11845 | CATGCCATGGCATGTTACTTTTTAGCATCTTTAGGACGCTTAGCACCG |
| oriT P1 | pEX18Gm | CCCAAGCTTCGCCTGATGCGGTATTTTCTCC |
| oriT P2 | pEX18Gm | ACATGCATGCCTAGAGTCGATCTTCGCCAGC |
| RA0C_1534 upP1 | RA ATCC11845 | CATGCCATGGCATGGAGACCTCACGCTGTTAATGGGGAG |
| RA0C_1534 upP2 | RA ATCC11845 | TCAGACCTTTAGTGTAACTTTACCTTTAATTTAAACC |
| RA0C_1534 downP1 | RA ATCC11845 | TAAATTAAAGGTAAAGTTACACTAAAGGTCTGAGC |
| RA0C_1534 downP2 | RA ATCC11845 | CCGCTCGAGCGGCCAAAATCTCTAGCACCCAAAGCG |
| Cfx P1 | pLMF03 | GGTGCTGCAATGTTGATG |
| Cfx P2 | pLMF03 | CCGCTAAGGTATAACTG |
| ErmRP1 | RA CH-1 | CGGGGTACCCCGACCACTTTCCAGTCTTACGAAG |
| ErmRP2 | RA CH-1 | GCTCTAGAGCCGACTTTGAACTACGAAGGATG |
| ErmRP1* | RA ATCC11845 | TGCGGTCGGCTTTCATTTTCTCTTC |
| ErmRP2* | RA ATCC11845 | GGGCTGATTTGACAGTTGGCGGT |
| rpsLP1* | RA ATCC11845 | GCTCTAGAGCATGCCTACTATTCAACAATTAG |
| rpsLP2* | RA ATCC11845 | ACGCGTCGACGTCGGCCATAGCGGTTACTTTTTAGCATCTTTAGGACGC |
| RA0C_1534 CompP1 | RA ATCC11845 | ACGCGTCGACGTCGCCTATCATTTTCGGTGGGAT |
| RA0C_1534 CompP2 | RA ATCC11845 | CATGCCATGGCATGCATAGAACGAAAAGCTCAGACC |
| RA0C_1534 up P1* | RA ATCC11845 | CATGCCATGGCATGGAGACCTCACGCTGTTAATGGGGAG |
| RA0C_1534 up P2* | RA ATCC11845 | CGGGGTACCCCGAACTTTACCTTTAATTTAAACC |
| RA0C_1534 down P1* | RA ATCC11845 | ACGCGTCGACGTCGGCCATAGCGGACACTAAAGGTCTGAGC |
| RA0C_1534 down P2* | RA ATCC11845 | ACATGCATGCATGTCCAAAATCTCTAGCACCCAAAGCG |
| RA0C_1534 up P1** | RA ATCC11845 | GAGACCTCACGCTGTTAATGGGGAG |
| RA0C_1534 up P2** | RA ATCC11845 | TCAGACCTTTAGTGTAACTTTACCTTTAATTTAAACC |
| RA0C_1534down P1** | RA ATCC11845 | TAAATTAAAGGTAAAGTTACACTAAAGGTCTGAGC |
| RA0C_1534down P2** | RA ATCC11845 | CCAAAATCTCTAGCACCCAAAGCG |
| fur ComP1 | RA ATCC11845 | CATGCCATGGAACATCAAGAGAAAG |
| fur ComP2 | RA ATCC11845 | GGACTAGTCCTTATGCTTTTTTATGACCGTAG |
| RA0C_1534 qRT P1 | RA ATCC11845 | GGCAAAGCGTATTGCTCAC |
| RA0C_1534 qRT P2 | RA ATCC11845 | CCGTAGGTTCGCTAATATGGTC |
| recA qRT P1 | RA ATCC11845 | TGAAACTAGGTGATGGTACG |
| recA qRT P2 | RA ATCC11845 | CTTAGGATAACCGCCTACTC |
| 16s rRNA P1 | RA ATCC11845 | ATGCGAAAGGAGGATTGC |
| 16s rRNA P2 | RA ATCC11845 | TTACACCTCAAATACCTC |
Isolation of the streptomycin-resistant rpsL mutant strain R. anatipestifer ATCCs
Streptomycin-resistant R. anatipestifer ATCC11845 cells were obtained by plating 108 wild-type cells on LB agar supplemented with 5% sheep blood containing 100 μg/mL streptomycin. Streptomycin-resistant clones were streaked for isolation on fresh medium, and rpsL from each clone was amplified by PCR using the primers rpsLP1 and rpsLP2 (Table 2) and then submitted for sequencing. Point mutations in rpsL that conferred streptomycin resistance were identified by comparison with the wild-type rpsL sequence.
Assessment of the sensitivity of R. anatipestifer ATCCs expressing wild-type rpsL to streptomycin
To determine whether wild-type rpsL was dominant compared to the mutant rpsL alleles, wild-type rpsL was cloned into the shuttle vector pLMF03 [12]. Specifically, a 551-bp fragment spanning wild-type rpsL and the presumed promoter region of this gene was amplified using the primers rpsLexpP1 and rpsLexpP2 (Table 2), which were designed to include SalI and NcoI restriction sites. The fragment was digested with SalI and NcoI and cloned into the corresponding sites of pLMF03 to generate pLMF03::rpsL. The plasmids pLMF03 and pLMF03::rpsL were introduced into streptomycin-resistant R. anatipestifer ATCCs separately by conjugation as previously described [12]. R. anatipestifer ATCCs harboring pLMF03 or pLMF03::rpsL was cultured overnight at 37°C in GCB agar containing 1 μg/mL Cfx. Then, the bacteria were reisolated on blood agar plates containing Cfx (1 μg/mL) as well as with streptomycin (0 and 100 μg/mL). The plates were incubated overnight at 37°C.
Construction of the rpsL-containing suicide vector pORS
The E. coli-Capnocytophaga canimorsus shuttle plasmid pMM47.A [29] was digested with PstI to remove the replication region and generate the plasmid pMM47.B, which has been constructed in previous study [24]. To increase the conjugation efficiency, oriT was amplified from the plasmid pEX18GM [30] using the primers oriTP1 (introducing a HindIII site) and oriTP2 (introducing a SphI site) (Table 2) and was cloned into pMM47.B to generate the plasmid pMM47.C. The wild-type rpsL with the native promoter was amplified from R. anatipestifer ATCC11845 using the primers rpsLexpP1 (introducing a SalI site) and rpsLexpP2 (introducing a NcoI site) and was cloned into pMM47.C to generate the suicide vector pORS.
Construction of the RA0C_1534 deletion mutant in the streptomycin-resistant strain ATCCs based on the suicide plasmid pORS
The process of knockout based on pORS was done as described in previous study with a little modification [24]. Briefly, the 780-bp upstream sequence of RA0C_1534 was amplified using the primers RA0C_1534 upP1 (containing a NcoI site) and RA0C_1534 upP2. The 796-bp downstream sequence of RA0C_1534 was amplified with the primers RA0C_1534 downP1 and RA0C_1534 downP2 (containing a XhoI site). The two PCR products were ligated using the overlap PCR method. The fused fragment was purified and digested with NcoI and XhoI. Then, the fragment was cloned into pORS, which had been digested with the same enzymes, to generate pORS::RA0C_1534 up-down. The plasmid pORS::RA0C_1534 up-down was introduced into streptomycin-resistant strain ATCCs by conjugation according to a previously described method [12, 24].
After the first recombination, the positive clone was isolated on the blood plate with Cfx (1 μg/mL) and tested by PCR using the primers CfxP1 and CfxP2 (Table 2). The correct clone was inoculated in GCB liquid medium for overnight. About 105 bacterial cells were spread on blood plates with 100 μg/mL streptomycin to screen the mutants which had lost the plasmid after a second recombination event. Mutation was identified by PCR using the primers RA0C_1534 CompP1 and RA0C_1534 CompP2 (Table 2).
Construction of the RA0C_1534 deletion mutant in ATCCs based on natural transformation
Similarly as described in previous study [24], the process for constructing the template plasmid pBAD24::ermR-rpsL was as follows. Briefly, the Erm-resistant (ermR) gene cassette was amplified from the genome of R. anatipestifer CH-1 [31] using the primers ErmRP1 (containing a KpnI site) and ErmRP2 (containing a XbaI site). The rpsL gene was amplified from R. anatipestifer ATCC11845 by PCR using the primers rpsLP1* (containing a XbaI site) and rpsLP2* (containing a SalI site). Two fragments were ligation with the plasmid pBAD24 generating pBAD24::ermR-rpsL. Gene deletion based on natural transformation was accomplished using a similar procedure as that described in a previous study [26]. The upstream (780 bp) and downstream (796 bp) regions of RA0C_1534 were amplified from ATCC11845 using the primers RA0C_1534 upP1* (containing a NcoI site), RA0C_1534 upP2* (containing a KpnI site); RA0C_1534 downP1*, (containing a SalI site) and RA0C_1534 downP2* (containing a SphI site), respectively. Two fragments were cloned into the plasmid pBAD24::ermR-rpsL, then the mutagenic PCR fragments RA0C_1534 upstream-ermR-rpsL-RA0C_1534 downstream were amplified from pBAD24::ermR-rpsL using the primers RA0C_1534 upP1* and RA0C_1534 downP2*. The purified fragments were used for the first natural transformation. The transformational bacteria were spread on blood agar plates with 1 μg/mL Erm to select for the first recombination. The correct Erm-resistant colonies were verified by PCR. For the second natural transformation, the fragments RA0C_1534 upstream and RA0C_1534 downstream were amplified using the primers RA0C_1534 upP1** and RA0C_1534 upP2**; RA0C_1534 downP1** and RA0C_1534 downP2**, respectively. The RA0C_1534 upstream fragments and RA0C_1534 downstream fragments were ligated using overlap PCR. The purified fragments were transformed into the Erm-resistant strain obtained in the first natural transformation. Transformants were plated on blood agar supplemented with 100 μg/mL streptomycin. Mutation was identified by PCR.
Construction of the complementation plasmid and strains
Plasmids carrying RA0C_1534 were constructed using the shuttle vector pLMF03 [12]. The primers RA0C_1534 CompP1 (introducing a SalI site) and RA0C_1534 CompP2 (introducing a NcoI site) were used to amplify the 828-bp fragment. The product was digested with SalI and NcoI, and the fragments were ligated with the shuttle vector pLMF03, which was digested with SalI and NcoI, to generate pLMF03::RA0C_1534. The plasmids were transferred to various R. anatipestifer strains by conjugation and selected on a blood agar plate containing 1 μg/mL Cfx as previously described for complementation studies [12]. Consistent with the above method, pLMF03::fur was constructed by ligating pLMF03 with fur fragment which was amplified using the primers fur CompP1 and fur CompP2.
H2O2 challenge
The assay of H2O2 challenge was performed as described in previous study with a little modification [32]. Briefly, the strains R. anatipestifer ATCCs pLMF03, ATCCsΔRA0C_1534 pLMF03 and ATCCsΔRA0C_1534 pLMF03::RA0C_1534 were grown in GCB medium until the exponential phase. Bacteria were collected and washed twice in PBS. The cell suspension was then diluted to an OD600 of 0.5. Before H2O2 challenge, several dilutions of the tested cell suspensions were spread on blood agar plates (T0). For the challenge assay, the bacteria were incubated for 30 min in PBS in the presence of H2O2 (5 or 10 mM) at 37°C. After exposure to H2O2, the bacteria were washed twice with PBS, and several dilutions were spread onto blood agar plates (T1). After a 1-day incubation at 37°C, the colonies were counted. The survival rate was calculated as (T1/T0) ×100%. All the experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software for Windows. Statistical significance was ascertained using Student’s T-test. P<0.05 was considered significant.
Results
Isolation and characteristics of the rpsL mutants
Spontaneous mutants of R. anatipestifer ATCC11845 that were resistant to streptomycin were selected on blood agar plates supplemented with 100 μg/mL streptomycin. The frequency of the spontaneous mutation was approximately 10−8. Ten spontaneous streptomycin-resistant mutants of wild-type R. anatipestifer ATCC11845 were isolated, and the rpsL gene was amplified and sequenced. Seven of the mutants had an adenine-to-guanine point mutation at position 128 of the rpsL coding sequence, resulting in a K43R substitution in RpsL. The mutant strain was named R. anatipestifer ATCCs.
Development of rpsL as a counterselectable marker for R. anatipestifer ATCCs
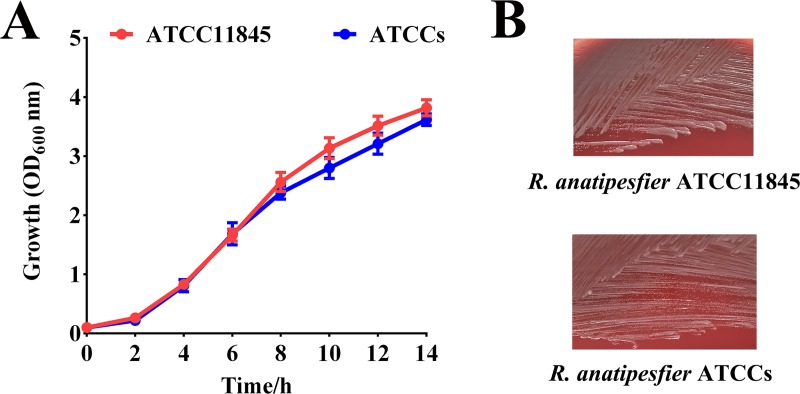
rpsL has been used as a counterselectable marker for Flavobacterium johnsoniae [33] and Flavobacterium columnare [23]. In Flavobacterium johnsoniae, wild-type rpsL is dominant compared to the mutant allele, and merodiploids are sensitive to streptomycin [33]. We tested whether this was also the case for R. anatipestifer ATCCs. The plasmid pLMF03::rpsL, harboring wild-type R. anatipestifer ATCC11845 rpsL, was introduced into R. anatipestifer ATCCs. R. anatipestifer ATCCs pLMF03::rpsL failed to grow on blood agar plates containing 100 μg/mL streptomycin, whereas R. anatipestifer ATCCs pLMF03 grew well in the presence of streptomycin (Fig 1). The results suggest that wild-type rpsL can function as a counterselectable marker in R. anatipestifer ATCCs. In addition, the rpsL mutation had no effect on the growth of R. anatipestifer ATCC11845 on solid medium or liquid medium (Fig 2).
Fig 1. Evaluation of wild-type rpsL as a counterselectable marker in the streptomycin-resistant strain R. anatipestifer ATCCs.
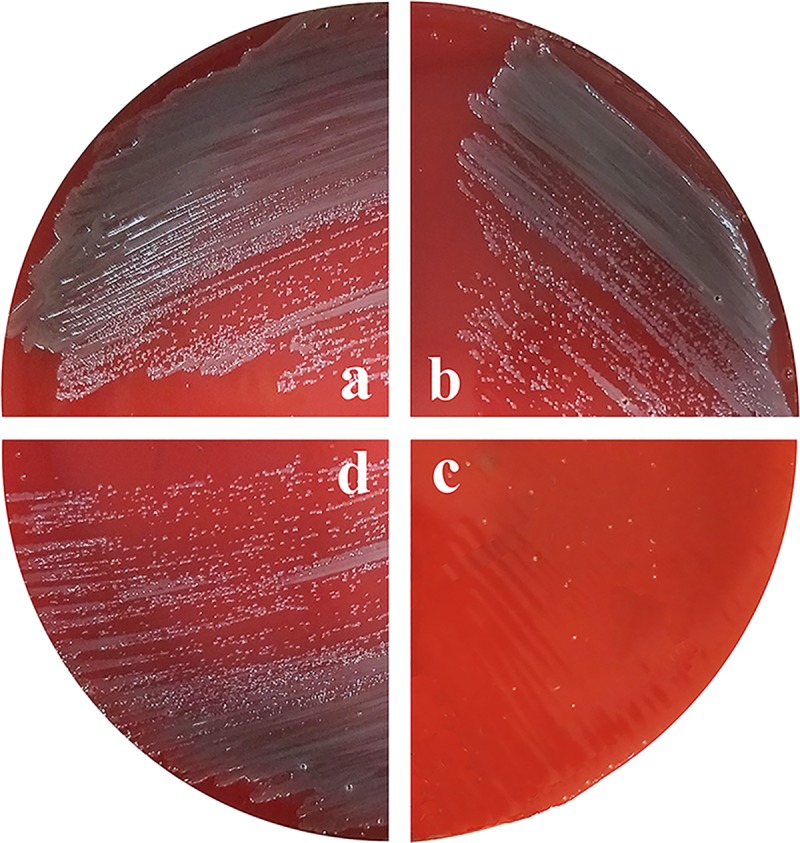
Single colonies of R. anatipestifer ATCCs harboring either pLMF03 or pLMF03::rpsL streaked onto blood agar plates or blood agar plates supplemented with 100 μg/mL streptomycin. (a) R. anatipestifer ATCCs pLMF03 cultured on blood agar plates. (b) R. anatipestifer ATCCs pLMF03 cultured on blood agar plates supplemented with streptomycin (100 μg/mL). (c) R. anatipestifer ATCCs pLMF03::rpsL cultured on blood agar plates supplemented with streptomycin (100 μg/mL). (d) R. anatipestifer ATCCs pLMF03::rpsL cultured on blood agar plates.
Fig 2. The growth of R. anatipestifer ATCC11845 and ATCCs on solid medium and liquid medium.
(A) The growth of R. anatipestifer ATCC11845 and ATCCs in GCB liquid medium was monitored by measuring the optical density at 600 nm every 2 h for 14 h. The data shown are the averages and SDs from three experiments. (B) Single colonies of R. anatipestifer ATCC11845 and ATCCs streaked on blood agar plates.
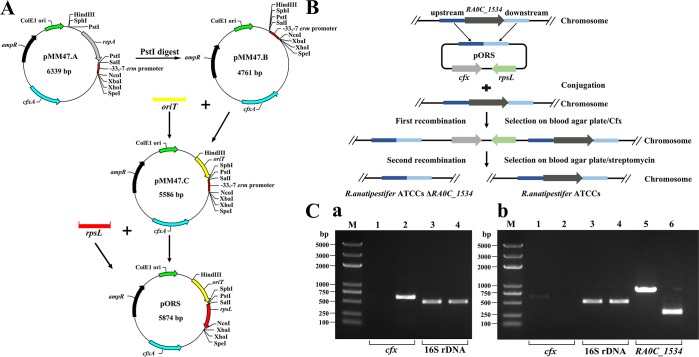
Development of a suicide plasmid carrying rpsL and construction of the RA0C_1534 deletion mutant in R. anatipestifer ATCCs
To investigate the feasibility of constructing in-frame deletion mutants of R. anatipestifer using rpsL as a counterselectable marker, the suicide vector pORS with rpsL as constructed as described in “Materials and Methods” (Fig 3A). Then, we performed a markerless RA0C_1534 gene deletion from the genome of R. anatipestifer ATCCs. The procedure for markerless deletion of the RA0C_1534 gene using a suicide vector is shown in Fig 3B. The clones grown on plates with Cfx were screened by PCR. cfx could be amplified from the merodiploids but not the wild-type strain (Fig 3C, a). For the first recombination, Cfx-resistant colonies were generated at a frequency of 10−5. For the second recombination, streptomycin-resistant colonies were generated at a frequency of 10−3. Finally, the correct deletion clones were identified by PCR with a frequency of ~30%. Deletion of RA0C_1534 in R. anatipestifer ATCCs was verified by PCR. The RA0C_1534 gene with its native promoter was amplified using the primers RA0C_1534 ComP1 and RA0C_1534 ComP2 to obtain an approximately 800-bp product from R. anatipestifer ATCCs. However, the product amplified from R. anatipestifer ATCCsΔRA0C_1534 was approximately 250 bp in size (Fig 3C, b). The cfx fragment was not amplified from R. anatipestifer ATCCsΔRA0C_1534, and 16S rDNA could be amplified from all the strains (Fig 3C, b). All these results suggest that the suicide plasmid pORS can be used for construction of the markerless mutant in R. anatipestifer ATCCs.
Fig 3. Schematic depiction of markerless gene deletion of RA0C_1534 from R. anatipestifer ATCCs based on the suicide vector pORS, and PCR verification.
(A) Schematic depiction of the generation of the counterselection vector pORS. (B) Schematic depiction of markerless gene deletion from R. anatipestifer ATCCs. (C) PCR followed by agarose gel electrophoresis was performed to verify deletion of the RA0C_1534 locus. a, Lane 1 and lane 2: the cfx sequence (638 bp) was amplified from R. anatipestifer ATCCs and Cfx-resistant clones, respectively. Lane 3 and lane 4: the 16S rDNA (525 bp) sequence was amplified from R. anatipestifer ATCCs and Cfx-resistant clones, respectively. b, Lane 1 and lane 2: the cfx sequence (638 bp) was amplified from R. anatipestifer ATCCs and the RA0C_1534 mutant strains, respectively. Lane 3 and lane 4: the 16S rDNA (525 bp) sequence was amplified from R. anatipestifer ATCCs and the RA0C_1534 mutant strain, respectively. Lane 5 and lane 6: the RA0C_1534 gene containing the native promoter region was amplified from R. anatipestifer ATCCs (828 bp) and the RA0C_1534 mutant strain (approximately 250 bp). M indicates a 100-bp DNA ladder.
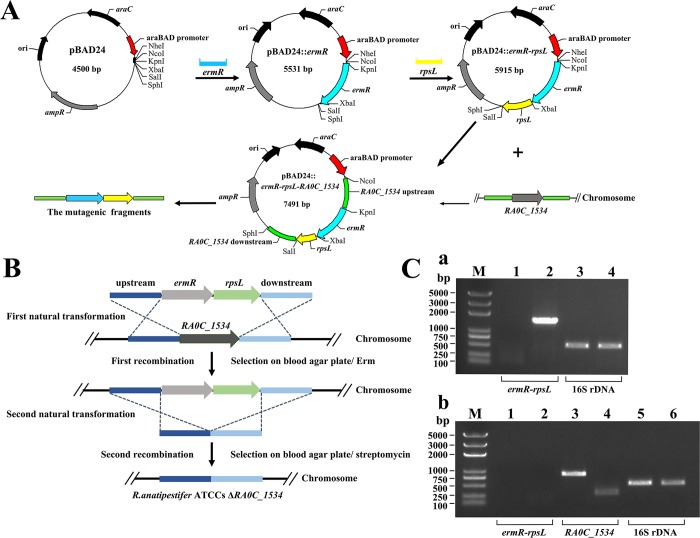
Construction of a markerless RA0C_1534 mutant based on natural transformation
As described in “Materials and Methods”, we first constructed the ermR-rpsL cassette and then ligated the RA0C_1534 upstream and downstream fragments (Fig 4A). The recombinant fragment was transferred into R. anatipestifer ATCCs as the first recombination (Fig 4B). The clones grown on plates containing Erm were screened by PCR. The ermR-rpsL cassette could be amplified from the transformants, unlike the results for the wild-type strain (Fig 4C, a). Erm-resistant clones were generated at a frequency of 10−5. For the second recombination, the merodiploids were incubated with a fusion fragment containing the RA0C_1534 flanking regions (Fig 4B). Clones that could grow on blood agar plates containing streptomycin but not on blood agar plates containing Erm were verified by PCR. Streptomycin-resistant and Erm-sensitive colonies were generated at a frequency of 10−5. As shown in Fig 4C, b, compared with the wild-type strain, the product size obtained upon amplification of the RA0C_1534 gene from the candidate strains was approximately 250 bp. The 16S rDNA sequence could be amplified, and the ermR-rpsL fragments could not be amplified, from both strains. These results suggest that the RA0C_1534 gene was knocked out successfully.
Fig 4. Schematic depiction of markerless gene deletion of RA0C_1534 from R. anatipestifer ATCCs based on natural transformation, and PCR identification.
(A) The ermR gene was amplified from R. anatipestifer CH-1 and cloned into the plasmid pBAD24 to generate pBAD24::ermR. The wild-type rpsL of R. anatipestifer ATCC11845 was cloned into pBAD24::ermR to generate pBAD24::ermR-rpsL. Subsequently, the upstream and downstream regions of the RA0C_1534 gene were amplified and cloned into pBAD24::ermR-rpsL to generate pBAD24::RA0C_1534 upstream-ermR-rpsL-RA0C_1534 downstream. RA0C_1534 upP1* and RA0C_1534 downP2* were used to amplify the RA0C_1534 upstream-ermR-rpsL-RA0C_1534 downstream fragments. (B) Schematic depiction of markerless gene deletion based on natural transformation. (C) a, The clones after the first homologous recombination were verified by PCR and agarose gel electrophoresis. Lane 1 and lane 2: the ermR-rpsL cassette (1323 bp) sequence was amplified from R. anatipestifer ATCCs and Erm-resistant clones, respectively. Lane 3 and lane 4: the 16S rDNA (525 bp) sequence was amplified from R. anatipestifer ATCCs and Erm-resistant clones, respectively. b, PCR identification of the deletion mutant after the second homologous recombination. Lane 1 and lane 2: the ermR-rpsL cassette (1323 bp) sequence was amplified from R. anatipestifer ATCCs and transformants, respectively. Lanes 3 and 4: RA0C_1534 gene containing the native promoter region was amplified from R. anatipestifer ATCCs (828 bp) and transformants (approximately 250 bp). Lane 5 and lane 6: the 16S rDNA (525 bp) sequence was amplified from R. anatipestifer ATCCs and transformants, respectively. M indicates the BM5000 DNA marker.
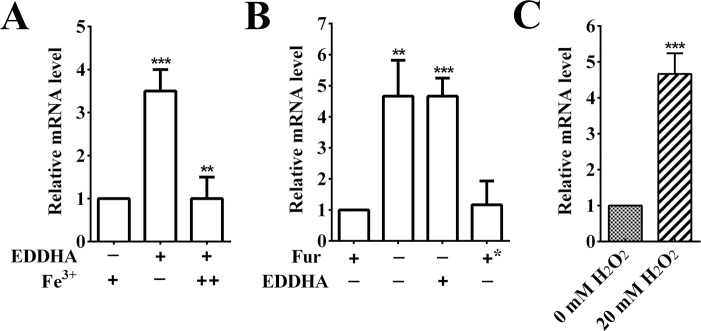
Transcription of RA0C_1534 is regulated by iron, fur and H2O2
A previous RNA-seq study showed that the B739_0625 of RA CH-1, the homolog of RA0C_1534, was upregulated under iron-limited conditions [25]. Generally, the regulation of iron on genes is mediated by the Fur protein [34], to further assess this effect and identify whether the regulation was mediated by Fur. Transcription was monitored by real-time PCR to measure the RA0C_1534 gene transcription level in the R. anatipestifer ATCC11845 and R. anatipestifer ATCC11845Δfur strains grown in GCB and GCB supplemented with 40 μM EDDHA, respectively. As shown in Fig 5A, the iron-limiting condition led to a more than 3-fold upregulation of RA0C_1534 expression in R. anatipestifer ATCC11845 compared to that under iron-rich conditions. This induction was repressed by exogenous Fe(NO3)3 (Fig 5A). Transcription of the RA0C_1534 gene increased 4-fold in the fur mutant strain compared to the wild type (Fig 5B). Moreover, the increased expression could be fully restored to the wild-type level by complementation of fur (Fig 5B). Many Fur regulated gene were demonstrated to be involved in the oxidative stress response [35]. Some bacteria employ sigma factors to adapt to oxidative stress in the environment [36, 37]. We investigated whether RA0C_1534 was regulated by exposure R. anatipestifer ATCC11845 to H2O2. The results showed that transcription of RA0C_1534 was upregulated 4-fold after treatment with 20 mM H2O2. These results suggest that transcription of RA0C_1534 is regulated by iron, fur and H2O2.
Fig 5. Transcription of the RA0C_1534 gene was regulated by iron, Fur and hydrogen peroxide.
(A) Iron-responsive transcription of the RA0C_1534 gene in R. anatipestifer ATCC11845. R. anatipestifer ATCC11845 was grown in GCB, GCB containing 40 μM EDDHA, or 40 μM EDDHA containing 50 μM Fe(NO3)3. Transcription was measured by qRT-PCR. Representative fold changes in comparison with growth in GCB medium. (B) The transcription levels of RA0C_1534 in R. anatipestifer ATCC11845 pLMF03 grown in GCB, in R. anatipestifer ATCC11845Δfur pLMF03 grown in GCB, in R. anatipestifer ATCC11845Δfur pLMF03 grown in GCB supplemented with 40 μM EDDHA, and in R. anatipestifer ATCC11845Δfur pLMF03::fur grown in GCB. (C) The transcription levels of RA0C_1534 in R. anatipestifer ATCC11845 and in R. anatipestifer ATCC11845 treated with 20 mM H2O2 for 30 min after growth to the exponential phase. The data shown are the averages and SDs from three independent experiments. ***p < 0.001, **p < 0.01, and *p < 0.05.
The R. anatipestifer ATCCsΔRA0C_1534 mutant is more sensitive to H2O2 than R. anatipestifer ATCCs
The H2O2 dependent regulation of RA0C_1534 was demonstrated above. This prompted us to investigate whether RA0C_1534 was involved in the R. anatipestifer response to H2O2 exposure. After exposure to 5 mM H2O2, the survival rate of R. anatipestifer ATCCs pLMF03 was approximately 80%; however, the survival rate of R. anatipestifer ATCCsΔRA0C_1534 pLMF03 was approximately 50%. After exposure to 10 mM H2O2, the survival rate of R. anatipestifer ATCCs pLMF03 was approximately 65%; however, the survival rate of R. anatipestifer ATCCsΔRA0C_1534 pLMF03 was approximately 20%. Compared with the control strain, the sensitivity of the deletion mutant strain to hydrogen peroxide increased approximately 2~3-fold (Fig 6). Moreover, the resistance to H2O2 of the mutant strain could be restored by expression of RA0C_1534 in trans (Fig 6). These results suggest that the deletion of RA0C_1534 in R. anatipestifer ATCCs significantly increased the sensitivity of this strain to H2O2.
Fig 6. Sensitivity of R. anatipestifer ATCCs-derived strains to hydrogen peroxide.
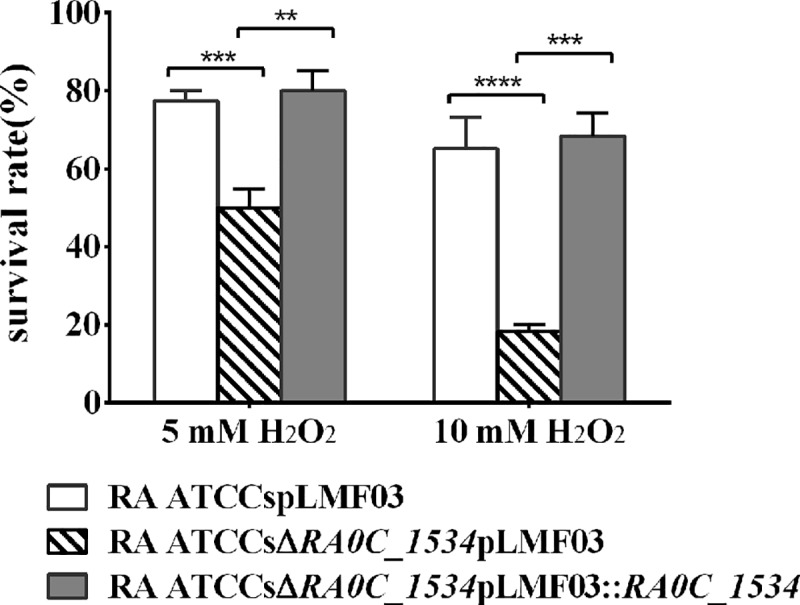
The survival rates of R. anatipestifer ATCCs pLMF03, R. anatipestifer ATCCsΔRA0C_1534 pLMF03 and R. anatipestifer ATCCsΔRA0C_1534 pLMF03::RA0C_1534 after treatment with 5 or 10 mM H2O2. The data shown are the averages and SDs from three independent experiments. ****p < 0.0001, ***p < 0.001, **p < 0.01, and *p < 0.05.
Discussion
The genetic tools were critical for the study on the physiology and pathogenic mechanism of the pathogens, however, it was imperfect for the R. anatipestifer, which is an important bacterial pathogen for duck industry. In a previous study, we established a markerless mutation method based on a pheS mutant as a counterselection marker [24]. However, the toxic effect of p-Cl-Phe on R. anatipestifer cells expressing the pheS mutant on blood agar plates was weak [24]. Here, we described a dominant gene marker encoding the S12 protein from R. anatipestifer ATCC11845, indicating the feasibility of this system for negative selection. A comparison of the rpsL alleles of several bacteria revealed that most of these alleles encoded a conserved lysine at position 43, which is precisely the amino acid that is known to confer streptomycin resistance when mutated in various species [22, 23, 33]. Consistent with this finding, we screened 7 of 10 mutants that exhibited A-to-G transversions at position 128. Furthermore, we showed that streptomycin-sensitive rpsL alleles were dominant compared to streptomycin-resistant rpsL alleles, and rpsL is a suitable counterselection marker for R. anatipestifer ATCCs. Subsequently, we developed a markerless mutant based on the constructed suicide plasmid. In this case, Cfx-resistant colonies were obtained for the first step (plasmid integration) at a frequency of 10−5. Streptomycin-resistant colonies were obtained for the second step (plasmid loss) at a frequency of 10−3, and approximately 30% of the streptomycin-resistant colonies carried the deletion. It's worth noting that the frequency for each step recombination should be various according the different target genes.
Alternatively, we also developed a markerless mutant based on natural transformation because it has been shown that R. anatipestifer cells are naturally competent [26]. For this method, a two-step transformation was required. The first natural transformation in the ATCCs strain using the ermR-rpsL cassette replaced the target gene on the chromosome by homologous recombination. In the second natural transformation, the ermR-rpsL cassette was deleted by homologous recombination. This step restored streptomycin resistance, allowing the generation of mutations without antibiotic markers. In this case, Erm-resistant colonies were obtained for the first natural transformation at a frequency of 10−5. Streptomycin-resistant colonies were obtained for the second natural transformation at a frequency of 10−5, and approximately 90% of the streptomycin-resistant colonies carried the deletion.
In addition to the construction of in-frame deletion mutants, in principle, the methods described here can also be used to insert any DNA fragment of interest into a desired location on the chromosome or to introduce site-directed point mutations in genes of interest, as described using the pheS mutant as a counterselection marker in a previous study [24]. However, one disadvantage of this method is that prior manipulation of the wild type for introduction of streptomycin resistance is required. Thus, it is required to establish the genetic manipulation system with wide range of application in the further study.
R. anatipestifer can infect ducks, thus, this species must have a specific mechanism for protection against the host-defense-associated oxidative burst. However, the mechanisms by which R. anatipestifer responds to oxidative stress remain unknown. Bacterial sigma factors are subunits of the RNA polymerase that play a fundamental role in the ability of bacteria to adapt to different environments [36]. In this study, we investigated whether the sigma factor, RA0C_1534, is involved in oxidative stress response. We showed that the RA0C_1534 mutant was highly sensitive to H2O2-induced oxidative stress and that transcription of RA0C_1534 was upregulated by H2O2 treatment. This gene is the first factor identified in R. anatipestifer to be involved in the oxidative stress response. It is unclear which genes are regulated by RA0C_1534. The regulation mechanism of RA0C_1534 needs further study.
In conclusion, we developed a markerless mutation method in R. anatipestifer based on rpsL as a counterselection marker. The simple and efficient method presented in this work expands the genetic toolbox for R. anatipestifer. This approach will facilitate future studies on the gene functions of R. anatipestifer and may also be adapted for use with other members of the large and diverse phylum Bacteroidetes.
Acknowledgments
We thank Guy R Cornelis (Biozentrum der Universität Basel, CH-4056 Basel, Switzerland) for generously providing plasmid pMM47.A.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 31772772, http://www.nsfc.gov.cn/), the China Agricultural Research System (CARS-42-17) and Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (CARS-SVDIP). The funders (MF Liu and AC Cheng) had a role in study design, decision to publish and perparation of the manuscript.
References
- 1.Segers P, Mannheim W, Vancanneyt M, Brandt KD, Hinz KH, Kersters K, et al. Riemerella anatipestifer gen. nov., comb. nov., the causative agent of septicemia anserum exudativa, and its phylogenic affiliation within the Flavobacterium-Cytophaga rRNA homology group. Int J Syst Bacteriol. 1993;43(4):768–76. 10.1099/00207713-43-4-768 [DOI] [PubMed] [Google Scholar]
- 2.Pathanasophon P, Sawada T, Tanticharoenyos T. New serotypes of Riemerella anatipestifer isolated from ducks in Thailand. Avian Pathology Journal of the Wvpa. 1995;24(1):195–9. [DOI] [PubMed] [Google Scholar]
- 3.Pathanasophon P, Sawada T, Pramoolsinsap T, Tanticharoenyos T. Immunogenicity of Riemerella anatipestifer broth culture bacterin and cell-free culture filtrate in ducks. Avian Pathology. 1996;25(4):705–19. 10.1080/03079459608419176 [DOI] [PubMed] [Google Scholar]
- 4.Pathanasophon P, Phuektes P, Tanticharoenyos T, Narongsak W, Sawada T. A potential new serotype of Riemerella anatipestifer isolated from ducks in Thailand. Avian Pathology. 2002;31(3):267–70. 10.1080/03079450220136576 [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Yuan H, Liu MF, Zhao XX, Wang MS, Jia RY, et al. Type B Chloramphenicol Acetyltransferases Are Responsible for Chloramphenicol Resistance in Riemerella anatipestifer, China. Frontiers in Microbiology. 2017;8:297 10.3389/fmicb.2017.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo HY, Liu MF, Wang MS, Zhao XX, Jia RY, Chen S, et al. A novel resistance gene, lnu(H), confers resistance to lincosamides in Riemerella anatipestifer CH-2. International Journal of Antimicrobial Agents. 2018;51(1). [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Wang MS, Liu MF, Zhu DK, Biville F, Jia RY, et al. Contribution of RaeB, a Putative RND-Type Transporter to Aminoglycoside and Detergent Resistance in Riemerella anatipestifer. Frontiers in Microbiology. 2017;8:2435 10.3389/fmicb.2017.02435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong CY, Cheng AC, Wang MS, Zhu DK, Luo QH, Zhong CD, et al. Antibiotic susceptibility of Riemerella anatipestifer field isolates. Avian Diseases. 2009;53(4):601 10.1637/8552-120408-ResNote.1 [DOI] [PubMed] [Google Scholar]
- 9.Zhu DK, Luo HY, Liu MF, Zhao XX, Jia RY, Chen S, et al. Various Profiles of tet Genes Addition to tet(X) in Riemerella anatipestifer Isolates From Ducks in China. Frontiers in Microbiology. 2018;9:585 10.3389/fmicb.2018.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Q, Han X, Zhou X, Ding C, Zhu Y, Yu S. OmpA is a virulence factor of Riemerella anatipestifer. Veterinary Microbiology. 2011;150(3–4):278–83. 10.1016/j.vetmic.2011.01.022 [DOI] [PubMed] [Google Scholar]
- 11.Liao HB, Cheng XJ, Zhu DK, Wang MS, Jia RY, Chen S, et al. TonB Energy Transduction Systems of Riemerella anatipestifer Are Required for Iron and Hemin Utilization. Plos One. 2014;10(5):e0127506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M, Wang M, Zhu D, Wang M, Jia R, Chen S, et al. Investigation of TbfA in Riemerella anatipestifer using plasmid-based methods for gene over-expression and knockdown. Scientific Reports. 2016;6:37159 10.1038/srep37159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao S, Xing L, Qi J, Yu H, Jiang P, Sun B, et al. Roles of the TonB1 and TonB2 proteins in haemin iron acquisition and virulence in Riemerella anatipestifer. Microbiology. 2015;161(8):1592 10.1099/mic.0.000123 [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Zhang P, Zhu D, Wang M, Jia R, Chen S, et al. Identification of the ferric iron utilization gene B739_1208 and its role in the virulence of R. anatipestifer CH-1. Veterinary Microbiology. 2017;201. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Huang M, Shui Y, Biville F, Zhu D, Wang M, et al. Roles of B739_1343 in iron acquisition and pathogenesis in Riemerella anatipestifer CH-1 and evaluation of the RA-CH-1ΔB739_1343 mutant as an attenuated vaccine. Plos One. 2018;13(5):e0197310 10.1371/journal.pone.0197310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi H, Yuan B, Liu J, Zhu D, Wu Y, Wang M, et al. Identification of a wza-like gene involved in capsule biosynthesis, pathogenicity and biofilm formation in Riemerella anatipestifer. Microbial Pathogenesis. 2017;107:442 10.1016/j.micpath.2017.04.023 [DOI] [PubMed] [Google Scholar]
- 17.Wang XL, Ding C, Wang SH, Han XG, Hou WW, Yue JP, et al. The AS87_04050 gene is involved in bacterial lipopolysaccharide biosynthesis and pathogenicity of Riemerella anatipestifer. PLoS One. 2014;9(10):e109962 10.1371/journal.pone.0109962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou J, Wang X, Tian M, Cao S, Hou W, Wang S, et al. The M949_1556 gene plays a role on the bacterial antigenicity and pathogenicity of Riemerella anatipestifer. Veterinary Microbiology. 2015;177(1–2):193–200. 10.1016/j.vetmic.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 19.Dou Y, Wang X, Yu G, Wang S, Tian M, Qi J, et al. Disruption of the M949_RS01915 gene changed the bacterial lipopolysaccharide pattern, pathogenicity and gene expression of Riemerella anatipestifer. Veterinary Research. 2018;48(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung CK, Li H, Claverys JP, Morrison DA. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol. 2001;67(11):5190–5196. 10.1128/AEM.67.11.5190-5196.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emilio BG, Felipe C, Eduardo LVA, Jesús M, José B. Use of a dominant rpsL allele conferring streptomycin dependence for positive and negative selection in Thermus thermophilus. Applied & Environmental Microbiology. 2007;73(16):5138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Ram G, Yoong P, Penadés JR, Shopsin B, Novick RP. An rpsL-based allelic exchange vector for Staphylococcus aureus. Plasmid. 2015;79:8–14. 10.1016/j.plasmid.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Qin T, Zhang XL, Huang B, Liu ZX, Xie HX, et al. Gene Deletion Strategy To Examine the Involvement of the Two Chondroitin Lyases in Flavobacterium columnare Virulence. Appl Environ Microbiol. 2015;81(21):7394–402. 10.1128/AEM.01586-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu MF, Huang Y, Liu JJ, Biville F, Zhu DK, Wang MS, et al. Multiple genetic tools for editing the genome of Riemerella anatipestifer using a counterselectable marker. Applied Microbiology & Biotechnology. 2018;(14):1–14. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, Huang M, Zhu D, Wang M, Jia R, Chen S, et al. Identifying the Genes Responsible for Iron-Limited Condition in Riemerella anatipestifer CH-1 through RNA-Seq-Based Analysis. BioMed Research International,2017,(2017-4-30). 2017;2017(2):8682057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M, Zhang L, Huang L, Biville F, Zhu D, Wang M, et al. Use of Natural Transformation To Establish an Easy Knockout Method in Riemerella anatipestifer. Applied & Environmental Microbiology. 2017;83(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon R, Priefer U, Pühler A. A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Nature Biotechnology. 1983;1(9):784–91. [Google Scholar]
- 28.Wang X, Zhu D, Wang M, Cheng A, Jia R, Zhou Y, et al. Complete genome sequence of Riemerella anatipestifer reference strain. Journal of Bacteriology. 2012;194(12):3270–1. 10.1128/JB.00366-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mally M, Cornelis GR. Genetic tools for studying Capnocytophaga canimorsus. Applied & Environmental Microbiology. 2008;74(74):6369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoang TT, Karkhoff-Schweizer RAR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212(1):77–86. [DOI] [PubMed] [Google Scholar]
- 31.Luo H, Liu M, Wang L, Zhou W, Wang M, Cheng A, et al. Identification of ribosomal RNA methyltranferase gene ermf in Riemerella anatipestifer. Avian Pathology. 2015;44(3):162–8. 10.1080/03079457.2015.1019828 [DOI] [PubMed] [Google Scholar]
- 32.Liu MF, Ferrandez Y, Bouhsira E, Monteil M, Franc M, Boulouis HJ, et al. Heme Binding Proteins of Bartonella henselae Are Required when Undergoing Oxidative Stress During Cell and Flea Invasion. Plos One. 2012;7(10):e48408 10.1371/journal.pone.0048408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhodes RG, Pucker HG, Mcbride J. M. Development and use of a gene deletion strategy for Flavobacterium johnsoniae to identify the redundant gliding motility genes remF, remG, remH, and remI. Journal of Bacteriology. 2011;193(10):2418–28. 10.1128/JB.00117-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troxell B, Hassan HM. Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front Cell Infect Microbiol. 2013;3(59):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Askoura M, Sarvan S, Couture JF, Stintzi A. The Campylobacter jejuni Ferric Uptake Regulator Promotes Acid Survival and Cross-Protection against Oxidative Stress. Infection & Immunity. 2016;84(5):1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ndamukong IC, Gee J, Smith CJ. The extracytoplasmic function sigma factor EcfO protects Bacteroides fragilis against oxidative stress. Journal of Bacteriology. 2013;195(1):145–55. 10.1128/JB.01491-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez-Martinez CE, Lourenço RF, Baldini RL, Laub MT, Gomes SL. The ECF sigma factor σT is involved in osmotic and oxidative stress responses in Caulobacter crescentus. Molecular Microbiology. 2010;66(5):1240–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.