Abstract
Diaphorin is a polyketide produced by Candidatus Profftella armatura (Betaproteobacteria), an organelle-like defensive symbiont harbored by a plant sap-sucking insect, Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae). Diaphorin belongs to the pederin family, a group of compounds that share much of their core structure with that of pederin, which is characterized by two dihydropyran rings bridged by an N-acyl aminal. Most members of this family have potent antitumor activity, making them promising anticancer drug candidates. The present study assessed the therapeutic potential of diaphorin for its antitumor activity against 39 human cancer cell lines including those from breast, brain, colon, lung, skin, ovary, kidney, stomach, and prostate. The results showed that diaphorin had inhibitory activity against all 39 cancer cell lines tested. The GI50, TGI, and LC50 values ranged from 0.28 μM– 2.4 μM, 1.6 μM –11 μM, and 7.5 μM–> 100 μM, respectively. These values are among the highest in the pederin family, indicating that the anticancer activity of diaphorin is milder than those of other pederin congeners. The inhibitory effects of diaphorin significantly differed among the distinct cancer types. The maximum difference was about 10-fold, which was similar to those of most other pederin congeners.
Introduction
A variety of invertebrate animals harbor endosymbiotic microbes to deter natural enemies, which makes them among the richest sources of biologically active secondary metabolites [1–6]. Pederin family compounds, mostly isolated from marine sponges, are polyketides that share much of their core structure with that of pederin, which is characterized by two dihydropyran rings bridged by an N-acyl aminal [7–12]. Most compounds in this family, including pederin, mycalamides, onnamides, theopederins, and psymberin, exhibit potent antitumor activity, making them promising as anticancer drug candidates [7–10,13–20]. Mycalamide A not only inhibited replication of various cancer cells in vitro but also improved the survival of mice bearing ascitic lymphomas and a variety of other ascitic and solid tumors [15]. Moreover, this compound exhibited cancer preventive properties, inhibiting epidermal growth factor (EGF)-induced neoplastic transformation in murine cells [20]. Analysis using 60 human cancer cell lines revealed that psymberin, also known as irciniastatin A [21], has an remarkably selective activity toward solid tumor cells, making it one of the most promising lead drug candidates among a variety of other possible leads from different structural classes [22].
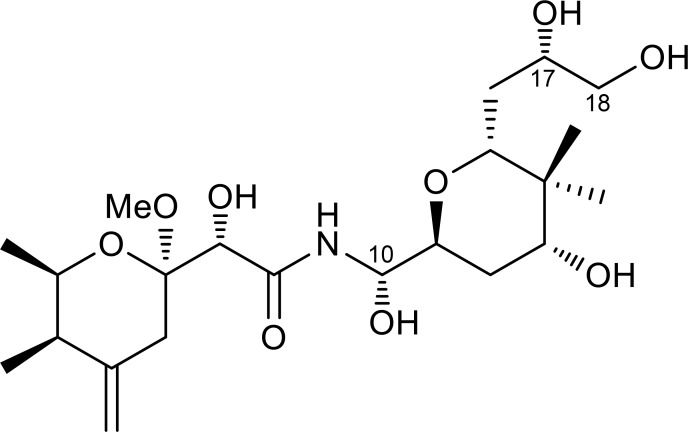
Although most pederin congeners are derived from marine invertebrates, pederin itself is found exclusively from rove beetles of the genera Paederus and Paederidus (Coleoptera: Staphylinidae) [7–10,23,24]. Since the discovery of pederin in the mid 20th century [25], these beetles have long been a sole terrestrial source of pederin family members until diaphorin, a tri-O-desmethyl analog of pederin (Fig 1), was discovered from a plant sap-sucking insect, the Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae)[26]. D. citri is an important pest of citrus worldwide and harbors the vertically transmitted intracellular symbiont Candidatus Profftella armatura (Betaproteobacteria) [26–30]. Profftella is an unprecedented organelle-like defensive symbiont with a drastically reduced genome of 460 bp, which produces diaphorin using the polyketide synthase (PKS) biosynthetic gene clusters [26]. A previous study showed that diaphorin has significant inhibitory activity against the rat neuroblastoma B104 and human HeLa cells [26]. In the present study, in an effort to further evaluate the therapeutic potential of diaphorin, we assessed the antitumor activities of diaphorin against various human cancers using a panel of 39 cancer cell lines prepared by the Japanese Foundation for Cancer Research.
Fig 1. Chemical structure of diaphorin.
Materials and methods
Preparation of diaphorin
Diaphorin was extracted from D. citri, purified, and quantified as previously described [30]. Briefly, adult D. citri were treated twice with methanol, and the extracts were combined and concentrated in vacuo. The residue was resuspended in methanol and purified in a Shimadzu (Kyoto, Japan) LC10 high-performance liquid chromatography (HPLC) system with an Inertsil ODS-3 C18 reversed-phase preparative column [5 μm, 7.6 × 150 mm, GL Science (Tokyo, Japan)] heated to 35°C. The mobile phase was isocratic 20% acetonitrile in water, with a flow rate of 1.5 mL/min. Diaphorin was detected at a wavelength of 200 nm. The purified samples were combined and dried in vacuo. Diaphorin was re-dissolved in methanol and quantified in an HPLC system as described above, except the mobile phase was 15% acetonitrile in water, with a flow rate of 1.0 mL/min, and an Inertsil ODS-3 analytical column (5 μm, 4.0 × 250 mm, GL Science) was used.
Cell lines and cell culture
The human cancer cell line panel prepared by the Japanese Foundation for Cancer Research (Tokyo, Japan) consisted of 39 cell lines as follows [31]: breast cancer, BSY-1, HBC-4, HBC-5, MCF-7, and MDA-MB-231; central nervous system (CNS) cancer, SF-268, SF-295, SF-539, SNB-75, SNB-78, and U251; colon cancer, HCC-2998, HCT-116, HCT-15, HT-29, and KM-12; lung cancer, A549, DMS114, DMS273, NCI-H226, NCI-H23, NCI-H460, and NCI-H522; melanoma, LOX-IMVI; ovarian cancer, OVCAR-3, OVCAR-4, OVCAR-5, OVCAR-8, and SK-OV-3; renal cancer, ACHN and RXF-631L; stomach cancer, MKN-A, MKN-B, MKN1, MKN45, MKN74, and St-4; prostate cancer, DU-145 and PC-3. Normal cell types were not included in the assay because they tend to respond to anticancer drugs with extreme phenotypes (e.g., fibroblasts being pan-resistant and renal epithelial cells being pan-sensitive), making them unsuitable as controls [32]. The cells were cultured in RPMI 1640 medium supplemented with 5% fetal bovine serum, 2 mM L-glutamine, penicillin (100 units/mL), and streptomycin (100 mg/mL) at 37°C in humidified air containing 5% CO2.
Analysis of cell growth inhibition by diaphorin
Inhibition of the growth of human cancer cell lines was assessed using a standard sulforhodamine B (SRB) assay as described previously [33]. Briefly, cells in culture medium were inoculated into 96-well plates and incubated at 37°C for 24 h. Subsequently, 12 replicates of each cell line were fixed with trichloroacetic acid (TCA) to measure the cell population at the time of adding diaphorin (Tz: time zero). Serial dilutions (10−8, 10−7, 10−6, 10−5, or 10−4 M final concentration) of diaphorin were then added to each cell line. After 48 h of additional incubation at 37°C, the plates were fixed with TCA, stained with SRB, and read with an automated microplate reader at a wavelength of 515 nm. Percentage growth inhibition was calculated as:
[(Ti-Tz)/(C-Tz)] × 100, when Ti≥Tz
[(Ti-Tz)/Tz] × 100, when Ti<Tz
(Tz: the SRB absorbance at time zero, C: the SRB absorbance of the control cells receiving no diaphorin, Ti: the SRB absorbance of the test cells treated with diaphorin at the five concentration levels). The concentration of diaphorin resulting in a 50% reduction in the cell number increase as compared with the control (GI50: 50% growth inhibition) was calculated from [(Ti-Tz)/(C-Tz)] × 100 = 50. The concentration of diaphorin resulting in total growth inhibition (TGI) was calculated from Ti = Tz. The concentration of diaphorin resulting in a 50% reduction of the number of cells at the end of the treatment as compared with that at the beginning (LC50: 50% lethal concentration) was calculated from [(Ti-Tz)/Tz] × 100 = -50.
Statistical analysis
Data were analyzed using R statistical computing software (version 3.4.2) [34].
Results and discussion
Growth inhibitory activity of diaphorin against 39 human cancer cell lines
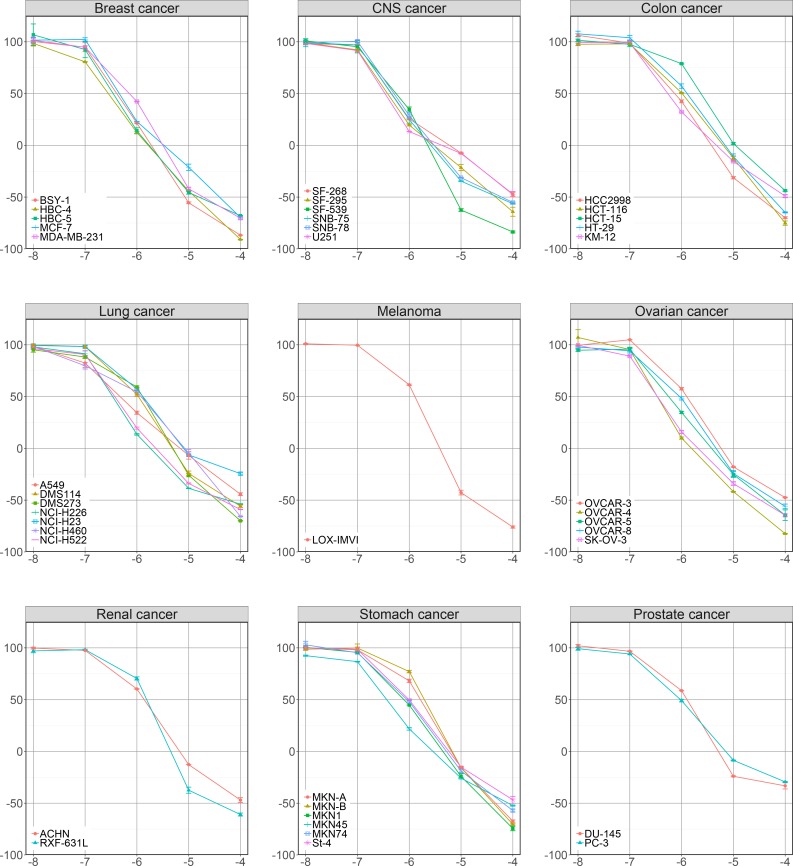
A total of 39 human tumor cell lines were treated with diaphorin at final concentrations of 10−8, 10−7, 10−6, 10−5, and 10−4 M (S1 Table). The dose-response curves (Fig 2) showed that diaphorin had significant dose-dependent patterns of inhibitory activity against all 39 cancer cell lines tested (Tukey-Kramer test, p < 0.05). The concentrations of diaphorin required to exert the growth inhibitory activity were at the micromolar level, which is much higher than those reported for other members of the pederin family including pederin, mycalamides, onnamides, theopederins, psymberin, and their synthetic analogs that exhibit antitumor activity at nanomolar or even subnanomolar concentrations [9,10,13–20]. The anticancer activities of pederin congeners are primarily attributed to their ability to bind to ribosomes and inhibit protein synthesis [9,18,19,35,36]. Moreover, the C10 methoxy group of pederin and its analogs is postulated to be important for ribosome binding through its hydrogen bonding and its effects on conformation [9]. Diaphorin is a tri-O-desmethyl analog of pederin (Fig 1), where the methoxy groups at the C10, C17, and C18 of pederin are replaced by hydroxyl groups in diaphorin [26,37]. This alteration increases the hydrophilicity of the compound while enabling the occurrence of putative conformational changes and hydrogen bonding required for ribosome binding. Analyses of structure–activity relationships in this family support the idea that greater affinity toward ribosomes is associated with lower hydrophilicity [10], which most likely accounts for the notably milder activity of diaphorin compared with other pederin congeners.
Fig 2. Dose response curves of the 39 human cancer cell lines treated with diaphorin.
The x-axis represents log10 molar concentrations of diaphorin added to the culture medium. The y-axis represents the percentage growth of the cells during the diaphorin treatment. The growth percentage value of 100 represents the growth of the control cells receiving no diaphorin, the value of 0 represents no net growth throughout the treatment period, and the value of -100 indicates that all cells are killed at the end of the experiment. Error bars represent standard errors.
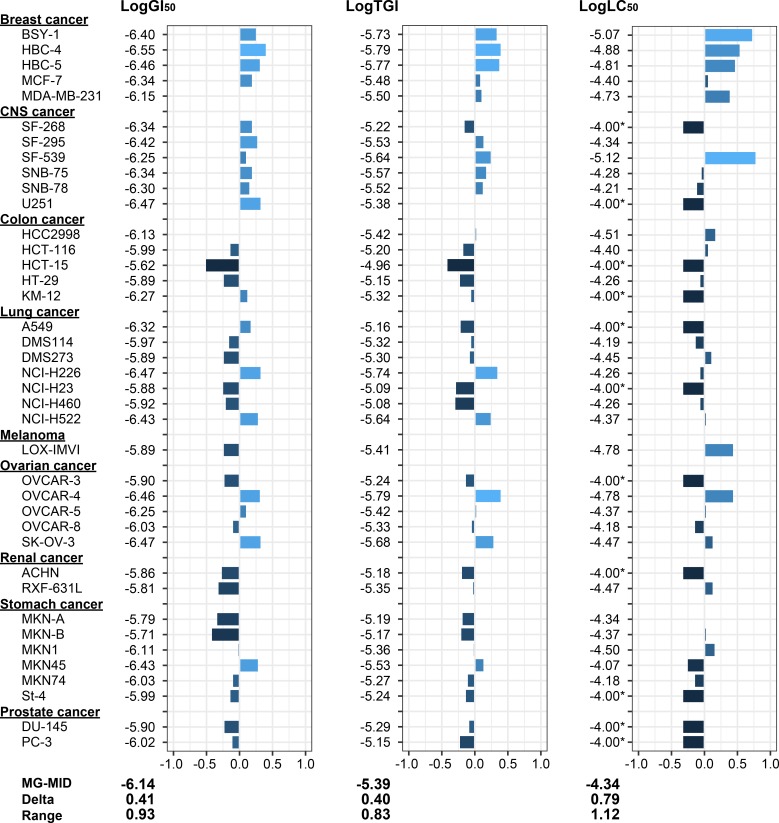
The GI50, TGI, and LC50 values are summarized in Table 1. The GI50 values varied 10-fold, ranging from 0.28 μM for the HBC-4 breast cancer cells to 2.4 μM for the HCT-15 colon cancer cells. The TGI values ranged from 1.6 μM for the HBC-4 breast cancer cells and the OVCAR-4 ovarian cancer cells to 11 μM for the HCT-15 colon cancer cells. The LC50 values varied more than 10-fold; the most sensitive cell line was the SF-539 CNS cancer cells with an LC50 value of 7.5 μM followed by the BSY-1 breast cancer cells with an LC50 value of 8.4 μM. The least potent inhibitory activities with LC50 values greater than 100 μM were observed for the SF-268 and U251 CNS cancer cells, the HCT-15 and KM-12 colon cancer cells, the A549 and NCI-H23 lung cancer cells, the OVCAR-3 ovarian cancer cells, the ACHN renal cancer cells, the St-4 stomach cancer cells, and the DU-145 and PC-3 prostate cancer cells. In these cell lines, treatment with 100 μM diaphorin did not result in a 50% reduction of the cells at the end of the treatment as compared with that at the beginning. In terms of the three parameters, GI50, TGI, and LC50, the HCT-15 colon cancer cells were the least sensitive of the 39 cell lines. This is reasonable because HCT-15 is a multidrug-resistant cell line, which expresses the multidrug-resistance related genes MDR1 and MRP that are involved in the efflux of cytotoxic substances including therapeutic drugs [38]. The mean graphs of log GI50, TGI, and LC50 visualize the relative sensitivity of each cell line against diaphorin (Fig 3).
Table 1. Indices showing the inhibitory activity of diaphorin against the 39 human cancer cell lines.
| Cell line | GI50 (μM) | TGI (μM) | LC50 (μM) |
|---|---|---|---|
| Breast cancer | |||
| BSY-1 | 0.40 | 1.9 | 8.4 |
| HBC-4 | 0.28 | 1.6 | 13 |
| HBC-5 | 0.35 | 1.7 | 16 |
| MCF-7 | 0.46 | 3.3 | 40 |
| MDA-MB-231 | 0.71 | 3.2 | 19 |
| CNS cancer | |||
| SF-268 | 0.46 | 6.0 | >100 |
| SF-295 | 0.38 | 2.9 | 46 |
| SF-539 | 0.56 | 2.3 | 7.5 |
| SNB-75 | 0.45 | 2.7 | 52 |
| SNB-78 | 0.51 | 3.0 | 61 |
| U251 | 0.34 | 4.2 | >100 |
| Colon cancer | |||
| HCC2998 | 0.74 | 3.8 | 31 |
| HCT-116 | 1.0 | 6.3 | 40 |
| HCT-15 | 2.4 | 11 | >100 |
| HT-29 | 1.3 | 7.1 | 54 |
| KM-12 | 0.54 | 4.7 | >100 |
| Lung cancer | |||
| A549 | 0.47 | 7.0 | >100 |
| DMS114 | 1.1 | 4.8 | 65 |
| DMS273 | 1.3 | 5.0 | 35 |
| NCI-H226 | 0.34 | 1.8 | 55 |
| NCI-H23 | 1.3 | 8.1 | >100 |
| NCI-H460 | 1.2 | 8.3 | 55 |
| NCI-H522 | 0.37 | 2.3 | 43 |
| Melanoma | |||
| LOX-IMVI | 1.3 | 3.9 | 17 |
| Ovarian cancer | |||
| OVCAR-3 | 1.3 | 5.8 | >100 |
| OVCAR-4 | 0.35 | 1.6 | 16 |
| OVCAR-5 | 0.56 | 3.8 | 43 |
| OVCAR-8 | 0.93 | 4.7 | 67 |
| SK-OV-3 | 0.34 | 2.1 | 34 |
| Renal cancer | |||
| ACHN | 1.4 | 6.6 | >100 |
| RXF-631L | 1.5 | 4.5 | 34 |
| Stomach cancer | |||
| MKN-A | 1.6 | 6.4 | 45 |
| MKN-B | 2.0 | 6.8 | 43 |
| MKN1 | 0.78 | 4.4 | 32 |
| MKN45 | 0.37 | 2.9 | 84 |
| MKN74 | 0.93 | 5.3 | 67 |
| St-4 | 1.0 | 5.8 | >100 |
| Prostate cancer | |||
| DU-145 | 1.3 | 5.1 | >100 |
| PC-3 | 0.96 | 7.1 | >100 |
Fig 3. Mean graphs showing the relative sensitivity of each cell line against diaphorin.
The logarithms of GI50, TGI, and LC50 are indicated for each cell line. The x-axis represents the difference between the mean of the parameters for all 39 cell lines and the parameters for each cell line. Namely, columns extending to the right of center (mean) indicate that the corresponding cell lines are more sensitive to diaphorin than the average of the 39 cell lines, and columns extending to the left indicate that the cell lines are more resistant to diaphorin than the average. MG-MID: the mean of log GI50, TGI, or LC50 for the 39 cell lines. Delta: the difference between the MG-MID and the log GI50, TGI, or LC50 of the most sensitive cell line. Range: the difference between the log GI50, TGI, or LC50 of the most resistant cell line and those of the most sensitive one. Asterisks (*) indicate that the log LC50 values were greater than -4.00 (10−4 M), the maximum concentration of diaphorin tested, at which the treatment did not result in 50% reduction of the cells at the end of the treatment as compared with those at the beginning.
The sensitivity toward diaphorin significantly differed among the distinct cancer types (Friedman test: Friedman chi-squared = 9.4821, df = 1, p = 0.002075). The maximum difference was about 10-fold, which is similar to those observed for most other pederin congeners. Psymberin is the only exception to this within the pederin family thus far, showing more than a 10,000-fold difference in its inhibitory activity toward distinct cancer types. Indeed, several melanoma, breast, and colon cancer cell lines were highly sensitive (LC50 < 2.5 nM) to psymberin, whereas six leukemia cell lines were invariably insensitive (LC50 > 25 μM), indicating a >104-fold difference in inhibitory activities [22]. Psymberin is the only pederin congener that lacks the A-ring oxane and the 1, 3-dioxane ring, which may account for the remarkably selective inhibitory profile of this compound [22].
The present study revealed that diaphorin inhibits a wide variety of human cancer cells. The selectivity of the inhibitory effect of diaphorin against distinct cancer types was only moderate, suggesting that this compound is less promising as a drug lead compared with psymberin. However, the notably milder inhibitory activity of diaphorin compared with other pederin analogs may make it worthwhile to explore its use for other potential pharmaceutical applications.
Supporting information
(XLSX)
Acknowledgments
This research was done with technical support from the Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, Koto, Tokyo, Japan.
Data Availability
All relevant data are within the manuscript and Supporting Information files.
Funding Statement
This work was supported by Japan Society for the Promotion of Science (https://www.jsps.go.jp) KAKENHI grant number 26292174 to AN. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schwartsmann G, Brondani da Rocha A, Berlinck RG, Jimeno J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001;2: 221–225. [DOI] [PubMed] [Google Scholar]
- 2.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42: 165–190. 10.1146/annurev.genet.41.110306.130119 [DOI] [PubMed] [Google Scholar]
- 3.Hentschel U, Piel J, Degnan SM, Taylor MW. Genomic insights into the marine sponge microbiome. Nat Rev Microbiol. 2012;10: 641–654. 10.1038/nrmicro2839 [DOI] [PubMed] [Google Scholar]
- 4.Freeman MF, Gurgui C, Helf MJ, Morinaka BI, Uria AR, Oldham NJ, et al. Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science (80-). 2012;338: 387–390. 10.1126/science.1226121 [DOI] [PubMed] [Google Scholar]
- 5.Wilson MC, Mori T, Rückert C, Uria AR, Helf MJ, Takada K, et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature. 2014;506: 58–62. 10.1038/nature12959 [DOI] [PubMed] [Google Scholar]
- 6.Engl T, Kroiss J, Kai M, Nechitaylo TY, Svatos A, Kaltenpoth M. Evolutionary stability of antibiotic protection in a defensive symbiosis. Proc Natl Acad Sci U S A. 2018;115: E2020–E2029. 10.1073/pnas.1719797115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piel J, Butzke D, Fusetani N, Hui D, Platzer M, Wen G, et al. Exploring the chemistry of uncultivated bacterial symbionts: antitumor polyketides of the pederin family. J Nat Prod. 2005/03/25. 2005;68: 472–479. 10.1021/np049612d [DOI] [PubMed] [Google Scholar]
- 8.Piel J. Bacterial symbionts: prospects for the sustainable production of invertebrate-derived pharmaceuticals. Curr Med Chem. 2006;13: 39–50. 10.2174/092986706775197944 [DOI] [PubMed] [Google Scholar]
- 9.Wan S, Wu F, Rech JC, Green ME, Balachandran R, Horne WS, et al. Total synthesis and biological evaluation of pederin, psymberin, and highly potent analogs. J Am Chem Soc. 2011;133: 16668–16679. 10.1021/ja207331m [DOI] [PubMed] [Google Scholar]
- 10.Mosey RA, Floreancig PE. Isolation, biological activity, synthesis, and medicinal chemistry of the pederin/mycalamide family of natural products. Nat Prod Rep. 2012;29: 980–995. 10.1039/c2np20052j [DOI] [PubMed] [Google Scholar]
- 11.Kampa A, Gagunashvili AN, Gulder TAM, Morinaka BI, Daolio C, Godejohann M, et al. Metagenomic natural product discovery in lichen provides evidence for a family of biosynthetic pathways in diverse symbioses. Proc Natl Acad Sci U S A. 2013;110: E3129–E3137. 10.1073/pnas.1305867110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kust A, Mareš J, Jokela J, Urajová P, Hájek J, Saurav K, et al. Discovery of a pederin family compound in a non-symbiotic bloom-forming cyanobacterium. ACS Chem Biol. 2018;13: 1123–1129. 10.1021/acschembio.7b01048 [DOI] [PubMed] [Google Scholar]
- 13.Soldati M, Fioretti A, Ghione M. Cytotoxicity of pederin and some of its derivatives on cultured mammalian cells. Experientia. 1966;22: 176–178. [DOI] [PubMed] [Google Scholar]
- 14.Brega A, Falaschi A, De Carli L, Pavan M. Studies on the mechanism of action of pederine. J Cell Biol. 1968/03/01. 1968;36: 485–496. Available: http://www.ncbi.nlm.nih.gov/pubmed/5650903 10.1083/jcb.36.3.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burres NS, Clement JJ. Antitumor activity and mechanism of action of the novel marine natural products mycalamide-A and -B and onnamide. Cancer Res. 1989;49: 2935–2940. [PubMed] [Google Scholar]
- 16.Matsunaga S, Fusetani N, Nakao Y. Eight new cytotoxic metabolites closely related to onnamide A from two marine sponges of the genus Theonella. Tetrahedron. 1992;48: 8369–8376. [Google Scholar]
- 17.Richter A, Kocienski P, Raubo P, Davies DE. The in vitro biological activities of synthetic 18-O-methyl mycalamide B, 10-epi-18-O-methyl mycalamide B and pederin. Anticancer Drug Des. 1997;12: 217–227. [PubMed] [Google Scholar]
- 18.Lee K, Nishimura S, Matsunaga S, Fusetani N, Horinouchi S, Yoshida M. Inhibition of protein synthesis and activation of stress-activated protein kinases by onnamide A and theopederin B, antitumor marine natural products. Cancer Sci. 2005;96: 357–364. 10.1111/j.1349-7006.2005.00055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang Y, Schneider-poetsch T, Eyler DE, Jewett JC, Bhat S, Rawal VH, et al. Inhibition of eukaryotic translation elongation by the antitumor natural product Mycalamide B. RNA. 2011;17: 1578–1588. 10.1261/rna.2624511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyshlovoy SA, Fedorov SN, Kalinovsky AI, Shubina LK, Bokemeyer C, Stonik VA, et al. Mycalamide A shows cytotoxic properties and prevents EGF-induced neoplastic transformation through inhibition of nuclear factors. Mar Drugs. 2012;10: 1212–1224. 10.3390/md10061212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettit GR, Xu J, Chapuis J, Pettit RK, Tackett LP, Doubek DL, et al. Antineoplastic agents. 520. Isolation and structure of irciniastatins A and B from the Indo-Pacific marine sponge Ircinia ramosa. J Med Chem. 2004;47: 1149–1152. 10.1021/jm030207d [DOI] [PubMed] [Google Scholar]
- 22.Cichewicz RH, Valeriote FA, Crews P. Psymberin, a potent sponge-derived cytotoxin from Psammocinia distantly related to the pederin family. Org Lett. 2004;6: 1951–1954. 10.1021/ol049503q [DOI] [PubMed] [Google Scholar]
- 23.Kellner RLL, Dettner K. Differential efficacy of toxic pederin in deterring potential arthropod predators of Paederus (Coleoptera: Staphylinidae) offspring. Oecologia. 1996;107: 293–300. 10.1007/BF00328445 [DOI] [PubMed] [Google Scholar]
- 24.Piel J. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc Natl Acad Sci U S A. 2002;99: 14002–14007. 10.1073/pnas.222481399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavan M, Bo G. Pederin, toxic principle obtained in the crystalline state from the beetle Paederus fuscipes Curt. Physiol Comp Oecol. 1953;3: 307–312. [Google Scholar]
- 26.Nakabachi A, Ueoka R, Oshima K, Teta R, Mangoni A, Gurgui M, et al. Defensive bacteriome symbiont with a drastically reduced genome. Curr Biol. 2013;23: 1478–1484. 10.1016/j.cub.2013.06.027 [DOI] [PubMed] [Google Scholar]
- 27.Nakabachi A, Nikoh N, Oshima K, Inoue H, Ohkuma M, Hongoh Y, et al. Horizontal gene acquisition of Liberibacter plant pathogens from a bacteriome-confined endosymbiont of their psyllid vector. PLoS One. 2013;8: e82612 10.1371/journal.pone.0082612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakabachi A. Horizontal gene transfers in insects. Curr Opin Insect Sci. 2015;7: 24–29. 10.1016/j.cois.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 29.Dan H, Ikeda N, Fujikami M, Nakabachi A. Behavior of bacteriome symbionts during transovarial transmission and development of the Asian citrus psyllid. PLoS One. 2017;12: e0189779 10.1371/journal.pone.0189779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada T, Hamada M, Floreancig P, Nakabachi A. Diaphorin, a polyketide synthesized by an intracellular symbiont of the Asian citrus psyllid, is potentially harmful for biological control agents. PLoS One. 2019; 14: e0216319 10.1371/journal.pone.0216319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamori T. Panel of human cancer cell lines provides valuable database for drug discovery and bioinformatics. Cancer Chemother Pharmacol. 2003;52: S74–S79. 10.1007/s00280-003-0649-1 [DOI] [PubMed] [Google Scholar]
- 32.Shoemaker RH. The NCl60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6: 813–823. 10.1038/nrc1951 [DOI] [PubMed] [Google Scholar]
- 33.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83: 757–766. 10.1093/jnci/83.11.757 [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. [Internet]. 2017.
- 35.Nishimura S, Matsunaga S, Yoshida M, Hirota H, Yokoyama S, Fusetani N. 13-Deoxytedanolide, a marine sponge-derived antitumor macrolide, binds to the 60S large ribosomal subunit. Bioorganic Med Chem. 2005;13: 449–454. 10.1016/j.bmc.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 36.Gürel G, Blaha G, Steitz TA, Moore PB. Structures of triacetyloleandomycin and mycalamide A bind to the large ribosomal subunit of Haloarcula marismortui. Antimicrob Agents Chemother. 2009;53: 5010–5014. 10.1128/AAC.00817-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szebenyi DM, Kriksunov I, Howe KJ, Ramsey JS, Hall DG, Heck ML, et al. Crystal structure of diaphorin methanol monosolvate isolated from Diaphorina citri Kuwayama, the insect vector of citrus greening disease. Acta Cryst. 2018;E74: 445–449. 10.1107/S2056989018002992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwahashi T, Okochi E, Ono K, Sugawara I, Tsuruo T, Mori S. Establishment of multidrug resistant human colorectal carcinoma HCT-15 cell lines and their properties. Anticancer Res. 1991;11: 1309–1312. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and Supporting Information files.