α1-antitrypsin (α1-AT) deficiency is a hereditary disorder characterised by an abnormally low concentration of functional α1-AT in blood and tissues [1]. The primary role of α1-AT is to protect elastin-containing tissues, most notably the lung, against the destructive activity of proteolytic enzymes [2]. Patients with severe α1-AT deficiency present with serum α1-AT concentrations <11 μM and are prone to destruction of the lung tissue, often developing respiratory symptoms and emphysema in the fourth or fifth decade of life [3, 4].
Short abstract
Administration of 120 mg·kg−1 α1-antitrypsin on a biweekly basis was safe and well tolerated http://ow.ly/CVbz30lUBum
To the Editor:
α1-antitrypsin (α1-AT) deficiency is a hereditary disorder characterised by an abnormally low concentration of functional α1-AT in blood and tissues [1]. The primary role of α1-AT is to protect elastin-containing tissues, most notably the lung, against the destructive activity of proteolytic enzymes [2]. Patients with severe α1-AT deficiency present with serum α1-AT concentrations <11 μM and are prone to destruction of the lung tissue, often developing respiratory symptoms and emphysema in the fourth or fifth decade of life [3, 4].
For three decades, treatment with intravenous α1-AT has been available, yet only recently was the RAPID (Randomised Trial of Augmentation Therapy in Alpha-1 Proteinase Inhibitor Deficiency)-Randomised Controlled Trial (RCT), a 2-year placebo-controlled trial in 180 α1-AT deficiency patients, able to demonstrate that weekly therapy with 60 mg·kg−1 body weight α1-AT reduces the loss of lung tissue [5] and slows disease progression [6]. The RAPID-Open Label Extension (OLE) study followed 130 of these patients for a further 2 years [6].
The licenced dose recommended by current guidelines is the weekly infusion of 60 mg·kg−1 body weight α1-AT [7–9]. This regimen can be time consuming, costly and inconvenient and alternative dosing regimens have been proposed [10]. However, there is a paucity of clinical trial data supporting the efficacy, safety and tolerability of alternative dosing regimens [11].
In a post hoc analysis we investigated the safety and tolerability of 60 mg·kg−1 and placebo infusions to 120 mg·kg−1 infusions administered in the RAPID-RCT; biweekly infusions of either placebo or 120 mg·kg−1 were administered to cover planned drug holidays. In addition, a pooled comparison of all biweekly 120 mg·kg−1 infusions during the 4-year RAPID programme was performed.
Treatment emergent adverse events (TEAEs) were analysed and the severity graded as mild (did not interfere with routine activities), moderate (interfered with routine activities) or severe (impossible to perform routine activities). Serious adverse events (SAEs) were defined as life-threatening TEAEs resulting in hospitalisation, prolonged hospitalisation, significant disability or death.
Two different measures to compare the adverse event rates were calculated. The exposure-adjusted event rates (EAERs) divided all TEAEs during the study conduct by the cumulative exposure of the respective group (60 or 120 mg·kg−1 α1-AT or matching placebo). The infusion-adjusted event rates (IAERs) compared the TEAE rates occurring in the 7-day period prior to and following a biweekly 120 mg·kg−1 infusion or matching placebo, i.e. an infusion sequence.
Differences in EAERs were assessed using a normal approximation test. The McNemar test was used for the intra-individual comparison of the occurrence of TEAEs in the 7 days before the first double dose (i.e. after 60 mg·kg−1) and in the 7 days after the last 120 mg·kg−1 α1-AT dose. Reported p-values are two-sided without adjustment for multiple testing.
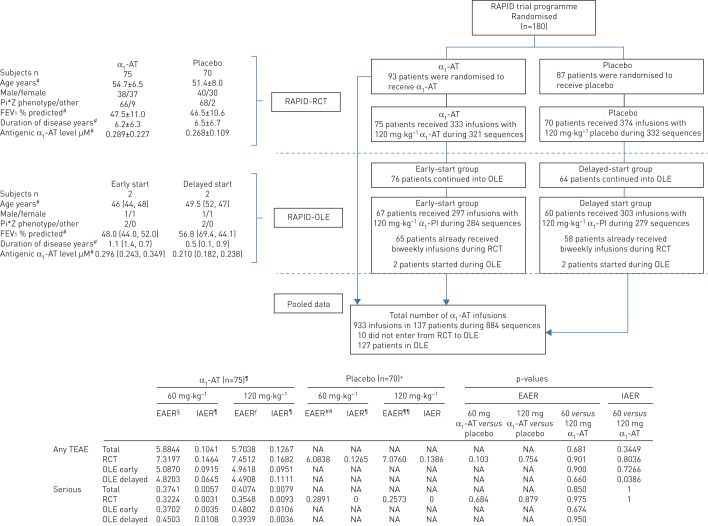
75 (80.6% of the α1-AT group) of 93 patients had at least one biweekly infusion of α1-AT during RAPID-RCT, compared to 70 (80.5%) of 87 patients who received biweekly placebo infusions. Baseline characteristics are given in figure 1.
FIGURE 1.
Patient flow, demographics and disease characteristics at baseline for patients who received at least one biweekly 120 mg·kg−1 α1-antitrypsin (α1-AT) infusion or matching placebo during RAPID (Randomised Trial of Augmentation Therapy in Alpha-1 Proteinase Inhibitor Deficiency)-Randomised Controlled Trial (RCT) or RAPID-Open Label Extension (OLE), and safety of α1-AT infusion sequences compared to placebo in RAPID-RCT occurring 7 days prior to and within 7 days after the administration of 120 mg·kg−1. Data are presented as mean±sd or n. Where only two data points were available, the individual data are shown in brackets. EAER: exposure-adjusted event rates (events per subject-years), parameter that takes into account all treatment-emergent adverse events (TEAEs) during the trial except the periods with unknown infusion volumes, which were omitted (64 out of 29 076 infusions); IAER: infusion-adjusted event rate (events per n infusion sequences), intra-individual comparison of TEAEs that includes individuals that have received 120 mg·kg−1 biweekly infusions. An infusion sequence consisted of a 60 mg·kg−1 weekly infusion or matching placebo followed by one or more infusions of biweekly 120 mg·kg−1 or matching placebo. In this analysis the number of TEAEs occurring within the 7-day period prior to a double-dose infusion while exposed to 60 mg·kg−1 or matching placebo was compared with those occurring in the 7-day period following the biweekly 120 mg·kg−1 infusion or matching placebo; Pi*Z: α1-AT deficiency; FEV1: forced expiratory volume in 1 s; PI: proteinase inhibitor; NA: not applicable. #: data obtained at baseline of RCT. ¶: biweekly infusion sequences with α1-AT during RCT n=321 and during OLE n=563, total n=884. +: biweekly infusion sequences with placebo during RCT n=332. §: drug exposure for α1-AT group 60 mg weekly in RAPID-RCT was 158.20 subject-years; number of infusions (I) 8098; RAPID-OLE early treatment group 135.02 subject-years, I=6948; RAPID-OLE late treatment group 113.27 subject-years, I=5814. ƒ: drug exposure for α1-AT group 120 mg weekly in RAPID-RCT was 14.09 subject-years, I=333; RAPID-OLE early treatment group 12.50 subject-years, I=297; RAPID-OLE late treatment group 12.69 subject-years, I=303. ##: drug exposure for placebo group 120 mg weekly in RAPID-RCT was 134.92 subject-years, I=6845. ¶¶: drug exposure for placebo group 120 mg weekly in RAPID-RCT was 15.55 subject-years, I=374.
Biweekly infusions comprised 4.3% of all α1-AT and 5.2% of all placebo administrations across both studies. The calculated drug exposure for 60 mg·kg−1 infusions weekly was >10 times higher than for the biweekly 120 mg·kg−1 infusions (α1-AT 158.20 versus 14.09 subject-years, placebo 134.92 versus 15.55 subject-years). When all α1-AT biweekly doses were pooled across RAPID-RCT and -OLE, 933 infusions over 884 infusion sequences in 137 patients were identified (mean±sd 6.45±4.26, range 1–21) (figure 1).
The EAERs were 7.32 versus 6.08 for weekly 60 mg·kg−1 α1-AT and placebo (p=0.103) and 7.45 versus 7.08 for biweekly 120 mg·kg−1 α1-AT and placebo infusions (p=0.754), respectively (figure 1). The proportion of patients who experienced a TEAE during the infusion sequences was 33.3% in the biweekly 120 mg·kg−1 group compared to 35.7% in biweekly placebo and 37.3% in weekly 60 mg·kg−1 or 37.1% in weekly placebo. The corresponding IAER were 0.1682 versus 0.1386 for 120 mg·kg−1 α1-AT and placebo infusions (p=0.754) and 0.1464 versus 0.1265 for 60 mg·kg−1 α1-AT versus weekly placebo (p=0.103). Within 24 h of infusion, when volume-related TEAEs would be expected, IAERs were 0.0374 for 120 mg·kg−1 α1-AT versus 0.0301 for biweekly placebo, and 0.0343 for 60 mg·kg−1 α1-AT versus 0.0361 for weekly placebo. IAERs in the delayed-start OLE subgroup were 0.0645 in the 60 mg compared to 0.1111 in the 120 mg periods (p=0.0386). During all double-dose infusion sequences four TEAEs were reported in association with the biweekly 120 mg·kg−1 infusions (one patient reported joint pain and headache, one had an exacerbation and one complained of headache; cumulative IAER=0.0125), compared to a single treatment-related report of rash in the placebo biweekly regimen (IAER=0.0030).
During RAPID-RCT and -OLE 16% of patients reported a TEAE within 24 h of infusion and 25% reported TEAEs within 72 h in either treatment group. A higher proportion of patients experienced a TEAE within 7 days following a biweekly 120 mg·kg−1 infusion (43.8%) than 7 days prior to double-dosing (34.3%). The number of TEAEs per infusion was 0.1267 for 120 mg·kg−1 and 0.1041 for 60 mg·kg−1. Most TEAEs were mild or moderate in intensity: 94.6% with 60 mg·kg−1 and 92.9% with biweekly 120 mg·kg−1. An analysis of TEAEs by system organ class showed similar distributions in all subgroups (data not shown).
In RAPID-RCT four SAEs were reported during the infusion sequences three SAEs with biweekly 120 mg·kg−1 (malignant tumour in the bladder, transurethral resection of the prostate and small bowel obstruction); one SAE was reported with 60 mg·kg−1 (chest pain); and none were reported with placebo. In RAPID-OLE eight SAEs occurred (exacerbations n=4; three adverse events (pneumonia, abscess and chronic carnification of the lobe) in a single patient and brain thrombus n=1). One of the 12 SAEs was considered to be drug related (chronic carnification).
To our knowledge this is the first systematic analysis of the adverse event rates and profile for two different α1-AT dosing patterns compared to matching placebo. Rates of TEAEs were comparable between all subgroups. In particular, there was no difference in event rates within 72 h after infusion or causally related events, demonstrating that doubling the dose and infusion volume is not associated with adverse volume effects in the investigated population. The significant difference in the OLE delayed-start group in favour of the 60 mg group was driven by a low rate of reported TEAEs compared to 60 mg RCT and early-start subgroups in an analysis that was not adjusted for multiple testing.
Biweekly dosing with 120 mg·kg−1 α1-AT is a convenient alternative to the recommended weekly dosing pattern, and which considerably reduces the organisational and medicinal burdens of weekly infusions, e.g. travelling to an infusion site, lifestyle disruptions and all procedures associated with infusions including scheduling, set-up and administration. This study is the largest body of evidence supporting the safety and tolerability of the biweekly 120 mg·kg−1 pattern. There were no indications of changes in the type, frequency, duration or severity of TEAEs reported. This is in line with previously published reports of the safety of augmentation therapy at doses >60 mg·kg−1 obtained in smaller cohorts [12, 13].
Data from population pharmacokinetic simulations predicted that trough levels above the 11 µM protective threshold will be maintained in the majority of patients treated with biweekly 120 mg·kg−1 infusions [14], an important prerequisite when considering alternative administration regimens. However, biweekly 120 mg·kg−1 infusions were administered infrequently and sporadically throughout the RAPID programme due to planned drug holidays, and no conclusions can be drawn regarding the impact of the biweekly regimen on rates of lung density decline. The evaluation of long-term weekly 120 mg·kg−1 infusions for efficacy, safety and tolerability outcomes is currently being evaluated in clinical trials [15].
In conclusion, biweekly infusions of 120 mg·kg−1 α1-AT has a similar safety and tolerability profile to 60 mg·kg−1 and both are comparable to placebo in patients with severe α1-AT deficiency. Biweekly dosing is expected to enhance the convenience of treatment with α1-AT.
Acknowledgements
Michael Riley and Barbara Boggetti (Trilogy Writing and Consulting GmbH, Frankfurt, Germany) provided medical writing support on behalf of CSL Behring.
Footnotes
This study is registered at ClinicalTrials.gov with identifier numbers NCT00261833 and NCT00670007. CSL Behring has previously published both the RAPID and RAPID OLE protocols with the Lancet and would be happy to provide these upon request in conjunction with this publication. This is a straightforward, post hoc safety analysis from these trials. At this time, CSL Behring does not plan to release the de-identified individual patient data upon which these analyses were conducted.
RAPID Trial Group. Australia: J.G.W. Burdon, R. Edwards, A. Glanville, M. Holmes, P. Thompson, M. Philipps, P.A. Wark. Canada: R.T. Abboud, K.R. Chapman, R.K. Elwood, P. Hernandez. Czech Republic: J. Chlumsky. Denmark: N. Seersholm, T. Skjold. Estonia: A. Altraja. Finland: R. Mäkitaro. Germany: J. Ficker, J.F. Herth, K. Schulze, H. Teschler. Ireland: N.G. McElvaney. Poland: J. Chorostowska-Wynimko, M. Sanak, A. Szczeklik, W.Z. Tomkowsk. Romania: P.I. Stoicescu. Russia: T. Martynenko. Sweden: E. Piitulainen. USA: M. Campos, T.J. Craig, R.A. Sandhaus, J.M. Stocks.
Conflict of interest: T. Greulich reports personal fees from AstraZeneca and GSK (lecture fees), Boehringer-Ingelheim, Chiesi, CSL Behring and Novartis (lecture fees, advisory board and travel support), and Mundipharma (lecture fees and advisory board); and grants and personal fees from Grifols (AATD-Labor, lecture fees and travel support), all outside the submitted work.
Conflict of interest: J. Chlumsky reports honoraria for lectures organised by CSL Behring, and for lectures organised by Boehringer Ingelheim, outside the submitted work.
Conflict of interest: M. Wencker reports support from conresp as a consultant to CSL Behring, during the conduct of the study.
Conflict of interest: O. Vit reports support from CSL Behring, as employer and sponsor of the study.
Conflict of interest: M. Fries reports support from CSL Behring, as employer and sponsor of the study.
Conflict of interest: T. Chung reports support from CSL Behring, as employer and sponsor of the study.
Conflict of interest: A. Shebl reports support from CSL Behring, as employer and sponsor of the study.
Conflict of interest: C. Vogelmeier reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols and Novartis; personal fees from CSL Behring, Chiesi, Menarini, Mundipharma, Teva and Cipla; and grants from Bayer-Schering, MSD and Pfizer, all outside the submitted work.
Conflict of interest: K.R. Chapman reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Grifols, Sanofi, Genentech, Kamada, Roche and Novartis; grants from Baxter, GlaxoSmithKline, Amgen, Shire and Octapharma; personal fees from Merck; and the CIHR-GSK Research Chair in Respiratory Health Care Delivery, UHN, all during the conduct of the study.
Conflict of interest: N.G. McElvaney reports research grants for carrying out the original RAPID and RAPID OLE studies from CSL Behring, during the conduct of the study; and personal fees from CSL Behring (advisory board), outside the submitted work.
Support statement: Funding was received from CSL Behring. Funding information for this article has been deposited with the Crossref Funder Registry.
Contributor Information
Collaborators: RAPID Trial Group, J.G.W. Burdon, R. Edwards, A. Glanville, M. Holmes, P. Thompson, M. Philipps, P.A. Wark, R.T. Abboud, K.R. Chapman, R.K. Elwood, P. Hernandez, J. Chlumsky, N. Seersholm, T. Skjold, A. Altraja, R. Mäkitaro, J. Ficker, J.F. Herth, K. Schulze, H. Teschler, N.G. McElvaney, J. Chorostowska-Wynimko, M. Sanak, A. Szczeklik, W.Z. Tomkowsk, P.I. Stoicescu, T. Martynenko, E. Piitulainen, M. Campos, T.J. Craig, R.A. Sandhaus, and J.M. Stocks
References
- 1.Greene CM, Marciniak SJ, Teckman J, et al. . α1-Antitrypsin deficiency. Nat Rev Dis Primers 2016; 2: 16051. [DOI] [PubMed] [Google Scholar]
- 2.Gadek JE, Klein HG, Holland PV, et al. . Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest 1981; 68: 1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piras B, Ferrarotti I, Lara B, et al. . Clinical phenotypes of Italian and Spanish patients with α1-antitrypsin deficiency. Eur Respir J 2013; 42: 54–64. [DOI] [PubMed] [Google Scholar]
- 4.Greulich T, Nell C, Herr C, et al. . Results from a large targeted screening program for alpha-1-antitrypsin deficiency: 2003 - 2015. Orphanet J Rare Dis 2016; 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman KR, Burdon JG, Piitulainen E, et al. . Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 386: 360–368. [DOI] [PubMed] [Google Scholar]
- 6.McElvaney NG, Burdon J, Holmes M, et al. . Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med 2017; 5: 51–60. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168: 818–900. [DOI] [PubMed] [Google Scholar]
- 8.Sandhaus RA, Turino G, Brantly ML, et al. . The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis 2016; 3: 668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miravitlles M, Dirksen A, Ferrarotti I, et al. . European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Respir J 2017; 50: 1700610. [DOI] [PubMed] [Google Scholar]
- 10.Vidal R, Blanco I, Casas F, et al. . Diagnóstico y tratamiento del déficit de alfa-1-antitripsina [Guidelines for the diagnosis and management of alpha-1 antitrypsin deficiency]. Arch Bronconeumol 2006; 42: 645–659. [DOI] [PubMed] [Google Scholar]
- 11.Dirksen A, Dijkman JH, Madsen F, et al. . A randomized clinical trial of α1-antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999; 160: 1468–1472. [DOI] [PubMed] [Google Scholar]
- 12.Campos MA, Kueppers F, Stocks JM, et al. . Safety and pharmacokinetics of 120 mg/kg versus 60 mg/kg weekly intravenous infusions of alpha-1 proteinase inhibitor in alpha-1 antitrypsin deficiency: a multicenter, randomized, double-blind, crossover study (SPARK). COPD 2013; 10: 687–695. [DOI] [PubMed] [Google Scholar]
- 13.Barker AF, Iwata-Morgan I, Oveson L, et al. . Pharmacokinetic study of α1-antitrypsin infusion in α1-antitrypsin deficiency. Chest 1997; 112: 607–613. [DOI] [PubMed] [Google Scholar]
- 14.Tortorici MA, Rogers JA, Vit O, et al. . Quantitative disease progression model of α-1 proteinase inhibitor therapy on computed tomography lung density in patients with α-1 antitrypsin deficiency. Br J Clin Pharmacol 2017; 83: 2386–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorrells S, Camprubi S, Griffin R, et al. . SPARTA clinical trial design: exploring the efficacy and safety of two dose regimens of alpha1-proteinase inhibitor augmentation therapy in alpha1-antitrypsin deficiency. Respir Med 2015; 109: 490–499. [DOI] [PubMed] [Google Scholar]