Abstract
Objective:
To determine whether men with unexplained infertility (UI) and low total testosterone (TT) have abnormal spermatogenesis and lower fecundity.
Design:
Secondary analysis of the prospective, randomized, multicenter clinical trial: Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS).
Setting:
Reproductive Medicine Network infertility clinics.
Patients:
900 couples with UI enrolled in AMIGOS. Semen analysis with an ejaculate of at least 5 million total motile sperm was required for enrollment. For inclusion in this secondary analysis, a fasting TT was required.
Interventions:
None
Main Outcome Measure:
Logistic regression, adjusted for age and BMI, assessed the association between low TT (defined as <264 ng/dL), semen parameters, and pregnancy outcome.
Results:
781 men (mean age 34.2 ± 5.7 years) with a median (IQR) TT of 411 (318–520) ng/dL were included. Men with TT < 264 ng/dL were less likely to have normal (≥4% strict Kruger) morphology (unadjusted OR 0.56, 95% CI (0.34, 0.92); adjusted odds ratio (OR) 0.59, 95% confidence intervals (CI) (0.35, 0.99)). There was no association between low TT and semen volume < 1.5 mL, sperm concentration < 15 106/mL, or motility < 40%. Among couples whose male partner had low TT, 21 (18.8%) had a live birth, compared to 184 (27.5%) live births in couples with a male partner having TT > 264 ng/dL. The odds of live birth decreased by 40% in couples whose male partner had low TT (unadjusted OR 0.60, 95% CI (0.36, 1.00); adjusted OR 0.65, 95% CI (0.38, 1.12)).
Conclusion:
In couples with UI, low TT in the male partner was associated with abnormal sperm morphology and lower live birth rates.
Clinical Trial Registration Number:
Capsule:
In couples with unexplained infertility, low total testosterone in the male partner was associated with abnormal strict morphology and lower live birth rate.
Introduction:
A significant male factor is found in 50% of infertile couples and is implicated as the sole cause for infertility in up to 30% of couples (1). Low testosterone is found in approximately 15% of male infertility cases (2); however, the impact of low testosterone on a man’s fecundity is less known. In animal models, the presence of a minimum testosterone level within the testis is clearly shown to be necessary for spermatogenesis (3) with rising serum testosterone concentrations associated with increasing sperm production (4). In humans, low serum total testosterone (TT), a surrogate for intratesticular levels, has previously been linked [sometimes inconsistently (5)] to abnormal semen parameters such as lower sperm concentration and total sperm motility (6). Furthermore, studies evaluating the relationship between low TT and pregnancy or live birth outcomes are lacking.
To address the relationship between TT, semen parameters and live birth rate in couples with unexplained infertility, we performed a secondary analysis of the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) trial. This large, multicenter clinical trial determined pregnancy, live birth, and multiple gestation rates following up to four cycles of ovarian stimulation with intrauterine insemination in couples with unexplained infertility (7–8). We tested the hypothesis that low TT would be associated with abnormal semen parameters (sperm concentration, total sperm motility, and Kruger strict morphology) and lower live birth rate.
Materials and Methods:
Study sample.
The AMIGOS trial was designed to discern if ovarian stimulation with letrozole would result in a significantly lower frequency of multiple gestations when compared to gonadotropins and/or clomiphene citrate (7–8). This IRB approved study enrolled 900 couples with unexplained infertility. The female partner was required to be between 18 and 40 years of age, with regular menstrual cycles, a normal uterus, and at least one patent fallopian tube (8). Male partners were required to have a baseline semen analysis with at least 5 million motile sperm, recorded within 1 year of study initiation (7). This minimum required sperm count was considered sufficient for sperm washing and intrauterine insemination—which was performed in all AMIGOS treatment cycles. While fasting, male partners also provided blood samples, which were processed for TT levels at the University of Virginia Ligand Assay and Analysis Core (Charlottesville, VA). TT was determined by radioimmunoassay (Siemens; functional sensitivity = 10 ng/dL; intra-assay coefficient of variation = 4.3%; inter-assay coefficient of variation = 7.4%). The assay performance was validated with determination of accuracy, linearity, precision, and functional sensitivity (i.e., the lowest concentration with accuracy to a known standard within 20% and intra-assay coefficient of variation <20%).
Statistical Analysis:
Bivariate analyses were conducted to examine the relationship between baseline demographics and low TT using chi-square, Fisher’s exact, t-tests or non-parametric Wilcoxon rank-sum test where appropriate. We defined low TT as <264 ng/dL, based on recently published data that represents the reproductive age group of our cohort (9). Normal semen parameters were defined as semen volume ≥ 1.5 mL, sperm concentration ≥15 ×106/mL, total sperm motility ≥40%, and Kruger strict morphology ≥4%. Conception was defined as having a rising serum level of hCG on two consecutive tests. Clinical pregnancy was defined as an intrauterine pregnancy with fetal heart motion detected by transvaginal ultrasound. Lastly, live birth was defined as the delivery of a viable infant. Logistic regression was used to investigate the relationship between low TT (<264 ng/dL) and the outcomes of normal semen parameters and pregnancy including conception, clinical pregnancy, and live birth. The logistic regression analyses were adjusted for age and BMI. The pregnancy outcome models adjusted for treatment group (i.e. clomiphene citrate, letrozole, and gonadotropins). All hypothesis tests were two-sided and all analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results:
Of the 900 couples enrolled, 781 men with both TT and a baseline semen analysis with at least 5 million total motile sperm were included in our present study, whereas 119 men were excluded due to missing TT. The male partners had a mean age of 34.2 (+/− 5.7) years, with a mean BMI of 28.9 (+/− 5.7) kg/m2. Over half of the male partners were non-smokers and had obtained a college or graduate degree (Table 1). Men with low TT had a higher BMI’s. There were no statistically significant differences in age, smoking status, or education level between those in the lower and higher TT groups. There was also no difference in the age of the female partners.
Table 1.
Patient characteristics of male partners of couples with unexplained infertility
| All | TT<264 ng/dL |
TT≥264 ng/dL |
p-value | |
|---|---|---|---|---|
| Age (years) | 34.2 (±5.7) | 34.6 (±4.9) | 34.2 (±5.8) | 0.45 |
| BMI (kg/m2) | 28.9 (±5.7) | 31.7 (±6.8) | 28.4 (±5.3) | <0.001 |
| n (%) | n (%) | n (%) | ||
| Reporting secondary infertility | 286 (37.2%) | 34 (31.5%) | 252 (38.2%) | 0.18 |
| Education: College/graduate degree | 426 (55.6%) | 62 (57.4%) | 364 (55.3%) | 0.69 |
| Current | 100 (13.0%) | 15 (13.9%) | 85 (12.9%) |
The median (IQR) TT for the cohort was 411 (318–520) ng/dL. There were 112 subjects (14.3%) with a TT less than 264 ng/dL. Baseline median (IQR) sperm parameters included semen volume 2.8 (2.0–3.7) mL, sperm concentration 49 (27–85) × 106/mL, motility 56 (49–65) %, and strict morphology 9 (5–18) %. Of these 781 couples, 295 (37.8%) conceived, 234 (30.0%) had a clinical pregnancy, and 205 (26.3%) had a live birth.
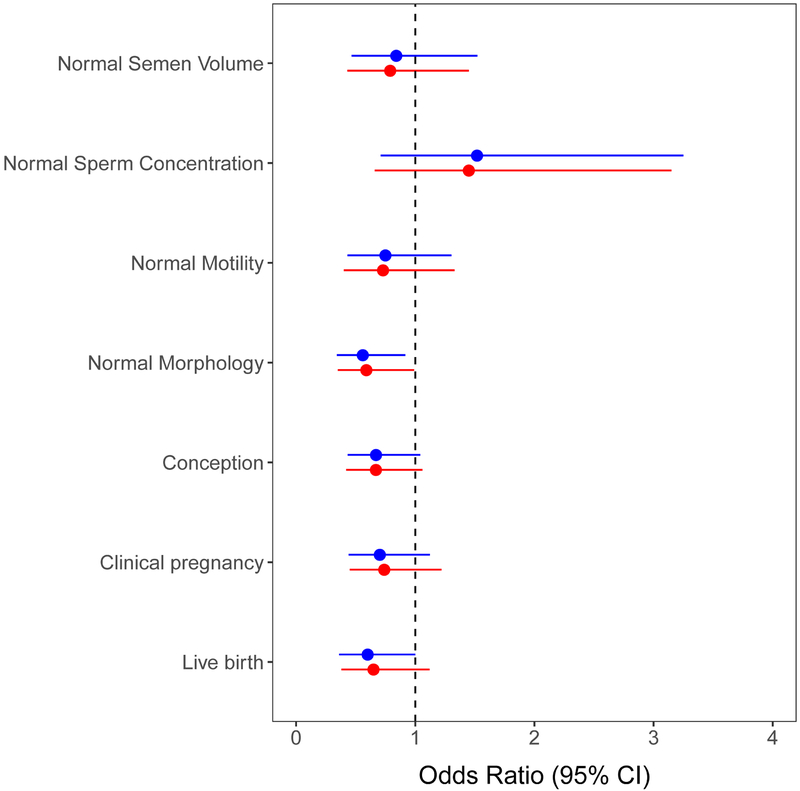
Men with low TT and at least 5 million total motile sperm were less likely than men with TT ≥ 264 ng/dL to have normal Kruger strict morphology (unadjusted odds ratio (OR) 0.56, 95% confidence interval (CI) (0.34, 0.92)) (Table 2) (Figure 1). The result remained statistically significant after adjusting for age and BMI (OR 0.59, 95% CI (0.35, 0.99). This corresponds to 41% decreased odds of normal morphology in men with low TT. No association between low TT and semen volume, sperm concentration or sperm motility was found.
Table 2.
Semen analysis and pregnancy outcomes (n=781)
| TT<264 ng/dL (N=112) |
TT≥264 ng/dL (N=669) |
TT<264 vs. TT≥264 Unadjusted |
TT<264 vs. TT≥264 Adjusted for age and BMI |
|||
|---|---|---|---|---|---|---|
| N (%) | N (%) | Odds Ratio (95% CI) |
p-value | Odds Ratio (95% CI) |
p-value | |
| Normal Semen Volume (≥ 1.5 mL) |
97 (86.6) | 592 (88.5) | 0.84 (0.46, 1.52) | 0.57 | 0.79 (0.43, 1.45) | 0.45 |
| Normal Sperm Concentration (≥ 15 106/mL) |
104 (92.9) | 599 (89.5) | 1.52 (0.71, 3.25) | 0.28 | 1.45 (0.66, 3.15) | 0.35 |
| Normal Total Sperm Motility (≥ 40%) |
94 (83.9) | 585 (87.4) | 0.75 (0.43, 1.30) | 0.31 | 0.73 (0.40, 1.33) | 0.31 |
| Normal Strict Morphology (≥ 4%) |
71 (72.4) | 474 (82.4) | 0.56 (0.34, 0.92) | 0.02 | 0.59 (0.35, 0.99) | 0.05 |
| Conception* | 34 (30.4) | 261 (39.0) | 0.67 (0.43, 1.04) | 0.08 | 0.67 (0.42, 1.06) | 0.09 |
| Clinical pregnancy (heart beat)* | 27 (24.1) | 207 (30.9) | 0.70 (0.44, 1.12) | 0.14 | 0.74 (0.45, 1.22) | 0.24 |
| Live birth* | 21 (18.8) | 184 (27.5) | 0.60 (0.36, 1.00) | 0.05 | 0.65 (0.38, 1.12) | 0.12 |
Odds ratio (95% CI) and p-value for pregnancy outcomes are adjusted for treatment group.
Figure 1:
Comparison of TT<264 vs. TT ≥ 264 with respect to semen analysis and pregnancy outcomes. Blue represents unadjusted models for semen parameters and models adjusted for treatment for pregnancy outcomes. Red represents models adjusted for age and BMI for semen parameters and models adjusted for treatment, age and BMI for pregnancy outcomes.
In couples with a male partner with low TT, 21 (18.8%) had a live birth, compared to 184 (27.5%) in couples with a male partner having a TT ≥ 264 ng/dL. This difference demonstrated a lower live birth for couples with a male partner with low TT (unadjusted OR 0.60, 95% CI (0.36, 1.00); adjusted OR 0.65, 95% CI (0.38, 1.12)) (Table 2) (Figure 1). Similarly, conception and clinical pregnancy rates were lower in women partnered with a male with low TT; however, these differences did not reach statistical significance (Table 2).
Discussion:
In this cohort of couples with unexplained infertility, men with low TT (< 264 ng/dL) and at least 5 million total motile sperm, had greater odds of having abnormal strict morphology sperm when compared to men with TT ≥ 264 ng/dL. Additionally, low TT was associated with lower live birth rates than that observed in couples with a male partner with normal TT (>264 ng/dL). These data suggest that optimal spermatogenesis and fecundity may require a minimum level of testosterone and underscore the importance of identifying men with low TT in couples with unexplained infertility.
The group of men with lower TT had a higher median BMI. This inverse relationship between testosterone and BMI is well established (1), yet complex. BMI is potentially on the causal pathway to developing low testosterone but was not independently associated with AMIGOS pregnancy outcomes (10). While we reported the odds ratios both unadjusted as well as adjusted for age and BMI, we believe the unadjusted data is actually the best representation of the relationship we sought to describe. Both increasing BMI and age are associated with lower testosterone levels. However, neither were independently associated with pregnancy outcomes in the original AMIGOS trial (10). Therefore, adjusting for one or both may attenuate the relationship between serum testosterone and treatment outcomes.
Low TT is present in approximately 15% of infertile men (2). Consistent with this previously published data, the present analysis found 14% of the male partners in couples with unexplained infertility had TT levels < 264 ng/dL. Both the American Urological Association’s best practice statement and the American Society for Reproductive Medicine’s Committee Opinion regarding the evaluation of the infertile male recommend testing total testosterone as part of the routine workup when the sperm concentration is low, a degree of sexual dysfunction is present, or other findings suggest an endocrinopathy. Many experts advocate that all infertile males should have endocrine testing (5–6).
Serum TT is a surrogate marker for intratesticular levels. In humans, intratesticular testosterone concentrations are 30–100 times higher than serum levels (11–15), and this elevated testosterone concentration is necessary for spermatogenesis to occur. Although testing in some animal models has shown minimum testosterone levels are needed for spermatogenesis (3), a minimum level for human spermatogenesis is not well defined. Along this line, studies have not shown a consistent pattern of abnormal semen parameters in men whose testosterone is low, but adequate for spermatogenesis to occur (5). One of the primary aims of the present study was to determine if semen parameters were associated with lower serum testosterone. Aside from idiopathic infertility, the main inclusion criterion for these men was to have a minimum total motile sperm count of 5 million, which potentially selects men with higher sperm concentrations and/or motility than an unselected group of men with infertility. No association was seen with sperm concentration or motility, but sperm morphology was decreased in the men with low TT.
More important than any semen parameter for couples suffering with infertility are reproductive outcomes, such as pregnancy and live birth. Unfortunately, studies relating these outcomes to the male partner’s endocrine status are lacking. To our knowledge, this is the first study evaluating the association of testosterone to reproductive outcomes. Conception, clinical pregnancy and live birth rates were all lower when male partners had lower testosterone. A lower live birth rate for couples with a male partner with low TT is a compelling finding that merits further study.
This study has several limitations. As a secondary analysis of a previously completed, randomized controlled trial performed with a different purpose in mind, many of the limitations for this present study are simply for this reason alone. Both male and female participants came in for blood draws in a fasting state, which predominantly occurred in the morning hours, but were not strictly controlled for time of day. This may have affected TT measurements, as males with later blood draws may be falsely identified as having low TT due to the diurnal secretion of testosterone production in men. Additionally, only one baseline TT was collected, and symptoms of hypogonadism were not assessed. Therefore, we cannot assign the diagnosis of symptomatic hypogonadism. Further limitations include an absence of free testosterone or calculated bioavailable testosterone from the present analysis. The AMIGOS study did not query for possible use of selective estrogen receptor modulators (SERMS), or other androgenic agents that could artificially raise the serum TT level. Moreover, our cohort excluded 119 male partners who were missing a baseline TT. Therefore, we considered multiple imputation to account for missing data. Although the association for the outcomes of morphology and live birth were slightly weaker after imputation (p=0.04 and p=0.07, respectively), the results were relatively consistent with the analysis of 781 men with measured TT (Supplemental Table 1). Moreover, although men were required to have 5 million total motile sperm for enrollment, a physical exam or additional evaluation of men with oligoasthenozoospermia was not a part of the AMIGOS protocol. Lastly, this study was not specifically designed to address outcomes on a per cycle (IUI) basis, and variations in the total motile sperm count for each IUI cycle may affect fecundity.
The study had many strengths, including a randomized controlled trial setting, a large sample size, systematic male partner phenotyping, and a standardized methodology for conducting semen analyses that was adopted across all study sites. Serum testosterone measurements were performed in a centralized, high-volume laboratory of excellence. Additionally, all participant couples were carefully followed through pregnancy and delivery, with complete capture of study end points.
This study highlights the importance of testosterone for male reproduction, not only for male sexual function, but also for semen quality and ultimately reproductive outcomes. The results underscore the importance of a complete evaluation of the sub-fertile male, which should include a physical examination, an endocrine assessment and treatment of underlying endocrinopathies. Efforts to further evaluate and optimize even asymptomatic low testosterone levels in male partners should be considered in the treatment of the infertile couple. Future studies should seek to determine if there is a threshold of TT below which semen parameters are more negatively impacted or if there are other male factor variables which may expand the predictive value of total testosterone and the semen analysis.
Conclusion:
In couples with unexplained infertility and baseline semen analyses with at least 5 million total motile sperm, low TT (< 264 ng/dL) in the male partner was associated with low sperm morphology and a lower live birth rate.
Supplementary Material
Acknowledgement:
We thank Esther Eisenberg, MD, acting as the NICHD Medical Officer, for her assistance with protocol development and manuscript review.
Financial Support: The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): U10 HD077844, U10 HD077680, U10 HD39005, U10 HD38992, U10 HD27049, U10 HD38998, U10 HD055942, HD055944, U10 HD055936, and U10HD055925.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Where the work was done: Reproductive Medicine Network (RMN).
References:
- 1.Sigman M, Jarow JP. Endocrine evaluation of infertile men. Urology. 1997;50:659–64. [DOI] [PubMed] [Google Scholar]
- 2.Ventimiglia E, Capogrosso P, Boeri L, Ippolito S, Scano R, et al. Validation of the American Society for Reproductive Medicine guidelines/recommendations in White European men presenting for couple’s infertility. Fertil Steril. 2016;106:1076–82. [DOI] [PubMed] [Google Scholar]
- 3.Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, et al. The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total (ARKO) or Sertoli cell-selective (SCARKO) ablation of the androgen receptor. Endocrinology. 2005;146:2674–2683. [DOI] [PubMed] [Google Scholar]
- 4.Ramaswamy S, Weinbauer G. Endocrine control of spermatogenesis: Role of FSH and LH/Testosterone. Spermatogenesis. 2014; 4;e996025 Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keskin MZ, Budak S, Zeyrek T, Celik O, Mertoglu O, Yoldas M, Ilbey YO. The relationship between serum hormone levels (follicle-stimulating hormone, luteinizing hormone, total testosterone) and semen parameters. Arch Ital Urol Androl. 2015;87:194–7 [DOI] [PubMed] [Google Scholar]
- 6.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl 2007;28:397–406. [DOI] [PubMed] [Google Scholar]
- 7.Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, et al. Letrozole, Gonadotropin, or Clomiphene for Unexplained Infertility. NEJM 2015;373:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, et al. Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) Trial: baseline characteristics. Fert Steril; 2015;103:962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, et al. Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. JCEM. 2017;102:1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen KR, He AL, Styper AK, Wild RA, Butts S, et al. Predictors of pregnancy and live-birth in couples with unexplained infertility after ovarian stimulation-intrauterine insemination. Fertil Steril. 2016;105:1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heller CG, Rowley MJ, Heller GV. Clomiphene citrate: a correlation of its effect on sperm concentration and morphology, total gonadotropins, ICSH, estrogen and testosterone excretion, and testicular cytology in normal men. JCEM. 1969;29:638–649. [DOI] [PubMed] [Google Scholar]
- 12.Morse HC, Horike N, Rowley MJ, Heller CG. Testosterone concentrations in testes of normal men: effects of testosterone propionate administration. JCEM. 1973;37:882–886. [DOI] [PubMed] [Google Scholar]
- 13.McLachlan RI, O’Donnell L, Stanton PG, Balourdos G, Frydenberg M, et al. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. JCEM. 2002;87:546–556. [DOI] [PubMed] [Google Scholar]
- 14.Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl 2004;25:931–8. [DOI] [PubMed] [Google Scholar]
- 15.Turner TT, Jones CE, Howards SS, Ewing LL, Zegeye B, Gunsalus GL. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–1932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.