Abstract
Purpose
To understand the mechanism through which obesity in breast cancer patients is associated with poorer outcome, we evaluated body mass index (BMI) and response to neoadjuvant chemotherapy (NC) in women with operable breast cancer.
Patients and Methods
From May 1990 to July 2004, 1,169 patients were diagnosed with invasive breast cancer at M. D. Anderson Cancer Center and received NC before surgery. Patients were categorized as obese (BMI ≥ 30 kg/m2), overweight (BMI of 25 to < 30 kg/m2), or normal/underweight (BMI < 25 kg/m2). Logistic regression was used to examine associations between BMI and pathologic complete response (pCR). Breast cancer–specific, progression-free, and overall survival times were examined using the Kaplan-Meier method and Cox proportional hazards regression analysis. All statistical tests were two-sided.
Results
Median age was 50 years; 30% of patients were obese, 32% were overweight, and 38% were normal or underweight. In multivariate analysis, there was no significant difference in pCR for obese compared with normal weight patients (odds ratio [OR] = 0.78; 95% CI, 0.49 to 1.26). Overweight and the combination of overweight and obese patients were significantly less likely to have a pCR (OR = 0.59; 95% CI, 0.37 to 0.95; and OR = 0.67; 95% CI, 0.45 to 0.99, respectively). Obese patients were more likely to have hormone-negative tumors (P < .01), stage III tumors (P < .01), and worse overall survival (P = .006) at a median follow-up time of 4.1 years.
Conclusion
Higher BMI was associated with worse pCR to NC. In addition, its association with worse overall survival suggests that greater attention should be focused on this risk factor to optimize the care of breast cancer patients.
INTRODUCTION
Obesity as measured using body mass index (BMI) is generally regarded as a poor prognostic factor for breast cancer;1-5 however, there is inconsistency in the literature.6 Conflicting reports on the prognostic role of obesity have been attributed to variations in chemotherapy dosing of obese patients7,8 and failure to adjust for treatment and tumor characteristics that strongly predict for clinical outcome.9 In addition, the biologic mechanism of action through which obesity may contribute to breast cancer prognosis remains unclear.
It has been proposed that obesity influences breast cancer prognosis by increasing circulating plasma levels of estrogen, insulin, insulin-like growth factor, and other hormonal factors that act to promote the growth of occult metastatic disease.10,11 It is also possible that obesity may affect response to chemotherapy because the conversion to active metabolite and/or clearance of cytotoxic drugs such as doxorubicin and cyclophosphamide may be altered by higher body weight without a corresponding increase in toxicity.12,13 An assessment of tumor response to neoadjuvant chemotherapy (NC) may serve as a surrogate measure for understanding how obesity influences breast cancer prognosis.
There is a lack of studies that have examined the influence of obesity on response to NC for the treatment of primary breast tumors. Therefore, we used a cohort of 1,169 patients with operable breast cancer treated with NC at The University of Texas M. D. Anderson Cancer Center (MDACC) to evaluate the relationship between BMI at diagnosis and the end points of pathologic complete response (pCR) and breast cancer–specific and progression-free survival. Because pCR to NC is considered a marker of improved progression-free survival,14,15 we hypothesized that decreased rates of pCR among obese patients should also predict for worse progression-free survival. Understanding the specific biologic mechanisms through which being overweight or obese contributes to breast cancer prognosis is essential for individualizing care for improving outcomes among overweight and obese breast cancer patients.
PATIENTS AND METHODS
Patient Selection
The Breast Cancer Management System database of MDACC was searched to identify women with nonmetastatic, primary invasive ductal or lobular noninflammatory breast cancer who were treated with NC before being eligible for surgical treatment at MDACC between May 1990 and July 2004. The database contains detailed information on patient (race, age, and menopausal status at start of NC), clinical (height and weight at start of NC, chemotherapy and endocrine treatment, surgery type, and assessment of pathologic response in the breast and axilla), and tumor (clinical stage, estrogen receptor [ER] and progesterone receptor [PR] status, histologic grade, and HER-2/neu status) characteristics at diagnosis and has been previously described.15 Follow-up information for patients in the Breast Cancer Management System database is obtained every 2 years by direct review of the medical records and linkage to the MDACC Tumor Registry, which mails annual follow-up letters to each patient registered at MDACC known to be alive to determine the patient's clinical status. The MDACC Tumor Registry checks the Social Security Death Index and the Texas Bureau of Vital Statistics for the status of patients who do not respond to the letters.
One thousand one hundred ninety-three patients were identified who met study criteria. Twenty-four patients were excluded for the following reasons: partial surgery before receiving NC (n = 21); patient refused surgery after NC (n = 1); concomitant pregnancy (n = 1); and time between NC and response assessment of more than 1 year (n = 1). The final study population consisted of 1,169 breast cancer patients.
BMI was calculated as weight (kg) divided by height (m2), and groups were separated into obese (BMI ≥ 30 kg/m2), overweight (BMI between 25 and 30 kg/m2), and normal/underweight (BMI < 25 kg/m2) as described by the National Institutes of Health and National Heart, Lung, and Blood Institute.16 The study was approved by the MDACC institutional review board.
Pathology
Breast cancer diagnosis was made by core needle biopsy, and diagnostic tissue was evaluated by pathology before the initiation of NC. The histologic type of all tumors was defined according to the WHO classification system.17 Nuclear grade was defined according to the Black's nuclear grading system with modification of numbers (1 represents well-differentiated tumors, and 3 represents poorly differentiated tumors).18 Immunohistochemistry was used to determine ER and PR status after 1993. HER-2/neu status was evaluated by immunohistochemistry or by fluorescence in situ hybridization in breast cancer tissue. HER-2/neu–positive tumors were defined as 3+ receptor overexpression on immunohistochemistry staining and/or gene amplification found on fluorescence in situ hybridization testing. pCR for this study was defined as no residual invasive carcinoma in either the breast or the axillary lymph nodes. Residual ductal carcinoma in situ was included in the pCR group.14,15,19
Treatment
Ninety-one percent of patients (n = 1,066) received an anthracycline-based regimen, and of these patients, 72% received the addition of taxane (n = 738) or trastuzumab (n = 31). Other systemic therapies included cyclophosphamide, methotrexate, and fluorouracil (n = 3); taxane with cyclophosphamide, methotrexate, and fluorouracil (n = 1); taxane with trastuzumab (n = 1); single-agent taxane (n = 97); and other investigational agents (n = 1). We grouped our chemotherapy categories into those regimens that either included or excluded a taxane based on data that taxanes improve response to NC.20 Our institutional policy is to dose chemotherapy by actual weight; however, data on dose of chemotherapy were not available for the study participants. At the completion of the NC, the majority of patients underwent definitive surgery. Surgical intervention was breast-conserving surgery for 38% of patients (n = 447) and mastectomy for 61% of patients (n = 714); 1% of the patients (n = 8) did not have surgery as a result of the development of metastatic disease. All patients included in this analysis who had definitive surgery received axillary node dissection (83%) or sentinel node biopsy (17%). Radiation therapy was included in the treatment plan for patients who underwent breast-conserving surgery or had locally advanced disease as per preoperative tumor characteristics. Postmenopausal women who were hormone receptor positive were offered 5 years of endocrine adjuvant therapy. Starting in 1997, adjuvant tamoxifen was also recommended to premenopausal women with hormone receptor–positive (ER-positive and/or PR-positive) disease.
Statistical Methods
Normal and underweight patients were grouped together because of the small number of patients in the underweight category (n = 17, 1.5%). The χ2 test was used to compare groups with respect to categoric variables. The Wilcoxon rank sum test and Kruskal-Wallis test were used to examine associations between categoric and ordinal variables, and Spearman's correlation coefficient was used to test for associations between two ordinal variables. Associations between clinical factors at diagnosis and BMI were analyzed using logistic regression. Survival curves were constructed using the Kaplan-Meier product-limit method and compared between BMI groups with the log-rank test. Cox proportional hazards regression analysis was performed to calculate hazard ratios and 95% CIs for demographic and clinical characteristics and treatment variables.
Overall survival was calculated from the date of NC initiation to the date of death or last follow-up. Progression-free survival was calculated from the time of treatment initiation to the time of disease recurrence or metastasis or, if no recurrence or metastasis was recorded, to the time of last follow-up. Patients who had not experienced progression or died by the last follow-up were censored. To address whether BMI was associated with breast cancer–specific mortality, we used a classification system that has been used by other investigators21 with high concordance for documented cause of death. We classified deaths as caused by breast cancer if the death occurred after a report of a recurrence. Deaths were classified as not being breast cancer related if no recurrence was recorded before the death.
Initially, univariate models were fit to evaluate the predictive effect of each factor alone, and then a backward selection procedure was used to determine the most parsimonious multivariate model. Variables considered in modeling of the probability of pCR to NC and the survival analyses included BMI, race, age at treatment start, menopausal status, ER status, PR status, HER-2/neu status, tumor histology, nuclear grade, clinical stage, lymphatic or vascular invasion, chemotherapy, and duration of NC. Number of positive nodes, number of nodes removed, adjuvant endocrine therapy, and pathologic response to NC were also considered in the survival analyses. All reported P values are two-sided, and P < .05 was considered statistically significant. Analyses were performed using SAS for Windows (release 9.1; SAS Institute, Cary, NC).
RESULTS
Relationship Between Patient and Tumor Characteristics and BMI Categories
Table 1 lists the patient and tumor characteristics by BMI categories. African American race, older age, and postmenopausal status at start of NC were significantly associated with overweight and obese status. Obese patients had a higher percentage of tumors that were ER negative (46% in obese v 38% in overweight and 36% in normal/underweight patients; P = .01). A higher percentage of obese patients (41%) had stage III tumors compared with overweight (31%) or normal/underweight patients (28%; P < .01). Specifically, more obese patients (40%) had stage T3 or T4 tumors compared with overweight (31%) or normal/underweight patients (25%; P < .01). BMI did not show a significant association with tumor histology, PR status, HER-2 status, nuclear grade, lymph node involvement, and presence of vascular or lymphatic invasion. A subgroup analysis was performed to evaluate the distribution of BMI category among patients with triple-negative (ER, PR, and HER-2/neu negative) breast cancers (n = 208). There was a trend of a higher percentage of triple-negative breast cancers among overweight (23%) and obese (25%) patients compared with normal weight patients (18%; P = .05; data not shown).
Table 1.
Patient and Tumor Characteristics by BMI Category (N = 1,169)
| Characteristic | Normal/Underweight |
Overweight |
Obese |
Total |
P* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | ||||||
| Race | < .0001 | ||||||||||||
| White | 332 | 75.3 | 266 | 70.9 | 220 | 62.3 | 818 | 70 | |||||
| Hispanic | 49 | 11 | 55 | 14.7 | 40 | 11.3 | 144 | 12.3 | |||||
| African American | 25 | 5.7 | 33 | 8.8 | 84 | 23.8 | 142 | 12.1 | |||||
| Asian/other | 35 | 8 | 21 | 5.6 | 9 | 2.62 | 65 | 5.6 | |||||
| Age at treatment start, years | < .0001† | ||||||||||||
| Median | 47.3 | 50.9 | 52.1 | 49.9 | |||||||||
| Range | 25.0-77.4 | 22.8-83.7 | 25.4-79.5 | 22.8-83.7 | |||||||||
| Menopausal status | < .0001 | ||||||||||||
| Premenopausal | 245 | 55.8 | 162 | 43.3 | 115 | 32.9 | 522 | 44.9 | |||||
| Perimenopausal | 18 | 4.1 | 11 | 3 | 15 | 4.3 | 44 | 3.8 | |||||
| Postmenopausal | 176 | 40.1 | 201 | 53.7 | 220 | 62.8 | 597 | 51.3 | |||||
| Cancer histology | .30 | ||||||||||||
| Ductal | 412 | 93.44 | 342 | 91.2 | 329 | 93.7 | 1,083 | 92.9 | |||||
| Lobular | 28 | 6.56 | 33 | 8.8 | 22 | 6.3 | 83 | 7.1 | |||||
| ER status of primary tumor | .01 | ||||||||||||
| Negative | 161 | 36.5 | 143 | 38.1 | 163 | 46.2 | 467 | 39.9 | |||||
| Positive | 280 | 63.5 | 232 | 61.9 | 190 | 53.8 | 702 | 60.1 | |||||
| PR status of primary tumor | .47 | ||||||||||||
| Negative | 204 | 46.5 | 186 | 50.4 | 174 | 49.9 | 564 | 48.8 | |||||
| Positive | 235 | 53.5 | 183 | 49.6 | 175 | 50.1 | 593 | 51.2 | |||||
| Nuclear grade | .11 | ||||||||||||
| 1 | 18 | 4.1 | 14 | 3.8 | 4 | 1.2 | 36 | 3.1 | |||||
| 2 | 156 | 35.9 | 123 | 33.5 | 116 | 33.6 | 395 | 34.5 | |||||
| 3 | 261 | 60 | 230 | 62.7 | 225 | 65.2 | 716 | 62.4 | |||||
| HER-2 status of primary tumor | .17 | ||||||||||||
| Negative | 256 | 74 | 252 | 80 | 230 | 78 | 738 | 77.2 | |||||
| Positive | 90 | 26 | 63 | 20 | 65 | 22 | 218 | 22.8 | |||||
| Lymphatic invasion | .24 | ||||||||||||
| Negative | 314 | 72.7 | 284 | 77.4 | 264 | 76.7 | 862 | 75.4 | |||||
| Positive | 118 | 27.3 | 83 | 22.6 | 80 | 23.3 | 281 | 24.6 | |||||
| Cancer stage | .0002 | ||||||||||||
| I | 15 | 3.4 | 23 | 6.1 | 10 | 2.8 | 48 | 4.1 | |||||
| II | 303 | 68.9 | 235 | 62.7 | 198 | 56.1 | 736 | 63 | |||||
| III | 122 | 27.7 | 117 | 31.2 | 145 | 41.1 | 384 | 32.9 | |||||
| Tumor stage | .0006 | ||||||||||||
| T0 | 1 | 0.2 | 0 | 0 | 1 | 0.3 | 2 | 0.2 | |||||
| T1 | 56 | 12.7 | 45 | 12 | 33 | 9.4 | 134 | 11.5 | |||||
| T2 | 273 | 62.1 | 211 | 56.3 | 175 | 49.9 | 656 | 56.5 | |||||
| T3 | 71 | 16.1 | 62 | 16.5 | 73 | 20.8 | 206 | 17.7 | |||||
| T4 | 39 | 8.9 | 57 | 15.2 | 69 | 19.6 | 165 | 14.1 | |||||
| Positive lymph nodes | .91 | ||||||||||||
| No | 189 | 42.9 | 166 | 44.3 | 155 | 43.9 | 510 | 43.6 | |||||
| Yes | 252 | 57.2 | 209 | 55.7 | 198 | 56.1 | 659 | 56.4 | |||||
| Total No. of nodes removed | .88† | ||||||||||||
| Median | 5.55 | 5.55 | 5.55 | 5.5 | |||||||||
| Range | 2.0-10.7 | 1.7-9.6 | 2.00-11.8 | 1.7-11.8 | |||||||||
Abbreviations: BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
P values are from the χ2 test unless otherwise indicated.
Kruskal-Wallis test.
BMI and pCR to NC
The time from initiation of NC to definitive surgical management ranged from 1.7 to 11.8 months (median, 5.5 months). Approximately 15% (14.5%, n = 170) of patients had a pCR to NC. In the univariate model, there was no association between pCR and BMI as either a categoric or continuous variable. In the multivariate model, there was no significant difference in pCR to NC for obese compared with normal/underweight patients (odds ratio [OR] = 0.78; 95% CI, 0.49 to 1.26). However, overweight patients compared with normal/underweight patients were less likely to have a pCR to NC (OR = 0.59; 95% CI, 0.37 to 0.95; Table 2). When the overweight and obese groups were combined and compared with the normal/underweight group, there was a significant association with pCR (OR = 0.67; 95% CI, 0.45 to 0.99).
Table 2.
Adjusted Logistic Regression Model of Clinical Factors and Odds of Pathologic Complete Response (n = 1,107)
| Factor | OR | 95% CI | P |
|---|---|---|---|
| BMI | |||
| Normal/underweight | 1.0 | ||
| Overweight | 0.59 | 0.37 to 0.95 | .03 |
| Obese | 0.78 | 0.49 to 1.26 | .31 |
| Race | |||
| White | 1.0 | ||
| Black | 1.02 | 0.58 to 1.79 | .94 |
| Hispanic | 0.99 | 0.56 to 1.78 | .98 |
| Asian | 1.72 | 0.84 to 3.50 | .13 |
| Age (years) at start of treatment (continuous) | 0.99 | 0.56 to 1.78 | .31 |
| Time (months) from start of NC to surgery | 1.34 | 1.17 to 1.54 | < .0001 |
| Menopausal status | |||
| Postmenopausal | 1.0 | ||
| Premenopausal | 0.80 | 0.46 to 1.40 | .44 |
| Perimenopausal | 0.60 | 0.21 to 1.69 | .33 |
| NC regimen | |||
| Taxane | 1.0 | ||
| No taxane | 1.45 | 0.86 to 2.44 | .16 |
| Hormone receptor status | |||
| ER positive | 1.0 | ||
| ER negative | 3.20 | 2.02 to 5.06 | < .0001 |
| PR negative | 1.0 | ||
| PR positive | 1.54 | 1.00 to 2.38 | .04 |
| Nuclear grade | |||
| Grade 1 or 2 | 1.0 | ||
| Grade 3 | 3.72 | 2.09 to 6.62 | < .0001 |
| Lymphatic invasion | |||
| Yes | 1.0 | ||
| No | 4.05 | 2.31 to 7.11 | < .0001 |
Abbreviations: OR, odds ratio; BMI, body mass index; NC, neoadjuvant chemotherapy; ER, estrogen receptor; PR, progesterone receptor.
BMI and Progression-Free Survival
Median time to progression was not attained in the follow-up of this study population. At 5 years, the estimated progression-free survival rate was 75% (95% CI, 72% to 77%). In multivariate analysis, progression-free survival was not associated with overweight or obese status (Table 3). The clinical factor most strongly related to an increased risk of progression was failure to obtain a pCR (hazard ratio = 4.76; 95% CI, 2.40 to 9.41; P < .001). Patients with a hormone receptor–positive tumor who received adjuvant endocrine therapy had a 66% reduced risk of progression compared with hormone receptor–negative patients (ER and PR negative; P < .001). Younger age at start of NC, clinical stage III at diagnosis, ER-negative status, nuclear grade 3, higher number of positive lymph nodes, and longer duration from start of neoadjuvant therapy to response assessment were all significantly associated with a decreased progression-free survival (Table 3).
Table 3.
Adjusted Cox Proportional Hazards Regression Model of Clinical Factors and Risk of Disease Progression (n = 1,088)
| Factor | OR | 95% CI | P |
|---|---|---|---|
| BMI | |||
| Normal/underweight | 1.0 | ||
| Overweight | 0.97 | 0.70 to 1.32 | .83 |
| Obese | 0.98 | 0.70 to 1.37 | .91 |
| Race | |||
| White | 1.00 | ||
| Black | 0.92 | 0.61 to 1.38 | .68 |
| Hispanic | 0.87 | 0.56 to 1.35 | .53 |
| Asian/other | 1.48 | 0.83 to 2.63 | .18 |
| Age (years) at start of treatment (continuous) | 0.98 | 0.96 to 0.99 | < .01 |
| Time (months) from start of NC to surgery | 1.10 | 1.01 to 1.20 | .02 |
| Menopausal status | |||
| Postmenopausal | 1.00 | ||
| Premenopausal | 0.79 | 0.54 to 1.16 | .23 |
| Perimenopausal | 0.52 | 0.24 to 1.14 | .10 |
| NC regimen | |||
| Nontaxane based | 1.00 | ||
| Taxane based | 1.12 | 0.80 to 1.56 | .50 |
| Hormone receptor status | |||
| ER and PR negative, no endocrine therapy | 1.00 | ||
| ER and/or PR positive, no endocrine therapy | 0.97 | 0.63 to 1.49 | .89 |
| ER and/or PR positive, endocrine therapy | 0.44 | 0.33 to 0.60 | < .01 |
| Nuclear grade | |||
| Grade 1 or 2 | 1.00 | ||
| Grade 3 | 1.76 | 1.27 to 2.45 | < .01 |
| No. of involved lymph nodes (continuous) | 1.10 | 1.08 to 1.13 | < .01 |
| Clinical stage | |||
| Stage I or II | 1.0 | ||
| Stage III | 1.43 | 1.08 to 1.90 | .01 |
| pCR | |||
| Yes | 1.0 | ||
| No | 4.72 | 2.40 to 9.41 | < .01 |
Abbreviations: OR, odds ratio; BMI, body mass index; NC, neoadjuvant chemotherapy; ER, estrogen receptor; PR, progesterone receptor; pCR, pathologic complete response.
BMI and Breast Cancer–Specific Survival
We evaluated patients for disease-specific survival based on BMI categories. There was a total of 194 deaths. Deaths were classified as caused by breast cancer if the death occurred after a report of a recurrence (n = 167). There were 18 deaths from other causes, and the cause of death was unknown in nine patients. The unadjusted breast cancer–specific survival percentages at 10 years were 74% for normal/underweight patients, 67% for overweight patients, and 62% for obese patients (P = .048). Adjusting for prognostic factors, there was no significant association between obese or overweight status and breast cancer–specific survival.
BMI and Overall Survival
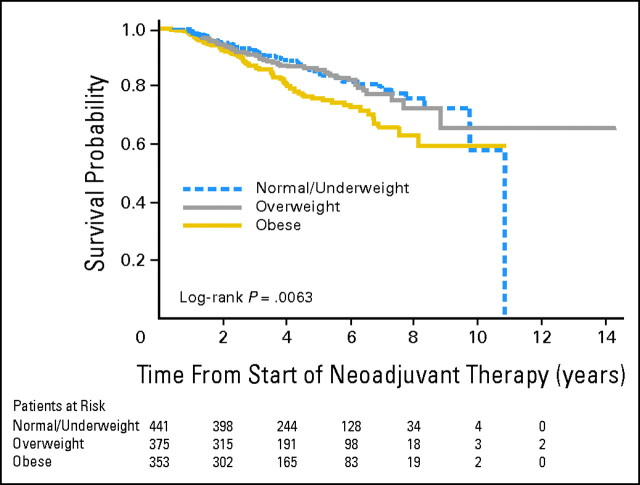
Median survival time was 10.8 years for normal/underweight patients but was not attained for overweight and obese patients. In univariate analysis, BMI was significantly associated with survival when considered as a continuous variable (P < .01) and as a categoric variable (obesity v normal weight, hazard ratio = 1.65; 95% CI, 1.18 to 2.30). Overall survival of normal weight patients did not differ significantly from that of overweight patients; however, survival of obese patients did seem to be significantly shorter than the survival of the other two BMI groups (P = .006). Figure 1 depicts overall survival from start of NC with only the normal/underweight group reaching a median survival.
Fig 1.
Kaplan-Meier curve of overall survival by body mass index category.
DISCUSSION
To our knowledge, this is the first study to evaluate the relationship between overweight and obese status and pathologic response to NC among patients with operable breast cancer. Patients with higher BMI were more likely to present with high-risk tumor characteristics and were less likely to obtain pCR to NC. Obese patients experienced a worse overall survival compared with normal or underweight patients, which is a consistent finding in the literature.
Randomized studies have shown that pCR to NC is a predictor of overall survival in breast cancer patients.19,22 In our study, higher BMI was associated with decreased pCR to NC and worse overall survival, but there was no association of overweight and obesity with breast cancer–specific or progression-free survival, as has been observed in some studies.21,23,24 It is possible that our study was underpowered to detect any small impact of BMI on breast cancer–specific or progression-free survival. In addition, differences in lymph node involvement at diagnosis, use of adjuvant endocrine therapy, and intrinsic tumor biology have been speculated to contribute to the heterogeneity in the disease-free survival of breast cancer patients who do not obtain a pCR to NC.22 Obese and overweight patients were more likely to present at diagnosis with larger tumors and more advanced clinical stage at diagnosis than normal or underweight patients. This association has been observed in some studies25 but not in others,26,27 and the conflicting reports may be a result of differences in study populations and access to early diagnosis. Although it has been reported that obese women are more likely to have hormone receptor–positive tumors,28,29 subgroups of premenopausal and postmenopausal obese women have been demonstrated to have hormone-negative tumors, as also shown in our study.30 Obese and overweight patients were also more likely to present with triple-negative breast cancers, which tend to respond better to NC.31 Despite having these tumors, obese and overweight patients were less likely to achieve a pCR to NC, highlighting the significance of BMI in this study as a predictive factor for pCR.
Several limitations of the study should be considered when interpreting the results. Although it is the standard of care at MDACC that breast cancer patients receive treatment according to their actual body weight, we were unable to verify the chemotherapy doses of the patients included in this study. Because clinicians tend to reduce doses in overweight and obese patients for fear of overdosing, this suggests that a less efficacious therapy would be more likely to be administered.7,8 Changes in chemotherapy dosing because of weight fluctuations or toxicities that occur during the course of NC treatments may also have influenced the study end points. We did not include data on clinical response to NC; however, all breast surgery after NC was performed at MDACC, and assessment of pathologic response was performed using uniform criteria.
In conclusion, this large single-institution study of breast cancer patients treated with NC demonstrates that higher BMI is associated with lower pCR to NC. This finding may be attributed to the influence of BMI on the clinical effectiveness of chemotherapy or the underdosing of overweight and obese patients by clinicians because of fears of toxicity despite randomized studies that have demonstrated that this practice contributes to worse disease-free survival.7,21 Efforts are currently underway to identify tumor gene expression profiles to better predict pCR and outcome in patients who do not experience a pCR.21 Clinicians should be aware of higher BMI status as a host risk factor influencing pCR to NC and overall survival for which attention to chemotherapy dosing based on actual body weight, investigations into chemotherapy pharmacokinetics, and management of comorbidities may yield significant benefits in improving the outcome of breast cancer patients.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer K. Litton, Ana M. Gonzalez-Angulo, Carla L. Warneke, Aman U. Buzdar, Melissa Bondy, Gabriel N. Hortobagyi, Abenaa M. Brewster
Administrative support: Jennifer K. Litton, Shu-Wan Kau
Provision of study materials or patients: Shu-Wan Kau
Collection and assembly of data: Jennifer K. Litton, Ana M. Gonzalez-Angulo, Carla L. Warneke, Aman U. Buzdar, Shu-Wan Kau, Abenaa M. Brewster
Data analysis and interpretation: Jennifer K. Litton, Carla L. Warneke, Abenaa M. Brewster
Manuscript writing: Jennifer K. Litton, Ana M. Gonzalez-Angulo, Carla L. Warneke, Aman U. Buzdar, Melissa Bondy, Somdat Mahabir, Gabriel N. Hortobagyi, Abenaa M. Brewster
Final approval of manuscript: Jennifer K. Litton, Ana M. Gonzalez-Angulo, Carla L. Warneke, Aman U. Buzdar, Melissa Bondy, Somdat Mahabir, Gabriel N. Hortobagyi, Abenaa M. Brewster
Footnotes
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Bastarrachea J, Hortobagyi GN, Smith TL, et al: Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann Intern Med 120::18,1994-25, [DOI] [PubMed] [Google Scholar]
- 2.Kroenke CH, Chen WY, Rosner B, et al: Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 23::1370,2005-1378, [DOI] [PubMed] [Google Scholar]
- 3.Galanis D, Kolonel L, Lee J, et al: Anthropomorphic predictors of breast cancer incidence and survival in a multi-ethnic cohort of female residents of Hawaii, United States. Cancer Causes Control 9::217,1998-224, [DOI] [PubMed] [Google Scholar]
- 4.Loi S, Milne RL, Friedlander ML, et al: Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 14::1686,2005-1691, [DOI] [PubMed] [Google Scholar]
- 5.Senie R, Rosen P, Rhodes P, et al: Obesity at diagnosis of breast carcinoma influences duration of disease-free survival. Ann Intern Med 116::26,1992-32, [DOI] [PubMed] [Google Scholar]
- 6.Obermair A, Kurz C, Hanzal E, et al: The influence of obesity on the disease-free survival in primary breast cancer. Anticancer Res 15::2265,1995-2269, [PubMed] [Google Scholar]
- 7.Rosner GL, Hargis JB, Hollis DR, et al: Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: Results from Cancer and Leukemia Group B study 8541. J Clin Oncol 14::3000,1996-3008, [DOI] [PubMed] [Google Scholar]
- 8.Madarnas Y, Sawka CA, Franssen E, et al: Are medical oncologists biased in their treatment of the large woman with breast cancer? Breast Cancer Res Treat 66::123,2001-133, [DOI] [PubMed] [Google Scholar]
- 9.Goodwin P: Body size and breast cancer prognosis: A critical review of the evidence. Breast Cancer Res Treat 16::205,1990-214, [DOI] [PubMed] [Google Scholar]
- 10.Modugno F, Kip K, Cochrane B, et al: Obesity, hormone therapy, estrogen metabolism and risk of postmenopausal breast cancer. Int J Cancer 118::1292,2006-1301, [DOI] [PubMed] [Google Scholar]
- 11.Goodwin PJ, Ennis M, Pritchard KI, et al: Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol 20::42,2002-51, [DOI] [PubMed] [Google Scholar]
- 12.Powis G, Reece P, Ahmann D, et al: Effect of body weight on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol 20::219,1987-222, [DOI] [PubMed] [Google Scholar]
- 13.Rodvold K, Rushing D, Tewksbury D: Doxorubicin clearance in the obese. J Clin Oncol 6::1321,1988-1327, [DOI] [PubMed] [Google Scholar]
- 14.Hennessy BT, Hortobagyi GN, Rouzier R, et al: Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 23::9304,2005-9311, [DOI] [PubMed] [Google Scholar]
- 15.Guarneri V, Broglio K, Kau S-W, et al: Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol 24::1037,2006-1044, [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health/National Heart, Lung, and Blood Institute: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View.ShowTOCrid=obesity.TOC
- 17.WHO: The World Health Organization Histological Typing of Breast Tumors–Second Edition. Am J Clin Pathol 78::806,1982-816, [DOI] [PubMed] [Google Scholar]
- 18.Black M, Speer F: Nuclear structure in cancer tissues. Surg Gynecol Obstet 105::97,1957-102, [PubMed] [Google Scholar]
- 19.Mazouni C, Peintinger F, Wan-Kau S, et al: Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol 25::2650,2007-2655, [DOI] [PubMed] [Google Scholar]
- 20.Bear HD, Anderson S, Smith RE, et al: Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 24::2019,2006-2027, [DOI] [PubMed] [Google Scholar]
- 21.Dignam J, Wieand K, Johnson K, et al: Obesity, tamoxifen use and outcomes in women with estrogen-receptor positive early-stage breast cancer. J Natl Cancer Inst 95::1467,2003-1476, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastogi T, Devesa S, Mangtani P, et al: Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project protocols. J Clin Oncol 26::778,2008-785, [DOI] [PubMed] [Google Scholar]
- 23.Dignam JJ, Wieand K, Johnson KA, et al: Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat 97::245,2006-254, [DOI] [PubMed] [Google Scholar]
- 24.Berclaz G, Li S, Price KN, et al: Body mass index as a prognostic feature in operable breast cancer: The International Breast Cancer Study Group experience. Ann Oncol 15::875,2004-884, [DOI] [PubMed] [Google Scholar]
- 25.Moorman PG, Jones BA, Millikan RC, et al: Race, anthropometric factors, and stage at diagnosis of breast cancer. Am J Epidemiol 153::284,2001-291, [DOI] [PubMed] [Google Scholar]
- 26.Wasserman L, Flatt SW, Natarajan L, et al: Correlates of obesity in postmenopausal women with breast cancer: Comparison of genetic, demographic, disease-related, life history and dietary factors. Int J Obes Relat Metab Disord 28::49,2004-56, [DOI] [PubMed] [Google Scholar]
- 27.Chagpar A, McMasters K, Saul J, et al: Body mass index influences palpability but not stage of breast cancer at diagnosis. Am Surg 73::555,2007-560, [PubMed] [Google Scholar]
- 28.Enger SM, Ross RK, Paganini-Hill A, et al: Body size, physical activity, and breast cancer hormone receptor status: Results from two case-control studies. Cancer Epidemiol Biomarkers Prev 9::681,2000-687, [PubMed] [Google Scholar]
- 29.Huang Z, Hankinson SE, Colditz GA, et al: Dual effects of weight and weight gain on breast cancer risk. JAMA 278::1407,1997-1411, [PubMed] [Google Scholar]
- 30.Maehle BO, Tretli S, Skjaerven R, et al: Premorbid body weight and its relations to primary tumour diameter in breast cancer patients; its dependence on estrogen and progesterone receptor status. Breast Cancer Res Treat 68::159,2001-169, [DOI] [PubMed] [Google Scholar]
- 31.Liedtke C, Mazouni C, Hess KR, et al: Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26::1275,2008-1281, [DOI] [PubMed] [Google Scholar]